Abstract
Purpose
Diagnosis of pneumothorax (PTX) in newborn infants has been reported as late. To explore diagnostic indices for early detection of progressing PTX, and offer explanations for delayed diagnoses.
Methods
Progressing PTX was created in rabbits (2.3 ± 0.5 kg, n = 7) by injecting 1 ml/min of air into the pleural space. Hemodynamic parameters, tidal volume, EtCO2, SpO2, blood gas analyses and chest wall tidal displacements (TDi) on both sides of the chest were recorded.
Results
(Mean ± SD): A decrease in SpO2 below 90 % was detected only after 46.6 ± 11.3 min in six experiments. In contrary to the expected gradual increase of CO2, there was a prolonged transient decrease of 14.2 ± 4.5 % in EtCO2 (p < 0.01), and a similar decrease in PaCO2 (p < 0.025). EtCO2 returned back to baseline only after 55.2 ± 24.7 min, and continued to rise thereafter. The decrease in CO2 was a mirror image of the 14.6 ± 5.3 % increase in tidal volume. The analysis of endotracheal flow and pressure dynamics revealed a paradoxical transient increase in the apparent compliance. Significant decrease in mean arterial blood pressure was observed after 46.2 ± 40.1 min. TDi provided the most sensitive and earliest sign of PTX, decreasing on the PTX side after 16.1 ± 7.2 min. The TDi progressively decreased faster and lower on the PTX side, thus enabling detection of asymmetric ventilation.
Conclusions
The counterintuitive transient prolonged decrease in CO2 without changes in SpO2 may explain the delay in diagnosis of PTX encountered in the clinical environment. An earlier indication of asymmetrically decreased ventilation on the affected side was achieved by monitoring the TDi.
Similar content being viewed by others
Introduction
Pneumothorax (PTX) is a serious complication that develops in mechanically ventilated and spontaneously breathing neonates. The incidence of PTX in very low birth weight infants (<1,500 g) is between 3 and 14 % [1–6]. The potential for irreversible brain damage or death is very high [7–10], with mortality rates as high as 43 % [5, 6, 11, 12].
McIntosh et al. [11, 13] have identified the PTX onset time from retrospective analysis of transcutaneous gas records. They have suggested that the probable delay from PTX onset time to diagnosis and treatment, using the current monitoring methods in the neonatal intensive care unit (NICU), ranges from 45 to 660 min (median 127 min). They have also suggested that closer surveillance and analysis of the trends in the monitored signals may decrease the time to diagnosis of PTX [11, 13].
Delayed diagnosis of PTX and its severe consequences has led others to suggest additional modalities for earlier detection of progressing PTX, based on breath sounds analysis [14, 15] and electrical impedance tomography (EIT) [15, 16]. These modalities are currently not in routine clinical use. These studies have used a large electronic stethoscope (1 in. in diameter) fastened to the chest by a relatively large load of 250–300 g [14]. EIT requires the application of multiple electrodes at equidistances around the thorax [15, 16]. These methods are not easy to apply on premature neonates in the NICU.
Our group recently described the feasibility and potential utility of monitoring the amplitude and symmetry of lung ventilation by miniature motion sensors attached to both chest wall sides, in infants ventilated with high frequency oscillatory ventilation (HFOV) [17]. This modality may enable an early recognition of deteriorating ventilation, as early as 22.4 ± 18.7 min before hypoxemia evolves during HFOV [17]. The high sensitivity to changes in lung ventilation were further validated in rabbits in which deceases in inspiratory pressure (simulates tube obstruction), one lung ventilation and PTX were induced [18].
The objectives of the present study were: (1) To decipher why the diagnosis of PTX is delayed by analyzing the trends of oxygenation, blood gas analysis, end-expiratory CO2 concentration (EtCO2), tidal volume (V T), endotracheal tube pressure and hemodynamic parameters; (2) To assess the utility of monitoring the amplitude and symmetry of the tidal displacement indices, for earlier diagnosis of a progressing PTX.
Methods
The experiments were performed on healthy male New Zealand rabbits, after approval by the Institutional Ethics Committee for the Care and Use of Animals. The rabbits were anesthetized by IM injection of xylazine (5 mg/kg), ketamine (35 mg/kg), and acepromazine (1 mg/kg). One-third of the dose was added every 20 min.
