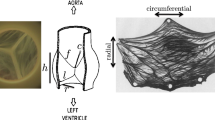
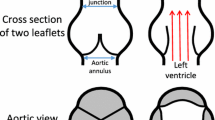
Abstract
It has long been recognized that aortic root elasticity helps to ensure efficient aortic valve closure, but our understanding of the functional importance of the elasticity and geometry of the aortic root continues to evolve as increasingly detailed in vivo imaging data become available. Herein, we describe a fluid–structure interaction model of the aortic root, including the aortic valve leaflets, the sinuses of Valsalva, the aortic annulus, and the sinotubular junction, that employs a version of Peskin’s immersed boundary (IB) method with a finite element description of the structural elasticity. As in earlier work, we use a fiber-based model of the valve leaflets, but this study extends earlier IB models of the aortic root by employing an incompressible hyperelastic model of the mechanics of the sinuses and ascending aorta using a constitutive law fit to experimental data from human aortic root tissue. In vivo pressure loading is accounted for by a backward displacement method that determines the unloaded configuration of the root model. Our model yields realistic cardiac output at physiological pressures, with low transvalvular pressure differences during forward flow, minimal regurgitation during valve closure, and realistic pressure loads when the valve is closed during diastole. Further, results from high-resolution computations indicate that although the detailed leaflet and root kinematics show some grid sensitivity, our IB model of the aortic root nonetheless produces essentially grid-converged flow rates and pressures at practical grid spacings for the high Reynolds number flows of the aortic root. These results thereby clarify minimum grid resolutions required by such models when used as stand-alone models of the aortic valve as well as when used to provide models of the outflow valves in models of left-ventricular fluid dynamics.
Similar content being viewed by others
References
Alastrué V., Garía A., Peña E., Rodríguez J., Martínez M., Doblaré M.: Numerical framework for patient-specific computational modelling of vascular tissue. Int. J. Numer. Methods Biomed. Eng. 26(1), 35–51 (2010)
Alastrué V., Peña E., Martínez M.Á., Doblaré M.: Assessing the use of the opening angle method to enforce residual stresses in patient-specific arteries. Ann. Biomed. Eng. 35(10), 1821–1837 (2007)
Azadani A.N., Chitsaz S., Matthews P.B., Jaussaud N., Leung J., Tsinman T., Ge L., Tseng E.E.: Comparison of mechanical properties of human ascending aorta and aortic sinuses. Ann. Thorac. Surg. 93(1), 87–94 (2012)
Bellhouse B.J., Bellhouse F.H.: Mechanism of closure of the aortic valve. Nature 217, 86–87 (1968)
Bols, J., Degroote, J., Trachet, B., Verhegghe, B., Segers, P., Vierendeels, J.: A computational method to assess the in vivo stresses and unloaded configuration of patient-specific blood vessels. J. Comput. Appl. Math. 246, 10–17 (2013). doi:10.1016/j.cam.2012.10.034
Bonow R.O., Carabello B.A., Chatterjee K., de Leon A.C., Faxon D.P., Freed M.D., Gaasch W.H., Lytle B.W., Nishimura R.A., O’Gara P.T., O’Rourke R.A., Otto C.M., Shah P.M., Shanewise J.S.: ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 114(5), E84–E231 (2006)
Borazjani I.: Fluid-structure interaction, immersed boundary-finite element method simulations of bio-prosthetic heart valves. Comput. Methods Appl. Mech. Eng. 257, 103–116 (2013)
Borazjani I., Ge L., Sotiropoulos F.: Curvilinear immersed boundary method for simulating fluid structure interaction with complex 3D rigid bodies. J. Comput. Phys. 227(16), 7587–7620 (2008)
Cai, M., Nonaka, A., Bell, J., Griffith, B., Donev, A.: Efficient variable-coefficient finite-volume Stokes solvers. Commun. Comput. Phys. 16(5), 1263–1297 (2014). doi:10.4208/cicp.070114.170614a
Cardamone L., Valentín A., Eberth J.F., Humphrey J.D.: Origin of axial prestretch and residual stress in arteries. Biomech. Model. Mechanobiol. 8(6), 431–446 (2009)
Carr J.A., Savage E.B.: Aortic valve repair for aortic insufficiency in adults: A contemporary review and comparison with replacement techniques. Eur. J. Cardio Thorac. Surg. 25(1), 6–15 (2005)
