Abstract
Purpose
Individualising drug dosing using model-informed precision dosing (MIPD) of beta-lactam antibiotics and ciprofloxacin has been proposed as an alternative to standard dosing to optimise antibiotic efficacy in critically ill patients. However, randomised clinical trials (RCT) on clinical outcomes have been lacking.
Methods
This multicentre RCT, including patients admitted to the intensive care unit (ICU) who were treated with antibiotics, was conducted in eight hospitals in the Netherlands. Patients were randomised to MIPD with dose and interval adjustments based on monitoring serum drug levels (therapeutic drug monitoring) combined with pharmacometric modelling of beta-lactam antibiotics and ciprofloxacin. The primary outcome was ICU length of stay (LOS). Secondary outcomes were ICU mortality, hospital mortality, 28-day mortality, 6-month mortality, delta sequential organ failure assessment (SOFA) score, adverse events and target attainment.
Results
In total, 388 (MIPD n = 189; standard dosing n = 199) patients were analysed (median age 64 [IQR 55–71]). We found no significant differences in ICU LOS between MIPD compared to standard dosing (10 MIPD vs 8 standard dosing; IRR = 1.16; 95% CI 0.96–1.41; p = 0.13). There was no significant difference in target attainment before intervention at day 1 (T1) (55.6% MIPD vs 60.9% standard dosing; p = 0.24) or at day 3 (T3) (59.5% vs 60.4%; p = 0.84). There were no significant differences in other secondary outcomes.
Conclusions
We could not show a beneficial effect of MIPD of beta-lactam antibiotics and ciprofloxacin on ICU LOS in critically ill patients. Our data highlight the need to identify other approaches to dose optimisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is currently no evidence for a clinical effect of model-informed precision dosing (MIPD) of beta-lactam antibiotics or ciprofloxacin in critically ill patients. Other approaches will need to be explored |
Introduction
Timely and appropriate antibiotic treatment is vital in treating critically ill patients [1, 2]. Beta-lactam antibiotics and fluoroquinolones are among the most prescribed antibiotics in the intensive care unit (ICU) [3, 4]. The pharmacokinetic (PK) behaviour of these antibiotics is different than in non-critically ill patients and subject to fast and significant changes over time [5]. Consequently, only 60% of patients receiving beta-lactam antibiotics and 30% of patients receiving ciprofloxacin achieve the adequate pharmacodynamic target (PDT) [6,7,8]. Not achieving the PDT is associated with poorer chances of clinical cure, bacteriological eradication, and an increased chance of antimicrobial resistance development [9, 10]. Preventing resistance development is important as resistance to beta-lactam antibiotics and fluoroquinolones account for more than 70% of deaths attributable to antimicrobial resistance [11].
The Surviving Sepsis Campaign advises precision dosing to optimise and individualise dosing strategies of antimicrobials based on pharmacokinetic/pharmacodynamic (PK/PD) principles [12]. Therapeutic drug monitoring (TDM), an individualised dosing strategy based on assessments of serum drug levels, has been proposed as a method to optimise exposure [13]. TDM increases target attainment of some beta-lactam antibiotics and potentially leads to better outcomes [14, 15]. Model-informed precision dosing (MIPD) is an emerging approach for TDM-guided dosing that addresses the limitations of traditional TDM, such as the need to wait for steady-state concentrations. Using population PK models and integrating TDM results, MIPD can assess the probability of target attainment for different dosing regimens [16, 17]. Therefore, MIPD could help to optimise dosing of beta-lactams and ciprofloxacin.
The effect of MIPD or TDM on clinical outcomes, especially in critically ill patients, has been scarcely studied in randomised clinical trials (RCT) [18]. The primary aim of this trial was to assess whether early MIPD using pharmacometric modelling of beta-lactam antibiotics and ciprofloxacin decreases ICU length of stay (LOS) compared to standard dosing.
Methods
Study design
The DOLPHIN multicentre, open-label, RCT was performed in ICUs at eight academic and teaching hospitals in the Netherlands. The trial design has been published before and is available in the trial protocol [19]. This trial was conducted between October 2018 and September 2021. Medical ethical board approval was obtained at the Erasmus University Medical Center (Erasmus MC) (MEC-2017-568; EudraCT 2017-004677-14). Additionally, the medical ethical board of each study site approved the protocol. All patients or their legal representatives provided written informed consent before randomisation.
Patients
Patients eligible for inclusion were adults (≥ 18 years) admitted to the ICU, expected to receive at least one of the target antibiotics using intermittent infusion for at least 2 days.
Patients were excluded in cases of pregnancy, antibiotic cessation before first blood sample collection, enrolment in another interventional trial, admittance for burn wounds or receiving study antibiotics only as part of selective decontamination of the digestive tract (SDD) prophylaxis (Supplement 1, Table S1) [20]. Patients admitted with a primary diagnosis of coronavirus disease 2019 (COVID-19) were excluded.
Randomisation
Patients were included and randomised within 36 h of the initial antibiotic dose. Randomisation took place in a 1:1 fashion, was stratified by site and antibiotic group (beta-lactam antibiotics and ciprofloxacin), computer-generated and used randomly permuted blocks of 2–8 allocations with increments of two. Patient allocation was not blinded to treating physicians due to the nature of the study.
