Abstract
Purpose
Awake prone positioning has been reported to reduce endotracheal intubation in patients with coronavirus disease 2019 (COVID-19)-related acute hypoxemic respiratory failure (AHRF). However, it is still unclear whether using the awake prone positioning for longer periods can further improve outcomes.
Methods
In this randomized, open-label clinical trial conducted at 12 hospitals in China, non-intubated patients with COVID-19-related AHRF were randomly assigned to prolonged awake prone positioning (target > 12 h daily for 7 days) or standard care with a shorter period of awake prone positioning. The primary outcome was endotracheal intubation within 28 days after randomization. The key secondary outcomes included mortality and adverse events.
Results
In total, 409 patients were enrolled and randomly assigned to prolonged awake prone positioning (n = 205) or standard care (n = 204). In the first 7 days after randomization, the median duration of prone positioning was 12 h/d (interquartile range [IQR] 12–14 h/d) in the prolonged awake prone positioning group vs. 5 h/d (IQR 2–8 h/d) in the standard care group. In the intention-to-treat analysis, intubation occurred in 35 (17%) patients assigned to prolonged awake prone positioning and in 56 (27%) patients assigned to standard care (relative risk 0.62 [95% confidence interval (CI) 0.42–0.9]). The hazard ratio (HR) for intubation was 0.56 (0.37–0.86), and for mortality was 0.63 (0.42–0.96) for prolonged awake prone positioning versus standard care, within 28 days. The incidence of pre-specified adverse events was low and similar in both groups.
Conclusion
Prolonged awake prone positioning of patients with COVID-19-related AHRF reduces the intubation rate without significant harm. These results support prolonged awake prone positioning of patients with COVID-19-related AHRF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The results of this prospective, multi-center randomised controlled trial showed that prolonged awake prone positioning reduced the incidence of intubation (the primary outcome) and mortality within 28 days of enrolment in patients with acute hypoxemic respiratory failure due to coronavirus disease 2019. Adverse effects were infrequent and occurred at similar rates between prolonged awake prone positioning and standard care with a shorter period of awake prone positioning |
Introduction
Placing intubated patients with acute respiratory distress syndrome (ARDS) into the prone position has been investigated for 5 decades [1]. It has been shown to improve oxygenation by recruiting dorsal lung regions and by improving the distribution of ventilation. It has also become the standard of care for patients with moderately-severe to severe ARDS (PaO2/FiO2 < 150 mmHg) based on studies demonstrating a decreased mortality, likely by decreasing ventilator-induced lung injury [2,3,4].
Coronavirus disease 2019 (COVID-19) primarily affects the respiratory system causing mild to severe respiratory illness [5, 6]. During the COVID-19 pandemic, several studies reported the benefit of using the prone position in non-intubated patients (an approach that is often termed “awake proning” or “awake prone positioning”) with acute hypoxemic respiratory failure (AHRF) to reduce the need for invasive mechanical ventilation [7,8,9]. Awake prone position has been suggested by ARDS guidelines as the standard of care for these patients to prevent intubation [10]. However, the daily median duration of the awake prone position varied substantially among studies (from 1.7 to 9 h per day) and there were uncertainties over the benefits of longer periods of awake proning. [11]
Longer durations of daily awake prone position appear to be associated with treatment success (survival without intubation) in patients with COVID-19-related AHRF [12], in line with a prospective cohort study, in which the awake prone position for ≥ 6 h/day reduced the risk of endotracheal intubation; and awake proning for ≥ 8 h/d reduced the risk of hospital mortality [13]. Two recent meta-analyses also suggested a reduction in intubation rate among those with longer durations of daily awake prone position [9, 13]. However, there is no high-quality randomized controlled trial (RCT) evidence demonstrating that prolonged awake prone positioning can further reduce intubation when compared to standard care which utilizes a short duration of awake prone position.
Given that the efficacy of proning appears to be time dependent in moderate to severe (intubated) ARDS patients [14], and in non-intubated COVID-19 AHRF patients [15], we conducted a prospective, multicenter, randomized, controlled trial to explore whether a strategy aimed at ensuring prolonged awake prone positioning (aiming for 12 h daily for 7 days) would reduce the rate of intubation at 28 days when compared with standard care with a shorter period of awake prone positioning in non-intubated patients with COVID-19-related AHRF.