Cheng A., Dagum P., Miller D.C.: Aortic root dynamics and surgery: from craft to science. Philos. Trans. R. Soc. B 362(1484), 1407–1419 (2007)
Cheng R., Lai Y.G., Chandran K.B.: Three-dimensional fluid–structure interaction simulation of bileaflet mechanical heart valve flow dynamics. Ann. Biomed. Eng. 32(11), 1471–1483 (2004)
Conti C.A., Votta E., Della Corte A., Del Viscovo L., Bancone C., Cotrufo M., Redaelli A.: Dynamic finite element analysis of the aortic root from MRI-derived parameters. Med. Eng. Phys. 32(2), 212–221 (2010)
Creane A., Kelly D.J., Lally C.: Patient specific computational modeling in cardiovascular mechanics. In: Lopez, B.C., Peña, E. Patient-Specific Computational Modeling, pp. 61–79. Springer, Berlin (2012)
Croft L.R., Mofrad M.R.K.: Computational modeling of aortic heart valves. In: De, S., Guilak, F., Mofrad, M.R.K. Computational Modeling in Biomechanics, pp. 221–252. Springer, Berlin (2010)
Crosetto P., Reymond P., Deparis S., Kontaxakis D., Stergiopulos N., Quarteroni A.: Fluid–structure interaction simulation of aortic blood flow. Comput. Fluids 43(1), 46–57 (2011)
Dagum P., Green G.R., Nistal F.J., Daughteres G.T., Timek T.A., Foppiano L.E., Bolger A.F., Ingels N.B., Miller D.C.: Deformational dynamics of the aortic root: modes and physiologic determinants. Circulation 100(2), II54–II62 (1999)
Hart J., Baaijens F.P.T., Peters G.W.M., Schreurs P.J.G.: A computational fluid–structure interaction analysis of a fiber-reinforced stentless aortic valve. J. Biomech. 36(5), 699–712 (2003)
Putter S., Wolters B.J.B.M., Rutten M.C.M., Breeuwer M., Gerritsen F.A., van de Vosse F.N.: Patient-specific initial wall stress in abdominal aortic aneurysms with a backward incremental method. J. Biomech. 40(5), 1081–1090 (2007)
Delfino A., Stergiopulos N., Moore J.E., Meister J.J.: Residual strain effects on the stress field in a thick wall finite element model of the human carotid bifurcation. J. Biomech. 30(8), 777–786 (1997)
Driscol T.E., Eckstein R.W.: Systolic pressure gradients across the aortic valve and in the ascending aorta. Am. J. Physiol. 209(3), 557–563 (1965)
Dumont K., Stijnen J.M.A., Vierendeels J., Van De Vosse F.N., Verdonck P.R.: Validation of a fluid–structure interaction model of a heart valve using the dynamic mesh method in fluent. Comput. Methods Biomech. Biomed. Eng. 7(3), 139–146 (2004)
Esmaily Moghadam M., Bazilevs Y., Hsia T.Y., Vignon-Clementel I.E., Marsden A.L.: A comparison of outlet boundary treatments for prevention of backflow divergence with relevance to blood flow simulations. Comput. Mech. 48(3), 277–291 (2011)
Fai T.G., Griffith B.E., Mori Y., Peskin C.S.: Immersed boundary method for variable viscosity and variable density problems using fast linear solvers. I: numerical method and results. SIAM J. Sci. Comput. 35(5), B1132–B1161 (2013)
Freeman R.V., Otto C.M.: Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111(24), 3316–3326 (2005)
Fung Y.C.: What principle governs the stress distribution in living organs?. In: Fung, Y.C., Fukada, E., Wang, J. Biomechanics in China, Japan and USA, Science Press, Beijing (1983)
Fung Y.C.: What are the residual stresses doing in our blood vessels?. Ann. Biomed. Eng. 19(3), 237–249 (1991)
Gao H., Wang H.M., Berry C., Luo X.Y., Griffith B.E.: Quasi-static image-based immersed boundary-finite element model of left ventricle under diastolic loading. Int. J. Numer. Methods Biomed. Eng. 30(11), 1199–1222 (2014)
Gasser T.C., Ogden R.W., Holzapfel G.A.: Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 3(6), 15–35 (2006)
Gee M.W., Reeps C., Eckstein H.H., Wall W.A.: Prestressing in finite deformation abdominal aortic aneurysm simulation. J. Biomech. 42(11), 1732–1739 (2009)
Govindjee S., Mihalic P.A.: Computational methods for inverse deformations in quasi-incompressible finite elasticity. Int. J. Numer. Methods Eng. 43(5), 821–838 (1998)
Griffith B.E.: An accurate and efficient method for the incompressible Navier–Stokes equations using the projection method as a preconditioner. J. Comput. Phys. 228(20), 7565–7595 (2009)