Conventional mandatory ventilation was used, via tracheostomy, with a pressure controlled ventilator (SLE 2000, SLE, Surrey, UK). Initial parameters were: respiratory rate (RR): 20/min; peak inspiratory pressure (PIP): 18 cmH2O; and positive end expiratory pressure (PEEP): 3 cmH2O. These parameters were adjusted to maintain normal arterial blood gases tensions [19] during baseline measurements (Roche OPTI CCA, Mannheim, Germany). Inspired oxygen fraction (FiO2 = 0.21) and inspiratory time (0.36 s) were kept constant in all the experiments.
Lung ventilation dynamics were measured by attaching two miniature (<1 g, 5 mm diameter) motion sensors to both sides of the chest, at the mid-clavicular line at the level of the 4th or 5th intercostal spaces. A third sensor was attached to the epigastrium, as described previously [17, 18]. The motion signals were acquired using the Pneumonitor® (Pneumedicare, Yokneam, Israel).
Study protocol
Slowly progressing PTX was induced by inserting a chest tube (10 Fr) into the left (n = 5) or right (n = 2) pleural space, in the tenth intercostal space at the mid-axillary line. The tube was connected to an automatic syringe pump (Graseby 3100, SIMS Graseby Ltd., UK) which injected air at a constant rate of 1 ml/min. Injection continued at least 10 min after a persistent decrease in SpO2 below 90 % and an increase in the EtCO2, or until overt respiratory distress with spontaneous breathing and cardiovascular decompensation appeared. During the experiment, PTX size was assessed using transillumination of the chest. The animals were euthanized by pentobarbital overdose.
EtCO2 was measured by side-stream capnography (Datex Multicap, Helsinki, Finland). Air flow was measured by a Fleisch pneumotachograph (TSD137, Harvard Apparatus, Holliston, MA, USA). Vital signs (ECG, BP and SpO2) were monitored by an anesthesia/ICU monitor (Datex-Ohmeda, Type F-CU8, Instrumentarium Corp. Helsinki, Finland)
Data analysis
ECG, BP (via arterial line in the ear artery), SpO2, EtCO2, airway pressure (Millar Instruments Inc., Houston, Texas, USA), airway flow, and tidal displacements (TDi) of the right (TDiR) and left (TDiL) sides of the chest and abdomen (TDiA) were continuously acquired (5 kHz). The parameter ratioTDi, defined as the ratio of TDi on the PTX side to TDi on the contralateral side, characterized ventilation symmetry. The end expiratory tilt of the chest sensors (TiltEE) was also continuously monitored. Arterial blood gas tensions were measured at baseline, every 20–30 min thereafter, and at the end.
The lumped compliance and resistance of the respiratory system were assessed from the endotracheal tube flow and pressure [20]. The resistance was derived from the work enclosed within the pressure–volume loop during each breath. The compliance was calculated from the exponential decay of the expiratory flow during the late expiration phase, after finding the lumped resistance.
Statistical analysis
Values are presented as mean ± SD. Differences between specific data means were evaluated by the Wilcoxon signed-rank test and a p value <0.05 was considered significant. A 20 % change from baseline of all the trends studied was used as a threshold for denoting the occurrence of a significant change, excluding SpO2 in which a persistent drop below 90 % was considered as the threshold for a significant decrease. Matlab (Mathworks, Inc., Natick, Massachusetts, USA) and Excel (Microsoft Office 2003, Microsoft Corp., Redmond, Washington, US) were used for data and statistical analyses.
Results
The rabbits (n = 7, weight 2.3 ± 0.5 kg) were ventilated at a respiratory rate of 19.6 ± 2.8 (min−1), PIP of 17.4 ± 1.5 cmH2O, with PEEP of 3.14 ± 0.4 cmH2O. The measured V T at baseline was 21.5 ± 3.4 ml.
Figure 1 presents the trends from a single experiment of progressing PTX in the left side (starting at time zero). PTX caused a gradual decrease in mean arterial blood pressure (MABP) with an increase in heart rate (HR). The expected decrease in SpO2 and increase in EtCO2 appeared very late, 34.8 and 38 min after PTX onset, respectively. Interestingly, the EtCO2 initially decreased by 12.6 % from baseline, reaching a minimal value 22.9 min after PTX onset, and only then did it gradually increase. The trend of V T mirrored the changes in EtCO2, with a gradual initial increase of 13.5 % in V T relative to baseline.