Methods
Study design
We conducted an open-label randomized clinical trial at 12 hospitals (eTable 1, electronic supplementary material [ESM] 3) in mainland China during the COVID-19 outbreak from December 2022 to early 2023 which saw the largest incidence of COVID-19 during the pandemic (eFigure 1 ESM 3). The trial was registered prior to enrollment (ClinicalTrials.gov: NCT05677984), and was overseen by an independent data and safety monitoring board (see Acknowledgements for membership). The trial protocol (ESM 1) was approved by the ethics committee at each participating institution. Study coordinators obtained a priori or deferred consent for all enrolled patients. Subjects were screened and enrolled between January 11, 2023, and April 30, 2023 (eTable 2, ESM 3).
Patients
Non-intubated patients between 18 and 85 years of age with confirmed COVID-19 pneumonia-related AHRF were eligible for enrolment. AHRF was defined as SpO2 ≤ 93% with ambient air or a ratio of partial pressure of arterial oxygen (PaO2) to FiO2 (PaO2/FiO2) ≤ 300 mmHg. We excluded patients who had any of the following: expected intolerance of awake prone positioning (e.g., pregnancy, extremity deformity, recent fracture, open thoracic or abdominal surgery), morbid obesity (body-mass index > 40 kg/m2), hemodynamic instability (receiving norepinephrine > 20 ug/min), cardiac dysfunction (New York Heart Association [NYHA] Grade III or IV)), a consciousness disorder (Glasgow Coma Scale < 13, delirium, dementia), severe hemoptysis, had been on long-term home oxygenation or continuous positive airway pressure (CPAP); or who had a “do not intubate” or a “do not resuscitate” order. Detailed inclusion and exclusion criteria are presented in eMethods (ESM 3). For low flow oxygen delivery devices (for example nasal cannula), the formula: FiO2 = 0.20 + 0.04 * oxygen flow (L/min) was used to calculate FiO2 (overview of oxygen delivery devices. OpenCriticalCare.org/encyclopedia/overview-of-oxygen-delivery-devices.).
Randomization and masking
Participants were randomized to either prolonged awake prone positioning or to standard care with a shorter period of awake prone positioning with a 1:1 allocation using block randomization by study center with randomly selected block sizes (i.e., 2 and 4). The block sizes were not disclosed, to ensure concealment. The randomization sequence was generated by the trial statistician.
Participants were randomized using sealed opaque envelopes, and were enrolled after providing informed consent. Due to the nature of the intervention, patients, physicians, and study investigators were not blinded to treatment allocation. However, data input and analysis were performed by trained personnel who had not participated in patient care and were blinded to group allocation.
Procedures
Patients in the prolonged awake prone positioning group were instructed and assisted to lie in the prone position under the supervision of a caregiver to ensure that they were lying predominantly on their chest. The target cumulative duration of prone positioning was a minimum of 12 h daily with several breaks if needed, for up to 7 days. If SpO2 was ≥ 93% on room air within 7 days after randomization, the patient decided whether they wanted to continue prolonged awake prone positioning. The duration of each proning session was recorded by the nurses and the cumulative awake prone positioning time per day was calculated by researchers. Based on our previous experience and on studies in the literature, we knew that compliance of patients with the awake prone position might be difficult. As such, we educated patients and family members about the underlying treatment principles and effects of awake proning, assisted them in finding the most comfortable prone position, and provided appropriate supervision, analgesics and sedatives to improve compliance with prone positioning (Table 1).
Patients in the standard care groups were treated according to the same standard of care and could decide themselves whether to be prone or not, but were not encouraged to remain in the prone position for a prolonged period of time (> 12 h/day).
Both groups received the same standard of care, i.e., oxygenation support with standard oxygen, high-flow nasal oxygen or mask noninvasive ventilation as per the patient’s physician. FiO2 was titrated to maintain SpO2 > 90%. To harmonize triggers for intubation, predefined criteria for tracheal intubation were provided to all centers. Intubation was recommended if: (1) Glasgow Coma Scale < 12 points; (2) cardiac arrest or malignant arrhythmia; or severe hemodynamic instability (systolic blood pressure < 90 mmHg, mean arterial pressure < 65 mmHg, or the use of vasoactive drugs); (3) worsening respiratory failure with at least 2 of the following conditions being met: (i) target oxygenation not achieved (when FiO2 = 100% and flow rate 60 L/min for 5 min, SpO2 < 90% or PaO2 ≤ 60 mmHg); (ii) respiratory rate > 40 breaths/minute; (iii) respiratory acidosis (PaCO2 > 50 mmHg and pH < 7.35); (iv) retention of airway secretions.
