Abstract
Background
In patients with COVID-19-related acute respiratory failure (ARF), awake prone positioning (AW-PP) reduces the need for intubation in patients treated with high-flow nasal oxygen (HFNO). However, the effects of different exposure times on clinical outcomes remain unclear. We evaluated the effect of AW-PP on the risk of endotracheal intubation and in-hospital mortality in patients with COVID-19-related ARF treated with HFNO and analyzed the effects of different exposure times to AW-PP.
Methods
This multicenter prospective cohort study in six ICUs of 6 centers in Argentine consecutively included patients > 18 years of age with confirmed COVID-19-related ARF requiring HFNO from June 2020 to January 2021. In the primary analysis, the main exposure was awake prone positioning for at least 6 h/day, compared to non-prone positioning (NON-PP). In the sensitivity analysis, exposure was based on the number of hours receiving AW-PP. Inverse probability weighting–propensity score (IPW-PS) was used to adjust the conditional probability of treatment assignment. The primary outcome was endotracheal intubation (ETI); and the secondary outcome was hospital mortality.
Results
During the study period, 580 patients were screened and 335 were included; 187 (56%) tolerated AW-PP for [median (p25–75)] 12 (9–16) h/day and 148 (44%) served as controls. The IPW–propensity analysis showed standardized differences < 0.1 in all the variables assessed. After adjusting for other confounders, the OR (95% CI) for ETI in the AW-PP group was 0.36 (0.2–0.7), with a progressive reduction in OR as the exposure to AW-PP increased. The adjusted OR (95% CI) for hospital mortality in the AW-PP group ≥ 6 h/day was 0.47 (0.19–1.31). The exposure to prone positioning ≥ 8 h/d resulted in a further reduction in OR [0.37 (0.17–0.8)].
Conclusion
In the study population, AW-PP for ≥ 6 h/day reduced the risk of endotracheal intubation, and exposure ≥ 8 h/d reduced the risk of hospital mortality.
Similar content being viewed by others
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is responsible for causing coronavirus disease 2019 (COVID-19) [1]. While most patients are asymptomatic or mildly symptomatic, a subset develops acute respiratory failure and acute respiratory distress syndrome (ARDS) [2]. The use of invasive mechanical ventilation for the treatment of these conditions is associated with high mortality rates, with reports exceeding 50% in both developed and developing countries [3, 4]. Given this high mortality risk reported, there is a high value in identifying strategies that can mitigate the progression of lung injury and prevent the need for invasive ventilation [5, 6].
In patients with acute respiratory failure (ARF), the use of high-flow nasal oxygen therapy (HFNO) has been found to decrease the need for endotracheal intubation and mortality when compared to conventional oxygen therapy and noninvasive ventilation [7, 8]. Thus, it has been increasingly used to support respiratory failure in patients with COVID-19 during the pandemic [6]. Prone positioning has consistently been shown to reduce mortality in patients with moderate to severe ARDS receiving invasive mechanical ventilation [9, 10]. However, the effect of prone positioning on clinical outcomes in patients not receiving invasive mechanical ventilation remains unclear. This intervention has been applied to spontaneously breathing patients with COVID-19-related ARF [11]. The combination of HFNO and awake prone position (AW-PP) has been described to be associated with a low rate of endotracheal intubation (ETI) in case series of patients with COVID-19 [11,12,13]. These findings were confirmed in a recent meta-trial that compared HFNO plus AW-PP versus HFNO plus usual care [14]. However, the effects of exposure times in AW-PP on relevant clinical outcomes remain unclear.
We evaluated the effect of prone positioning on the risk of endotracheal intubation and in-hospital mortality in a cohort of patients admitted to ICU with COVID-19-related acute respiratory failure initially treated with high-flow nasal oxygen and analyzed the effects of different exposure times to AW-PP.
