Abstract
Purpose
Early recognition and effective treatment of sepsis improves outcomes in critically ill patients. However, antibiotic exposures are frequently suboptimal in the intensive care unit (ICU) setting. We describe the feasibility of the Bayesian dosing software Individually Designed Optimum Dosing Strategies (ID-ODS™), to reduce time to effective antibiotic exposure in children and adults with sepsis in ICU.
Methods
A multi-centre prospective, non-randomised interventional trial in three adult ICUs and one paediatric ICU. In a pre-intervention Phase 1, we measured the time to target antibiotic exposure in participants. In Phase 2, antibiotic dosing recommendations were made using ID-ODS™, and time to target antibiotic concentrations were compared to patients in Phase 1 (a pre–post-design).
Results
175 antibiotic courses (Phase 1 = 123, Phase 2 = 52) were analysed from 156 participants. Across all patients, there was no difference in the time to achieve target exposures (8.7 h vs 14.3 h in Phase 1 and Phase 2, respectively, p = 0.45). Sixty-one courses in 54 participants failed to achieve target exposures within 24 h of antibiotic commencement (n = 36 in Phase 1, n = 18 in Phase 2). In these participants, ID-ODS™ was associated with a reduction in time to target antibiotic exposure (96 vs 36.4 h in Phase 1 and Phase 2, respectively, p < 0.01). These patients were less likely to exhibit subtherapeutic antibiotic exposures at 96 h (hazard ratio (HR) 0.02, 95% confidence interval (CI) 0.01–0.05, p < 0.01). There was no difference observed in in-hospital mortality.
Conclusions
Dosing software may reduce the time to achieve target antibiotic exposures. It should be evaluated further in trials to establish its impact on clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This multi-centre study evaluated the feasibility of Bayesian dosing software to improve antibiotic exposures in children and adults with sepsis in intensive care unit. Dosing software reduced the time to achieve effective antibiotic concentrations and may improve clinical outcomes in critically ill patients with sepsis. |
Introduction
Sepsis is a significant contributor to mortality in critically ill children and adults [1, 2]. Prompt recognition and treatment, including the administration of effective antibiotics, improves outcomes and reduces mortality [3, 4]. There are several barriers to the early recognition and treatment of sepsis. Its clinical presentation is often non-specific, while the processes of pathogen identification and susceptibility testing using conventional culture-based methods are time-consuming [5]. Even when pathogen and susceptibility are known, patients in the intensive care unit (ICU) are exposed to subtherapeutic antibiotic exposures as a consequence of the pathophysiological changes that occur in critical illness, and the interventions they require [6, 7]. Overcoming these barriers and reducing the time to therapeutic antibiotic exposures is intended to lead to reductions in mortality and length of stay.
Consensus guidelines for the management of sepsis and septic shock in critically ill adults recommend strategies to optimise antibiotic dosing [8]. These include the use of therapeutic drug monitoring and dosing software to personalise dosing and increase the probability of achieving therapeutic drug exposures, while minimising toxicity [9]. Therapeutic drug monitoring (TDM) improves the probability of target attainment of beta lactam antibiotics [10]. Dosing software may complement TDM by assisting clinicians to identify a dosing strategy most likely to achieve target exposures. The place of TDM was articulated in a recent Position paper endorsed by specialist international societies which recommended it as standard of care for most antimicrobials in ICU [11], and was recommended for consideration in the 2021 Surviving Sepsis Campaign guidelines [8]. Software using Bayesian statistics has embedded pharmacokinetic (PK) models that use patient and TDM data to estimate the drug’s PK in that patient and, therefore, develop a dosing regimen that is optimal for the individual patient at that point in time [12, 13]. A small number of studies have demonstrated improved clinical response, reduced antibiotic associated adverse reactions, and decreased length of hospital stay and treatment costs using dosing software for aminoglycosides and vancomycin [14,15,16,17,18].
We set out to determine the feasibility and clinical impact of a personalised approach to antibiotic dosing using the Bayesian dosing software Individually Designed Optimum Dosing Strategies (ID-ODS™), in critically ill children and adults with sepsis in the ICU.