Procedures
Patients in both groups started with antibiotic treatment according to standard clinical practice [21]. Following randomisation, trough and peak concentrations were drawn up to 4 times per patient with intervals of 48 h.
Sampling moment one (T1) was the day of the first antibiotic sampling, which was at most 36 h after the first antibiotic dose (T0). Within 12 h of sampling, a dosing recommendation was given based on measured serum antibiotic concentration. Dosing was re-evaluated on day 3 (T3) and day 5 (T5) after initiation of the antibiotic. For patients still receiving the antibiotic on day 7 (T7), antibiotic sampling without a dosing recommendation was performed to analyse the effects of dose adjustments on T5.
Dosing of the antibiotics is based on the range in minimum inhibitory concentration (MIC) of expected pathogens. The epidemiological cut-off values (MICECOFF) describe the highest MIC for organisms devoid of phenotypically-detectable acquired resistance mechanisms: the upper end of the wild-type distribution [22]. For PD properties, the MICECOFF of EUCAST for the expected pathogens were used (Supplement 1, Table S2). The PDT is the minimum value of the PK/PD index that is based on preclinical and clinical drug/microorganism exposure–response relationships. Beta-lactam antibiotics exhibit time-dependent bacterial killing and a successful outcome is best associated with the time that the unbound (free, f) concentration remains above the MICECOFF as a function of the dosing interval (fT > MICECOFF). For ciprofloxacin, bactericidal activity is characterised by concentration-dependent activity. The ratio of the area under the drug serum concentration–time curve over 24 h to the MICECOFF (AUC0–24 h / MICECOFF) is a good predictor of ciprofloxacin efficacy. In the present study, the PDT for beta-lactam antibiotics and ciprofloxacin were fT > MIC for 100% of the dosing interval and an AUC0–24 h / MICECOFF ratio above 125, respectively (Supplement 1, Table S3). Above target was defined as a steady-state trough concentration (Ctrough,ss) of more than 10 times the MICECOFF in the case of beta-lactam antibiotics and an AUC0–24 h/MICECOFF of more than 500 h for ciprofloxacin.
All serum antibiotic concentrations were measured at the Erasmus MC, using a validated LC/MS–MS method [23]. Unbound concentrations were measured for antibiotics with high protein binding (ceftriaxone and flucloxacillin). Samples that were not collected at the primary study site were stored at + 4 to + 8 degrees Celsius and transported to the Erasmus MC for analysis within 6 h.
Target attainment is defined as achieving the PDT of the respective antibiotic after reaching a steady-state. Steady-state antibiotic exposure was predicted using Bayesian forecasting incorporating the measured serum antibiotic concentrations into previously published parametric population PK models of critically ill patients using InsightRx™ (version 1.15.16, San Francisco, California). An overview of the used models and their publications is included in the supplements (Supplement 1, Table S4). Both trough and peak concentrations were used to Bayesian fit these population PK models to the individual patient [17]. Concentrations from previous days were analysed in relation to changes in kidney function or albumin levels, since these were the variable covariates. From the fitted PK model, fT > MIC, area under the curve divided by MIC (AUC0-24 h/MICECOFF) and Ctrough,ss were calculated. For ceftriaxone and flucloxacillin, the unbound concentrations were estimated using the measured unbound fraction in combination with the Bayesian predicted total concentration. A dose recommendation was formulated based on these estimations for the relevant PDT.
Patients in the standard dosing group were dosed based on local guidelines and could freely be adjusted by the attending physician. Sampling continued at the same intervals as in the MIPD group, but without dosing recommendations. These samples were stored at -80 degrees Celsius and analysed in bulk.
Outcomes
The primary outcome was the intensive care unit length of stay (ICU LOS). This was calculated as the days between ICU admission and ICU discharge or death. The ICU LOS of patients transferred to another ICU was calculated as the days between ICU admission and transfer date.
The following secondary outcomes were assessed: 28-day mortality, ICU mortality, hospital mortality, 6-month mortality, delta-SOFA score (the difference between SOFA score at T0 and T5), delta-CRP (C-reactive protein) between T0 and T5, delta-WBC (white blood cell count) between T0 and T5, the incidence of adverse events within 7 days (including death and readmissions within 6 months of T0), antibiotic PDT attainment and quality of life expressed as VAS-score and QALY (assessed by EuroQol 5D-5L questionnaire) at 6 months.
A cost-effectiveness analysis was performed from a hospital perspective on the QALY at 6 months. The outcome of the analysis was the incremental cost-effectiveness ratio (ICER) per QALY defined as the costs, divided by the difference in effects between MIPD compared to standard therapy. Admission costs for the ICU were estimated using a recent micro-costing study [24]. Admission costs for the hospital wards were extracted from the Dutch guideline for economical evaluations in healthcare [25]. For patients in the MIPD group, the costs of the intervention were added to the admission costs. All costs were indexed and inflation-corrected to 2020.