Methods
Design and scope of the study
We conducted a prospective multicenter cohort study at 6 ICUs of 6 centers in Argentina. Four of these were University Affiliated Centers described in the Additional File. The study was conducted between June 2020 and January 2021, corresponding to the first wave in Argentina, and is reported following guidelines from Strengthening the Reporting of Observational studies in Epidemiology (STROBE) [15]. The Internal Review Board from the 6 centers approved the study including waived informed consent (Universidad Austral Comité Institucional de Evaluación N°20-072; Comité de Ética en Investigación SATI for Trinidad Palermo; Comité de Docencia e Investigación Sanatorio Clínica Modelo de Morón; Comité de Ética e Investigación Hospital Alemán; Consejo Institucional de Revisión de Estudios de Investigación Hospital Privado de Comunidad Nº 2919/2143/2020; Comité de Ética en Investigación Clínica Olivos). The interventions carried out were part of the usual practice at each center, and researchers guaranteed confidentiality of their patient’s information.
Study population
We included patients older than 17 years admitted to the ICU with a confirmed diagnosis of COVID-19 (real-time PCR) and receiving HFNO for at least 4 h. Patients received HFNO when any of the following criteria were present: (a) peripheral oxygen saturation (SpO2) < 92% with oxygen > 4 L/min; (b) increased work of breathing with use of accessory respiratory muscles, and a respiratory rate > 30/min; (c) PaO2/FiO2ratio < 200 mmHg. Patients with respiratory failure secondary to a different etiology, decreased level of consciousness, presence of shock requiring vasopressors, immediate need for intubation, use of positive-pressure ventilation prior to HFNO, and with do-not-intubate orders were excluded.
Study procedures
Immediately after admission to the ICU, the inclusion criteria regarding oxygenation and/or work of breathing were checked. Then, a high-flow nasal cannula sized according to the nares size (Optiflow, Fisher and Paykel Healthcare) was placed and connected to a specific device for the provision of high-flow O2 (Humidoflo HF-2900, GGM Co., Taiwan; or AIRVO, Fisher and Paykel Healthcare, Auckland, New Zealand) or to an ICU respirator in high flow mode (Neumovent GraphNet Advance, Tecme S.A., Córdoba, Argentina; or Monnal T75, Air Liquide Medical System, France). The initial flow was 50–70 L/min, with the FiO2 necessary to obtain an SpO2 > 92%. No maximum FiO2 limits were established. Once therapy with HFNO was started, participants were encouraged and assisted by the healthcare team to rotate from supine to prone position for as long as possible, taking breaks for personal hygiene and eating. No maximum time limits for prone position were established. Patients who tolerated HFNO for the next 4 h were included in the study. Where prone positioning was not tolerated by patients, they were assisted to remain in the lateral position, alternating right and left decubitus. These interventions were maintained during the study period until one of the following criteria was met: maintenance of SpO2 > 92% with FiO2 ≤ 40%, and flow ≤ 40 L/min for a period > 12 h in the supine position; or endotracheal intubation. Once clinical stability and gas stability were attained with HFNO, flow and oxygen were progressively weaned. The de-escalation was initiated with a gradual FiO2 decrease until ≤ 40% was reached. Subsequently, the flow level was progressively reduced until reaching ≤ 40 L/min. Once these requirements were met, a low-flow nasal cannula was placed with an O2 flow of 3–5 L/min in the supine position. Analgesic drugs (opioids, paracetamol) or light sedation (dexmedetomidine) was allowed and indicated according to the criteria of the healthcare team. (For more details about the procedures, see Additional file 1.)
Variables and measurements
We collected data on patients’ demographics (age, sex, body mass index [BMI]), comorbidities, severity scores upon ICU admission (APACHE II and SOFA), chronology of the disease (time from the onset of symptoms to hospital admission and ICU admission), vital signs, laboratory parameters, Respiratory rate Oxygenation index (ROX index) [16], and chest computed tomography score (CT score) determined as the sum of lung involvement, ranging from 0—no involvement to 25—maximum involvement [17].
The main exposure of this study was awake prone positioning. For analysis purposes, we defined awake prone positioning as remaining in this position for at least 6 h per day, based on recent evidence suggesting that this is the minimum time that could impact ETI rate [11]. We also collected information on the number of hours in prone, and the number of days with at least 6 h of prone position per patient. The following data were recorded: (a) the predominant position adopted by the patient, defined as the position in which the patient spent most h/day, i.e., prone, lateral or supine position; (b) the average number of h/day in that position; and (c) the number of days of exposure to said position for a period of ≥ 6 h/day (AW-PP). Patients who did not receive prone positioning for at least 6 h served as controls. Since AW-PP exposure was initially attempted in the total population of patients included, some patients in the control group could have had some degree of prone exposure (< 6 h/d).