Methods
Study design
The DIRECT (Optimising Treatment Outcomes for Children and Adults Through Rapid Genome Sequencing of Sepsis Pathogens) study was a prospective, non-randomised multicentre interventional trial combining rapid pathogen sequencing with dosing software to optimise antibiotic exposures in children and adults with sepsis in ICU [19]. We have separately reported the results of direct metagenomic sequencing from blood culture broth which indicate its feasibility and potential to reduce time to antibiotic susceptibility prediction [20].
The study was conducted at the following ICUs in Brisbane, Australia: Royal Brisbane and Women’s Hospital, The Prince Charles Hospital, Princess Alexandra Hospital and Queensland Children’s Hospital. The study protocol was approved by the Children’s Health Queensland Human Research Ethics Committee (HREC/19/CHQ/55177) and local governance approval was obtained from participating sites. The study protocol was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12620001122943) and is reported in line with the CONSORT extension to pilot and feasibility trials [21] with the recommended adaptions [22].
Participants
Inpatients in participating ICUs were considered eligible for inclusion if they developed sepsis or septic shock within 24 h of screening and were receiving antibiotic treatment with one of the following antibiotics: cefotaxime, ceftazidime, flucloxacillin, meropenem, piperacillin–tazobactam or vancomycin. The full inclusion and exclusion criteria are documented in supplementary Table 1.
Procedures
The methods for pathogen identification and antimicrobial susceptibility were those validated for clinical use at Pathology Queensland. Blood cultures were performed using the BACT/Alert Virtuo System. Species identification was performed using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF, Vitek MS, bioMérieux) on pure cultured isolates, with antimicrobial susceptibility testing (AST) performed by Vitek 2 automated broth microdilution (N-246 AST cards; bioMérieux), using EUCAST clinical breakpoints applicable at the time [23]. For certain species (e.g., Streptococcus pyogenes), AST was undertaken using disk diffusion according to EUCAST methods [24], or by Etest (bioMérieux) where appropriate (e.g., penicillin for Streptococcus pneumoniae).
In Phase 1, antibiotic dosing was solely determined by the treating team. In Phase 2, participants who failed to achieve target antibiotic exposures within the first 24 h of therapy underwent dose optimisation by clinicians trained to use the Bayesian dosing software ID-ODS™. ID-ODS™ incorporates both paediatric and adult PK antibiotic models developed in critically ill patients, and appropriate models were used in each patient group. Treating clinicians applied software-guided dosing recommendations at their discretion. Target exposures for the beta-lactam antibiotics were unbound concentrations exceeding the minimum inhibitory concentration (MIC) of the pathogen (in blood culture-confirmed sepsis). These were either trough concentrations for beta-lactams administered as an intermittent infusion or steady-state concentrations for beta-lactams administered as a continuous infusion. In the setting of culture-negative sepsis and accounting for the most common local pathogens, the following EUCAST clinical breakpoint MICs were used (www.eucast.org/clinical_breakpoints/):
• cefotaxime—2 mg/L
• ceftazidime—8 mg/L
• flucloxacillin—2 mg/L
• piperacillin/Tazobactam—16 mg/L
• meropenem—2 mg/L
For vancomycin, target exposures were defined as either trough concentrations between 15 mg/L and 20 mg/L for intermittent infusions, or steady-state concentrations between 20 mg/L and 25 mg/L for continuous infusions. ID-ODS™ allows a target range to be inputted and selects the lowest dosing option needed to achieve a trough concentration between the range. These target exposures were selected based on a recently published position paper describing target exposures for various antimicrobials in critically ill patients [11]. All antibiotic concentrations were collected every 24 h after antibiotic commencement for up to 96 h.
In both phases of the study, the time that target exposures were achieved was identified through a posteriori ID-ODS™ antibiotic exposure simulations as has previously been described [25]. Patients who did not achieve target concentrations by the end of their study enrolment were assigned a time to target of 96 h for data analysis purposes.
Outcomes
We compared study outcomes in participants before (Phase 1) and after (Phase 2) the study intervention. The primary outcome was time to target antibiotic exposures.
