Abstract
Purpose
Common opioids for analgesia and sedation of mechanically ventilated infants may tend to accumulate and cause prolonged sedation with an unpredictable extubation time. Remifentanil is a promising option due to its unique pharmacokinetic properties, which seem to be valid in adults as well as in infants.
Methods
In this double-blind, randomized, controlled trial mechanically ventilated neonates and young infants (<60 days) received either a remifentanil or fentanyl-based analgesia and sedation regimen with low dose midazolam. The primary endpoint of the trial was the extubation time following discontinuation of the opioid infusion. Secondary endpoints included efficacy and safety aspects.
Results
Between November 2006 and March 2010, we screened 431 mechanically ventilated infants for eligibility. The intention to treat group included 23 infants who were assigned to receive either remifentanil (n = 11) or fentanyl (n = 12). Although this was designed as a pilot study, median extubation time was significantly shorter in the remifentanil group (80.0 min, IQR = 15.0–165.0) compared to the fentanyl group (782.5 min, IQR = 250.8–1,875.0) (p = 0.005). Remifentanil and fentanyl provided comparable efficacy with more than two-thirds of the measurements indicating optimal analgesia and sedation (66.4 and 70.2 %, respectively; p = 0.743). Overall, both groups had good hemodynamic stability and a comparably low incidence of adverse events.
Conclusions
As neonates and young infants have a decreased metabolism of common opioids like fentanyl and are more prone to respiratory depression, remifentanil could be the ideal opioid for analgesia and sedation of mechanically ventilated infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanically ventilated infants usually receive an analgesic and sedative agent to control pain and anxiety and to facilitate mechanical ventilation. The most often used agents in pediatric intensive care patients are morphine or fentanyl in combination with midazolam [1, 2]. However, common opioids like fentanyl have a context-sensitive half-life that increases with time. Thus, these drugs can accumulate in the peripheral compartments during prolonged use and may cause prolonged sedation with an unpredictable extubation time after cessation of a continuous infusion [3]. Prolonged sedation and mechanical ventilation may have several negative consequences like an increased risk of lung injury, hemodynamic interference, or gastrointestinal motility disturbances [4, 5].
Remifentanil is a relative new synthetic opioid with a potency comparable to fentanyl but an exceptionally short context-sensitive half-life of only 3–5 min [6, 7]. The aim of this pilot study was to compare the efficacy and safety of a remifentanil-based regimen to a conventional fentanyl-based regimen for analgesia and sedation of medium-term mechanically ventilated neonates and young infants. The results of this pilot study were supposed to serve as a base for a larger confirmatory trial.
Patients and methods
This single-center study was conducted in accordance with good clinical practice and with the guidelines set out in the Declaration of Helsinki. Written informed consent was given by the children’s parents or legal guardian. After approval by the Ethical Review Board of the Medical Faculty of the University of Cologne, a total of 24 mechanically ventilated infants were recruited. Infants were randomized, in a double-blind manner, to receive either a remifentanil or a fentanyl-based regimen for analgesia and sedation at the Childrens Hospital, University of Cologne, Germany.
Inclusion and exclusion criteria
Infants were eligible for entry in the study if they had a gestational age of at least 36 weeks with a postnatal age no greater than 60 days, if they had been intubated within the last 12 h, and were expected to require analgesia and sedation due to mechanical ventilation for a further 12–96 h.
Infants were excluded from the study if they suffered a CNS insult (e.g., asphyxia) or a structural brain disorder affecting the ability to assess their level of sedation.
Treatment protocol
Premedication for endotracheal intubation included atropine 10 μg/kg, piritramide 0.1 mg/kg, thiopental 5 mg/kg, and vecuronium 0.1 mg/kg. Following intubation we applied further piritramide and thiopental boluses as needed to keep the infant sedated and pain free until the start of the study medication.