Progressing left pneumothorax produced an overt continuous decrease in left tidal chest wall displacement (TDiL), with a 20 % decrease in TDiL after 20.1 min. A 20 % change in the symmetry of ventilation (RatioTDi) was identified after 24.2 min, while the SpO2 decreased only after 34.8 min. Interestingly, the EtCO2 presented a prolonged transient initial decrease, with a mirror transient increase in tidal volume (V T). MABP Mean arterial blood pressure, HR heart rate, TDi R and TDi L right and left tidal displacement indices, respectively, Tilt EE the tilt of the chest sensors at end expiration
The trends of the chest wall displacement indices (TDis) depicted the development of asymmetric ventilation between both chest sides. The tidal-displacement on the PTX (left) side (TDiL) decreased faster and to lower values in comparison with the contralateral side (TDiR). The symmetry index (RatioTDi) decreased by 20 % relative to baseline 24.2 min after PTX onset. The tilt of the chest was measured at the end of the expiratory phase (TiltEE), and it showed monotonic increase in tilt due to gradual chest expansion.
Figure 2 presents a close-up of the signals at baseline (Fig. 2a) and at the time of minimal EtCO2, 23 min after the onset of PTX (Fig. 2b). Note the decrease in peak EtCO2 and increase in V T that were associated with a decrease in the amplitude of the left motion sensor signals (used to calculate TDiL).
Close-up of the raw signals at baseline (a) and at the time of minimal recorded EtCO2 (b). The paradoxical decrease in peak EtCO2 and the increase in the tidal volume (V T) were associated with a decrease in the amplitude of the left motion sensor signals (Left FILT filtered data that was used for calculating the TDiL). The motion sensor signals were normalized to the baseline levels
Figure 3 compiles the results from all the experiments (n = 7), where five PTX were induced on the left side and two on the right side. Baseline values of HR, EtCO2 and PaCO2 were 177 ± 23 BPM, 3.51 ± 0.30 % and 38.14 ± 4.22 mmHg, respectively. The normal value of PaCO2 in healthy rabbits is around 37.0 [19]. All experiments presented identical trends, independently of the PTX side. There was an initial decrease in EtCO2 associated with an increase in V T. A clear minimum in EtCO2 was obtained in all experiments, 14.2 ± 4.5 % lower than baseline (p < 0.01). Since there were variations in the time of occurrence of the phenomena, the time scale was normalized according to the time of minimum EtCO2 (indicated as normalized time 1). This minimum in EtCO2 was reached 28.2 ± 11.8 min after the onset of air injection. Thereafter the EtCO2 gradually increased and reached the baseline value again only after 55.2 ± 24.7 min, as detailed in Table 2.
Compiled data from all experiments (mean ± SD) revealed that the counterintuitive transient initial decrease in EtCO2 and increase in the tidal volume (V T) were observed in all experiments. The decrease in the TDi on the PTX side, and the development of asymmetric ventilation (ratioTDi) provided an early indication for the progressing PTX. The time scale in each experiment was normalized by the time it took to reach a minimum of EtCO2
Conspicuous biphasic changes in V T are also seen in Fig. 3, which mirrored the changes in EtCO2. V T gradually increased to a maximum after 22.5 ± 8.5 min. The maximal V T was 14.6 ± 5.3 % higher than baseline (p < 0.01). V T fell below baseline only after 41.2 ± 15.8 min. The transient decrease in EtCO2 was observed also in the blood gas results (Table 1). The PaCO2 decreased from 38.1 ± 4.2 mmHg at baseline to 32.9 ± 3.4 mmHg (p < 0.025) 29.2 ± 12.0 min after PTX onset. Thereafter, the PaCO2 increased back to 37.7 ± 4.8 mmHg, 49.8 ± 13.7 min after PTX onset.
Decreases in SpO2 and PaO2 came very late in all cases. In six experiments the SpO2 dropped below 90 % only after 46.6 ± 11.3 min. In one experiment the SpO2 remained around 95 %. This experiment was stopped after 96.5 min due to cardiorespiratory failure and severe respiratory distress with vigorous spontaneous breathing. The blood gas samples revealed a significant decrease in PaO2 from 84.6 ± 8.50 to 71.0 ± 13.3 mmHg (p < 0.05) after 49.8 ± 13.7 min (Table 1). The late decrease in the SpO2 and increase in EtCO2 were associated with the development of spontaneous breathing in all experiments, which appeared after 46.5 ± 17.9 min.
