Abstract
Purpose
Hypercapnia is frequent during mechanical ventilation for acute respiratory distress syndrome (ARDS), but its effects on morbidity and mortality are still controversial. We conducted a systematic review and meta-analysis to explore clinical consequences of acute hypercapnia in adult patients ventilated for ARDS.
Methods
We searched Medline, Embase, and the Cochrane Library via the OVID platform for studies published from 1946 to 2021. “Permissive hypercapnia” defined hypercapnia in studies where the group with hypercapnia was ventilated with a protective ventilation (PV) strategy (lower VT targeting 6 ml/kg predicted body weight) while the group without hypercapnia was managed with a non-protective ventilation (NPV); “imposed hypercapnia” defined hypercapnia in studies where hypercapnic and non-hypercapnic patients were managed with a similar ventilation strategy.
Results
Twenty-nine studies (10,101 patients) were included. Permissive hypercapnia, imposed hypercapnia under PV, and imposed hypercapnia under NPV were reported in 8, 21 and 1 study, respectively. Studies testing permissive hypercapnia reported lower mortality in hypercapnic patients receiving PV as compared to non-hypercapnic patients receiving NPV: OR = 0.26, 95% CI [0.07–0.89]. By contrast, studies reporting imposed hypercapnia under PV reported increased mortality in hypercapnic patients receiving PV as compared to non-hypercapnic patients also receiving PV: OR = 1.54, 95% CI [1.15–2.07]. There was a significant interaction between the mechanism of hypercapnia and the effect on mortality.
Conclusions
Clinical effects of hypercapnia are conflicting depending on its mechanism. Permissive hypercapnia was associated with improved mortality contrary to imposed hypercapnia under PV, suggesting a major role of PV strategy on the outcome.
Similar content being viewed by others
We found conflicting clinical effects of hypercapnia during ARDS depending on its mechanism. | |
The protective effects of permissive hypercapnia seemed driven by protective ventilation while the deleterious effects of imposed hypercapnia seemed mediated by pulmonary vascular dysfunction. |
Introduction
Mechanical ventilation is a frequently used supportive technique for acute respiratory distress syndrome (ARDS). The main purpose of mechanical ventilation in this setting is to maintain oxygenation, lower oxygen consumption and reduce respiratory work. Despite the clear benefits of this therapy, the mechanical forces generated by the ventilator can cause worsening injury in previously damaged lungs (ventilator induced lung injury, VILI). To minimize VILI, a strategy of lung protective ventilation (PV) involving lower tidal volume (VT targeting 6 ml/kg predicted body weight) is recommended [1]. In practice, PV may elevate carbon dioxide (CO2) levels in the blood inducing hypercapnia. “Permissive” hypercapnia, which results from lowering VT to achieve PV is, therefore, generally accepted to minimize VILI. In addition, some authors have suggested a specific beneficial role for hypercapnia in the experimental setting [2].
Recent evidence suggests that acute hypercapnia could have harmful physiological and clinical effects in patients with ARDS, particularly impacting the hemodynamic system [3,4,5]. The fact that hypercapnia is specifically driven by VT reduction from non-protective ventilation (NPV) to PV (“permissive” hypercapnia) or is rather the result of ARDS severity, may have a major role in its net clinical effect, given the associated benefits of PV on VILI and survival [1, 6].
The aim of the current review and meta-analysis was to summarize the clinical consequences of acute hypercapnia in mechanically ventilated patients while considering its mechanism (“permissive” or not). The primary objective was to determine the association between acute hypercapnia and mortality in adult patients mechanically ventilated for ARDS. The secondary objective was to identify association between acute hypercapnia and hemodynamics (systemic and pulmonary circulation) in adult patients mechanically ventilated for ARDS.