If any patient in either group was intubated, the subsequent management (including prone positioning) was left to the treating physician’s discretion; time in the prone position after intubation was not collected as part of this study.
Outcomes
The primary outcome was intubation within 28 days after randomization. Death without intubation was not included in the primary outcome. Key secondary outcomes (all censored at 28 days after enrolment) included mortality, days free of respiratory support, days free of invasive mechanical ventilation, and hospital-free days. Prespecified adverse events of interest were nausea, unintentional removal of intravenous access, pressure ulcers, and unexpected respiratory or cardiac arrest. Details regarding additional outcomes, including cumulative hours of awake prone positioning per day within 7 days after randomization was provided in eMethods (ESM 3).
Statistical analysis
According to previous studies from Ehrmann 2021 [7], and Alhazzani 2022 [16], we expected the intubation rate to be 35% in the standard care group. Initially, we determined that a total of 470 patients would provide 80% power to detect a 13% between-group difference with a two-sided alpha of 0.05 after allowing for 20% withdrawals or loss to follow-up. However, there were very few withdrawals and losses to follow-up, and after 307 patients, the sample size was changed to 409, based on a 10% withdrawal rate instead of the initial 20%.
The analyses were based on the intention-to-treat principle, including all participants who were randomized. Per-protocol analyses were also performed (ESM 2). Between-group differences in the primary outcome were calculated using a generalized linear model after controlling for center. Results are reported as the relative risk and risk difference with 95% confidence intervals. We also present the Kaplan–Meier curves for the time to intubation and for the time to death; and assessed the treatment difference using a Cox proportional-hazards model with the results reported as hazard ratios (HRs) with 95% confidence intervals (CIs). The Fine–Gray model was used as a sensitivity analysis for the time to intubation to account for the competing risk of death. We did prespecified subgroup analyses for the time to intubation and for the time to death by age (< 60, ≥ 60 years), respiratory support (standard oxygen, non-standard oxygen), and location at enrolment (ward, ICU/intermediate care unit [ICU/int]) with heterogeneity determined by fitting an interaction between treatment assignment and subgroup. These variables were also included in the adjusted analysis.
A mixed linear model with the subject as the random effect was used to compare repeated measurements of the duration of prone positioning. Safety was assessed in all participants excluding those who did not perform any prone positioning. Analyses were conducted using R software, version 4.0.2. The statistical analysis plan is available in ESM 2.
Results
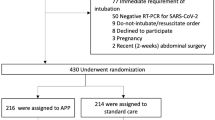
Of 815 patients screened for eligibility 409 underwent randomization (205 to the prolonged awake prone positioning group and 204 to the standard care group); 74 patients were excluded because they would likely not tolerate the prone position (e.g., pregnancy, fractures, etc.; details in ESM 1). Seven patients in each group withdraw consent (after randomization) for intubation and died subsequently. In the prolonged awake prone positioning group, 1 patient did not undergo any prone positioning due to severe kyphosis; 59 patients (29%) were in the prone position < 12 h on day 1. In the standard care group, 31 patients (15%) were in the prone positioning > 12 h on day 1. Thus, 408 subjects were included in the safety analysis (204 per group) and 305 per protocol analysis (139 in the prolonged awake prone positioning group and 166 in the standard care group) (Fig. 1). We had no loss to follow-up.
Patient characteristics at randomization were similar between groups. The mean (standard deviation, SD) age and was 67.6 (10.4) years in the prolonged awake prone positioning group and 68.9 (9.6) years in the standard care group, with similar median times from hospital admission to enrolment (Table 2). Most patients were recruited from the general medical ward (65%) and were supported with standard oxygen therapy (64%), i.e. oxygen by face mask or nasal prongs. The median FiO2 and SpO2/FiO2 at randomization were similar between groups. The most prevalent coexisting illnesses at baseline were hypertension (52%) and diabetes (34%). Most patients received corticosteroids (62%), antiviral drugs (63%) and prophylactic-dose anticoagulants (84%) at enrollment (Table 2). Other details of coexisting illness are provided in eTable 3 (ESM 3).