For post hoc analyses, the following variables were tested: ICU days after the first antibiotic dose (T0), ICU-free days alive (number of days not admitted to the ICU within 28 days after T0; Patients who died were defined as 0). Furthermore, the ICU LOS analysis was performed after a correction of patients who were transferred to another ICU: The correct discharge date was determined by contacting the receiving ICU. The different antibiotic groups (beta-lactam antibiotics and ciprofloxacin) and per-protocol were tested in post hoc analysis with the primary outcome of ICU LOS.
Statistical analysis
Sample size calculations were based on a decrease in ICU LOS from seven to 6 days with a standard deviation (SD) of 3.5 days. With an alpha level of 0.05 and power of 0.80, we aimed to include 382 patients [19]. With an expected dropout rate of 15%, we aimed to randomise 450 patients.
The data were analysed using the intention-to-treat (ITT) principle. Patients were not included in the analysis if they met the exclusion criteria after randomisation. First, the patient and clinical characteristics were described as mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables depending on their normality of distribution. The Shapiro–Wilk test was used to assess normality. Differences between MIPD and standard dosing were analysed using an independent sample t test or Mann–Whitney U test for normally distributed and non-normally distributed variables, respectively. Categorical variables were described using count with percentages and differences between MIPD and standard dosing groups were examined using Fisher’s exact test.
The primary outcome was presented as median with IQR. We used a negative binomial Poisson regression or Poisson regression, depending on the dispersion parameter, to adjust for patient and clinical characteristics that differ significantly with a p value < 0.15 in the univariate analysis. For the Poisson regression, the incidence rate ratio (IRR) and the 95% confidence interval were reported.
Statistically significant differences for continuous and categorical variables between study groups will be assessed using an independent sample t test and chi-square test, respectively. If a continuous variable is non-normally distributed, a Mann–Whitney U test will be employed to assess the statistical differences. In case of imbalances between the two groups, we will switch to Poisson regression, binary logistic regression or linear regression for count, binary outcome or continuous outcomes, respectively. Due to inflated zeros in the ICU-free days alive, a Mann–Whitney U test was used to test for significant differences.
Adverse events were described as count with percentage. Target attainment was described as count with percentage over time and visualised using an alluvial plot.
Post hoc analysis was performed on the primary outcome of ICU LOS and target attainment at T3 within the groups of interest using the same regression models as described under the primary outcome.
Results
Patient characteristics
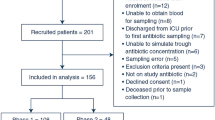
From October 2018 to September 2021, 450 patients were randomised (MIPD; n = 226, standard dosing group; n = 224). Due to not acquiring informed consent or retracting informed consent, 18 patients were excluded from analyses. Furthermore, 44 patients met the exclusion criteria between randomisation and any intervention and were therefore excluded, leaving 388 in the primary analysis (MIPD; n = 189, standard dosing group; n = 199) (Fig. 1). Of these patients, the median age was 64 (IQR 55–71), the median Acute Physiology and Chronic Health Evaluation version four (APACHE IV) score was 70 (IQR 51–90) and 65% of the patients were treated for a pulmonary infection. Most patients had sepsis without shock (31.2%) or septic shock (23.5%). The median SOFA score was 8 (IQR 5–10.3) at T0 and only 3 patients had a SOFA score of 0 (Supplement 1, Figure S2). Patient and clinical characteristics were well balanced between groups (Table 1). The only characteristic with a p value below 0.15 was the Charlson comorbidity index (CCI).
Patient flow in the DOLPHIN trial. AB antibiotic; SDD selective decontamination of the digestive track; T1 first moment of blood sampling. Patients were excluded from analyses if they did not comply to the inclusion criteria e.g. no informed consent was gathered. Patients were furthermore excluded if they met an exclusion criterium within the first 24 h of therapy, before sampling was performed
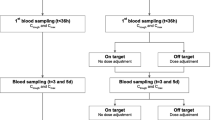
The median time of first sampling after the start of antibiotics was 19.6 h (IQR 14.5–31) in the MIPD group and 18.5 h (IQR 14.1–29) in the standard dosing group. A positive isolate was found in 206 patients (101 MIPD; 105 standard dosing). In 59 patients (31 MIPD; 28 standard dosing) the pathogen was intrinsically resistant to the researched antibiotic. In the intervention group, a dose adjustment was recommended for 71 (37.6%), 27 (23.1%), and 8 (13.3%) patients at T1, T3 and T5, respectively (Fig. 2).
Study flow and dose advice given. ICU intensive care unit; MIP model-informed precision dosing; MD medical doctor; T1, first moment of antibiotic sampling, 1 day after initiation of antibiotic; T3, second moment of sampling, 48 h after T1; T5, third moment of sampling, 48 h after T3. (1) Patients in the ICU were sampled in the morning, after a logistical route the sample was analysed in the laboratory and the analytical results were imported into the analysing software. (2) A Bayesian prediction was made using previously published population PK models. Dose adjustments were simulated, after which a final dose advice was chosen. (3) The bedside physician (MD) was informed of the dosing advice. (4) The dosing of the antibiotic was altered by the physician. In the bottom panel, the given dose advice are reported. Doses could be increased, decreased or kept equal. Equal doses were advised when the target was attained or when the maximum daily dose was achieved
Primary and secondary outcomes
The median ICU LOS was 10.0 days (IQR 5–20) in the MIPD group and 8 days (IQR 3–19) in the standard dosing group (Table 2). The dispersion parameter was 1.47 (χ2 = 568), indicating that a negative binomial Poisson regression is more appropriate. The unadjusted and adjusted IRR for ICU LOS were 1.12 (95% CI 0.92–1.36; p = 0.27) and 1.16 (95% CI 0.96–1.41; p = 0.13), respectively (Table 2). The CCI was the only parameter included in the adjusted analysis.