Methods
Search strategy and selection criteria
We performed this study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [7]. The following electronic databases were searched via the OVID platform on November 2018: MEDLINE® In-Process & Other Non-Indexed Citations, MEDLINE (1946 to present), Embase (1980 to present), The Cochrane Library, incorporating the Cochrane Database of Systematic Reviews (Cochrane Reviews), the Database of Abstracts of Reviews of Effects, the Cochrane Central Register of Controlled Trials, the Health Technology Assessment Database, and the NHS Economic Evaluation Database. To identify any recent studies for which there are currently no full publications, the following conference proceedings were examined for relevant abstracts (and posters/slide decks, if available) from 2011 to 2018: American Thoracic Society, European Respiratory Society, European Society of Intensive Care Medicine, International Symposium on Intensive Care and Emergency Medicine, International Society for Pharmacoeconomics and Outcomes Research (International and European meeting), and Society for Critical Care Medicine. Research was updated on November 2021 with the same research method. The search strategies used are detailed in online resource, Appendix A. Potentially relevant studies were screened by two independent reviewers in separate databases. We included all studies with mechanically ventilated patients reporting acute hypercapnia with no restriction on severity of hypercapnia, intervention, countries, or study design (we included cross-sectional studies, case–control studies, cohort studies, database/registries analyses, hospital records analyses, and randomized controlled trials). We excluded studies in children and animals and focused primarily on studies written in English. After the removal of the duplicates, two reviewers independently screened titles and abstracts to obtain relevant articles for full text analysis (first pass). Full-text publications of all potentially relevant citations identified at first pass were reviewed for eligibility (2nd pass). Eligible papers were then independently selected for inclusion if they involved adult patients fulfilling ARDS criteria as per the Berlin definition (considering “acute lung injury” as per the previous definition as mild ARDS; 3rd pass) [8]. Papers on the use of extracorporeal carbon dioxide removal for ultraprotective ventilation were excluded. Any disagreement was resolved by discussion with a third reviewer. This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (registration number CRD42020159018). Ethical approval was not required.
Data analysis
The following data were independently extracted by the review authors from each selected study: year of publication, study design, PaCO2, and ventilation strategy (defined as PV if targeting 6 ml/kg predicted body weight of VT [1] and NPV otherwise), hemodynamics (pulmonary and systemic circulation) and mortality.
For quality (risk of bias) assessment, we used the risk-of-bias tool (RoB2) [9] for randomized controlled trials (RCTs) and the Quality Assessment Tool for Quantitative Studies produced as part of the Effective Public Health Practice Project for observational studies (including prospective interventional studies) [10]. For every study, each component was rated as: strong, moderate or weak, and used to assign an overall rating for the study.
Definitions
We used the term “permissive hypercapnia” to define hypercapnia in studies where the group with hypercapnia was ventilated with a PV strategy (lower VT targeting 6 ml/kg predicted body weight) while the group without hypercapnia was managed with a NPV strategy. We used the term “imposed hypercapnia under PV” to define hypercapnia in studies were hypercapnic and non-hypercapnic patients were both managed with a PV strategy. We used the term “imposed hypercapnia under NPV” to define hypercapnia in studies were hypercapnic and non-hypercapnic patients were both managed with a NPV strategy [11]. Hypercapnia was primarily the result of the chosen ventilation strategy (PV or NPV), and the strategy was mostly guided irrespective of PaCO2 values.
Statistical analysis
We conducted a meta-analysis of observational prospective and retrospective studies. Data were summarized using medians and interquartile ranges (IQRs) or mean ± standard deviation (SD) where appropriate [12]. The odds ratio (OR) with 95% confidence interval (CI) was calculated for death.
We adopted a random effect model with Mantel–Haenszel method for individual study effects, to assess the population OR and 95% confidence interval for death according to hypercapnia. We used the Knapp–Hartung adjustment for test statistics and confidence intervals [13]. Between study variances and their square roots was adjusted by the Sidik–Jonkman estimator [14]. We quantified heterogeneity using I2 and Q statistics, with values greater than 50% regarded as being indicative of moderate-to-high heterogeneity [15]. To measure the dispersion of the pooled effect across study settings, we generated predictions intervals [16]. Results were visualized through forest plot.