Significant hemodynamic changes appeared in all experiments. The MABP decreased by 20 % after 46.2 ± 40.1 min. Only five animals showed increases in HR of more than 20 %, appearing 14.9 ± 8.30 min after PTX induction.
The progressing PTX had clear effects on tidal displacements. TDiR and TDiL decreased with the progression of the PTX, until spontaneous respiration occurred (Fig. 3). The decrease in TDi was faster and larger on the PTX side. A 20 % decrease in TDi on the PTX side was reached after 16.1 ± 7.2 min, while a similar 20 % decrease appeared only after 30.2 ± 27.9 min on the contralateral side. There was a significantly deeper drop in TDi on the PTX side relative to the contralateral side: to 32.0 ± 15.7 versus 59.7 ± 14.8 % of baseline values, respectively (p < 0.01). The symmetry index (ratioTDi) decreased by 20 % after 20.0 ± 12.4 min. The gradual accumulation of air within the pleural space was also associated with a slow and progressive increase in chest tilt.
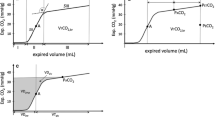
The apparent resistance and compliance of the respiratory system were evaluated from the analysis of the endotracheal tube flow and pressure (Fig. 4a), and the pressure–volume loop (Fig. 4b). The apparent resistance progressively increased with PTX size (Fig. 4c). Apparent compliance appeared to have a biphasic behavior, with a transient increase, resembling the biphasic changes in V T (Fig. 4c). Figure 5 compiles the calculated changes in the apparent resistance and compliance. The mean resistance and compliance at baseline were 0.081 ± 0.008 cmH2O s/ml and 2.62 ± 0.53 ml/cmH2O, respectively. In all the experiments, a gradual continuous increase in the resistance to flow was observed. The changes in compliance resembled the changes in V T, and presented a mirror image of the changes in EtCO2. The maximal compliance appeared after 21.3 ± 7.1 min, reaching a value 13.7 ± 5.2 % (p < 0.01) greater than baseline. The mean compliance dropped below baseline only after 40.9 ± 20.1 min.
The apparent resistance and compliance of the respiratory system were evaluated from the analysis of the endotracheal tube flow, flowETT, and pressure, PETT (a), and the related pressure–volume loops (b). While the apparent respiratory system resistance progressively increased, the apparent compliance showed a biphasic behavior. The transient increase in compliance resembled the biphasic changes observed in tidal volume (V T) (c)
Discussion
The study reveals the following significant findings that may explain the prolonged delay in the diagnosis of PTX, and may assist in earlier identification of PTX, especially in the NICU:
-
(1)
There was a counterintuitive initial significant decrease in EtCO2 and PaCO2, which returned to baseline levels only after a prolonged delay of approximately 1 h (55.2 ± 24.7 min).
-
(2)
The biphasic changes in EtCO2 were associated with mirror transient increases in V T.
-
(3)
The apparent compliance of the respiratory system exhibited a transient initial increase.
-
(4)
The delayed decrease in blood oxygenation (46.6 ± 11.3 min) appeared when PTX reached large dimensions.
-
(5)
Early identification (16.1 ± 7.2 min) of PTX was feasible by detecting progressive decreases in the TDis, especially on the PTX side.
-
(6)
TDi monitoring enabled detection of asymmetric ventilation, important diagnostic information.
-
(7)
Monitoring the chest wall tilt at end-expiration enabled detection of progressive chest cavity distention.
The counterintuitive initial prolonged decrease in CO2 concentration (PaCO2 and EtCO2) and the late decrease in saturation can explain the delay in diagnosing PTX. Interestingly, the delayed decrease in oxygen saturation, after the injection of approximately 20 mL/kg of body weight, has been previously reported [15]. McIntosh et al. [11, 13] have noticed that in many cases the initial PaCO2 level was relatively low, thus raising the possibility that these patients were over-ventilated, and that over-inflation might have been the cause for the development of PTX. However, the relationship between over-inflation and the development of PTX is not trivial. Watkinson et al. [3] investigated whether patients that were unintentionally over-ventilated and had subsequent hypocapnia suffered from high incidence of PTX, and did not find such a causative association.