Economic evaluation of the intervention
A cost–benefit approach was undertaken. The cost of the intervention was estimated using a combination of bottom-up and top-down costing. The cost components of the intervention include: the cost of antibiotic assay of patient blood samples (therapeutic drug monitoring), the clinician (pharmacist) cost of service based on simulation runs, obtained from Queensland Health wage rates for health practitioners (HP5, Level 1 https://www.health.qld.gov.au/hrpolicies/wage-rates/health-practitioners), the cost of dosing simulation with ID-ODS™, and the cost of the server line [A. Farkas, personal communication, February 6, 2023]. The cost of dosing simulation and server line were adjusted to 2022 AU$ following the guidelines of the Campbell and Cochrane Economics Methods Group (CCEGM) and the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre) https://eppi.ioe.ac.uk/costconversion/default.aspx. The benefit of the intervention was measured as the cost savings resulting from reduced length of ICU stay due to ID-ODS™ for patients in Phase 2. The cost of ICU stay per day was estimated from the Australian ICU registry report [26], and adjusted to 2022 value using 2022/2014 Health Price Index of Australia https://www.abs.gov.au/statistics/economy/price-indexes-and-inflation/consumer-price-index-australia/latest-release. Where the benefit–cost ratio (BCR) is > 1, it indicates that the benefits of the intervention outweigh the costs and vice versa.
Statistics
Characteristics of study participants and time to target antibiotic exposures are presented as medians with interquartile ranges (IQR). Time taken to target antibiotic exposure was compared between patients in Phase 1 and 2 using a Mann–Whitney U test. Kaplan–Meier plots were used to assess the likelihood of achieving target exposures at 96 h. A two-sided p value < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPadPrism version 9.
Results
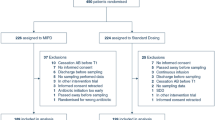
Screening and enrolment occurred from March 2020 to Feb 2021 for Phase 1 and March 2021 to December 2021 for Phase 2. A total of 201 patients were recruited into the trial. 156 patients were included in the analysis (108 patients with 123 antibiotic courses in Phase 1 and 48 patients with 52 antibiotic courses in Phase 2, see Fig. 1). A total of 321 antibiotic concentrations were collected in Phase 1 and 154 in Phase 2. The baseline characteristics of participants in each phase are reported in Table 1. Trough concentrations could not be accurately simulated in 6 patients (five in Phase 1 and one in Phase 2) as their antibiotic measurements were too far removed from the accepted range of possible concentrations set by ID-ODS™ based on the underlying PK model and patient covariate data. A bacterial pathogen was isolated from blood cultures of 48 patients (36/108 in Phase 1 and 12/48 in Phase 2).
No statistically significant difference in the primary outcome of median time taken to achieve target exposures was observed (8.7 h [interquartile range (IQR) 5.9–96] vs 14.3 h [IQR 7.3–32.0] in Phase 1 and Phase 2, respectively, p = 0.45, see Fig. 2A). However, in participants who did not achieve target exposures within the first 24 h of antibiotic therapy (n = 36 patients with 41 antibiotic courses in Phase 1, and n = 18 patients with 20 antibiotic courses in Phase 2), the use of ID-ODS™ was associated with a reduction in median time to target antibiotic exposure (96 h [IQR 96–96] vs 36.4 h [IQR 29.8–42.6] in Phase 1 and Phase 2, respectively, p < 0.01, see Fig. 2B). These findings were consistent in children and adults separately. A breakdown of adult and paediatric data is provided in the supplement.
Participants in Phase 2 who failed to achieve target antibiotic exposures within the first 24 h were much less likely to exhibit subtherapeutic antibiotic exposures at 96 h following the dosing intervention (hazard ratio (HR) 0.02, 95% confidence interval (CI) 0.01–0.05, see Fig. 3A). Again, this finding was consistent in both adults and children (HR in adults 0.08, 95% CI 0.01–0.55). The breakdown of adult and paediatric data is provided in supplementary Fig. 3B, C. There was no difference observed in in-hospital mortality (assessed up to 60 days) following the introduction of ID-ODS™ (11/108 vs 5/48 in Phase 1 and Phase 2, respectively; see supplementary Fig. 4 and supplementary Table 2).
Dosing Software recommendations
ID-ODS™ was used to generate dosing recommendations for 20 antibiotic courses (in 18 participants). All suggestions were accepted by the treating teams. Seventeen patients achieved target exposures after one dose change (85%), with one requiring two dose changes. Two patients did not achieve target antibiotic exposures after one dose change but were discharged from the ICU before a second recommendation could be made. The most common software recommendation was to extend the infusion time (8/20) (keeping the total daily antibiotic dose the same), or to convert from an intermittent infusion to a continuous infusion (4/20). A total daily dose increase was recommended in the remaining 8 patients. There were no adverse events associated with the dosing software recommendations.