The primary outcome was defined as the receipt of endotracheal intubation. The decision to intubate was based on the criteria of the attending healthcare team. However, intubation was recommended for anyone meeting the following criteria: deterioration of neurologic status, hemodynamic instability, or if two or more of the following criteria were met: decline in oxygen saturation with SpO2 < 90% for more than 5 min (not explained by technical failures), lack of improvement in the signs of respiratory muscle fatigue, impossibility to control airway secretions and respiratory acidosis with pH < 7.30 [7]. The secondary outcome was hospital mortality. Additionally, we described other clinical outcomes: ICU and hospital length of stay, time to endotracheal intubation, and severe complications related to AW-PP.
Statistical analysis
We used descriptive statistics to describe patients’ baseline characteristics. Standardized mean differences were used to assess the balance between baseline characteristics of patients who received prone positioning and controls. A standardized mean difference of 10% or less was considered to indicate good balance [18].
Since treatment allocation was not randomly assigned, inverse probability of treatment weighting (IPW) was used to control for potential confounding by indication (Additional file 1: Table E8). The IPW is an extension of the propensity score method used to summarize the conditional probability of treatment assignment [19, 20]. Based on subject matter knowledge and previous literature, we used a direct acyclic graph (DAG) to identify and select variables potentially associated with both awake prone positioning and study outcomes [21, 22] (Additional File 1: Fig. E3). First, we created a propensity score by fitting a multivariable logistic regression model with awake prone positioning as the binary outcome. The following potential confounders were included in the model: age, sex, BMI, comorbidities, smoking status, SOFA and APACHE II scores, days from symptoms onset to hospital admission, oxygen therapy previous to ICU admission (days and administration mode), antibiotic therapy, corticosteroid therapy, ROX index, TC score, C-reactive protein (CRP), and use of light sedation. Once the propensity score was created, each patient was assigned a weight which was inversely proportional to the probability of being treated as estimated by the propensity score. These weights were stabilized and trimmed by removing observations with extreme weights (i.e., percentile < 1% and > 99%) [23]. Finally, the primary analysis was performed by fitting a logistic regression model with awake prone position as the main exposure and endotracheal intubation as the dependent variable. This model was fitted using the weighted population by the previous procedure. Moreover, we included in the model the participant center and the chronological time since the onset of the pandemic as independent variables (double robust approach). Measures of association are expressed as odds ratios (OR) with 95% confidence intervals (CI), which were created using bootstrapping to account for the correlation of the weighted population [23].
The dynamics of the pandemic and the number of patients admitted to ICUs could not be predicted. Considering the lack of data on the effectiveness of the intervention, as many patients as possible were consecutively recruited without a predefined sample size.
Several post hoc sensitivity analyses were conducted in order to assess the robustness of our findings. First, we recategorized the exposure based on the number of hours receiving awake prone positioning (≥ 8, ≥ 12 and ≥ 16 h/d). Second, we performed restricted analyses according to hypoxemia severity (PaO2/FiO2 < 150 mmHg and < 100 mmHg) and the predominant body position (prone, lateral or supine). Finally, we performed a restricted analysis comparing AW-PP > 6 h versus patients with “zero” hours in prone position.
Given the likelihood of an unmeasured confounder, we estimated the e value as a way to determine how strong the association between such unmeasured confounder with both the exposure and outcome should be to fully explain the estimated effect [24].
Every test was two-sided, and a p value < 0.05 was considered statistically significant. All analyses were performed with STATA version 15.1. For more details about statistical analysis, see Additional file 1.