We performed prespecified subgroup analyses according to the mechanism of hypercapnia (permissive or imposed). Test for differences in effect sizes between subgroups was performed using mixed-effect model, with a random-effect model for the overall effect size for each subgroup, and a fixed-effect model for subgroup differences [17]. Mortality was also assessed after exclusion of outliers and after exclusion of studies with COVID-19-related ARDS.
Heterogeneity was assessed graphically through L’Abbé plot [18]. Data were pooled and analyzed using R 4.1.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Studies
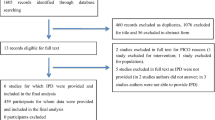
The overall flow of studies across the reviews is reported in the PRISMA flow diagram in Fig. 1. The electronic database searches identified a total of 5513 citations which were screened on the basis of title and abstract. At this stage, a total of 5786 articles were excluded, and 423 were deemed to be potentially relevant. These citations were retrieved for full publication review. Upon review of the full publications, a further 345 articles were excluded. Hand searching yielded eleven additional relevant papers, resulting in a total of 89 relevant publications from which 29 met the eligibility criteria of the review after the third pass. We, therefore, selected these 29 studies (10,101 patients) reporting the clinical consequences of hypercapnia in adults with ARDS for the present review [5, 11, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. All included studies were published as full publication. The sample size among the included studies varied from N = 4 [34] to N = 3642 [44]. An overview of all included studies is presented in Table 1. Two studies shared some patients [19, 20]: hemodynamic data were extracted from Amato 1995 [19], and mortality data from Amato 1998 [20]. Results from quality assessment checklist for included studies are presented in Table 2: for observational studies, 15 studies had overall weak rating (high risk of bias), eight had moderate rating and three had strong rating (low risk of bias). For RCTs, two studies had overall concerns, and one had low overall risk of bias. These bias are reported in Table 2. Due to the small number of studies, statistical tests to investigate for the presence of publication bias were not conducted.
Hypercapnia and tidal volumes
Definition of hypercapnia and VT used among the included studies have been captured in Table 1. A clear threshold for hypercapnia was reported in 11/29 of the included studies, that was defined as PaCO2 ≥ 38 mmHg [19], PaCO2 ≥ 45 mmHg [11, 25, 41], ≥ 48 mmHg [23], ≥ 50 mmHg [5, 28, 33, 35, 44] or ≥ 55 mmHg[34]. Information regarding VT were reported in all but three studies [5, 26, 27]. Permissive hypercapnia, imposed hypercapnia under PV, and imposed hypercapnia under NPV were reported in eight studies (218 patients) [19, 20, 22, 24, 25, 29, 31, 32], 21 studies (9514 patients) [5, 11, 21,22,23, 26,27,28, 30, 33,34,35,36,37,38,39,40,41,42,43,44] and one (369 patients) [11] study, respectively. The latter study [11] was unique for imposed hyperca pnia in NPV and reported < 1% (3/369) patients with sustained PaCO2 > 45 mmHg, precluding any further analysis of imposed hypercapnia under NPV.
Clinical consequenc es of acute hypercapnia
Mortality
Data for mortality were reported for hypercapnic and non-hypercapnic groups in three studies with permissive hypercapnia (157 patients) [19, 25, 45], and six others with imposed hypercapnia under PV (9,096 patients) [5, 11, 21, 23, 43, 44]. Studies testing permissive hypercapnia reported a lower mortality in hypercapnic patients receiving PV co mpared to non-hypercapnic patients receiving NPV (OR for random effect model = 0.26, 95% CI [0.07–0.89]). By contrast, studies reporting imposed hypercapnia under PV reported increased mortality in hypercapnic patients rece iving PV as compared to non-hypercapnic patients also receiving PV (OR for random ef fect model = 1.54, 95% CI [1.15–2.07]). There was a significant interaction between the mechanism of hypercapnia (permissive or imposed under PV) and the effect on mortality (p < 0.01) (Fig. 2), which persisted even after exclusion of outliers [43] (online resource, Appendix B) or exclusion of studies with ARDS related to coronavirus disease 2019 (COVID-19) [44] (online resource, Appendix C) (see Fig. 3).