The unadjusted and adjusted odds ratio for 28 day mortality was 1.10 (95% CI 0.7–1.74; p = 0.68) and 1.04 (95% CI 0.65–1.66; p = 0.87), respectively. For QALY at 6 months, the unadjusted and adjusted estimates were 0.03 (95% CI − 0.12 to 0.06; = 0.55) and 0.03 (95% CI − 0.12 to 0.06; = 0.49), respectively. There was no significant difference between study groups in all mortality outcomes, in delta-CRP, delta-WBC, or delta-SOFA scores (Table 2).
Target attainment was 55.6% at T1 in the MIPD group compared to 60.9% in the standard dosing group (Supplement 1, Table S10 and Fig. 3) (p = 0.24). At T3, target attainment was measured in 222 (57%) patients (116 MIPD group; 106 standard dosing group) and was similar with 59.5% and 60.4% in the MIPD and standard dosing group, respectively (p = 0.84). The differences in target attainment between the study groups at any timepoint were not significant (Table 2).
There were no major differences in adverse events between the groups and none were likely to be related to the study intervention (Supplement 1, Table S11).
The mean total costs were €52,056 (95% CI − 52,133 to 156,246) and €48,210 (95% CI − 64,077 to 160,498) in the MIPD group and standard dosing group, respectively. The incremental costs were €5,312 with an average decrease in 6 months QALY of 0.03 (range − 0.5 to 0.5) in MIPD compared to the standard dosing. The negative effect and increase in costs lead to a negative ICER of €-177,066, which means the intervention is not cost-effective.
Post hoc analyses
The unadjusted and adjusted IRR for ICU LOS after T0 were 1.09 (95% CI 0.9–1.31; p = 0.4) and 1.11 (95% CI 0.92–1.34; p = 0.27), respectively (Table 2). The ICU-free days alive was 16 (IQR 0–23) and 18 (0–25) for the MIPD group and standard therapy group, respectively.
Furthermore, when including the ICU LOS after patients were transferred to another ICU, the total ICU LOS was similar, albeit increased in the MIPD group (IRR = 1.13; 95% CI 0.93–1.38; p = 0.22) (Supplement 1, Table S12).
The estimates of treatment effect for post hoc subgroup analyses were similar to the main analysis (Supplement 1, Tables S13–S14). We observed no significant differences in ICU LOS between patients receiving beta-lactam antibiotics (IRR 1.20; 95% CI 0.96–1.52) or ciprofloxacin (IRR 0.96; 95% CI 0.65–1.4).
Discussion
In this multicentre RCT involving critically ill patients, we investigated whether early MIPD using pharmacometric modelling of beta-lactam antibiotics and ciprofloxacin can decrease ICU LOS compared to standard dosing. Contrary to our hypothesis, we found that the ICU LOS in the MIPD group and standard dosing group were similar, although the ICU LOS in the MIPD group was non-significantly increased by 2 days on average.
To our knowledge, this RCT is the first multicentre trial focusing on the effect of antibiotic MIPD on clinical outcomes. In our current study, no significant differences were found in mortality outcomes and adverse events between the groups. In the first 36 h, our target attainment was 64% for the beta-lactam antibiotics and 40% for ciprofloxacin, which seems to be within the margins of literature [6,7,8]. Furthermore, target attainment at different time points was not improved in the MIPD arm. Four smaller studies showed no significant clinical effect of TDM without using models of antibiotics on outcomes in critically ill patients [15, 26,27,28]. Two of these studies used retrospective TDM data that were applied in routine clinical treatment [27, 28]. The other two were smaller RCTs: De Waele and colleagues included patients receiving meropenem or piperacillin; [15] while Hagel and colleagues studied continuous infusion of piperacillin in patients with sepsis [26]. None of the trials showed a significant difference in clinical outcomes. However, three of these trials showed an increase in target attainment when applying TDM [15, 26, 28]. In contrary to our study, these trials did not use pharmacometric modelling to estimate future antibiotic exposure.
Out of all the patients, 59 were infected with a pathogen that was intrinsically resistant to the antibiotic they were randomised for. Empiric antibiotic therapy and our intervention are usually started before a pathogen is found. Usually, antibiotic combination therapy will cover the pathogen treatment. Alternatively, the choice of antibiotics will be altered after finding an intrinsically resistant pathogen.
We did not observe intervention-related side effects. Furthermore, no difference in liver or renal toxicity was found. However, diagnosing neurotoxicity on the ICU is challenging and probably results in underreporting of these side effects [29]. It is not easy to attribute neurological side effects to antibiotics due to comedication or comorbidities. Additional prospective registration of symptoms could aid in this diagnosis. Furthermore, to avoid potentially toxic effects, dose reduction is arbitrarily recommended when the unbound trough levels exceeds 8 × MIC [30].