Results
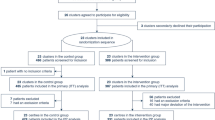
During the study period, 580 patients with COVID-19-related ARF were admitted to the participating ICUs (Fig. 1). Three hundred thirty-five patients met the inclusion criteria and were included in the analysis, of which 187 (56%) were treated with HFNO and ≥ 6 h/day of AW-PP (intervention group) and 148 (44%) were treated with HFNO but did not complete 6 h of awake prone position, and therefore served as the control group (NON-PP). Patients in the AW-PP group spent a median (p25–75) of 12 (9–16) h/day in the prone position, during 5 (3–8) days. In the control group, 84 patients (25%) remained predominantly in the lateral position for a median (p25–75) of 8 (6–10) hours and 3.5 (2–6) days; and 64 patients (19%) remained predominantly in the supine position (Fig. 1). The baseline characteristics of the study population and the balance between groups before and after IPW are described in Table 1. (More detailed data are shown in Additional file 1: Table E1.) Even though there were differences between groups (e.g., age, sex APACHE II score, comorbidities, previous days with oxygen therapy, and C-reactive protein), after weighting by IPW, the values of all variables were balanced, showing a standardized difference of less than 0.1 (Table 1 and Additional file 1: Fig. E1-E2). The variables related to oxygen therapy and exposure to prone positioning in the study groups are listed in Table 2 and Table E2 (Additional file 1). No patient received noninvasive ventilation with positive-pressure as respiratory support.
Forty-four patients in the AW-PP group (23%) and 79 (53%) in NON-PP group were intubated (Additional file 1: Table E3). In the weighted population, the OR for endotracheal intubation was 0.27 (95% CI 0.14–0.47) and the adjusted OR by center and pandemic time in the weighted population was 0.36 (0.2–0.7) (Fig. 2 and Additional file 1: Table E4). The e value for the primary analysis for the effects of AW-PP on ETI was 2.72, showing robustness to potential unmeasured confounding (Additional file 1: Fig. E4). The reasons for ETI in the 123 patients who required it were progression of respiratory failure [n = 120 (98%)] and hemodynamic failure [n = 3 (2%)]. In the sensitivity analysis for ETI, the adjusted OR decreased progressively with increasing exposure to the prone position (measured in h/day), resulting in a dose–response effect (Fig. 2 and Additional file 1: Table E4). The effects of exposure to AW-PP on ETI were maintained when they were evaluated according to the severity of respiratory failure defined by the PaO2/FiO2 ratio (Additional file 1: Fig. E5). When the populations were compared based on the predominant position they adopted during the study period, differences were found between the AW-PP versus supine-position groups, but not between the AW-PP versus the lateral-position groups (Additional file 1: Fig. E6). The results of the analysis restricted to patients with AW-PP > 6 h versus patients with “zero” hour in prone position (n = 103) showed an OR = 0.29 (0.3–0.6) (Additional file 1: Fig. E7).
Risk of intubation between groups in awake prone position and non-prone position. OR indicates odds ratio; 95% CI indicates confidence interval. Non-awake prone positioning group (supine or lateral) as reference. Abbreviations. AW-PP: awake prone positioning. Weighted population and adjusted by center and pandemic time
Twenty-one patients in the AW-PP group (11%) and 47 (32%) in the NON-PP group died while being hospitalized. In the weighted population, the OR for hospital mortality was 0.58 (0.19–1.77) and the adjusted OR by center and pandemic time in the weighted population was 0.50 (95% CI 0.19–1.31) (Fig. 3 and Additional file 1: Table E5). One death occurred in each group while patients remained on HFNO (prior to ETI). In the sensitivity analysis for hospital mortality, the adjusted OR progressively decreased with increasing exposure to the prone position (measured in h/day), with a true OR reduction when the exposure was ≥ 8 h/d [OR 0.37 (95% CI 95 0.17–0.8)] (Fig. 3 and Additional file 1: Table E5). The results of the analysis restricted to patients with AW-PP > 6 h versus patients with “zero” hours in prone position (n = 103) showed an OR = 0.37 (0.2–0.8) (Additional file 1: Fig. E7).