Prone positioning and intervention
As per the study design, the cumulative duration of prone positioning was longer in the prolonged awake prone positioning group compared to the standard care group across the treatment period (i.e., 7 days) (Fig. 2A). Cumulative duration of prone positioning in the per protocol population is provided in eFigure 2 (ESM 3). Prone position was performed in all except one patient in the prolonged awake prone positioning group from day 1 to day 7, while 85.4–94.9% of patients underwent prone position from day 1 to day 7 in the standard care group (eTable 4, ESM 3). The median duration of prone positioning on days in which it was indicated (up to 7 days after randomization) was 12 h/d (interquartile range [IQR] 12–14 h/d) in the prolonged awake prone positioning group vs 5 h/d (IQR 2–8 h/d) in the standard care group; the median cumulative duration of daily prone positioning and patient adherence for each day are in eTable 4 (ESN 3).
Duration of Awake Prone Positioning and Kaplan–Meier probabilities estimates in the intention-to-treat population. A The box plots display the median durations of prone positioning. The lines represent the median, the box edges represent the first and third quartiles, the whiskers represent the most extreme values up to 1.5 × IQR, and the dots represent the more extreme values. B Probability of endotracheal intubation*. The log-rank test demonstrated a significant between-group difference (P = 0.0092). *Patients who refused intubation after randomization were included as NOT being intubated. C Probability of death. The log-rank test demonstrated a significant between-group difference (P = 0.045)
During the study period, the use of pharmacological agents such as antivirals, corticosteroids, anticoagulants, vasoactive agents, sedation and antibiotics was similar between groups (eTable 4, ESM 3). After randomization, 150 patients (73%) in the prolonged awake prone positioning group and 146 patients (72%) in the standard care group received standard oxygen therapy; 69 patients (34%) vs 65 patients (32%) received high-flow oxygen, and 20 patients (10%) vs 30 patients (15%), respectively, received noninvasive positive pressure ventilation. SpO2/FiO2 (pre-proning) and FiO2 were comparable between groups and did not change during the 7 days of the study period. Details on the changes in oxygenation parameters are given in eFigure 3 and eFigure 4 (ESM 3).
Primary outcome
By day 28, 35 of 205 patients (17%) in the prolonged awake prone positioning group and 56 of 204 patients (27%) in the standard care group were intubated (relative risk [RR] 0.62 [95% CI 0.42–0.9]; absolute difference, − 10.75% [95% CI − 18.99 to − 2.49%] Table 3). The per-protocol analysis was consistent with the primary analysis [20/139(14%) versus 49/166(30%); RR, 0.49 [95% CI 0.3–0.76], eTable 6 (ESM 3). The adjusted analyses are given in eTable 7 (ESM 3).
Secondary outcomes
Within 28 days, 38 of 205 patients (19%) died in the prolonged awake prone positioning group and 55 of 204 patients (27%) died in the standard care group (RR 0.69 [95% CI 0.47–0.98]; absolute difference, − 10.38% [95% CI − 18.39 to − 2.36%]; Table 3). Patients in the prolonged awake prone positioning group had a mean of 20.5 days (SD 10.9 days) free from respiratory support compared with a mean of 18.4 days (SD 12.3 days) in the standard care group (mean difference, 2.05 days [95% CI − 0.14 to 4.23 days]). The difference between groups was statistically significant for invasive ventilation-free days (mean difference, 2.33 days [95% CI 0.06–4.61 days]), and hospital-free days (mean difference, 2.36 days [95% CI 0.76–3.93 days, Table 3), at 28 days.
The results of time-to-event analyses (using Cox regression) intubation or mortality are shown in Fig. 2B and C. We found a significantly lower risk between groups for intubations (hazard ratio [HR] 0.56(0.37–0.86) and mortality (HR = 0.63(0.42–0.96)). A similar finding was found for the risk of intubations after accounting for the competing risk from death (HR 0.58 (0.38–0.88)).
Subgroup analyses
A prespecified subgroup analysis by age yielded an HR of 0.24 (95% CI 0.06–0.94) for endotracheal intubation in patients < 60 years old and an HR of 0.67 (95% CI 0.43–1.07) in patients ≥ 60 years. The subgroup analysis by respiratory support modality yielded an HR of 0.49 (95% CI 0.29–0.82) for endotracheal intubation in patients who received high flow nasal cannula (HFNC) and non-invasive ventilation (NIV), and an HR of 0.53 (95% CI 0.24–1.20) in patients who received standard oxygen (eFigure 5, ESM 3).
Safety outcomes
Eight adverse events of interest were reported in 8 patients in the prolonged awake prone positioning group and 11 events in 10 patients were reported in the standard care group. The most reported adverse event was pressure ulcer (6 of 205 patients [3%] in the prolonged awake prone positioning group and 8 of 204 patients [4%] in the standard care group) eTable 8 (ESM 3).