Hemodynamics
The impact of hypercapnia on hemodynamic parameters was reported in seven studies [19, 22, 24, 28, 29, 31, 32] involving 102 patients (see Fig. 4). Permissive hypercapnia induced an increase in cardiac index/output [19, 24, 31, 32], which could be due to increased systemic vasodilation as evidenced by a decrease in systemic vascular resistances [29, 31, 32]. This increased cardiac index was associated with: (i) an increase in pulmonary shunt [24, 28, 31] (see Fig. 4), with deterioration in gas exchange [24, 28] in all but one [19] study reporting shunt data; (ii) increased pulmonary pressures [19, 24, 31, 32], but no significant change in pulmonary vascular resistances [24, 29, 31, 32]. During PV, imposed hypercapnia was associated with conflicting effects on cardiac index [22, 28] and worsened pulmonary vascular function [22, 28].
Forest plots of hemodynamic changes in hypercapnic and normocapnic patients according to their mechanism. Results are reported in mean difference (SD). Only two studies [22, 28] (N = 11 and N = 7) reported cardiac index in imposed hypercapnia under PV, and were not included in meta-analysis due to this limited number and high clinical heterogeneity
Discussion
To the best of our knowledge, we herein report the first review of the literature with meta-analysis on the clinical consequences of hypercapnia in adult patients with ARDS, with the following findings: (i) the clinical effects of hypercapnia were conflicting depending on the mechanism of hypercapnia; (ii) permissive hypercapnia was associated with improved survival whereas imposed hypercapnia under PV worsened mortality, suggesting a major role of the PV strategy on the outcome and indicating imposed hypercapnia as a marker of ARDS severity; (iii) permissive hypercapnia was associated with increased cardiac index whereas imposed hypercapnia yielded conflicting results with worsened lung vascular function.
Conflicting role of hypercapnia
Complex findings were observed across literature. From these findings, it appeared that hypercapnia is protective when driven by lower VT, but is associated with increased mortality when imposed at lower VT (targeting 6 ml/kg predicted body weight). Overall, PV is probably driving the protective effect of permissive hypercapnia, in accordance with observational cohorts [5], randomized trials [1] and recommendations [46]. By contrast, the association of imposed hypercapnia under PV with increased mortality indicates it could be a marker of ARDS severity and/or have own detrimental effects. The former point is in accordance with studies suggesting pulmonary dead space as a strong prognostic factor in ARDS [47]. The latter point is corroborated by the finding of more renal and cardiac failure in patients with imposed hypercapnia under PV [5]. The main hemodynamic effect of imposed hypercapnia under PV relates to pulmonary vascular dysfunction, with pulmonary hypertension and RV dysfunction, which could trigger or worsen renal failure via a decreased cardiac output and/or an increased congestion [48]. This pulmonary vasoconstrictive effect of hypercapnia is in accordance with previous data in critically ill patients with [49] or without [50] ARDS.
Altogether, our findings suggest that, in the clinical setting, (i) permissive hypercapnia to achieve PV should be preferred to normocapnia under NPV; (ii) normocapnia under PV could be preferred to imposed hypercapnia under PV. However, we are still lacking randomized trials to assess if mitigating imposed hypercapnia under PV via reduced CO2 production (e.g., hypothermia) or increased elimination (e.g., increased respiratory rate, and/or decreased instrumental dead space) alters clinical outcomes. Whether the use of extracorporeal CO2 removal for imposed hypercapnia under PV may improve outcomes [51] also require further studies. Future studies are similarly necessary to scrutinize the prognostic role of increased PaCO2 generated by ultra-protective ventilation (UPV i.e. VT targeting 3–4 ml/kg of predicted body weight), as compared to PV (i.e. VT targeting 6 ml/kg of predicted body weight), and its potential mitigation by extracorporeal CO2 removal [52]. In the recent REST randomized clinical trial, the use of extracorporeal CO2 removal to facilitate UPV, compared with PV, did not significantly reduce 90-day mortality and was associated with more serious adverse events [53].
