Abstract
Purpose
Lung transplantation (LTx) of patients on mechanical ventilation (MV) or extracorporeal support (ECS) is controversial because of impaired survival. Prognostic factors to predict survival should be identified.
Methods
A retrospective analysis was performed in a single centre of all ventilated LTx-candidates awarded an Eurotransplant (ET) high-urgency (HU) status between November 2004 and July 2009. Clinical data were collected on the first day of HU-status from intubated patients with an approved HU status. Single parameters as well as the lung allocation score (LAS), the Sequential Organ Failure Assessment score (SOFA) and the Simplified Acute Physiology Score (SAPS 2) were calculated. The association of these variables with survival was evaluated.
Results
A total of 100 intubated patients (median age 38 years, 56 % female) fulfilled the inclusion criteria, of whom 60 also required ECS. The main indications were cystic fibrosis (25 %) and idiopathic pulmonary fibrosis (24 %). Median time with HU status was 12 days [interquartile range (IQR) 6–21 days]. Sixty patients were transplanted, five were weaned from mechanical ventilation and 38 died while on the wait list. One-year-survival rates were 57, 36 and 5 % for transplanted patients, all candidates and non-transplanted candidates, respectively (p < 0.001). A SAPS score >24 (median 30, IQR 27–35), a procalcitonin level of >0.5 µg/l (median 0.4, IQR 0.1–1.4 µg/l) and any escalation of bridging strategy were independently associated with mortality (p = 0.021, = 0.003, and < 0.001, respectively). The LAS (median 88, IQR 8–90) did not predict survival (p = 0.92).
Conclusions
High-urgency LTx improves survival in critically ill intubated candidates. Higher SAPS scores, escalating therapy and an abnormal procalcitonin level were associated with a poor outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, transplant specialists have been reluctant to perform lung transplantation (LTx) in patients on invasive respiratory support. The prevailing argument has been that these patients had become too sick and thus no longer in the ‘‘transplant window’’ because survival after LTx was unlikely. Invasive respiratory support may be provided by mechanical ventilation (MV) with associated extracorporeal support (ECS) when necessary. Intensive care unit (ICU) complications, such as pressure ulcers, vascular complications, nosocomial infections, delirium, critical illness polyneuropathy/myopathy and airway colonization, might increase waiting list mortality as well as mortality after LTx in patients on invasive respiratory support. Lung transplant recipients requiring MV have a 1.59-fold relative risk of 1-year mortality according to a recent registry report [1]. Single-centre case series including 6–23 patients on MV who were subsequently transplanted [2–11] reported 1-year survival rates of between 25 and 87 %.
In contrast to this historical tendency, the lung allocation score (LAS), which was introduced in the USA in 2005, bestows ventilated candidates with a specific advantage because allocation is directed towards patients in more critical condition [12]. The need for continuous MV is a heavily weighted factor in the LAS model [13].
Our institution has a history of more than 20 years for accepting critically ill ventilated patients for LTx [11]. We therefore conducted a retrospective analysis of mechanically ventilated critically ill patients accepted for LTx in our programme to study the outcome of these candidates and to identify factors beneficial for survival following LTx.
Methods
A retrospective analysis was performed in a single university centre. Between November 2004 and July 2009 all intubated and mechanically ventilated patients listed for lung transplantation who had been awarded a high urgency (HU) status in Eurotransplant and fulfilled the following inclusion criteria were included:
-
inspiratory oxygen fraction of >0.5 or
-
presence of ECS
Patients on non-controlled ventilation were excluded.
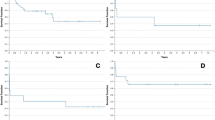
The study subjects were followed until June 30, 2010, or until death. Wait list survival was defined as surviving 1 year after HU status approval or until LTx. Overall survival was defined as surviving 1 year after HU status approval. Post-transplant survival was defined as surviving 1 year after LTx. Donor data, patient and graft survival of the transplanted group with a HU status on ventilator was compared to a cohort of 425 patients without prior ventilation transplanted between January 2005 and December 2009. The mean age of the control group was older (48 vs. 38 years in the MV group), while the MV group contained more patients with chronic obstructive pulmonary disease (COPD)/emphysema (30 vs. 7 %) and fewer patients with pulmonary fibrosis (22 vs. 34 %). In general, the elderly and COPD patients have worse outcome from LTx than patients with pulmonary fibrosis [1]. However, as shown in Fig. 3, the outcome of the patients on MV as a bridging treatment to transplant was worse than that of the controls.