Post-hoc analyses
We found that 31 of 35 patients (88.6%) who were intubated in the prolonged awake prone positioning group and 48 of 56 patients (85.7%) who were intubated in the standard car group died within days 28 after randomization (HR 0.924 [95% CI 0.587–1.456]). A post hoc analysis showed that 42 of 205 patients (20%) in the prolonged awake prone positioning group and 63 of 204 patients (31%) in the standard care group were intubated or died within 28 days after randomization (HR, 0.6 [95% CI 0.4–0.89]) (eFigure 6, ESM 3). A significant reduction in the incidence of intubation was also found when we allocated those patients who withdraw their consent for intubation (after randomization) into the intubated group (RR, 0.66 (95% CI 0.47, 0.93) (eTable 6 in ESM 3).
Discussion
In this multicenter, randomised, open-label trial, prolonged awake prone positioning decreased the incidence of intubation and mortality within 30 days after randomization in non-intubated patients with COVID-19 related AHRF when compared to standard care with a shorter period of awake prone positioning. Adverse effects of interests were infrequent and occurred at similar rates between the prolonged awake prone positioning and standard care groups.
The present study demonstrates that exposure time to awake prone positioning impacts clinical outcomes. Recent meta-analyses and reviews have shown the effectiveness of awake prone positioning in reducing intubation rate when compared with the supine position. In addition, various sub-group analyses have demonstrated that the duration of daily prone positioning was likely a crucial element determining the effectiveness of awake prone positioning [9, 13, 14, 17]. Our results obtained with a randomized clinical trial are in accord with a multicenter cohort study by Mariano and colleagues that showed that progressively increasing the length of the awake prone position positively impacted intubation rate and mortality, with a OR reduction for hospital mortality when the exposure was ≥ 8 h/d. [11]
Importantly, our study suggests that there may be a survival benefit of prolonged proning for patients who are less sick (e.g., patients on a general medical ward, or receiving standard oxygen therapy). This is in contrast to previous studies [7, 8] and may be due to the prolonged proning. Of note, this finding is based on a secondary outcome, and ideally should be replicated.
Prospective physiologic studies demonstrated that prone position improved pulmonary ventilation-perfusion matching in non-intubated COVID-19 patients [18, 19]. As well, SpO2/FiO2 has been shown to be significantly improved after proning and this improvement persisted after patients returned to the supine position [7]. We did not find a difference in SpO2/FiO2 (measured immediately before proning) between groups during the study period, consistent with a previous study [16]. We are not certain why there was no difference between groups, but it may relate to the use of prone positioning in many control patients. From the pathophysiological perspective, a prospective cohort study found that the beneficial physiological effects (increase of static and dynamic compliance) of prone positioning continued for 16 h and at least up to 24 h after return to the supine position in some intubated ARDS patients [20]. Similar pathophysiological mechanisms were likely operative in both groups after proning in our study [20,21,22].
We found that the awake prone position was generally well tolerated. Of note, the median cumulative duration of awake proning was about 12 h daily from day 1 to day 7 in the prolonged awake prone positioning group. This is greater than most other studies, and may help explain our positive results. We think that we were successful in this regard for a number of reasons. First, similar to a number of other studies, we used several short sessions of awake prone positioning, based on data demonstrating that most patients can tolerate awake prone positioning for 3 h at a time [23]. Second, we used a clinician-driven awake prone positioning strategy in which our clinicians were trained to improve compliance by helping patients adopt the most comfortable prone position, providing appropriate supervision, educating patients to establish their confidence in the intervention, and delivering analgesics and sedatives when necessary. The most common cited reason for interruption of awake prone positioning in previous studies was patient request, which might relate to discomfort from prone positioning or lack of understanding as to its potential benefits [16]. Finally, compared to previous studies [7, 11, 16, 24], we enrolled less severe patients (64% patients with standard oxygen), who might have better tolerance to prone positioning. Of note, our patients were almost 10 years older than in other trials [16]. However, this is unlikely to explain our results as there appeared a very weak correlation between proning time and age, with an R2 ranging from 0.013 to 0.023 during the study period. (eFigure 7 ESM 3).