Risk of hospital mortality between groups in the awake prone position versus non- prone position. OR indicates odds ratio; 95% CI indicates confidence interval. Non-prone positioning group (supine or lateral) as reference. Abbreviations. AW-PP: awake prone positioning. Weighted population and adjusted by center and pandemic time
Other clinical outcomes are shown in Table E3 (Additional file 1). ICU and hospital length of stay for AW-PP and NON-AW-PP were [median (p 25–75)] 9 (6–14) vs. 12 (7–20) days (p = 0.0012); and 15 (11–25) vs. 20 (13–32) days, (p = 0.002), respectively. The time until endotracheal intubation for AW-PP and NON-PP was 3 (1–4) 1 versus 1 (1–3) 3 days (p = 0.017), respectively (Additional file 1: Fig. E7). The functional results are listed in Table E6 (Additional file 1), 95% of the patients who remained prone for at least 6 h managed to ambulate upon discharge from the ICU. The variables related to the respiratory parameters of ventilated patients are listed in Table E7 (Additional file 1). Three patients had severe complications in the AW-PP group: syncope in the prone position requiring ETI, cardiorespiratory arrest requiring ETI, and arterial line displacement with bleeding and hypotension (not requiring ETI), respectively.
Discussion
In this multicenter prospective observational study of patients with COVID-19-related acute respiratory failure receiving initial treatment with HFNO, prone position for at least 6 h a day was associated with a lower risk of endotracheal intubation, even after adjustment for potential confounders. This association was more consistent as the exposure to prone position (measured in h/day) increased, thus evidencing a dose–response effect. Additionally, prone positioning was associated with a lower risk of hospital mortality when the exposure was > 8 h.
Since the beginning of the pandemic, decisions surrounding the proper time for initiation of invasive mechanical ventilation have been a matter of controversy [25, 26]. It has been suggested that delaying intubation could lead to patient self-induced lung injury. For this reason, some authors have recommended an early intubation strategy [27, 28]. Nonetheless, the potential benefits of such a strategy must be weighed against the complications associated with invasive mechanical ventilation, including higher risk of severe infections and death [6, 29]. Mortality in patients with COVID-19 receiving invasive mechanical ventilation is particularly high, with rates exceeding 70% in patients above 60 years [5], accentuating the need for caution when deciding which patients will benefit from this strategy.
Both prone position and HFNO may exert positive physiologic effects and improve outcomes. Prone position has been shown to improve ventilation–perfusion ratio [30], which may explain improved oxygenation, but also to decrease global lung strain and determine a more homogeneous distribution of regional strain [31, 32], which may prevent ventilation-induced lung injury. The prone position has been shown to reduce mortality in ventilated patients with moderate to severe ARDS when the exposure time is early and prolonged [9]. It is therefore recommended to use the prone position for a period > 12 h in these patients [33]. HFNO increases end-expiratory lung volume, decreases breathing effort and favors a more homogeneous ventilation distribution [34]. Besides, HFNO can reduce the rate of ETI in patients with acute hypoxemic respiratory failure and a PaO2/FiO2 ratio < 200 mmHg.[7].
The feasibility of applying HFNO and ventilation in AW-PP, on an independent or combined basis, has been evaluated in case series [11,12,13]. In our study, these interventions were applied concomitantly and systematically in a cohort of consecutive patients. Since study groups displayed differences in baseline variables, a causal approximation was carried out by weighting these variables using a propensity score method (IPW). The results displayed a balanced distribution of values of the variables between groups, thereby allowing the assumption of population interchangeability [18]. In view of the fact that the attending healthcare teams may have acted differently as they gained experience over the course of the pandemic [35], a second adjustment was made based on the time of the pandemic when the patients were included and the centers (“double robust”). The same method was used in the sensitivity analysis for restricted groups. The results obtained from the secondary outcome provide additional consistency to the results of the primary analysis.
A recent meta-trial comparing HFNO versus HFNO plus AW-PP showed a reduction in ETI risk in the combined treatment group. Importantly, the size of the effect was small and there were no benefits on mortality or other relevant clinical outcomes [14]. These results can be explained by the short exposure time to AW-PP in the treatment group (5 h). The rate of treatment failure (mainly ETI) was lower in patients who remained on AW-PP > 8 h/d. However, the study was not designed to evaluate the effect of exposure time in AW-PP, and no comparisons were made between intervention and control groups according to exposure time [14].