Strengths and limitations
Strengths of our study include the wide period of assessment and selection process. Our search ended in 2021, and little new information has been published since on this topic, including for COVID-19-related ARDS. One limitation is the lack of standardization in the definition and duration of hypercapnia. However, we performed a subgroup analysis to scrutinize the respective roles of permissive and imposed hypercapnia. We cannot exclude that some part of the permissive hypercapnia in studies of PV is due to ARDS severity. There was heterogeneity among studies concerning their design (prospective or retrospective), tidal volume under PV (especially in observational cohorts), reporting of tidal volume related to predicted body weight, and hypercapnia definition. In addition, other potential confounding factors that might be associated with both hypercapnia and mortality were not taken into account. Last, we used the Berlin definition for ARDS, which was published after many studies included in the meta-analysis. However, included patients with acute lung injury before the Berlin definition were considered as having mild ARDS.
Conclusion
We performed a systematic review and meta-analysis of a wide population of adult patients with ARDS, and found conflicting clinical effects of hypercapnia depending on its mechanism. The favorable effects of permissive hypercapnia seemed driven by the associated PV, with improved hemodynamics. On the contrary, imposed hypercapnia under PV was associated with a worse outcome.
References
Brower RG, Matthay MA, Morris A et al (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Curley GF, Laffey JG, Kavanagh BP (2013) CrossTalk proposal: there is added benefit to providing permissive hypercapnia in the treatment of ARDS. J Physiol 591:2763–2765. https://doi.org/10.1113/jphysiol.2013.252601
Tiruvoipati R, Pilcher D, Buscher H et al (2017) Effects of hypercapnia and hypercapnic acidosis on hospital mortality in mechanically ventilated patients. Crit Care Med 45:e649–e656. https://doi.org/10.1097/CCM.0000000000002332
Helmerhorst HJF, Roos-Blom M-J, van Westerloo DJ et al (2015) Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit Care Lond Engl 19:348. https://doi.org/10.1186/s13054-015-1067-6
Nin N, Muriel A, Peñuelas O et al (2017) Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med 43:200–208. https://doi.org/10.1007/s00134-016-4611-1
Morales-Quinteros L, Camprubí-Rimblas M, Bringué J et al (2019) The role of hypercapnia in acute respiratory failure. Intensive Care Med Exp 7:39. https://doi.org/10.1186/s40635-019-0239-0
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. https://doi.org/10.1016/j.jclinepi.2009.06.005
Ranieri V, Rubenfeld G, Thompson B et al (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Quality Assessment Tool for Quantitative Studies. In: Eff. Public Healthc. Panacea Proj. https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/. Accessed 25 Jul 2021
Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER (2006) Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med 34:1–7. https://doi.org/10.1097/01.CCM.0000194533.75481.03
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. https://doi.org/10.1186/1471-2288-14-135
Borenstein M, Hedges L, Higgins J, Rothstein H (2021) Knapp–Hartung Adjustment. pp 243–249. https://doi.org/10.1002/9781119558378.ch26
Sidik K, Jonkman JN (2002) A simple confidence interval for meta-analysis. Stat Med 21:3153–3159. https://doi.org/10.1002/sim.1262
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Riley RD, Higgins JPT, Deeks JJ (2011) Interpretation of random effects meta-analyses. BMJ. https://doi.org/10.1136/bmj.d549
Borenstein M, Higgins JPT (2013) Meta-analysis and subgroups. Prev Sci 14:134–143. https://doi.org/10.1007/s11121-013-0377-7
L’Abbé KA, Detsky AS, O’Rourke K (1987) Meta-analysis in clinical research. Ann Intern Med 107:224–233. https://doi.org/10.7326/0003-4819-107-2-224