The mortality in our control group (27%) was slightly higher than other studies (24%) [7, 16], although the baseline SpO2/FiO2 was somewhat higher than other studies. This is likely related to the fact our study population was almost 8–10 years older than other studies [7, 16]. In addition, the mortality of our intubated patients was quite high (82.2%) compared with that in Alhazzani’s (61.7%) and Ehrmann’s (51.9%) studies [7, 16]. However, our results are in keeping with a meta-analysis which demonstrated that the mortality of COVID-19 patients receiving invasive mechanical ventilation varied greatly, with a mortality rate of 70.2% (95% CI 60.8–79.7%) [25]. Of note, body mass index (BMI) has been demonstrated to be a protective factor for mortality in COVID-19 patients receiving invasive mechanical ventilation [26, 27]. Our patients had a lower BMI (23.8) than patients in Alhazzani’s (29.7) and Ehrmann’s (29.5) studies. As well, “too strict” intubation criteria that delayed intubation could have increased mortality of intubated patients. However, our guidance for initiation of intubation, and the median duration from randomization to intubation were similar to other studies [7, 16].
In this trial, only 19 adverse events of interest were reported and were comparable between groups; the majority (74%) were pressure ulcers. No patient had a cardiac arrest during awake prone positioning or in relation to proning. This low incidence was similar to the low incidence of pre-specified adverse events reported in other trials [7, 16].
The present study has several limitations. First, the variants circulating during the study period (Omicron BF.7 and BA.5.2) were less virulent compared to the beginning of the pandemic. However, considering the population density and a large elderly demographic, a significant number of patients experienced acute hypoxemic respiratory failure due to COVID-19 during the study period. Second, because of the nature of awake prone positioning, blinding patients and caregivers was not possible. Importantly both groups appeared to receive similar care in terms of pharmacological agents and modes of respiratory support. Third, only two-thirds of patients received corticosteroids, which might be a contributing factor to overall mortality. However, it was unlikely to have affected our main conclusion, since the number of patients who received corticosteroids was comparable between groups. Fourth, we did not specify the indications and standards for the use of NIV in the study, which might have led to inconsistent NIV application in different patients. However, NIV is a widely used respiratory support strategy in China and each center has their local protocols. As such, we did not think it was critical to specify the indications and standards for the use of NIV in the protocol. Fifth, although we set specific criteria for intubation, the decision to intubate any given patient has a large subjective component and the reasons for intubation were not recorded. Importantly, mortality at 28 days—clearly an objective endpoint—was lower in the active treatment group. Sixth, our primary endpoint was intubation, which was confounded because a number of patients changed their minds and refused intubation, when this was recommended by their physicians. Thus, the a priori primary endpoint was subject to potential bias. However, the number of refusals was identical in both groups and the post hoc analysis using intubation or death yielded similar results, as did 28-day mortality.
Conclusion
In non-intubated COVID-19 patients with acute hypoxemic respiratory failure, who had no contraindications to proning, prolonged awake prone positioning had a favourable effect on intubation and mortality within 28 days of enrolment when compared with shorter awake prone positioning.
Data availability
All authors had access to all the raw data sets and that at least two authors have verified the data. Two verifying authors were independent of the company or funder. Individual participant data that underlie the results reported in this article will be shared after de-identification (text, tables, figures, and appendices) to researchers conditional upon receipt of an approved study proposal along with evidence of approval of the proposal by an accredited ethics committee. Proposals should be directed to lingliudoctor@126.com. To gain access, data requestors will need to sign a data access agreement.