Study medication had to be started at the latest 12 h after intubation. The blinded study drug was diluted in such a way that an infusion rate of 0.5 ml/h corresponded to 3 μg/kg/h remifentanil or 1 μg/kg/h fentanyl, respectively. Study medication was started with 1.5 ml/h (9 μg/kg/h remifentanil or 3 μg/kg/h fentanyl) combined with midazolam 50 μg/kg/h. Subsequently, the opioid infusion was adjusted in steps of 0.5 ml/h (3 μg/kg/h remifentanil or 1 μg/kg/h fentanyl) to achieve and maintain a Hartwig score between 9 and 13. The Hartwig score is a validated score for assessment of pain and distress in mechanically ventilated infants and was evaluated at least every 6 h [8]. We allowed a maximum opioid infusion rate of 5.0 ml/h (30 μg/kg/h remifentanil or 10 μg/kg/h fentanyl). When the infant required supplementary sedation despite the maximum allowed opioid dosage, we increased midazolam in steps of 50 μg/kg/h up to a maximum dose of 400 μg/kg/h. Only in case of urgent need for deeper sedation to avoid accidental extubation or central venous line dislocation, we allowed a thiopental bolus of 5 mg/kg as rescue therapy followed by a subsequent increase of the study medication. If analgesia and sedation was insufficient despite the maximum allowed opioid and midazolam dosage, the infant was excluded from the study and treated with a high dose fentanyl, midazolam, and clonidine regimen.
When the starting dose of 1.5 ml/h study medication (9 μg/kg/h remifentanil or 3 μg/kg/h fentanyl) combined with midazolam 50 μg/kg/h caused excessive sedation, we first reduced the opioid infusion to 1.0 ml/h. In case the Hartwig score remained less than 9, we subsequently decreased midazolam in steps of 12.5 μg/kg/h.
During infusion of the study medication we continuously monitored heart rate, blood pressure, pulsoxymetrical oxygen saturation, transcutaneous or expiratory carbon dioxide, and body temperature. These vital signs were recorded every 6 h. In case of arterial hypotension, defined as mean blood pressure greater than 20 % below the physiologic range, we gradually infused up to 30 ml/kg crystalloids. If arterial hypotension persisted, we subsequently started catecholamines.
We kept our patients within the desired sedation range while weaning the ventilator settings. When the clinical condition had improved and the infant was judged ready for extubation, we discontinued the study drugs and midazolam infusion at the same time without prior dose reduction. To detect an influence of the midazolam concentration on our primary study endpoint, we obtained a blood sample immediately prior to discontinuation of the opioid and midazolam infusion. Extubation was performed as soon as possible following cessation of the opioid and midazolam infusion, when the infant demonstrated protective airway reflexes and a regular respiratory drive. We allowed a single naloxone bolus of 0.01 mg/kg in case of apnea following extubation. If apnea persisted or recurred, the infant had to be reintubated.
Study endpoints
Primary endpoint
The extubation time, defined as time from cessation of the opioid infusion until extubation, was the primary endpoint of our study.
Secondary endpoints
Secondary endpoints were to compare the efficacy and safety of the two regimens. Efficacy was indicated by the percentage of Hartwig scores within the desired range. Safety was indicated by hemodynamic stability and the incidence of adverse events.
Statistical analysis
Random allocation to the study groups was conducted by the central pharmacy of the university hospital on the basis of a computer-generated randomization list which realized balance points using blocks of varying length. Because adequate knowledge of baseline parameters and effect sizes needed for designing a confirmative study for neonates and young infants was not present at the time when the study was planned, the focus of this study was to yield estimates of effect sizes and distributional characteristics related to clinical endpoints reflecting the efficacy and safety of the regimens to be compared. Under these requirements a sample size of n = 2 × 12 for the intention to treat (ITT) population was chosen to minimize the exposure to experimental interventions for the study patients, taking into account aspects of feasibility in a single-center study environment and to give reasonable precise estimates for population parameters and effect sizes.
Owing to the pilot character of our clinical study the efficacy and safety of the regimens under study were assessed for the ITT as well as for the per protocol (PP) study population using exploratory tests (citing p values as computed) and descriptive statistics, suitable for observed distributional characteristics of the primary and secondary endpoints, respectively; nevertheless, we used the reading “significant” in a colloquial manner whenever a p value was less than 0.05. Unless otherwise stated results are presented for the ITT population.
Sample characteristics were given by usual descriptive statistics (e.g., median and quartiles, ranges, means and standard deviations). Efficacy of remifentanil versus fentanyl was explored by means of a non-parametric test (Mann–Whitney U test) because of skewness of the observed data for the primary endpoint. The p values for secondary endpoints (two-sided alternatives) have to be interpreted in an explorative manner. Computations were performed using the IBM SPSS 19.0 program (SPSS, USA).