The “golden” window of antibiotic treatment is within the first hours [31]. Therefore, the essence of performing MIPD studies in critically ill patients is a short turnaround time between concentration assessment and clinical intervention in the early phase of treatment. Especially as there are rapid and frequent changes in illness severity and treatment of the critically ill patient. To ensure the right antibiotic exposure, the dose should be personalised even before the first administration and updated frequently using MIPD.
Target attainment did not significantly increase in the MIPD group. This finding can have multiple possible explanations. The large variability within a single patient over time could make the PK changes—for example, due to dialysis, volume resuscitation, or organ failure—unpredictable within the timeframe between intervention and measurement of the target attainment outcome. When applying model-based dose adjustments, the used models were unlikely able to predict these PK changes within the following 48 h. Additionally, we only used traditional restrictive dose adjustments according to our protocol. These dose adjustments may not have been vigorous enough to attain the PDT, as there was a maximum to the dose increase or decrease for every 48-h interval. Furthermore, a maximum in daily dosing was adhered which limited the possibilities of dose increases.
There are some directions for future research on dose optimisation of beta-lactam antibiotics in the critically ill. Dreesen and colleagues recently described a simulation that target attainment could be improved by doubling daily doses of ceftriaxone for patients with a prior increased risk of augmented renal clearance [32]. They showed that a machine learning approach could aid in dose optimisation. A recent article by Yang and colleagues showed that model-based forecasting of meropenem may be possible using Bayesian estimations in combination with TDM information in a critically ill population [33]. They also showed the importance of external validation of these population PK models before use in the clinical setting. However, this study did not specify the time difference between samples that were predicted and insights into the accuracy of 48-h predictions are needed. The use of live bedside measurements could be used in MIPD frameworks to quickly optimise dosing during the entire admission [34, 35]. Moreover, finding the appropriate subpopulation that may benefit from dose optimisation strategies will be needed, as not all patients are at equal risk of not reaching the PDT [36]. Those can be patients who are at risk for having a resistant pathogen or those with augmented renal clearance [18]. Selection of patients at risk for target non-attainment could aid in the power of future trials.
We could not find an explanation for the non-significant increase of ICU LOS in the MIPD group in our data. We considered the non-blinding approach could lead to a bias. Another explored explanation was toxicity of beta-lactam, but could not find this in our data. As discussed earlier, beta-lactams toxicity is difficult to assess. Dose increases leading to excessive dosing and dose decreases leading to below target were found once in the intervention group: this patient received an advice for dose increase at T1 and was because of acute changes in renal function above target at T5.
This study has several limitations. First, due to a maximum delay of 36 h after initiation of antibiotics to gather informed consent, the dose optimisation was delayed. Future studies should research dose personalisation at the first administration. Second, the current PDTs of 100% fT > MIC and AUC/MIC > 125 were based on expert opinion and retrospective studies. However, there is no conclusive evidence that advocates for other PDTs. Furthermore, the exposure was examined in blood serum, which might not have the same concentrations as on the site of infection. Novel techniques to examine the exposure in tissue should be explored for future trials. Third, the non-blinded design might have introduced some bias in the decision to lengthen the ICU stay. However, we expect little effect due to a persistent high ICU turnover of patients and high rotation of ICU physicians. Fourth, both patients receiving beta-lactam antibiotics and patients receiving ciprofloxacin were analysed as one group. To avoid significant imbalances between centres and these two antibiotic groups, the randomisation was stratified by these two variables. In post hoc analyses, we also found no differences concerning ICU LOS when analysed separately. Finally, the MIC values used for assessing PDTs were based on ECOFF values of the expected pathogens. Due to this approach, there is a chance that PDTs are overestimated in our study. The causing pathogen will often not be available at the time of TDM, and if the pathogen is available, the measurement of a relatively reliable MIC should be performed using the golden standard method several times. These methods are not available and feasible in routine clinical laboratories [37].
To conclude, we could not show a beneficial effect of MIPD of beta-lactam antibiotics and ciprofloxacin on ICU LOS in critically ill patients in this RCT. MIPD in the current approach cannot be recommended for implementation. Further studies are needed to explore other approaches to dose optimisation of these antibiotics.
Data sharing
The deidentified participant data used and/or analysed during the current trial are available from the corresponding author on reasonable request 3 months after publication. The data will need to be requested in the context of research approved by a medical ethical committee, will need to follow the General Data Protection Regulation and not have the same objective as a planned analysis.