Cost and benefit of the intervention
The cost of the intervention per patient was estimated to be $71.13. The intervention was associated with a reduction in the length of ICU stay by 2.13 days, which translates to a potential benefit of $12,324.28 (95% CI $9,520.93–$15,127.62) from averted ICU stay (supplementary Table 3).
Discussion
In this prospective, multi-centre trial of critically ill children and adults with sepsis, we demonstrated that it is feasible to employ Bayesian dosing software (ID-ODS™) in the ICU. Use of dosing software in patients who did not achieve target antibiotic exposures within 24 h of commencing antibiotics was associated with a reduction in the time taken to achieve target exposures. This finding was consistent in both children and adults separately.
Emerging evidence supports the potential for TDM in combination with dosing software to attain target antibiotic exposures and improve clinical outcomes. In the TARGET clinical trial, the use of TDM-guided antibiotic therapy increased the proportion of patients that exceeded the minimum target exposure from 65% to 77%, though the trial failed to confirm any improved clinical outcomes. The study was limited by a marginal increase in minimum target exposure attainment which may have been amenable to improvement through the addition of dosing software [27]. The recently published DOLPHIN trial is the first multicentre randomised controlled trial (RCT) to measure the impact of dosing software on clinical outcomes. Use of the model informed precision dosing (MIPD) software, InsightRX™, in combination with TDM failed to reduce ICU length of stay. There are several things to consider when interpreting the results of this important trial. Firstly, the use of MIPD software was delayed by up to 36 h. A conservative dosing strategy was used, limiting dose changes proposed by the MIPD software and restricting the maximum daily dose allowed (for example, a maximum cumulative dose of 16 g–2 g of piperacillin–tazobactam within a 24-h period). Participants were included in the analysis even if the infectious pathogen was not susceptible to the study drug. Finally, some of the models used in the MIPD software may have been unable to accurately predict target exposures as the models used total antibiotic concentrations while the investigators used unbound concentrations for TDM measurements [28]. Commentators have highlighted the absence of external validation of some of the models used by the software [29], while a recent multicentre evaluation of 24 popPK piperacillin models illustrated substantial inter-model variability leading to highly variable predictive performance [30]. However, meta-analyses of individualised antimicrobial dose optimisation confirm the achievement of higher rates of target exposures and have suggested reductions in treatment failure and adverse outcomes, such as nephrotoxicity [31, 32]. Practical barriers to dose optimisation include the time required to measure and report antibiotic concentrations in the laboratory. Innovative approaches such as wearable biosensors that provide rapid quantification of antibiotic concentrations, could reduce these delays and facilitate earlier TDM and dose optimisation [33]. More work is undoubtedly necessary to assess the benefit of the approach [34], and it is hoped that findings from our study provide further impetus to examine this important research question in the context of a suitably powered randomised clinical trial.
We observed a reduction in costs associated with the dosing software intervention, attributable to a reduction in LOS in patients in Phase 2. For every $1 spent to optimise treatment outcomes with the intervention, this translated into a potential cost saving of $173.27 (95% CI $133.86–$212.69). Our cost analysis assumed the cost of ICU each day was the same by applying the average cost of ICU per day from the Australia ICU registry data, whereas the actual cost per day may vary [35]. Our inability to collect patient-level cost data during the study led to the use of an average national estimate to value the cost of ICU stay in this study. The estimated cost saving is indicative only and will vary from country to country depending on patient case-mix and local healthcare costs. There was unmeasured variation between the two phases of the study which we did not adjust for (including factors unrelated to infection), and so our economic evaluation should be interpreted with caution.
Strengths
Our study presents a real-world evaluation of Bayesian dosing software to optimise antibiotic exposures in four ICUs, including a children’s ICU. We prospectively recruited more than 200 critically ill patients with sepsis. The dosing software intervention was successfully implemented by clinical pharmacists trained in its use, and dosing decisions made at the discretion of the treating clinical team. All dosing recommendations were accepted by the treating clinicians, and while this may reflect the expertise in the present academic ICU setting, our multi-centre design provides assurance that the use of dosing software can be feasibly applied in a range of ICU settings.