The manifestation of a progressing PTX with mild hypocapnia has not yet been described in the clinic. There can be several explanations for this: (1) the PTX-induced decrease in CO2 is very mild and can be easily overlooked in the clinical arena; (2) various changes in the metabolism and the ventilation parameters may cause variability in the CO2 levels in humans and mask the hypocapnia; (3) when a decrease in CO2 was detected in premature infants that suffered from PTX, it was not recognized as part of the manifestation of the PTX, but rather suspected to be associated with hyperventilation causing the PTX [11, 13]; and (4) the accuracy of EtCO2 is lower in the presence of lung disease with nonuniform or dynamic changes in the ventilation/perfusion relationship within the lungs [21].
The mechanism for the decrease in CO2 in the early stages of PTX is unclear. It was associated with parallel increases in tidal volume and apparent respiratory system compliance, suggesting a cause-and-effect relationship. The first possible mechanism relates to nonlinearity and the dependence of lung compliance on the functional residual lung capacity (FRC). PTX increases the pleural pressure and decreases the transpulmonary pressure, therefore also reducing the FRC. A second plausible explanation relates to the effects of the circulatory system on lung compliance. Decreased lung compliance due to increased pulmonary blood volume has been described before in the presence of a patent ductus arteriosus [22–24] and with lung congestion due to heart failure [25]. PTX may represent the opposite effect, where lung compliance increases due to a decrease in pulmonary blood volume. Pulmonary blood volume decreases due to the increase in the intrapleural pressure. A third plausible mechanism may be the activation of the sympathetic system. Activation of the sympathetic system leads to hyperventilation, by increasing both the breath rate and the tidal volume [26, 27]. Interestingly, the increase in tidal volume is the dominant result [26, 28]. Transient changes in EtCO2 may also result from variations in ventilation/perfusion relationships during the emergence of PTX.
Another conspicuous observation is the ability to detect the development of PTX by monitoring the amplitude and symmetry of the chest wall tidal displacement. Progressing PTX causes gradual decreases in TDis, especially on the side of PTX. This enables an early detection of deteriorating ventilation almost half an hour before SpO2 decreases (Table 2). Monitoring the changes in TDi may also facilitate diagnosis, since it reveals the development of asymmetric ventilation. Monitoring the changes in sensor tilts shows that the chest cage gradually expands during the development of progressive PTX, as has been described previously by others [29, 30]. The potential of the suggested modality was discussed in a recent editorial [31].
Detection of deterioration before the development of life threatening conditions is of great importance, as prevention may be more desirable than a cure [31]. Moreover, several studies described a conservative approach for asymptomatic infants [32, 33]. However, it is currently impossible to predict who will develop tension PTX, and when it will occur. Monitoring the symmetry and amplitude of ventilation may provide key information whether PTX is resolving and a conservative approach is appropriate, or when there is a continuously progressing deterioration and treatment should be considered.
Other modalities, such as respiratory inductive plethysmography [34–36], total body plethysmography [37] and EIT [15, 16] monitor the changes in the chest cavity volume. However these modalities are cumbersome and therefore are rarely used in the NICU setting. Measurement of pressure and flow at the endotracheal tube are insensitive to the development of asymmetric ventilation. Recent studies suggest that lung ultrasound is more accurate than X-ray particularly in ruling out PTX [38, 39]. Therefore lung ultrasound may be used as an initial diagnostic study in patients with suspected PTX. [38, 39].
Limitations
This study was performed in ventilated rabbits with normal lungs. It should be also investigated in a model with sick lungs. The identification of asymmetric ventilation by monitoring the chest wall dynamics may be limited with a stiff chest wall, as in adults. However, the chest wall of neonates and infants is very flexible and has a significantly larger compliance than the underlying lungs [40].
Conclusions
The present study has revealed novel and counterintuitive observations. PTX induced a prolonged transient decrease in CO2 in ventilated animals, with a mirror transient increase in the tidal volume and lung compliance, while hypoxemia developed at a very late stage. These observations masked the commonly accepted pathognomonic signs of PTX leading to delayed diagnosis. It has been shown that the early detection of PTX was feasible by monitoring the amplitude and symmetry of the chest wall tidal displacement. The method can facilitate correct diagnosis by localizing the PTX side and quantifying the severity of the progressing PTX. The potential clinical utility should be explored in a clinical study in NICUs.