Results
Study participants
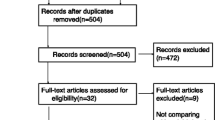
From November 2006 through March 2010, a total of 431 mechanically ventilated infants admitted to the pediatric intensive care unit of the University of Cologne were screened for eligibility. After written informed consent was obtained, 24 infants were randomized (Fig. 1).
In the remifentanil group, a 44-day-old infant with respiratory syncytial virus (RSV) pneumonia had to be withdrawn from the ITT group according to the study protocol because of insufficient analgesia and sedation despite the maximum allowed remifentanil and midazolam dosage. Two patients in the fentanyl group had to be withdrawn from the PP group because of protocol violations. In one patient, insufficient sedation was erroneously treated with repeated thiopental boluses instead of an adjustment of the study medication and the other patient accidentally received a tenfold midazolam dosage (500 μg/kg/h) for nearly 7 h. Thus, 23 patients were analyzed in the ITT group and 21 patients in the PP group. Demographic and clinical baseline characteristics for the ITT group are summarized in Table 1.
Primary endpoint
Extubation time was significantly shorter in the remifentanil group compared to the fentanyl group. In the ITT population, median extubation time was 80.0 min (IQR = 15.0–165.0) in the remifentanil and 782.5 min (IQR = 250.8–1,875.0) in the fentanyl group (p = 0.005). For the PP population, we found a comparable difference with 80.0 min (IQR = 15.0–165.0) in the remifentanil group and 782.5 min (IQR = 340.3–1,863.8) in the fentanyl group (p = 0.004).
Midazolam data of the ITT group are presented in Table 2. Including the infant who erroneously received a tenfold midazolam dosage, the midazolam dose was slightly higher in the fentanyl group compared to the remifentanil group (86.5 vs. 47.9 μg/kg/h) (p = 0.33). In the PP group, excluding this infant, the midazolam doses were comparable with 48.8 μg/kg/h in the fentanyl group and 47.9 μg/kg/h in the remifentanil group (p = 0.76). Median midazolam concentration of the ITT group prior to discontinuation of the study medication was 0.20 mg/l (IQR = 0.10–0.80) in the remifentanil group and 0.22 mg/l (IQR = 0.11–0.53) in the fentanyl group (p = 0.89).
One patient in the remifentanil group and two patients in the fentanyl group received naloxone because of apnea; however, no infant had to be reintubated.
Secondary endpoints
Efficacy
Remifentanil and fentanyl provided comparably good sedation and analgesia in combination with low dose midazolam. The mean individual percentage of measurements indicating optimal analgesia and sedation (Hartwig score 9–13) was 66.4 % in the remifentanil group and 70.2 % in the fentanyl group (t test: p = 0.743). The mean Hartwig score in the course of applied study medication is demonstrated in Fig. 2.
In both groups, no infant required thiopental as rescue therapy to avoid accidental extubation or central venous line dislocation due to inadequate sedation.
Safety
Mean duration of study medication and mean opioid dose are illustrated in Table 2. There was a tendency for a longer need of mechanical ventilation and analgesic/sedative medication in the remifentanil group compared to the fentanyl group (mean difference 25 h, 95 % CI from −2.3 to 52.3 h).
The mean heart rate in the course of applied study medication is demonstrated in Fig. 3. While the remifentanil group had considerably higher heart rates within the first 24 h of study medication (p = 0.002), the rates became comparable for both groups in the next 3 days. There were no episodes of bradycardia with an absolute range of 95–208 bpm for remifentanil and 95–150 bpm for fentanyl.
Heart rate during study medication. Analysis of heart rate was based on repeated measurements during infusion of study medication. Individual repeated measurements were averaged for each study patient within every 24-h interval resulting in one mean heart rate measurement for every study patient and each 24-h interval. Box plots represent median, IQR, minimum, and maximum. Values greater than 1.5 IQRs but less than 3 IQRs from the end of the box are labelled as outliers (circles)
The mean blood pressure in the course of applied study medication is illustrated in Fig. 4. All infants had mean blood pressures in a physiological range and we could detect no significant differences between both groups. The absolute range was 31–77 mmHg in the remifentanil group and 26–59 mmHg in the fentanyl group. Five infants of the remifentanil group and six infants of the fentanyl group received catecholamines.