We elected to include both children and adults in our trial for several reasons. Children are often excluded from clinical trials, including antimicrobial clinical trials, resulting in persisting knowledge gaps [36]. The study investigators share considerable clinical and research expertise in paediatric and adult critical care, infectious diseases, and antimicrobial optimisation, and considered that as the trial was designed to test feasibility and measure endpoints that were suitable for both adults and children, there was a valuable opportunity to explore the feasibility of the intervention in the children’s ICU. The inclusion of clinical endpoints such as mortality or length of hospital stay would have made this more challenging [37]. The software-guided dosing recommendations for children were all derived from paediatric pharmacokinetic models and the successful adoption of the dosing software, including the demonstration of its impact in children, are valuable learnings. Our results were consistent across the adult and paediatric cohorts, and we believe our findings are broadly applicable to both adults and children critically unwell with sepsis.
Limitations
The limitations of our pre–post-quasi-experimental design are acknowledged. In addition, although dosing software was associated with a significant reduction in time to target exposures, we acknowledge the small number of participants who did not achieve target concentrations and accept there is residual uncertainty over the finding. The largely effective nature of empiric dosing in this study may contribute to the similar clinical outcomes observed, noting that the study was not powered to assess these. Our findings suggest, however, that dosing software (in this case ID-ODS™) is a suitable dosing intervention that can achieve high rates of target attainment and should be evaluated in trials for its impact on clinical outcomes. Approximately 30% of participants had culture-confirmed sepsis, which is slightly lower than other ICU sepsis studies [2]. Though this is broadly representative of sepsis in high-income ICUs, the inclusion of patients with non-bacterial sepsis may reduce the impact of the intervention. Advanced infection diagnostics, particularly the clinical application of pathogen metagenomics [20, 38] could improve the selection of patients and better target antibiotic therapy, thereby enhancing the impact of TDM and dosing interventions.
As our study performed convenience sampling for TDM within each 24-h interval, we had to perform a posteriori pharmacokinetic modelling using ID-ODS™ to estimate the time target exposures were achieved. This method has previously demonstrated low bias at predicting actual antibiotic exposures [39, 40]. Finally, we were unable to include data from 6 patients as their clinical characteristics were too far removed from the underlying PK model in ID-ODS™.
Conclusion
In a multi-centre study of critically ill children and adults with sepsis, the introduction of Bayesian dosing software (ID-ODS™) reduced time to target antibiotic exposures in those who failed to achieve target exposure in the first 24 h. The impact of this observation on clinical outcomes in sepsis should be evaluated further in the context of a randomised clinical trial. Work to improve the speed of pathogen identification that can be rapidly linked to dose optimisation remains a priority.
Data availability
De-identified clinical data are stored in the UQ Research Data Management system and are not publically available. They may be made available upon request to the corresponding authors.
Change history
02 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00134-024-07393-9
References
Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd KE, Schlattmann P, Allegranzi B, Reinhart K (2020) Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intens Care Med 46:1552–1562. https://doi.org/10.1007/s00134-020-06151-x
Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, Slater A, Group APS (2015) Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis 15:46–54. https://doi.org/10.1016/S1473-3099(14)71003-5
Kumar A, Haery C, Paladugu B, Kumar A, Symeoneides S, Taiberg L, Osman J, Trenholme G, Opal SM, Goldfarb R, Parrillo JE (2006) The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis 193:251–258. https://doi.org/10.1086/498909