After February 2007, quality of life, as measured by the Short Form 36 (SF36) questionnaire, and the activity of daily living (ADL) index were recorded at the end of the patients’ rehabilitation programme. The results of a subgroup of 125 not previously intubated lung transplant recipients were available for a comparison of the quality of life and ADL measurements during this period.
The rules for LTx in EUROTRANSPLANT and the management of patients on the transplant waiting list are described in detail in the Electronic Supplementary Material (ESM).
Day 1 of support was defined as the first day that the patient was on invasive respiratory support with accepted HU status. Data on the following variables were collected on day 1 of the HU listing: age, height, weight, underlying disease, pulmonary hypertension as estimated by echocardiography (right ventricular pressure >50 mmHg) or pulmonary artery catheterization (mean pulmonary artery pressure >30 mm Hg), date of intubation, team decision vote, bilirubin, creatinine, glomerular filtration rate (GFR) as calculated according to the modification of diet in Modification of Diet in Renal Disease (MDRD) Study Group [14], need for vasopressors, type of ECS, escalation of support, duration of support, urea, leukocyte count, platelets, bicarbonate, partial pressure carbon dioxide (pCO2), maximum and minimum pCO2 during current hospital stay, fraction of inspired oxygen (FiO2), sodium, potassium, C-reactive protein (CRP) and procalcitonin. Donor variables were weight, height, ventilator days, smoking history, chest X-ray opacities, bronchoscopy findings and oxygenation index and cold ischemic time.
In transplanted patients, days on ventilator post-transplant, hospital length of stay, airway complications, quality of life (SF36) and activity index at end of rehabilitation, use of cardiopulmonary bypass, primary graft dysfunction 48 h after LTx, graft size reduction, tracheostomy and need for renal replacement therapy were recorded.
The Sequential Organ Failure Assessment score (SOFA) was calculated from raw data on the first day of HU approval as previously published [15].
The Simplified Acute Physiology Score (SAPS 2) was calculated on the first day of HU approval according its original description [16]. To calculate SAPS and SOFA scores, a Glasgow coma scale of 15 was assumed in the case of sedation to calculate the SOFA score. The LAS was calculated according to its modification of 1 July 2009 [13]. Default values for pulmonary artery and wedge pressures were used if a pulmonary artery catheter was not performed. A “zero” was entered for the 6-min walk distance and forced vital capacity.
A donor score (0–16 points) consisting of donor age, oxygenation index, chest X-ray, bronchoscopy finding and smoking history was calculated for each transplanted patient as previously described [17]. This score was modified by truncating the donor’s smoking history to a maximum of 1, if present. A score of >7 was defined as an extended donor.
Cold ischemic time was defined as the time interval between the application of the aortic cross-clamp during harvesting and reperfusion of the graft in the recipient. In the case of a double lung transplantation (DLTx), the maximal raft ischemic time of the second side was used. Patients usually on veno–venous ECS were switched to veno-arterial mode intraoperatively. LTx performed on extracorporeal membrane oxygenation (ECMO) was rated as being performed on cardiopulmonary bypass.
Primary graft dysfunction (PGD) was graded according to the International Society of Heart and Lung Transplantation [18]. The presence of PGD was recorded (grade 0–3) at 48 h after LTx.
The activities of daily living (ADL) index as a measure of functional independence was calculated as previously described with a maximum of 100 points [19]. The SF-36 as a measure of functional health and well-being scores was used in its validated German form [20, 21]. Quality of life and ADL at end of rehabilitation period was compared to that of 125 non-ventilated patients transplanted successfully between April 2007 and July 2009.