References
Bryan AC (1974) Conference on the scientific basis of respiratory therapy. Pulmonary physiotherapy in the pediatric age group. Comments of a devil’s advocate. Am Rev Respir Dis 110:143–144. https://doi.org/10.1164/arrd.1974.110.6P2.143
Guérin C, Reignier J, Richard JC et al (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368(23):2159–2168. https://doi.org/10.1056/NEJMoa1214103
Guérin C, Albert RK, Beitler J et al (2020) Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med 46(12):2385–2396. https://doi.org/10.1007/s00134-020-06306-w
Gorman EA, O’Kane CM, McAuley DF (2022) Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet 400(10358):1157–1170. https://doi.org/10.1016/S0140-6736(22)01439-8
Berlin DA, Gulick RM, Martinez FJ (2020) Severe Covid-19. N Engl J Med 383(25):2451–2460. https://doi.org/10.1056/NEJMcp2009575
Nichol AD, O’Kane C, McAuley DF (2022) Respiratory support in the time of COVID-19. JAMA 328(12):1203–1205. https://doi.org/10.1001/jama.2022.15229
Ehrmann S, Li J, Ibarra-Estrada M et al (2021) Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med 9(12):1387–1395. https://doi.org/10.1016/S2213-2600(21)00356-8
Li J, Luo J, Pavlov I et al (2022) Awake prone positioning for non-intubated patients with COVID-19-related acute hypoxaemic respiratory failure: a systematic review and meta-analysis. Lancet Respir Med 10(6):573–583. https://doi.org/10.1016/S2213-2600(22)00043-1
Qin S, Chang W, Peng F et al (2023) Awake prone position in COVID-19-related acute respiratory failure: a meta-analysis of randomized controlled trials. BMC Pulm Med 23(1):145. https://doi.org/10.1186/s12890-023-02442-3
Grasselli G, Calfee CS, Camporota L et al (2023) ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med 49(7):727–759. https://doi.org/10.1007/s00134-023-07050-7
Mariano E, Marina B, Nora A et al (2022) Impact of exposure time in awake prone positioning on clinical outcomes of patients with COVID-19-related acute respiratory failure treated with high-flow nasal oxygen: a multicenter cohort study. Crit Care 26(1):16. https://doi.org/10.1186/s13054-021-03881-2
Ibarra-Estrada M, Li J, Pavlov I et al (2022) Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial. Crit Care 26(1):84. https://doi.org/10.1186/s13054-022-03950-0
Weatherald J, Parhar KKS, Al Duhailib Z et al (2022) Efficacy of awake prone positioning in patients with covid-19 related hypoxemic respiratory failure: systematic review and meta analysis of randomized trials. BMJ 379:e071966. https://doi.org/10.1136/bmj-2022-071966
Hu SL, He HL, Pan C et al (2014) The effect of prone positioning on mortality in patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Crit Care 18(3):R109. https://doi.org/10.1186/cc13896
Piciucchi S, Ravaglia C, Vizzuso A et al (2022) Awake prone positioning for COVID-19 acute respiratory failure: imaging and histological background. Lancet Respir Med 10(2):e14. https://doi.org/10.1016/S2213-2600(21)00554-3
Alhazzani W, Parhar KKS, Weatherald J et al (2022) Effect of awake prone positioning on endotracheal intubation in patients with COVID-19 and acute respiratory failure: a randomized clinical trial. JAMA 327(21):2104–2113. https://doi.org/10.1001/jama.2022.7993
Rampon GL, Simpson SQ, Agrawal R (2023) Prone positioning for acute hypoxemic respiratory failure and ARDS: a review. Chest 163(2):332–340. https://doi.org/10.1016/j.chest.2022.09.020
Anesi GL (2023) Awake-Prone Positioning in COVID-19: new data on efficacy, timing, and mechanism. Crit Care Med 51(9):1270–1272. https://doi.org/10.1097/CCM.0000000000005935
Liu L, Xie J, Wang C et al (2022) Prone position improves lung ventilation-perfusion matching in non-intubated COVID-19 patients: a prospective physiologic study. Crit Care 26(1):193. https://doi.org/10.1186/s13054-022-04069-y
Jochmans S, Mazerand S, Chelly J et al (2020) Duration of prone position sessions: a prospective cohort study. Ann Intensive Care 10(1):66. https://doi.org/10.1186/s13613-020-00683-7
Thompson BT (2013) Prone positioning for 16 h/d reduced mortality more than supine positioning in early severe ARDS. Ann Intern Med 159(6):JC2. https://doi.org/10.7326/0003-4819-159-6-201309170-02002
Gattinoni L, Busana M, Giosa L et al (2019) Prone positioning in acute respiratory distress syndrome. Semin Respir Crit Care Med 40(1):94–100. https://doi.org/10.1055/s-0039-1685180
Coppo A, Bellani G, Winterton D et al (2020) Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med 8:765–774. https://doi.org/10.1016/S2213-2600(20)30268-X
Fralick M, Colacci M, Munshi L et al (2022) Prone positioning of patients with moderate hypoxaemia due to covid-19: multicentre pragmatic randomised trial (COVID-PRONE). BMJ 376:e068585. https://doi.org/10.1136/bmj-2021-068585
Elsayed HH, Hassaballa AS, Ahmed TA et al (2021) Variation in outcome of invasive mechanical ventilation between different countries for patients with severe COVID-19: a systematic review and meta-analysis. PLoS One 16(6):e0252760. https://doi.org/10.1371/journal.pone.0252760
Osawa EA, Maciel AT (2022) Characteristics and risk factors for mortality in critically Ill patients with COVID-19 receiving invasive mechanical ventilation: the experience of a private network in Sao Paulo, Brazil. J Crit Care Med (Targu Mures) 8(3):165–175. https://doi.org/10.2478/jccm-2022-0015
Khokher W, Iftikhar S, Abrahamian A et al (2023) Association between Body Mass Index and hospital outcomes for COVID-19 patients: a nationwide study. J Clin Med 12(4):1617. https://doi.org/10.3390/jcm12041617
Acknowledgements
The authors thank Shu Lu, Chenliang Sun, Ruiqiang Zheng, Jun Wang, Qindong Shi, Hongliang Wang, Huaguang Ye, Bingwei Chen and all the members of Chi-ARDS Net for their generous help for this research.