Evans IVR, Phillips GS, Alpern ER, Angus DC, Friedrich ME, Kissoon N, Lemeshow S, Levy MM, Parker MM, Terry KM, Watson RS, Weiss SL, Zimmerman J, Seymour CW (2018) Association between the New York sepsis care mandate and in-hospital mortality for pediatric sepsis. JAMA 320:358–367. https://doi.org/10.1001/jama.2018.9071
Tabak YP, Vankeepuram L, Ye G, Jeffers K, Gupta V, Murray PR (2018) Blood culture turnaround time in U.S. acute care hospitals and implications for laboratory process optimization. J Clin Microbiol. https://doi.org/10.1128/jcm.00500-18
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J (2014) DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. https://doi.org/10.1093/cid/ciu027
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL (2014) Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. https://doi.org/10.1016/s1473-3099(14)70036-2
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Moller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 47:1181–1247
Tangden T, Ramos Martin V, Felton TW, Nielsen EI, Marchand S, Bruggemann RJ, Bulitta JB, Bassetti M, Theuretzbacher U, Tsuji BT, Wareham DW, Friberg LE, De Waele JJ, Tam VH, Roberts JA, Infection Section for the European Society of Intensive Care Medicine tP, Pharmacodynamics Study Group of the European Society of Clinical M, Infectious Diseases tISoA-IP, the Critically Ill Patients Study Group of European Society of Clinical M, Infectious D (2017) The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med 43:1021–1032. https://doi.org/10.1007/s00134-017-4780-6
De Waele JJ, Carrette S, Carlier M, Stove V, Boelens J, Claeys G, Leroux-Roels I, Hoste E, Depuydt P, Decruyenaere J, Verstraete AG (2014) Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med 40:380–387. https://doi.org/10.1007/s00134-013-3187-2
Abdul-Aziz MH, Alffenaar JC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, Neely MN, Paiva JA, Pea F, Sjovall F, Timsit JF, Udy AA, Wicha SG, Zeitlinger M, De Waele JJ, Roberts JA, Infection Section of European Society of Intensive Care M, Pharmacokinetic/pharmacodynamic, Critically Ill Patient Study Groups of European Society of Clinical M, Infectious D, Infectious Diseases Group of International Association of Therapeutic Drug M, Clinical T, Infections in the ICU, Sepsis Working Group of International Society of Antimicrobial C (2020) Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med 46:1127–1153. https://doi.org/10.1007/s00134-020-06050-1
Chai MG, Cotta MO, Abdul-Aziz MH, Roberts JA (2020) What are the current approaches to optimising antimicrobial dosing in the intensive care unit? Pharmaceutics 12:638. https://doi.org/10.3390/pharmaceutics12070638
Jager NGL, Chai MG, van Hest RM, Lipman J, Roberts JA, Cotta MO (2022) Precision dosing software to optimise antimicrobial dosing: a systematic search and follow-up survey of available programs. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2022.03.041
Burton ME, Ash CL, Hill DP Jr, Handy T, Shepherd MD, Vasko MR (1991) A controlled trial of the cost benefit of computerized bayesian aminoglycoside administration. Clin Pharmacol Ther 49:685–694. https://doi.org/10.1038/clpt.1991.86
Neely MN, Kato L, Youn G, Kraler L, Bayard D, van Guilder M, Schumitzky A, Yamada W, Jones B, Minejima E (2018) Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother 62:e02042-e2017. https://doi.org/10.1128/AAC.02042-17
Zhang Y, Wang TT, Zhang D, You HS, Dong YZ, Liu Y, Du Q, Sun D, Zhang T, Dong YL (2020) Therapeutic drug monitoring coupled with bayesian forecasting could prevent vancomycin-associated nephrotoxicity in renal insufficiency patients: a prospective study and pharmacoeconomic analysis. Therapeutic Drug Monitor 42:600–609. https://doi.org/10.1097/Ftd.0000000000000750
Sabourenkov PE, McLeay RC (2019) 1599. AUC24 vancomycin bayesian-based dosing: increasing therapeutic target attainment with decreased TDM cost. Open Forum Infect Dis 6:S583–S583. https://doi.org/10.1093/ofid/ofz360.1463
van Lent-Evers NA, Mathot RA, Geus WP, van Hout BA, Vinks AA (1999) Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit 21:63–73. https://doi.org/10.1097/00007691-199902000-00010