Amato MB, Barbas CS, Medeiros DM et al (1995) Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med 152:1835–1846. https://doi.org/10.1164/ajrccm.152.6.8520744
Amato MB, Barbas CS, Medeiros DM et al (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354. https://doi.org/10.1056/NEJM199802053380602
Bellani G, Laffey JG, Pham T et al (2016) EPidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315:788–800. https://doi.org/10.1001/jama.2016.0291
Mekontso Dessap A, Charron C, Devaquet J et al (2009) Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med 35:1850–1858. https://doi.org/10.1007/s00134-009-1569-2
Mekontso Dessap A, Boissier F, Charron C et al (2016) Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 42:862–870. https://doi.org/10.1007/s00134-015-4141-2
Feihl F, Eckert P, Brimioulle S et al (2000) Permissive hypercapnia impairs pulmonary gas exchange in the acute respiratory distress syndrome. Am J Respir Crit Care Med 162:209–215. https://doi.org/10.1164/ajrccm.162.1.9907119
Gentilello LM, Anardi D, Mock C et al (1995) Permissive hypercapnia in trauma patients. J Trauma 39:846–852. https://doi.org/10.1097/00005373-199511000-00007
Hickling KG, Henderson SJ, Jackson R (1990) Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 16:372–377. https://doi.org/10.1007/BF01735174
Hickling KG, Walsh J, Henderson S, Jackson R (1994) Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 22:1568–1578. https://doi.org/10.1097/00003246-199422100-00011
Kalfon P, Rao GS, Gallart L et al (1997) Permissive hypercapnia with and without expiratory washout in patients with severe acute respiratory distress syndrome. Anesthesiology 87:6–17. https://doi.org/10.1097/00000542-199707000-00003
McIntyre RC, Haenel JB, Moore FA et al (1994) Cardiopulmonary effects of permissive hypercapnia in the management of adult respiratory distress syndrome. J Trauma 37:433–438. https://doi.org/10.1097/00005373-199409000-00017
Aguirre-Bermeo H, Morán I, Bottiroli M et al (2016) End-inspiratory pause prolongation in acute respiratory distress syndrome patients: effects on gas exchange and mechanics. Ann Intensive Care 6:81. https://doi.org/10.1186/s13613-016-0183-z
Pfeiffer B, Hachenberg T, Wendt M, Marshall B (2002) Mechanical ventilation with permissive hypercapnia increases intrapulmonary shunt in septic and nonseptic patients with acute respiratory distress syndrome. Crit Care Med 30:285–289. https://doi.org/10.1097/00003246-200202000-00003
Thorens JB, Jolliet P, Ritz M, Chevrolet JC (1996) Effects of rapid permissive hypercapnia on hemodynamics, gas exchange, and oxygen transport and consumption during mechanical ventilation for the acute respiratory distress syndrome. Intensive Care Med 22:182–191. https://doi.org/10.1007/BF01712235
Ding X, Chen H, Zhao H et al (2021) ECCO2R in 12 COVID-19 ARDS patients with extremely low compliance and refractory hypercapnia. Front Med 8:654658. https://doi.org/10.3389/fmed.2021.654658
Husain-Syed F, Birk H-W, Wilhelm J et al (2020) Extracorporeal carbon dioxide removal using a renal replacement therapy platform to enhance lung-protective ventilation in hypercapnic patients with coronavirus disease 2019-associated acute respiratory distress syndrome. Front Med 7:598379. https://doi.org/10.3389/fmed.2020.598379
Kahl U, Yu Y, Nierhaus A et al (2021) Cerebrovascular autoregulation and arterial carbon dioxide in patients with acute respiratory distress syndrome: a prospective observational cohort study. Ann Intensive Care 11:47. https://doi.org/10.1186/s13613-021-00831-7
Liu X, Liu X, Xu Y et al (2020) Ventilatory ratio in hypercapnic mechanically ventilated patients with COVID-19-associated Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 201:1297–1299. https://doi.org/10.1164/rccm.202002-0373LE
Lotz C, Muellenbach RM, Meybohm P et al (2021) Effects of inhaled nitric oxide in COVID-19-induced ARDS - Is it worthwhile? Acta Anaesthesiol Scand 65:629–632. https://doi.org/10.1111/aas.13757
Pan C, Chen L, Lu C et al (2020) Lung recruitability in COVID-19–associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med 201:1294–1297. https://doi.org/10.1164/rccm.202003-0527LE