References
Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, Phibbs R, Soll RF, Members of the Vermont Oxford Network (2002) Trends in mortality and morbidity for very low birth weight infants 1991–1999. Pediatrics 110:143–151
Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, COIN Trial Investigators (2008) Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 358:700–708
Watkinson M, Tiron I (2008) Events before the diagnosis of a pneumothorax in ventilated neonates. Arch Dis Child Fetal Neonatal Ed 85:F201–F203
Sinha SK, Lacaze-Masmonteil T, Valls i Soler A, Wiswell TE, Gadzinowski J, Hajdu J, Bernstein G, Sanchez-Luna M, Segal R, Schaber CJ, Massaro J, d’Agostino R, Surfaxin Therapy Against Respiratory Distress Syndrome Collaborative Group (2005) A multicenter, randomized, controlled trial of lucinactant versus poractant alfa among very premature infants at high risk for respiratory distress syndrome. Pediatrics 115:1030–1038
Klinger G, Ish-Hurwitz S, Osovsky M, Sirota L, Linder N (2008) Risk factors for pneumothorax in very low birth weight infants. Pediatr Crit Care Med 9:398–402
Bhatia R, Davis PG, Doyle LW, Wong C, Morley CJ (2011) Identification of pneumothorax in very preterm infants. J Pediatr 159:115–120
Linder N, Haskin O (2003) Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Pediatrics 111:e590–e595
Gammon CM, Wiswell TE, Spitzer AR (1998) Volutrauma, PaCO2 levels, and neuro-developmental sequelae following assisted ventilation. Clin Perinatol 25:75–159
Hill A, Perlman JM, Volpe JJ (1982) Relationship of pneumothorax to occurrence of intraventricular hemorrhage in the premature newborn. Pediatrics 69:144–149
Powers WF, Clemens JD (1993) Prognostic implications of age at detection of air leak in very low birth weight infants requiring ventilatory support. J Pediatr 123:611–617
McIntosh N, Becher JC, Cunningham S, Stenson B, Laing IA, Lyon AJ, Badger P (2000) Clinical diagnosis of pneumothorax is late: use of trend data and decision support might allow preclinical detection. Pediatr Res 48:408–415
Walker MW, Shoemaker M, Riddle K, Crane MM, Clark R (2002) Clinical process improvement: reduction of pneumothorax and mortality in high-risk preterm infants. J Perinatol 22:641–645
McIntosh N (2002) Intensive care monitoring: past, present and future. Clin Med 2:349–355
Mansy HA, Royston TJ, Balk RA, Sandler RH (2002) Pneumothorax detection using computerized analysis of breath sounds. Med Biol Eng Comput 40:526–532
Bhatia R, Schmölzer GM, Davis PG, Tingay DG (2012) Electrical impedance tomography can rapidly detect small pneumothoraces in surfactant-depleted piglets. Intensive Care Med 38:308–315
Costa EL, Chaves CN, Gomes S, Beraldo MA, Volpe MS, Tucci MR, Schettino IA, Bohm SH, Carvalho CR, Tanaka H, Lima RG, Amato MB (2008) Real-time detection of pneumothorax using electrical impedance tomography. Crit Care Med 36:1230–1238
Waisman D, Levy C, Faingersh A, Klotzman FIC, Konyukhov E, Kessel I, Rotschild A, Landesberg A (2011) A new method for continuous monitoring of the chest wall movement to characterize hypoxemic episodes during HFOV. Intensive Care Med 37:1174–1181
Waisman D, Faingersh A, Levy C, Konyukhov E, Klotzman FI, Rotschild A, Landesberg A (2012) Early detection of deteriorating ventilation by monitoring bilateral chest wall dynamics in the rabbit. Intensive Care Med 38:120–127
Kiwull-Schöne H, Kalhoff H, Manz F, Kiwull P (2005) Food mineral composition and acid–base balance in rabbits. Eur J Nutr 44:499–508
Khoo MCK (2000) Physiological control system, analysis, simulation and estimation. IEEE press, New York, pp 206–208
Chock VY, Wong RJ, Hintz SR, Stevenson DK (2011) Biomedical engineering aspects of neonatal monitoring. In: Martin RJ, Fanaroff AA, Walsh MC (eds) Fanaroff and Martin’s neonatal-perinatal medicine: diseases of the fetus and infant, 9th edn. Mosby, Elsevier, St. Louis, Missouri, pp 584–585