Escalation of mechanical support was defined as intubation for >24 h after prior ECS in spontaneously breathing patients, installation of ECS for >24 h after intubation or any switch of ECS mode (arterio–venous, veno–venous, veno–arterial).
Statistics
Continuous variables are here reported as the median and interquartile ranges (IQRs). All reported P values are two-sided, unless otherwise indicated. Medians were compared with the Mann–Whitney U test, and means were analysed with Student’s t test. Category variables were analysed using either a χ2 test or Fisher’s exact test. For all analyses, P values <0.05 were considered to be statistically significant.
Survival analysis was limited to 1 year after HU approval or after transplantation. Kaplan–Meier curves were plotted to compare survival. The log-rank test was applied to compare survival. A multivariate analysis using Cox stepwise forward proportional hazards analysis was used to compare overall, wait list and transplant survival for each significant variable from the univariate analysis separately. All variables with a P value ≤0.10 were included, and variables with a P value of >0.10 were excluded in this multivariate analysis.
Results
From among the 296 lung transplant candidates for whom a HU status had been requested during the study period in our centre, 100 (34 %) fulfilled the inclusion criteria of our study. In all but four patients (96 %), HU status was awarded upon first request. In the remaining candidates a second HU application was successful within a couple of days. No application for HU was refused upon re-evaluation. Patient characteristics and modes of ECS are given in Table 1. Of 12 candidates with bronchiolitis obliterans syndrome (BOS), five developed this syndrome after allogenic hematopoietic stem cell transplantation and seven had a previous lung transplant and were listed for re-do transplantation.
All patients were intubated, and 60 were on ECS. Of all LTx candidates, 26% were intubated in an external hospital without any prior evaluation as LTx candidate.
Details of patient outcome are displayed in Fig. 1. Thirty-eight patients died before an organ was available. Of the five patients who were weaned from the ventilator, three were transplanted 21, 40 and 74 days, respectively, after HU approval, and two of these survived the first postoperative year. The remaining two weaned patients recovered; one patient, with Wegener’s granulomatosis, remained free of oxygen, and the second patient, who suffered from bleomycin-induced fibrosis, remained on oxygen during the follow-up but refused LTx. Both patients were subsequently removed from the waiting list.
Sixty patients (including the three who had been weaned from support prior to LTx) were transplanted during their hospital stay. Of these, 37 (62 %) survived to hospital discharge, with 34 (57 %) surviving 1 year after LTx. Three patients died 127, 197 and 327 days after initial hospital discharge from graft failure and sepsis (n = 2). Invasive respiratory support was escalated in 24 patients: ten patients were put on ECS more than 24 h after intubation, eight patients were switched onto ECS mode and six candidates were intubated after prior ECMO.
No patient was lost to follow-up. Of the 60 transplanted patients, 57, 48 and 42 % survived 1, 3 and 5 years, respectively, compared to 82, 69 and 60 % of the non-critically ill reference population (n = 425) (Fig. 3). Graft survival in the ventilated cohort was 55, 46 and 40 % after 1, 3 and 5 years, respectively, compared to 82, 66 and 56 % in the reference cohort.
Survival analysis
Details of the univariate and multivariate analysis are shown in Figs. 2 and 3 (and in Figs. 5–8 in the ESM) and Table 2. Overall survival of lung transplant candidates was significantly improved compared to patients who were not transplanted (Fig. 2). The wait list survival was lower for cystic fibrosis patients. Patients with BOS survived more frequently to transplant and post-transplant. Overall survival and post-transplant survival were not associated with underlying diseases. Age, body mass index (BMI), gender and duration of bridging to transplant did not influence survival. A split team decision was associated with lower overall survival and increased mortality after LTx and wait list survival. In the multivariate analysis, neither of these effects was demonstrated to be independent. The LAS was unable to identify survivors, and the SAPS 2 score (cut-off >24 points) was independently associated with overall survival. A procalcitonin (PCT) level of ≥0.5 mg/ml was independently associated with overall mortality (Figs. 6–8 in ESM).