Irwin AD, Coin LJM, Harris PNA, Cotta MO, Bauer MJ, Buckley C, Balch R, Kruger P, Meyer J, Shekar K, Brady K, Fourie C, Sharp N, Vlad L, Whiley D, Beatson SA, Forde BM, Paterson D, Clark J, Hajkowicz K, Raman S, Bialasiewicz S, Lipman J, Schlapbach LJ, Roberts JA (2021) Optimising treatment outcomes for children and adults through rapid genome sequencing of sepsis pathogens. A study protocol for a prospective, multi-centre trial (DIRECT). Front Cell Infect Microbiol 11:667680. https://doi.org/10.3389/fcimb.2021.667680
Harris PNA, Bauer MJ, Luftinger L, Beisken S, Forde BM, Balch R, Cotta M, Schlapbach L, Raman S, Shekar K, Kruger P, Lipman J, Bialasiewicz S, Coin L, Roberts JA, Paterson DL, Irwin AD (2024) Rapid nanopore sequencing and predictive susceptibility testing of positive blood cultures from intensive care patients with sepsis. Microbiol Spectr. https://doi.org/10.1128/spectrum.03065-23
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA, Group PC (2016) CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 355:i5239. https://doi.org/10.1136/bmj.i5239
Lancaster GA, Thabane L (2019) Guidelines for reporting non-randomised pilot and feasibility studies. Pilot Feasibil Stud 5:114. https://doi.org/10.1186/s40814-019-0499-1
European Committee on Antimicrobial Susceptibility Testing (2019) Breakpoint tables for interpretation of MICs and zone diameters version 9. In: Editor (ed)^(eds) Book Breakpoint tables for interpretation of MICs and zone diameters version 9. EUCAST, City, pp
Matuschek E, Brown DF, Kahlmeter G (2014) Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect 20:O255–O266. https://doi.org/10.1111/1469-0691.12373
Chai MG, Roberts JA, Farkas A, Cotta MO (2022) Accuracy of a precision dosing software program for predicting antibiotic concentrations in critically ill patients. J Antimicrob Chemother 78:354–358. https://doi.org/10.1093/jac/dkac392
Hicks P, Huckson S, Fenney E, Leggett I, Pilcher D, Litton E (2019) The financial cost of intensive care in Australia: a multicentre registry study. Med J Aust 211:324–325. https://doi.org/10.5694/mja2.50309
Hagel S, Bach F, Brenner T, Bracht H, Brinkmann A, Annecke T, Hohn A, Weigand M, Michels G, Kluge S, Nierhaus A, Jarczak D, König C, Weismann D, Frey O, Witzke D, Müller C, Bauer M, Kiehntopf M, Neugebauer S, Lehmann T, Roberts JA, Pletz MW (2022) Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: a randomized controlled trial. Intensive Care Med 48:311–321. https://doi.org/10.1007/s00134-021-06609-6
Ewoldt TMJ, Abdulla A, Rietdijk WJR, Muller AE, de Winter BCM, Hunfeld NGM, Purmer IM, van Vliet P, Wils EJ, Haringman J, Draisma A, Rijpstra TA, Karakus A, Gommers D, Endeman H, Koch BCP (2022) Model-informed precision dosing of beta-lactam antibiotics and ciprofloxacin in critically ill patients: a multicentre randomised clinical trial. Intensive Care Med. https://doi.org/10.1007/s00134-022-06921-9
Liebchen U, Briegel J, Brinkmann A, Frey O, Wicha SG (2023) Individualised dosing of antibiotics in ICU patients: timing, target and model selection matter. Intensive Care Med 49:475–476. https://doi.org/10.1007/s00134-023-06990-4
Greppmair S, Brinkmann A, Roehr A, Frey O, Hagel S, Dorn C, Marsot A, El-Haffaf I, Zoller M, Saller T, Zander J, Schatz LM, Scharf C, Briegel J, Minichmayr IK, Wicha SG, Liebchen U (2023) Towards model-informed precision dosing of piperacillin: multicenter systematic external evaluation of pharmacokinetic models in critically ill adults with a focus on Bayesian forecasting. Intensive Care Med 49:966–976. https://doi.org/10.1007/s00134-023-07154-0
Codina MS, Bozkir H, Jorda A, Zeitlinger M (2023) Individualised antimicrobial dose optimisation: a systematic review and meta-analysis of randomised controlled trials. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2023.03.018
Pai Mangalore R, Ashok A, Lee SJ, Romero L, Peel TN, Udy AA, Peleg AY (2022) Beta-lactam antibiotic therapeutic drug monitoring in critically ill patients: a systematic review and meta-analysis. Clin Infect Dis 75:1848–1860. https://doi.org/10.1093/cid/ciac506
Brasier N, Ates HC, Sempionatto JR, Cotta MO, Widmer AF, Eckstein J, Goldhahn J, Roberts JA, Gao W, Dincer C (2023) A three-level model for therapeutic drug monitoring of antimicrobials at the site of infection. Lancet Infect Dis 23:e445–e453. https://doi.org/10.1016/s1473-3099(23)00215-3