References
Bassetti M, Rello J, Blasi F, Goossens H, Sotgiu G, Tavoschi L, Zasowski EJ, Arber MR, McCool R, Patterson JV, Longshaw CM, Lopes S, Manissero D, Nguyen ST, Tone K, Aliberti S (2020) Systematic review of the impact of appropriate versus inappropriate initial antibiotic therapy on outcomes of patients with severe bacterial infections. Int J Antimicrob Agents 56:106184
Kollef MH, Shorr AF, Bassetti M, Timsit J-F, Micek ST, Michelson AP, Garnacho-Montero J (2021) Timing of antibiotic therapy in the ICU. Crit Care 25:360
Axente C, Licker M, Moldovan R, Hogea E, Muntean D, Horhat F, Bedreag O, Sandesc D, Papurica M, Dugaesescu D, Voicu M, Baditoiu L (2017) Antimicrobial consumption, costs and resistance patterns: a two year prospective study in a Romanian intensive care unit. BMC Infect Dis 17:358
Dulhunty JM, Webb SAR, Paterson DL, Bellomo R, Myburgh J, Roberts JA, Lipman J (2010) A survey of antibiotic prescribing practices in Australian and New Zealand intensive care units. Crit Care Resusc 12:162–170
Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J (2012) Introduction to drug pharmacokinetics in the critically III patient. Chest 141:1327–1336
Abdulla A, Dijkstra A, Hunfeld NGM, Endeman H, Bahmany S, Ewoldt TMJ, Muller AE, van Gelder T, Gommers D, Koch BCP (2020) Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: a two-center prospective study (EXPAT). Crit Care 24:558
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen K-M, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, Study D, Roberts JA, Lipman J, Starr T, Wallis SC, Paul SK, MargaritRibas A, De Waele JJ, De Crop L, Spapen H, Wauters J, Dugernier T, Jorens P, Dapper I, De Backer D, Taccone FS, Rello J, Ruano L, Afonso E, Alvarez-Lerma F, Gracia-Arnillas MP, Fernández F, Feijoo N, Bardolet N, Rovira A, Garro P, Colon D, Castillo C, Fernado J, Lopez MJ, Fernandez JL, Arribas AM, Teja JL, Ots E, Carlos Montejo J, Catalan M, Prieto I, Gonzalo G, Galvan B, Blasco MA, Meyer E, Del Nogal F, Vidaur L, Sebastian R, Garde PM, Martin Velasco MDM, Zaragoza Crespo R, Esperatti M, Torres A, Montravers P, Baldesi O, Dupont H, Mahjoub Y, Lasocki S, Constantin JM, Payen JF, Martin C, Albanese J, Malledant Y, Pottecher J, Lefrant J-Y, Jaber S, Joannes-Boyau O, Orban C, Ostermann M, McKenzie C, Berry W, Smith J, Lei K, Rubulotta F, Gordon A, Brett S, Stotz M, Templeton M, Rhodes A, Ebm C, Moran C, Kaukonen K-M, Pettilä V, Dimopoulos G, Koulenti D, Xristodoulou A, Theodorou V, Kouliatsis G, Sertaridou E, Anthopoulos G, Choutas G, Rantis T, Karatzas S, Balla M, Papanikolaou M, Myrianthefs P, Gavala A, Fildisis G, Koutsoukou A, Kyriakopoulou M, Petrochilou K, Kompoti M, Michalia M, Clouva-Molyvdas F-M, Gkiokas G, Nikolakopoulos F, Psychogiou V, Malliotakis P, Akoumianaki E, Lilitsis E, Koulouras V, Nakos G, Kalogirou M, Komnos A, Zafeiridis T, Chaintoutis C, Arvaniti K, Matamis D, Chaintoutis C, Kydona C, Gritsi-Gerogianni N, Giasnetsova T, Giannakou M, Soultati I, Chytas I, Antoniadou E, Antipa E, Lathyris D, Koukoubani T, Paraforou T, Spiropoulou K, Bekos V, Spring A, Kalatzi T, Nikolaou H, Laskou M, Strouvalis I, Aloizos S, Kapogiannis S, Soldatou O, Bassetti M, Adembri C, Villa G, Giarratano A, Maurizio Raineri S, Cortegiani A, Montalto F, Strano MT, Ranieri VM, Sandroni C, De Pascale G, Molin A, Pelosi P, Montagnani L, Urbino R, Mastromauro I, De Rosa FG, Ranieri VM, Cardoso T, Afonso S, Gonçalves-Pereira J, Baptista JP, Akova M, Özveren A (2014) DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083
Abdulla A, Rogouti O, Hunfeld NGM, Endeman H, Dijkstra A, van Gelder T, Muller AE, de Winter BCM, Koch BCP (2020) Population pharmacokinetics and target attainment of ciprofloxacin in critically ill patients. Eur J Clin Pharmacol 76:957–967
McKinnon PS, Paladino JA, Schentag JJ (2008) Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351
Abdul-Aziz MH, Lipman J, Mouton JW, Hope WW, Roberts JA (2015) Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: optimizing efficacy and reducing resistance development. Semin Respir Crit Care Med 36:136–153
Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC, Browne AJ, Chipeta MG, Fell F, Hackett S, Haines-Woodhouse G, KashefHamadani BH, Kumaran EAP, McManigal B, Agarwal R, Akech S, Albertson S, Amuasi J, Andrews J, Aravkin A, Ashley E, Bailey F, Baker S, Basnyat B, Bekker A, Bender R, Bethou A, Bielicki J, Boonkasidecha S, Bukosia J, Carvalheiro C, Castañeda-Orjuela C, Chansamouth V, Chaurasia S, Chiurchiù S, Chowdhury F, Cook AJ, Cooper B, Cressey TR, Criollo-Mora E, Cunningham M, Darboe S, Day NPJ, De Luca M, Dokova K, Dramowski A, Dunachie SJ, Eckmanns T, Eibach D, Emami A, Feasey N, Fisher-Pearson N, Forrest K, Garrett D, Gastmeier P, Giref AZ, Greer RC, Gupta V, Haller S, Haselbeck A, Hay SI, Holm M, Hopkins S, Iregbu KC, Jacobs J, Jarovsky D, Javanmardi F, Khorana M, Kissoon N, Kobeissi E, Kostyanev T, Krapp F, Krumkamp R, Kumar A, Kyu HH, Lim C, Limmathurotsakul D, Loftus MJ, Lunn M, Ma J, Mturi N, Munera-Huertas T, Musicha P, Mussi-Pinhata MM, Nakamura T, Nanavati R, Nangia S, Newton P, Ngoun C, Novotney A, Nwakanma D, Obiero CW, Olivas-Martinez A, Olliaro P, Ooko E, Ortiz-Brizuela E, Peleg AY, Perrone C, Plakkal N, Ponce-de-Leon A, Raad M, Ramdin T, Riddell A, Roberts T, Robotham JV, Roca A, Rudd KE, Russell N, Schnall J, Scott JAG, Shivamallappa M, Sifuentes-Osornio J, Steenkeste N, Stewardson AJ, Stoeva T, Tasak N, Thaiprakong A, Thwaites G, Turner C, Turner P, van Doorn HR, Velaphi S, Vongpradith A, Vu H, Walsh T, Waner S, Wangrangsimakul T, Wozniak T, Zheng P, Sartorius B, Lopez AD, Stergachis A, Moore C, Dolecek C, Naghavi M (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399:629–655
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, HylanderMøller M, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med 49:1063–1143
Muller AE, Huttner B, Huttner A (2018) Therapeutic drug monitoring of beta-lactams and other antibiotics in the intensive care unit: which agents, which patients and which infections? Drugs 78:439–451
Sime FB, Roberts MS, Tiong IS, Gardner JH, Lehman S, Peake SL, Hahn U, Warner MS, Roberts JA (2015) Can therapeutic drug monitoring optimize exposure to piperacillin in febrile neutropenic patients with haematological malignancies? A randomized controlled trial. J Antimicrob Chemother 70:2369–2375
De Waele JJ, Carrette S, Carlier M, Stove V, Boelens J, Claeys G, Leroux-Roels I, Hoste E, Depuydt P, Decruyenaere J, Verstraete AG (2014) Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med 40:380–387
Tängdén T, Ramos Martín V, Felton TW, Nielsen EI, Marchand S, Brüggemann RJ, Bulitta JB, Bassetti M, Theuretzbacher U, Tsuji BT, Wareham DW, Friberg LE, De Waele JJ, Tam VH, Roberts JA, Infection Section for the European Society of Intensive Care Medicine tP, Pharmacodynamics Study Group of the European Society of Clinical M, Infectious Diseases tISoA-IP, the Critically Ill Patients Study Group of European Society of Clinical M, Infectious D (2017) The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med 43:1021–1032
Wicha SG, Märtson A-G, Nielsen EI, Koch BCP, Friberg LE, Alffenaar J-W, Minichmayr IK, The International Society of Anti-Infective Pharmacology tPKPDsgotESoCMID (2021) From therapeutic drug monitoring to model-informed precision dosing for antibiotics. Clin Pharmacol Ther 109:928–941
Fratoni AJ, Nicolau DP, Kuti JL (2021) A guide to therapeutic drug monitoring of β-lactam antibiotics. Pharmacotherapy 41:220–233
Abdulla A, Ewoldt TMJ, Hunfeld NGM, Muller AE, Rietdijk WJR, Polinder S, van Gelder T, Endeman H, Koch BCP (2020) The effect of therapeutic drug monitoring of beta-lactam and fluoroquinolones on clinical outcome in critically ill patients: the DOLPHIN trial protocol of a multi-centre randomised controlled trial. BMC Infect Dis 20:57
Wittekamp BHJ, Oostdijk EAN, Cuthbertson BH, Brun-Buisson C, Bonten MJM (2020) Selective decontamination of the digestive tract (SDD) in critically ill patients: a narrative review. Intensive Care Med 46:343–349
SWAB Guidelines (2022) SWAB Guidelines. Stichting Werkgroep Antibioticabeleid.
Committee ES (2021) MIC distributions and the setting of epidemiological cut-off (ECOFF) values. MIC distributions and the setting of epidemiological cut-off (ECOFF) values. EUCAST
Bahmany S, Abdulla A, Ewoldt TMJ, Oehlers PL, de Winter BCM, Koch BCP (2022) High-throughput analysis for the simultaneous quantification of nine beta-lactam antibiotics in human plasma by UPC2-MS/MS: method development, validation, and clinical application. J Pharm Biomed Anal 219:114904
Ewoldt TMJ, Abdulla A, Hunfeld NGM, Muller AE, Gommers D, Polinder S, Koch BCP, Endeman H (2022) Health care costs of target attainment for beta-lactam antibiotics in critically ill patients: a retrospective analysis of the EXPAT study. Ther Drug Monit 44:224–229
Hakkaart-van Roijen L, Van der Linden N, Bouwmans C, Kanters T, Tan S (2016) Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg. Zorginstituut Nederland.