Of 16 supported candidates without escalation of support, with a PCT <0.5 µg/l and a SAPS ≤24 at time of HU approval, 15 (94 %) reached LTx and 13 of these 15 (87 %) survived LTx. None of the four patients with escalation therapy, a PCT >0.5 µg/l and a SAPS >24 survived, and a single patient of these reached LTx.
Two patients were re-transplanted for PGD (6 and 32 days after primary LTx, respectively) during the 1 year after being awarded HU status.
Post-transplant outcome
Details of transplant and early postoperative results are given in Table 3. There was a trend (P < 0.10) towards shorter cold ischemia times in transplanted patients (Table 3) with prior mechanical ventilation compared to our total cohort of 2005–2009. In our total cohort, cold ischemic times were 462 (range 380–598) min. The proportion of extended donors in our total cohort was 24 % compared to 15 % in intubated recipients.
Quality of life
After a median hospital stay of 60 (29–89) days post-LTx, surviving patients were discharged to a rehabilitation facility. The median time spent in in-patient rehabilitation was 27 days; in comparison, in the control group of 125 patients, the median length of stay was 21 days (p < 0.001). The SF36 and ADL results were available for 21/34 patients at the end of rehabilitation program. The results of the SF-36 questionnaire are given in Fig. 4 in comparison to the patients without prior MV before LTx. Significant differences were noted for the subscales physical functioning, general health perception, vitality and mental health.
Quality of life as measured by the Short Form 36 (SF36) at the end of the rehabilitation programme for LTx patients on invasive respiratory support in comparison to a non-ventilated cohort (n = 125). The eight sections of the SF-36 are: PF physical functioning, RP physical role functioning, BP bodily pain, GHP general health perceptions, VITA vitality, SF social role functioning, RE emotional role functioning, MH mental health
All but one patient (94 %) had no activity limitations with an ADL index of 100 at the end of rehabilitation programme. A single female patient had an ADL of 65.
Discussion
In this analysis we demonstrated that 59 % of lung transplant candidates on invasive respiratory support survive up to transplant and that more than every second organ recipient survives the first postoperative year. LTx significantly improved the 1-year survival in this critically ill cohort, and prognostic factors could be identified.
Ten small single-centre case series reported on between 6 and 23 patients on MV who were subsequently transplanted [2–11]. These publications describe ventilator times of between 8 and 162 days before transplantation compared to 12 days in our cohort. The authors of these studies reported 1-year survival rates of between 25 and 87 %. Very long pre-transplant ventilator times and a 1-year survival rate comparable to that of non-intubated transplant recipients can be explained by the inclusion of stable ventilator patients who are usually non-sedated and have had less invasive respiratory support. In our study, stable long-term ventilated patients were excluded. Previous publications have shown that stable patients have much better survival rates than critically ill transplant recipients [4].
In the United Network for Organ Sharing (UNOS) analysis carried out between 1987 and 2008 [22], 587 ventilated patients and 51 patients on ECS had a 1-year survival rate of 62 and 50 %, respectively, which is comparable to the 59 % reported in our study. While only 8.5 % of the patients in the UNOS analysis were on ECS, 60 % of our cohort were on ECS. The mean LAS in the UNOS study was 54 compared to 87 in our study, with 88% of our candidates having a LAS score of >75, which is above the 98 % percentile of candidates in a recent registry analysis [23]. The use of ECS did not significantly affect overall post-transplant survival, but any escalating use of ECS resulted in a dismal survival on the wait list (33 %) and post-transplantation (25 %).
In a recent analysis, Smits et al. [24] demonstrated that, overall, the LAS accurately predicted mortality in a cohort of candidates in Eurotransplant with HU status. In this published study, which included 317 patients (median LAS 35), 18 % were ventilated and 7 % were on ECS. An adjustment of the original LAS may, however, be indicated according to Smits et al. [24] in order to accurately predict wait list mortality in the sickest patients. A major problem is that the LAS does not include ECMO.