Different physiological parameters improved from the beginning of the prone position and progressively increased with exposure time within each session, up to 16–24 h in ventilated patients [36, 37]. On the other hand, evidence from observational studies suggests that a period > 24 h/session may not add additional clinical benefits [38, 39]. The rate of adherence in our study was high. Fifty-six percent of patients met the definition of AW-PP (≥ 6 h/d), placed in the prone position for a large number of hours (12 h/d), thereby resulting in a greater reduction in ETI risk. That exposure time could explain the differences in the effect size (risk of ETI) in our study, with respect to the meta-trial. Additionally, our results suggest that ≥ 8 h/d may be the time necessary to impact on death risk reduction.
Our study has several strengths: (a) a representative sample of patients with COVID-19-related ARF from different ICUs with consecutive inclusion, and similar initial treatment (HFNO), thus minimizing selection bias; (b) exhaustive treatment of confounders using causal inference and a sensitivity analysis that showed consistent results in different sub-populations. The main limitations of this study are those inherent to establishing causality by an observational design, i.e., the possibility of not considering unmeasured confounders. Several strategies were employed to minimize the potential biases inherent to the design: The prospective nature of the cohort allowed us to consider most of the known confounders; the use of IPW in the adjustment permitted to reduce multidimensionality and balance the factors that could influence the hypothesis [23], and the estimation of the e value [24]. All the patients included were treated in an ICU setting. Therefore, these results cannot be extrapolated to patients seen in a less complex settings (a general ward), or with less severe diseases. Finally, another limitation was the potential bias derived from the non-blinded position of the attending team to intubation order. Nonetheless, the healthcare team followed the recommendations pre-established in the protocol.
Conclusions
The results of our study indicate that, in patients with Covid-19 and acute respiratory failure admitted to the ICU and initially treated with HFNO, prone positioning for at least 6 h/day reduces the risk of endotracheal intubation, and for ≥ 8 h/d the risk of death.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory failure and acute respiratory distress syndrome
- ARF:
-
Acute respiratory failure
- AW-PP:
-
Awake prone positioning
- CI:
-
Confidence intervals
- CRP:
-
C-reactive protein
- CT score:
-
Chest computed tomography score
- DAG:
-
Direct acyclic graph
- ETI:
-
Endotracheal intubation
- H/DAY:
-
Hours per day
- HFNO:
-
High-flow nasal oxygen
- ICU:
-
Intensive care unit
- IPW-PS:
-
Inverse probability weighting–propensity score
- NON-PP:
-
Non-prone positioning
- OR:
-
Odds ratio
- ROX index:
-
Respiratory rate oxygenation index
- SpO2 :
-
Peripheral oxygen saturation
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
References
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–62.
Ranzani OT, Bastos LSL, Gelli JGM, Marchesi JF, Baião F, Hamacher S, et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9:407–18.
Lim ZJ, Subramaniam A, Reddy MP, Blecher G, Kadam U, Afroz A, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respiratory Crit Care Med. 2021. https://doi.org/10.1164/rccm.202006-2405oc.
Investigators C-IG on B of TRNATC-I, COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-06294-x
Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–96.
Rochwerg B, Granton D, Wang DX, Helviz Y, Einav S, Frat JP, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019. https://doi.org/10.1007/s00134-019-05590-5.
Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013. https://doi.org/10.1056/nejmoa1214103.
Beitler JR, Shaefi S, Montesi SB, Devlin A, Loring SH, Talmor D, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med. 2014;40:332–41.
Tan W, Xu D-Y, Xu M-J, Wang Z-F, Dai B, Li L-L, et al. The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis. Ther Adv Respir Dis. 2021;15:17534666211009408.
Reddy MP, Subramaniam A, Afroz A, Billah B, Lim ZJ, Zubarev A, et al. Prone positioning of nonintubated patients with coronavirus disease 2019—A systematic review and meta-analysis. Crit Care Med. 2021;49:e1001–14. https://doi.org/10.1097/CCM.0000000000005086.
Cardona S, Downing J, Alfalasi R, Bzhilyanskaya V, Milzman D, Rehan M, et al. Intubation rate of patients with hypoxia due to COVID-19 treated with awake proning: A meta-analysis. Am J Emerg Med. 2021;43:88–96.