Griffith B.E.: Immersed boundary model of aortic heart valve dynamics with physiological driving and loading conditions. Int. J. Numer. Methods Biomed. Eng. 28(3), 317–345 (2012)
Griffith B.E., Hornung R.D., McQueen D.M., Peskin C.S.: An adaptive, formally second order accurate version of the immersed boundary method. J. Comput. Phys. 223(1), 10–49 (2007)
Griffith B.E., Hornung R.D., McQueen D.M., Peskin C.S.: Parallel and adaptive simulation of cardiac fluid dynamics. In: Parashar, M., Li, X. Advanced Computational Infrastructures for Parallel and Distributed Adaptive Applications, Wiley, Hoboken (2009)
Griffith B.E., Lim S.: Simulating an elastic ring with bend and twist by an adaptive generalized immersed boundary method. Commun. Comput. Phys. 12(2), 433–461 (2012)
Griffith B.E., Luo X., McQueen D.M., Peskin C.S.: Simulating the fluid dynamics of natural and prosthetic heart valves using the immersed boundary method. Int. J. Appl. Mech. 1(1), 137–177 (2009)
Griffith, B.E., Luo, X.Y.: Hybrid finite difference/finite element version of the immersed boundary method (submitted)
Guy R.D., Phillip B., Griffith B.E.: Geometric multigrid for an implicit-time immersed boundary method. Adv. Comput. Math. 41(3), 635–662 (2015)
Humphrey J.D.: Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. Springer, Berlin (2002)
Kim Y., Peskin C.S.: Penalty immersed boundary method for an elastic boundary with mass. Phys. Fluids 19, 053103 (2007)
Kim, Y., Zhu, L., Wang, X., Peskin C.S.: On various techniques for computer simulation of boundaries with mass. In: Bathe, K.J. (ed.) Proceedings of the Second MIT Conference on Computational Fluid and Solid Mechanics, pp. 1746–1750. Elsevier, Amsterdam (2003)
Kovács S.J., McQueen D.M., Peskin C.S.: Modelling cardiac fluid dynamics and diastolic function. Philos. Trans. R. Soc. Lond. A 359(1783), 1299–1314 (2001)
Lansac E., Lim H.S., Shomura Y., Lim K.H., Rice N.T., Goetz W., Acar C., Duran C.M.G.: A four-dimensional study of the aortic root dynamics. Eur. J. Cardio Thorac. Surg. 22(4), 497–503 (2002)
Lu J., Zhou X., Raghavan M.L.: Inverse elastostatic stress analysis in pre-deformed biological structures: demonstration using abdominal aortic aneurysms. J. Biomech. 40(3), 693–696 (2007)
Luo X.Y., Griffith B.E., Ma X.S., Yin M., Wang T.J., Liang C.L., Watton P.N., Bernacca G.M.: Effect of bending rigidity in a dynamic model of a polyurethane prosthetic mitral valve. Biomech. Model. Mechanobiol. 11(6), 815–827 (2012)
Ma X.S., Gao H., Griffith B.E., Berry C., Luo X.Y.: Image-based fluid–structure interaction model of the human mitral valve. Comput. Fluids 71, 417–425 (2013)
Marom G., Haj-Ali R., Raanani E., Schäfers H.J., Rosenfeld M.: A fluid–structure interaction model of the aortic valve with coaptation and compliant aortic root. Med. Biol. Eng. Comput. 50(2), 173–182 (2012)
May-Newman K., Lam C., Yin F.C.P.: A hyperelastic constitutive law for aortic valve tissue. J. Biomech. Eng. 131(8), 081009 (2009)
McQueen D.M., Peskin C.S.: Shared-memory parallel vector implementation of the immersed boundary method for the computation of blood flow in the beating mammalian heart. J. Supercomput. 11(3), 213–236 (1997)
McQueen D.M., Peskin C.S.: A three-dimensional computer model of the human heart for studying cardiac fluid dynamics. Comput. Graph. 34(1), 56–60 (2000)
McQueen, D.M., Peskin, C.S.: Heart simulation by an immersed boundary method with formal second-order accuracy and reduced numerical viscosity. In: Aref, H., Phillips J.W. (eds.) Mechanics for a New Millennium, Proceedings of the 20th International Conference on Theoretical and Applied Mechanics (ICTAM 2000). Kluwer Academic Publishers (2001)
Mori Y., Peskin C.S.: Implicit second order immersed boundary methods with boundary mass. Comput. Methods Appl. Mech. Eng. 197(25–28), 2049–2067 (2008)
Murgo J.P., Westerhof N., Giolma J.P., Altobelli S.A.: Aortic input impedance in normal man: relationship to pressure wave forms. Circulation 62(1), 105–116 (1980)
Nichols W.W., O’Rourke M.F.: McDonald’s Blood Flow in Arteries: Theoretical, Experimental, and Clinical Principles. CRC Press, Boca Raton (2011)
Peskin C.S.: Flow patterns around heart valves: a numerical method. J. Comput. Phys. 10(2), 252–271 (1972)