To the best of our knowledge, our study is the first in which risk stratification of LTx candidates on invasive respiratory support has been analysed. The factors we identified as having an impact on 1-year survival are clinically relevant. The level of PCT in the blood stream may be increased in response to a proinflammatory stimulus, especially that of bacterial origin, and can be used as a marker of severe sepsis—a potential contraindication to transplantation. It is important to stress that our study cohort represents a highly selected group of candidates. General contraindications to LTx, such as history of malignancy, morbid obesity, airway colonization with Burkholderia cenocepacia, smoking, and non-adherence, should be strictly enforced [25]. A further concern of performing LTx in ventilated patients is uncertain neurological status, especially after emergency intubation or even cardiopulmonary resuscitation. Previously unlisted candidates are usually unacceptable because they cannot be completely evaluated and they cannot provide informed consent. A consensus in the transplant team should be reached, and the possibility of the patient surviving without LTx should be discussed, especially when the patient has “non-classical” lung transplant indications. An individual escalation strategy is necessary after intubated candidates have been accepted for LTx, and daily re-evaluation of candidates on invasive respiratory support is mandatory.
Even brief periods of MV can cause diaphragmatic weakness, which impairs recovery after LTx. Weaning from the ventilator postoperatively becomes more difficult, and delayed mobilization increases the risk of postoperative complications. Although survival is markedly reduced in this high-risk population, in our opinion, LTx is not futile in selected candidates. To limit as much as possible any negative impact on the use of resources and overall transplant outcomes, these patients should only represent a reasonable proportion of patients admitted to LTx programmes (10 % in our centre during recent years).
ECS in intubated patients with end-stage lung disease can improve oxygenation, remove CO2 and reduce or even avoid ventilatory support [26]. In the case of right-sided heart failure, ECS can provide circulatory support. The disadvantages of ECS include bleeding complications, thromboembolism, limb perfusion deficits, infections, hemolysis and vascular damage. Long-term biliary consequences potentially associated with ECS (ischemic cholangiopathy) after prolonged support are of concern, especially because of the importance of hepatic metabolism after LTx [27].
Our group has recently published its first experience with percutaneous insertion of ECMO in non-intubated lung transplant candidates with right-sided heart failure [28]. In a case series of five patients in which this technique was applied, 80 % of patients were successfully bridged and 75 % survived transplant. These rates compared favorable to the results of intubated LTx candidates in the present study and demonstrate a promising new approach of respiratory support.
The major limitations of our study are its retrospective design and data being only available only on ICU admission. Future studies may include longitudinal changes in variables and scores.
In conclusion, HU LTx improves survival in critically ill candidates requiring invasive respiratory support. An increasing number of ventilated candidates are being accepted for LTx, which indicates the necessity to formulate guidelines for acceptance of this special cohort of patients. The combination of normal levels of PCT, a low SAPS 2 score and the absence of treatment escalation during bridging can help identify candidates on invasive respiratory support who are suitable for LTx with acceptable 1-year survival rates. With the majority of patients surviving the first year after transplantation it is still ethical to accept these candidates.