Hagel S, Bach F, Brenner T, Bracht H, Brinkmann A, Annecke T, Hohn A, Weigand M, Michels G, Kluge S, Nierhaus A, Jarczak D, König C, Weismann D, Frey O, Witzke D, Müller C, Bauer M, Kiehntopf M, Neugebauer S, Lehmann T, Roberts JA, Pletz MW, Braune A, Schmidt K, Motsch J, Pinder N, Richter D, Schlattmann P, von Ameln-Mayerhofer A, Schappacher M, Fuchs T, Röhr A, Kurlbaum M, Schreiner O, Hüter L, Gründling M, Angermair S, Deja M, Bloos F, Fiedler S, Chkirni H (2022) Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: a randomized controlled trial. Intensive Care Med 48:311–321
Machado AS, Oliveira MS, Sanches C, Silva Junior CVd, Gomez DS, Gemperli R, Santos SRCJ, Levin AS (2017) Clinical outcome and antimicrobial therapeutic drug monitoring for the treatment of infections in acute burn patients. Clin Ther 39:1649-1657.e1643
Wong G, Briscoe S, McWhinney B, Ally M, Ungerer J, Lipman J, Roberts JA (2018) Therapeutic drug monitoring of β-lactam antibiotics in the critically ill: direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J Antimicrob Chemother 73:3087–3094
Roger C, Louart B (2021) Beta-lactams toxicity in the intensive care unit: an underestimated collateral damage? Microorganisms 9:1505
Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, Goutelle S, Lefeuvre S, Mongardon N, Roger C, Scala-Bertola J, Lemaitre F, Garnier M (2019) Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit Care 23:104
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock*. Crit Care Med 34:1589–1596
Dreesen E, Gijsen M, Elkayal O, Annaert P, Debaveye Y, Wauters J, Karlsson MO, Spriet I (2022) Ceftriaxone dosing based on the predicted probability of augmented renal clearance in critically ill patients with pneumonia. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkac209
Yang N, Wang J, Xie Y, Ding J, Wu C, Liu J, Pei Q (2022) External evaluation of population pharmacokinetic models to inform precision dosing of meropenem in critically ill patients. Front Pharmacol 13:838205
Visser EWA, Yan J, van Ijzendoorn LJ, Prins MWJ (2018) Continuous biomarker monitoring by particle mobility sensing with single molecule resolution. Nat Commun 9:2541
Ates HC, Roberts JA, Lipman J, Cass AEG, Urban GA, Dincer C (2020) On-Site Therapeutic Drug Monitoring. Trends Biotechnol 38:1262–1277
Abdulla A, Ewoldt TMJ, Purmer IM, Muller AE, Gommers D, Endeman H, Koch BCP (2021) A narrative review of predictors for β-lactam antibiotic exposure during empirical treatment in critically ill patients. Expert Opin Drug Metab Toxicol 17:359–368
Mouton JW, Muller AE, Canton R, Giske CG, Kahlmeter G, Turnidge J (2017) MIC-based dose adjustment: facts and fables. J Antimicrob Chemother 73:564–568
Acknowledgements
We acknowledge Alicija Vileito, Chafika Anakhrouch, Christa Kruger, Ditty van Duijn, Femke van der Horst, Kiran Bhansing, Lettie van den Berg, Nils van Willigenburg, Manizha Mir, Melanie Glasbergen, Patricia Ormskerk, Puck van den Broek, Robin van der Klip, Soma Bahmany and Stanley Hau for their contribution in carrying out the trial. We acknowledge all patients that participated in the study and their representatives for their contribution to the study. We acknowledge the patient representatives for their contributions during the planning of the study. Furthermore, we acknowledge all the ICU nurses, analysts and research nurses of the research centres for their contribution.
Funding
This project has received funding from the Dutch Organization for Health Research and Development ZonMw (Grant 848017008), Stichting de Merel and Erasmus MC MRace Grant. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
TE and AA contributed equally. Concept and design were performed by TE, AA, AM, NH, HE and BK. Statistical analysis was performed by TE and WR. Facilitation of inclusions was performed by DG, IP, PvV, EJW, JH, AD, TR and AK. The initial interpretation of the data was performed by TE, AA, WR, AM, BdW, HE and BK. Thereafter, the first draft was critically reviewed and revised by all authors. All authors had full access to the data, verified the underlying data and contributed to data interpretation and review, revision and approval of the report.
Corresponding author
Ethics declarations
Conflicts of interest
All authors reported no possible competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ewoldt, T.M.J., Abdulla, A., Rietdijk, W.J.R. et al. Model-informed precision dosing of beta-lactam antibiotics and ciprofloxacin in critically ill patients: a multicentre randomised clinical trial. Intensive Care Med 48, 1760–1771 (2022). https://doi.org/10.1007/s00134-022-06921-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-022-06921-9