Gerhardt T, Bancalari E (1980) Lung compliance in newborns with patent ductus arteriosus before and after surgical ligation. Biol Neonate 38:96–105
Szymankiewicz M, Hodgman JE, Siassi B, Gadzinowski J (2004) Mechanics of breathing after surgical ligation of patent ductus arteriosus in newborns with respiratory distress syndrome. Biol Neonate 85:32–36
McCurnin DC, Yoder BA, Coalson J, Grubb P, Kerecman J, Kupferschmid J, Breuer C, Siler-Khodr T, Shaul PW, Clyman R (2005) Effect of ductus ligation on cardiopulmonary function in premature baboons. Am J Respir Crit Care Med 172:1569–1574
Gehlbach BK, Geppert E (2004) The pulmonary manifestations of left heart failure. Chest 125:669–682
Young M (1957) Some observations on the mechanism of adrenaline hyperpnoea. J Physiol 137:374–395
Clark AL, Galloway S, MacFarlane N, Henderson E, Aitchison T, McMurray JJ (1997) Catecholamines contribute to exertional dyspnoea and to the ventilatory response to exercise in normal humans. Eur Heart J 18:1829–1833
Stewart JM, Rivera E, Clarke DA, Baugham IL, Ocon AJ, Taneja I, Terilli C, Medow MS (2011) Ventilatory baroreflex sensitivity in humans is not modulated by chemoreflex activation. Am J Physiol Heart Circ Physiol 300:H1492–H1500
Maranhão E, Barboza AP, Ciminelli PB, Alcântara BJ, Berti M, Oliveira-Neto J, Capelozzi VL, Zin WA, Rocco PR (2000) Temporal evolution of pneumothorax: respiratory mechanical and histopathological study. Respir Physiol 119:41–50
Sousa AS, Moll RJ, Pontes CF, Saldiva PH, Zin WA (1995) Mechanical and morphometrical changes in progressive bilateral pneumothorax and pleural effusion in normal rats. Eur Respir J 8:99–104
Gama De Abreu M, Güldner A (2012) Early detection of deteriorating ventilation: prevention is better than cure! Intensive Care Med 38:7–8
Litmanovitz I, Carlo WA (2008) Expectant management of pneumothorax in ventilated neonates. Pediatrics 122:e975–e979
Katar S, Devecioglu C, Kervancioglu M, Ulku R (2006) Symptomatic spontaneous pneumothorax in term newborns. Pediatr Surg Int 22:755–758
Warren RH, Horan SM, Robertson PK (1997) Chest wall motion in preterm infants using respiratory inductive plethysmography. Eur Respir J 10:2295–2300
Esquer C, Claure N, D’Ugard C, Wada Y, Bancalari E (2008) Mechanisms of hypoxemia episodes in spontaneously breathing preterm infants after mechanical ventilation. Neonatology 94:100–104
Ali N, Claure N, Alegria X, D’Ugard C, Organero R, Bancalari E (2007) Effects of non-invasive pressure support ventilation (NI-PSV) on ventilation and respiratory effort in very low birth weight infants. Pediatr Pulmonol 42:704–710
Gappa M, Pillow JJ, Allen J, Mayer O, Stocks J (2006) Lung function tests in neonates and infants with chronic lung disease: lung and chest-wall mechanics. Pediatr Pulmonol 41:291–317
Volpicelli G (2011) Sonographic diagnosis of pneumothorax. Intensive Care Med 37:224–232
Volpicelli G, Elbarbary M, Blaivas M et.al, International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS) (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38:577–591
Papastamelos C, Panitch HB, England SE, Allen JL (1995) Developmental changes in chest wall compliance in infancy and early childhood. J Appl Physiol 78:179–184
Acknowledgments
This work was supported in part by the Dr. Y. Rabinovitz Research Fund and the Ruth and Allen Ziegler Fund for Pediatric Research (DW), and by a grant for the promotion of research at the Technion (AL). Chest wall dynamics were measured by equipment provided by Pneumedicare Ltd. The authors thank Idit Lavi, MA, for her kind advice on the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Waisman and A. Faingersh equally contributed to performance of this work.
Rights and permissions
About this article
Cite this article
Waisman, D., Faingersh, A., Levy, C. et al. Transient decrease in PaCO2 and asymmetric chest wall dynamics in early progressing pneumothorax. Intensive Care Med 39, 137–145 (2013). https://doi.org/10.1007/s00134-012-2749-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2749-z