Ehrmann S, Li J, Ibarra-Estrada M, Perez Y, Pavlov I, McNicholas B, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021. https://doi.org/10.1016/S2213-2600(21)00356-8.
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24.
Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199:1368–76.
Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808–17.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
Xian Y, O’Brien EC, Fonarow GC, Olson DM, Schwamm LH, Hannah D, et al. Patient-centered research into outcomes stroke patients prefer and effectiveness research: implementing the patient-driven research paradigm to aid decision making in stroke care. Am Heart J. 2015;170(36–45):45.e1-11.
Etminan M, Collins GS, Mansournia MA. Using causal diagrams to improve the design and interpretation of medical research. Chest. 2020;158:S21–8.
Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH. Robust causal inference using directed acyclic graphs: the R package “dagitty.” Int J Epidemiol. 2017. https://doi.org/10.1093/ije/dyw341.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74.
Tobin MJ. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020;201:1319–20.
Tobin MJ, Jubran A, Laghi F. Noninvasive strategies in COVID-19: epistemology, randomised trials, guidelines, physiology. Eur Respir J. 2021;57:2004247. https://doi.org/10.1183/13993003.04247-2020.
Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–30. https://doi.org/10.1001/jama.2020.6825.
Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–300. https://doi.org/10.1164/rccm.202003-0817LE.
Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160:454–65. https://doi.org/10.1016/j.chest.2021.04.002.
Richter T, Bellani G, Scott Harris R, Vidal Melo MF, Winkler T, Venegas JG, et al. Effect of prone position on regional shunt, aeration, and perfusion in experimental acute lung injury. Am J Respir Crit Care Med. 2005;172:480–7.
Mentzelopoulos SD, Roussos C, Zakynthinos SG. Prone position reduces lung stress and strain in severe acute respiratory distress syndrome. Eur Respir J. 2005;25:534–44.
Scaramuzzo G, Ball L, Pino F, Ricci L, Larsson A, Guérin C, et al. Influence of positive end-expiratory pressure titration on the effects of pronation in acute respiratory distress syndrome: a comprehensive experimental study. Front Physiol. 2020;11:179.
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2017;195:1253–63.
Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195:1207–15.
Kurtz P, Bastos LSL, Dantas LF, Zampieri FG, Soares M, Hamacher S, et al. Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months. Intensive Care Med. 2021;47:538–48.
Jochmans S, Mazerand S, Chelly J, Pourcine F, Sy O, Thieulot-Rolin N, et al. Duration of prone position sessions: a prospective cohort study. Ann Intensive Care. 2020;10:66. https://doi.org/10.1186/s13613-020-00683-7.
McAuley DF, Giles S, Fichter H, Perkins GD, Gao F. What is the optimal duration of ventilation in the prone position in acute lung injury and acute respiratory distress syndrome? Intensive Care Med. 2002;28:414–8.
Page DB, Vijaykumar K, Russell DW, Gandotra S, Chiles JW 3rd, Whitson MR, et al. Prolonged prone positioning for COVID-19-induced acute respiratory distress syndrome: a randomized pilot study. Am Thorac Soc. 2021. https://doi.org/10.1513/AnnalsATS.202104-498RL.
Rezoagli E, Mariani I, Rona R, Foti G, Bellani G. Difference between prolonged versus standard duration of prone position in COVID-19 patients: a retrospective study. Minerva Anestesiol. 2021. https://doi.org/10.23736/S0375-9393.21.15864-X.