Petran J, Muelly T, Dembinski R et al (2020) Validation of RESP and PRESERVE score for ARDS patients with pumpless extracorporeal lung assist (pECLA). BMC Anesthesiol 20:102. https://doi.org/10.1186/s12871-020-01010-0
Schmidt M, Hajage D, Lebreton G et al (2020) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med 8:1121–1131. https://doi.org/10.1016/S2213-2600(20)30328-3
Shimoda T, Sekino M, Higashijima U et al (2021) Removal of a catheter mount and heat-and-moisture exchanger improves hypercapnia in patients with acute respiratory distress syndrome: A retrospective observational study. Medicine (Baltimore) 100:e27199. https://doi.org/10.1097/MD.0000000000027199
Winiszewski H, Aptel F, Belon F et al (2018) Daily use of extracorporeal CO2 removal in a critical care unit: indications and results. J Intensive Care 6:36. https://doi.org/10.1186/s40560-018-0304-x
Pereira Romano ML, Maia IS, Laranjeira LN et al (2020) Driving pressure-limited strategy for patients with acute respiratory distress syndrome. a pilot randomized clinical trial. Ann Am Thorac Soc 17:596–604. https://doi.org/10.1513/AnnalsATS.201907-506OC
COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators (2021) Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 47:60–73. https://doi.org/10.1007/s00134-020-06294-x
Jardin F, Fellahi JL, Beauchet A et al (1999) Improved prognosis of acute respiratory distress syndrome 15 years on. Intensive Care Med 25:936–941. https://doi.org/10.1007/s001340050985
Papazian L, Aubron C, Brochard L et al (2019) Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care 9:69. https://doi.org/10.1186/s13613-019-0540-9
Nuckton TJ, Alonso JA, Kallet RH et al (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346:1281–1286. https://doi.org/10.1056/NEJMoa012835
Audard V, Homs S, Habibi A et al (2010) Acute kidney injury in sickle patients with painful crisis or acute chest syndrome and its relation to pulmonary hypertension. Nephrol Dial Transpl 25:2524–2529
Puybasset L, Stewart T, Rouby JJ et al (1994) Inhaled nitric oxide reverses the increase in pulmonary vascular resistance induced by permissive hypercapnia in patients with acute respiratory distress syndrome. Anesthesiology 80:1254–1267
Combes JC, Nicolas F, Lenfant F et al (1996) Hemodynamic changes induced by apnea test in patients with brain death. Ann Fr Anesthèsie Rèanimation 15:1173–1177
İnal V, Efe S (2021) Extracorporeal carbon dioxide removal (ECCO2R) in COPD and ARDS patients with severe hypercapnic respiratory failure. A retrospective case-control study. Turk J Med Sci 51:2127–2135. https://doi.org/10.3906/sag-2012-151
Combes A, Fanelli V, Pham T et al (2019) Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med 45:592–600. https://doi.org/10.1007/s00134-019-05567-4
McNamee JJ, Gillies MA, Barrett NA et al (2021) Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: the REST randomized clinical trial. JAMA 326:1013–1023. https://doi.org/10.1001/jama.2021.13374
Acknowledgements
We thank Baxter for support for data extraction and the Reva network and COVID-ICU investigators for data provision.
Author information
Authors and Affiliations
Contributions
AMD and AVB designed the meta-analysis. SG, TP, GG and AMD searched for the articles, screened titles and abstracts and extracted data. SG, TP, GG and AMD performed statistical analysis and interpretation of data. SG, TP, GG and AMD drafted the manuscript, and all authors revised it for important intellectual content. Final approval of the version submitted for publication was obtained for all authors.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gendreau, S., Geri, G., Pham, T. et al. The role of acute hypercapnia on mortality and short-term physiology in patients mechanically ventilated for ARDS: a systematic review and meta-analysis. Intensive Care Med 48, 517–534 (2022). https://doi.org/10.1007/s00134-022-06640-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-022-06640-1