Cotta MO, Lipman J, De Waele J (2023) Advancing precision-based antimicrobial dosing in critically ill patients. Intensive Care Med. https://doi.org/10.1007/s00134-022-06969-7
Gershengorn HB, Garland A, Gong MN (2015) Patterns of daily costs differ for medical and surgical intensive care unit patients. Annals Am Thor Soc 12:1831–1836. https://doi.org/10.1513/AnnalsATS.201506-366BC
Thompson G, Barker CI, Folgori L, Bielicki JA, Bradley JS, Lutsar I, Sharland M (2017) Global shortage of neonatal and paediatric antibiotic trials: rapid review. BMJ Open 7:e016293. https://doi.org/10.1136/bmjopen-2017-016293
Tanaudommongkon I, John Miyagi S, Green DJ, Burnham JM, van den Anker JN, Park K, Wu J, McCune SK, Yao L, Burckart GJ (2020) Combined pediatric and adult trials submitted to the US food and drug administration 2012–2018. Clin Pharmacol Ther 108:1018–1025. https://doi.org/10.1002/cpt.1886
Edgeworth JD (2023) Respiratory metagenomics: route to routine service. Curr Opin Infect Dis 36:115–123. https://doi.org/10.1097/QCO.0000000000000909
Felton TW, Roberts JA, Lodise TP, Van Guilder M, Boselli E, Neely MN, Hope WW (2014) Individualization of piperacillin dosing for critically ill patients: dosing software to optimize antimicrobial therapy. Antimicrob Agents Chemother 58:4094–4102. https://doi.org/10.1128/AAC.02664-14
Wong G, Farkas A, Sussman R, Daroczi G, Hope WW, Lipman J, Roberts JA (2015) Comparison of the accuracy and precision of pharmacokinetic equations to predict free meropenem concentrations in critically ill patients. Antimicrob Agents Chemother 59:1411–1417. https://doi.org/10.1128/AAC.04001-14
Acknowledgements
The authors would like to thank the patients and families for consenting to participate in this study. In addition, we would like to acknowledge the support provided by the intensive care clinical pharmacy team at the Royal Brisbane and Women’s Hospital, The Princess Alexandra Hospital, The Prince Charles Hospital and Queensland Children’s Hospital. The authors would like to acknowledge Scott Beatson for his valuable contribution to the original design of the study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by the Queensland Genomics Health Alliance (QGHA) Round 2 (now Queensland Genomics) clinical implementation, innovation and incubation program and by a Brisbane Diamantina Health Partners Health System Improvement Ideas Grant (MRFF Rapid Applied Research Translation Program). MGC is supported by a NHMRC Postgraduate scholarship (APP2002981). ADI is supported by a NHMRC Emerging Leadership Investigator Award (APP1197743). LJS is supported by a NHMRC practitioner Fellowship (APP1161657) and by Children’s Hospital Foundation, Brisbane, Australia. PNAH is supported by a NHMRC Early Career Fellowship (APP1157530). JAR is supported by a NHMRC Centre for Research Excellence Award (APP2007007) and an Investigator Award (APP2009736). None of the funding bodies had any involvement of the design, undertaking or interpretation of the study.
Author information
Authors and Affiliations
Contributions
ADI, JAR, LJS, LC, PNAH and DLP conceived the study. LJS and JAR secured funding. ADI, JAR, LJS, LC, PNAH, DLP, SB, JL, SR, KS, PK, LV were members of the steering committee. KB, CF, NS, JM led study recruitment in each participating ICU. MOC, MGC, QT, KB led the dosing software implementation. RB, SB, LC developed the methods for and performed metagenomic sequencing. JU, BM, MJB, DW oversaw laboratory analysis. AF designed the dosing software. CO and TC carried out the health economic evaluation. MGC, MOC, JAR, LC, RB, SB, PNAH, BNF analysed laboratory data. MGC, MOC, ADI and JAR drafted the manuscript. All authors contributed to the review and editing of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
AF is the developer of ID-ODS™.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chai, M.G., Tu, Q., Cotta, M.O. et al. Achievement of therapeutic antibiotic exposures using Bayesian dosing software in critically unwell children and adults with sepsis. Intensive Care Med 50, 539–547 (2024). https://doi.org/10.1007/s00134-024-07353-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-024-07353-3