Peskin C.S.: The immersed boundary method. Acta Numer. 11, 479–517 (2002)
Peskin C.S., McQueen D.M.: Mechanical equilibrium determines the fractal fiber architecture of aortic heart valve leaflets. Am. J. Physiol. Heart Circ. Physiol. 266(1), H319–H328 (1994)
Peskin C.S., McQueen D.M.: Fluid dynamics of the heart and its valves. In: Othmer, H.G., Adler, F.R., Lewis, M.A., Dallon, J.C. Case Studies in Mathematical Modeling: Ecology, Physiology, and Cell Biology, pp. 309–337. Prentice-Hall, Englewood Cliffs (1996)
Reul H., Vahlbruch A., Giersiepen M., Schmitz-Rode T.H., Hirtz V., Effert S.: The geometry of the aortic root in health, at valve disease and after valve replacement. J. Biomech. 23(2), 181–191 (1990)
Roy, S., Heltai, L., Costanzo, F.: Benchmarking the immersed finite element method for fluid–structure interaction problems. ArXiv preprint arXiv:1306.0936
Sacks M.S., Yoganathan A.P.: Heart valve function: a biomechanical perspective. Philos. Trans. R. Soc. B Biol. Sci. 362(1484), 1369–1391 (2007)
Sauren, A.A.H.J.: The mechanical behaviour of the aortic valve. Ph.D. thesis, Technische Universiteit Eindhoven (1981)
Sellier M.: An iterative method for the inverse elasto-static problem. J. Fluids Struct. 27(8), 1461–1470 (2011)
Shunk K.A., Garot J., Atalar E., Lima J.A.: Transesophageal magnetic resonance imaging of the aortic arch and descending thoracic aorta in patients with aortic atherosclerosis. J. Am. Coll. Cardiol. 37(8), 2031–2035 (2001)
Singh I.M., Shishehbor M.H., Christofferson R.D., Tuzcu E.M., Kapadia S.R.: Percutaneous treatment of aortic valve stenosis. Clevel. Clin. J. Med. 75(11), 805–812 (2008)
Sotiropoulos F., Borazjani I.: A review of state-of-the-art numerical methods for simulating flow through mechanical heart valves. Med. Biol. Eng. Comput. 47(3), 245–256 (2009)
Stergiopulos N., Westerhof B.E., Westerhof N.: Total arterial inertance as the fourth element of the windkessel model. Am. J. Physiol. Heart Circ. Physiol. 276(1), H81–H88 (1999)
Swanson W.M., Clark R.E.: Dimensions and geometric relationships of the human aortic valve as a function of pressure. Circ. Res. 35(6), 871–882 (1974)
Thubrikar M.: The Aortic Valve. CRC Press, Boca Raton (1989)
Vaishnav R.N., Vossoughi J.: Estimation of residual strain in aortic segment. In: Hall, C.W. Biomedical Engineering II: Recent Developments, Pergamon Press, Oxford (1983)
Vavourakis V., Papaharilaou Y., Ekaterinaris J.A.: Coupled fluid–structure interaction hemodynamics in a zero-pressure state corrected arterial geometry. J. Biomech. 44(13), 2453–2460 (2011)
Viscardi F., Vergara C., Antiga L., Merelli S., Veneziani A., Puppini G., Faggian G., Mazzucco A., Luciani G.B.: Comparative finite element model analysis of ascending aortic flow in bicuspid and tricuspid aortic valve. Artif. Organs 34(12), 1114–1120 (2010)
Weinberg E.J., Kaazempur Mofrad M.R.: A multiscale computational comparison of the bicuspid and tricuspid aortic valves in relation to calcific aortic stenosis. J. Biomech. 41(16), 3482–3487 (2008)
Westerhof N., Stergiopulos N., Noble M.I.M.: Snapshots of Hemodynamics: An Aid for Clinical Research and Graduate Education. Springer, Berlin (2010)
Wittek A., Karatolios K., Bihari P., Schmitz-Rixen T., Moosdorf R., Vogt S., Blase C.: In vivo determination of elastic properties of the human aorta based on 4D ultrasound data. J. Mech. Behav. Biomed. Mater. 27, 167–183 (2013)
Yao J., Liu G.R., Narmoneva D.A., Hinton R.B., Zhang Z.Q.: Immersed smoothed finite element method for fluid–structure interaction simulation of aortic valves. Comput. Mech. 50(6), 789–804 (2012)
Zhu L., Peskin C.S.: Simulation of a flapping flexible filament in a flowing soap film by the immersed boundary method. J. Comput. Phys. 179(2), 452–468 (2002)
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Mittal.
Rights and permissions
About this article
Cite this article
Flamini, V., DeAnda, A. & Griffith, B.E. Immersed boundary-finite element model of fluid–structure interaction in the aortic root. Theor. Comput. Fluid Dyn. 30, 139–164 (2016). https://doi.org/10.1007/s00162-015-0374-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00162-015-0374-5