Acknowledgements
The authors wish to thank the Research Department of the Hospital Italiano Buenos Aires (HIBA), especially Diego Giunta MD. PhD. and Sebastian Marciano. MD We also acknowledge the collaboration of Horacio Abonna. Argentine Collaborative Group on High Flow and Prone Positioning, Anabel Miranda Tirado (Intensive Care Unit. Hospital Privado de Comunidad. Mar del Plata. Argentina), María Constanza Viñas (Intensive Care Unit. Hospital Privado de Comunidad. Mar del Plata. Argentina), Juan Manuel Pintos (Intensive Care Unit. Hospital Privado de Comunidad. Mar del Plata. Argentina), Maria Eugenia Gonzalez (Intensive Care Unit. Hospital Privado de Comunidad. Mar del Plata. Argentina. Escuela Superior de Medicina. Universidad Nacional de Mar del Plata. Mar del Plata. Argentina.), Maite Mateos (Intensive Care Unit. Hospital Privado de Comunidad. Mar del Plata. Argentina), Mariela Marisol Laiz (Intensive Care Unit, Clínica Olivos SMG. Olivos. Buenos Aires. Argentina.), Jose Garcia Urrutia (Intensive Care Unit, Clínica Olivos SMG. Olivos. Buenos Aires. Argentina.), Micaela Ruiz Seifert (Intensive Care Unit. Sanatorio de la Trinidad Palermo. Ciudad Autónoma de Buenos Aires. Argentina.), and Emilce Mastroberti (Intensive Care Unit. Sanatorio de la Trinidad Palermo. Ciudad Autónoma de Buenos Aires. Argentina).
Funding
No funding source.
Author information
Authors and Affiliations
Consortia
Contributions
ME, MB, NAF, MO, and HK contributed to conception and study design. MB, AG, JO, AV, EGW, JO, SNA, AIL, FJA, MO, HK, NER, AM, MAM, GM, CNM, GRN, and VB contributed to study execution, acquisition of data, and interpretation. ME, NAF, BLF, ABC, and AT contributed to analysis and interpretation of data. ME, NAF, and BLF contributed to writing the manuscript. ABC and AT contributed to revision. ME, NAF, and BLF contributed to final drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Internal Review Board from the 6 centers approved the study including waived informed consent (Universidad Austral Comité Institucional de Evaluación N°20-072; Comité de Ética en Investigación SATI for Trinidad Palermo; Comité de Docencia e Investigación Sanatorio Clínica Modelo de Morón; Comité de Ética e Investigación Hospital Alemán; Consejo Institucional de Revisión de Estudios de Investigación Hospital Privado de Comunidad Nº 2919/2143/2020; Comité de Ética en Investigación Clínica Olivos). The interventions carried out were part of the usual practice at each center, and the researchers guaranteed confidentiality of their patient’s information.
Consent for publication
The manuscript does not contain any individual person’s data in any form.
Competing interests
AT is Consultant of Pfizer, Poliphor, MSD, Jansen OM Pharma. MB received lecture fees from Tecme S.A. and MEDIX Medical Devices S.R.L. Argentina. The rest of the authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
1. Further details on the procedures. 2. Further details on statistical analysis. TABLE E1. Baseline characteristics of the study population and the balance between groups. TABLE E2. Treatment with oxygen therapy and prone positioning. TABLE E3. Outcomes of patients in awake prone positioning versus non awake prone positioning. TABLE E4. Risk of intubation in awake prone positioning versus non awake prone positioning. OR indicates odds ratio; 95% CI indicates confidence interval. Non-prone positioning group as reference. TABLE E5. Risk of hospital mortality in awake prone positioning versus non-prone positioning. TABLE E6. Functional outcomes at discharge. TABLE E7. Variables related to invasive mechanical ventilation in ventilated patients at day 1 after starting invasive mechanical ventilation. TABLE E8. Selection of variables for adjustment of confounding. Figure E1. Diagnosis of inverse probability weights-propensity score (graphic and statistical). Figure E2. Standardized differences before and after applying inverse probability weighting. Figure E3. Directed acyclic graph (DAG). Figure E4. E-value calculation for primary outcome of interest (ETI). Figure E5. Risk of intubation between groups in the awake prone position vs. non-awake prone position according to severity of respiratory failure. Figure E6. Risk of intubation between groups in the awake prone position vs. non-awake prone position according to predominant body position. Figure E7. Cumulative incidence of endotracheal intubation over time in the study population.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Esperatti, M., Busico, M., Fuentes, N.A. et al. Impact of exposure time in awake prone positioning on clinical outcomes of patients with COVID-19-related acute respiratory failure treated with high-flow nasal oxygen: a multicenter cohort study. Crit Care 26, 16 (2022). https://doi.org/10.1186/s13054-021-03881-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-021-03881-2