Principal Investigators. Ling Liu, Haibo Qiu: Jiangsu Provincial Key Laboratory of Critical Care Medicine, Department of Critical Care Medicine, School of Medicine, Zhongda Hospital, Southeast University, Nanjing, Jiangsu, 210009, China.
Co-Investigators. Hongsheng Zhao, Shu Lu, Chenliang Sun: Intensive Care Unit, Affiliated Hospital of Nantong University, Nantong, China; Weili Liu: Department of Intensive Care Unit, Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, Jiangsu 225001, China; Yali Chao: Department of Critical Care Medicine, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, 320300, Jiangsu, China; Ying Zhu: Department of Critical Care Medicine, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou 310006, China; Ruiqiang Zheng, Jiangquan Yu: Department of Critical Care Medicine, Northern Jiangsu People's Hospital, Yangzhou, Jiangsu, China; Jun Wang, Jun Jin: Department of Critical Care Medicine, The First Affiliated Hospital of Soochow University, Suzhou, 215000, Jiangsu, China; Xuehua Pu: Department of Intensive Care Unit, Taizhou People's Hospital, Taizhou, Jiangsu, China; Yu Liu, Qindong Shi: Department of Critical Care Medicine, The First Affiliated Hospital of Xi'an Jiaotong University, Xi’an, 710061, Shaanxi, China; Hongliang Wang, Sicong, Wang: Department of Intensive Care Unit, Second Affiliated Hospital of Harbin Medical University, Harbin, China; Bin Wu, Huaguang Ye: Department of Intensive Care Unit, Third Hospital of Xiamen, Xiamen, Fujian, China; Jibin Han: Department of Critical Care Medicine, The First Hospital of Shanxi Medical University, Taiyuan, Shanxi, China; Tao Chen: Global Health Trials Unit, Liverpool School of Tropical Medicine, Center for Health Economics, University of York.
Coordinating Site Staff
Database Lead. Qin Sun, Wei Chang: Jiangsu Provincial Key Laboratory of Critical Care Medicine, Department of Critical Care Medicine, School of Medicine, Zhongda Hospital, Southeast University, Nanjing, Jiangsu, 210009, China. Trial statistician. Bingwei Chen: Department of Epidemiology and Biostatistics, School of Public health, Southeast University, Nanjing, 210009, China; Tao Chen: Global Health Trials Unit, Liverpool School of Tropical Medicine, Center for Health Economics, University of York.
Funding
This work was supported, in part, by National Key Research and Development Program of China (2022YFC2504405), the National Natural Science Foundation of China (82341032, 81930058, 82270083), the Second Level Talents of the “333 High Level Talents Training Project” in the sixth phase in Jiangsu (LGY2022025), and Jiangsu Provincial Medical Key Laboratory (ZDXYS202205).
Author information
Authors and Affiliations
Consortia
Contributions
LL, HQ, YY, JX and QS contributed to the conception and design. HZ, WL, XP, JY, JH, JJ, YC, SW, YL, BW, YL and YZ contributed to data acquisition. TC, YL, QS, LL, WC and HQ contributed to data analysis and interpretation. LL, QS, YL, TC, HQ, YY and AS contributed to drafting the manuscript for important intellectual content. All authors revised the report and approved the final version before submission.
Corresponding author
Ethics declarations
Conflicts of interest
All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work. All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All Investigators, Collaborators, and Contributors are listed in the Acknowledgement section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, L., Sun, Q., Zhao, H. et al. Prolonged vs shorter awake prone positioning for COVID-19 patients with acute respiratory failure: a multicenter, randomised controlled trial. Intensive Care Med (2024). https://doi.org/10.1007/s00134-024-07545-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00134-024-07545-x