References
Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Taylor DO, Kucheryavaya AY, Hertz MI (2009) The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report—2009. J Heart Lung Transplant 28:1031–1049
Adams DH, Cochrane AD, Khaghani A, Smith JD, Yacoub MH (1994) Retransplantation in heart-lung recipients with obliterative bronchiolitis. J Thorac Cardiovasc Surg 107:450–459
Bartz RR, Love RB, Leverson GE, Will LR, Welter DL, Meyer KC (2003) Pre-transplant mechanical ventilation and outcome in patients with cystic fibrosis. J Heart Lung Transplant 22:433–438
Baz MA, Palmer SM, Staples ED, Greer DG, Tapson VF, Davis DD (2001) Lung transplantation after long-term mechanical ventilation: results and 1-year follow-up. Chest 119:224–227
Elizur A, Sweet SC, Huddleston CB, Gandhi SK, Boslaugh SE, Kuklinski CA, Faro A (2007) Pre-transplant mechanical ventilation increases short-term morbidity and mortality in pediatric patients with cystic fibrosis. J Heart Lung Transplant 26:127–131
Flume PA, Egan TM, Westerman JH, Paradowski LJ, Yankaskas JR, Detterbeck FC, Mill MR (1994) Lung transplantation for mechanically ventilated patients. J Heart Lung Transplant 13:15–21
Hammainen P, Schersten H, Lemstrom K, Riise GC, Kukkonen S, Sward K, Sipponen J, Silverborn M, Dellgren G (2011) Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: a descriptive study. J Heart Lung Transplant 30:103–107
Low DE, Trulock EP, Kaiser LR, Pasque MK, Ettinger NA, Dresler C, Cooper JD (1992) Lung transplantation of ventilator-dependent patients. The Washington University Lung Transplantation Group. Chest 101:8–11
Massard G, Shennib H, Metras D, Camboulives J, Viard L, Mulder DS, Tchervenkov CI, Morin JF, Giudicelli R, Noirclerc M (1993) Double-lung transplantation in mechanically ventilated patients with cystic fibrosis. Ann Thorac Surg 55:1087–1091
Meyers BF, Lynch JP, Battafarano RJ, Guthrie TJ, Trulock EP, Cooper JD, Patterson GA (2000) Lung transplantation is warranted for stable, ventilator-dependent recipients. Ann Thorac Surg 70:1675–1678
Niedermeyer J, Hoffmeyer F, Strueber M, Hamm M, Harringer W, Haverich A (1999) Lung transplantation in ventilated patients. Intensivmedizin Notfallmedizin 36:183–189
Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, Grover FL (2006) Development of the new lung allocation system in the United States. Am J Transplant 6:1212–1227
Internet Communication (2009) A guide to calculating the lung allocation score. Available at: http://www.unos.org/docs/lung_allocation_score.pdf
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Oto T, Levvey BJ, Whitford H, Griffiths AP, Kotsimbos T, Williams TJ, Snell GI (2007) Feasibility and utility of a lung donor score: correlation with early post-transplant outcomes. Ann Thorac Surg 83:257–263
Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D (2005) Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 24:1454–1459
Mahoney FI, Barthel DW (1965) Functional evaluation: the Barthel index. MD State Med J 14:61–65
Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L (1992) Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J 305:160–164
Bullinger M (1996) Assessment of health related quality of life with the SF-36 Health Survey. Rehabilitation (Stuttg) 35:17–27
Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH (2010) Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg 139:765–773
Russo MJ, Iribarne A, Hong KN, Davies RR, Xydas S, Takayama H, Ibrahimiye A, Gelijns AC, Bacchetta MD, D’Ovidio F, Arcasoy S, Sonett JR (2010) High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest 137:651–657
Smits JM, Nossent GD, De Vries E, Rahmel A, Meiser B, Strueber M, Gottlieb J (2011) Evaluation of the lung allocation score in highly urgent and urgent lung transplant candidates in Eurotransplant. J Heart Lung Transplant 30:22–28
Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Kotloff R, Martinez FJ, Nathan S, Palmer S, Patterson A, Singer L, Snell G, Studer S, Vachiery JL, Glanville AR (2006) International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 25:745–755
Strueber M (2010) Extracorporeal support as a bridge to lung transplantation. Curr Opin Crit Care 16:69–73
Gelbmann CM, Rummele P, Wimmer M, Hofstadter F, Gohlmann B, Endlicher E, Kullmann F, Langgartner J, Scholmerich J (2007) Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol 102:1221–1229
Olsson KM, Simon A, Strueber M, Hadem J, Wiesner O, Gottlieb J, Fuehner T, Fischer S, Warnecke G, Kuhn C, Haverich A, Welte T, Hoeper MM (2010) Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 10:2173–2178
Acknowledgments
This work was supported by a grant from the German Federal Ministry of Education and Research (reference number: 01EO0802). The contents of this article are the sole responsibility of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Haverich and T. Welte contributed equally to the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gottlieb, J., Warnecke, G., Hadem, J. et al. Outcome of critically ill lung transplant candidates on invasive respiratory support. Intensive Care Med 38, 968–975 (2012). https://doi.org/10.1007/s00134-012-2551-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2551-y