Mean blood pressure during study medication. Analysis of mean blood pressure was based on repeated measurements during infusion of study medication. Individual repeated measurements were averaged for each study patient within every 24-h interval resulting in one averaged mean arterial blood pressure measurement for every study patient and each 24-h interval. Box plots represent median, IQR, minimum, and maximum. Values greater than 3 IQRs from the box are labelled as extreme, denoted by an asterisk. Values greater than 1.5 IQRs but less than 3 IQRs from the end of the box are labelled as outliers (circles)
Overall we observed no difference between remifentanil and fentanyl in terms of adverse events. One patient in each group suffered from pneumothorax. None of these adverse events was judged to be study drug related. One infant of the remifentanil group had a serious adverse event, which was also not study drug related. Prior to study entry, this infant already suffered from cyanotic spells due to RSV pneumonia combined with congenital tracheobronchomalacia. Eighteen days after extubation, the infant had to be hospitalized for a second time, as he again suffered from cyanotic spells.
Discussion
The remifentanil-based analgesia and sedation of pediatric intensive care patients (RAPIP) trial was designed as a randomized pilot study to compare remifentanil and fentanyl for analgesia and sedation of mechanically ventilated neonates and young infants. Concerning our primary study endpoint, the extubation time following cessation of the opioid infusion, we found that patients of the remifentanil group could be extubated significantly faster. This is in contrast to a previous study in mechanically ventilated neonates comparing sufentanil with fentanyl, where both groups had comparably long extubation times [9].
Most experience with remifentanil in pediatric patients is as maintenance anesthesia during surgery [10–12]. However, very limited data exist on remifentanil for analgesia and sedation of mechanically ventilated pediatric intensive care patients. Two observational studies without control group described remifentanil as safe and effective for analgesia and sedation of mechanically ventilated preterm infants [13, 14]. Apart from that, only two further trials investigated the short-term use of remifentanil in mechanically ventilated children. In the study of Pereira et al. [15], 20 preterm infants with respiratory distress syndrome received either remifentanil or morphine while they were mechanically ventilated. The average infusion time was 8 h with efficient analgesia and sedation in both groups. There were no major side effects and the mean extubation time was significantly shorter in the remifentanil group (106 vs. 1,320 min). Akinci et al. [16] compared remifentanil with fentanyl for postoperative analgesia in 22 mechanically ventilated postoperative orthopedic patients aged 3–16 years. Some patients additionally received propofol and the average duration of mechanical ventilation was 19 h. Overall both regimens provided comparable analgesia and there were no differences regarding adverse events.
Our study on remifentanil is the first double-blind, randomized trial in medium-term mechanically ventilated children. With our opioid-based analgesia and sedation regimen, we found a significantly shorter extubation time in the remifentanil group compared to the fentanyl group, which is in contrast to results in adults. Muellejans et al. [17] compared remifentanil with fentanyl for analgesia and sedation of mechanically ventilated adult ICU patients and found quite similar extubation times of 1.1 h for remifentanil and 1.3 h for fentanyl. Spies et al. [18] stopped their trial in mechanically ventilated adult patients ahead of time as a planned interim analysis could detect no advantage of remifentanil compared to fentanyl. The significant differences for remifentanil and fentanyl in our study demonstrate that neonates and young infants seem to benefit more than any other subgroup of patients from the unique pharmacokinetic properties of remifentanil. In neonates and young infants, age-specific body composition and immature hepatic metabolism result in accumulation of common opioids like fentanyl and prolonged side effects [19]. Remifentanil, however, does not accumulate during continuous infusion and is metabolized by nonspecific esterases, which have a high metabolic activity even in very preterm infants [6, 20, 21].
Three out of 23 infants received naloxone because of apnea. This relatively high number might be explained by the fact that we tried to extubate our patients as soon as possible. Usually extubation is somewhat delayed on a pediatric ICU, as infants mostly get extubated when they start to struggle against the respirator.
Our remifentanil starting dosage was based on recommendations for adults, whereas starting dosages of fentanyl and midazolam were based on our own prior experiences and pediatric data in the literature [3, 22–24]. Combined with low dose midazolam we required a mean remifentanil infusion rate of 13.8 μg/kg/h and a mean fentanyl rate of 4.9 μg/kg/h to maintain optimal analgesia and sedation for the mechanically ventilated infants. Daily costs of the required opioid doses mounted up to 5.14 euros for remifentanil compared to 0.30 euros for fentanyl. Although remifentanil is more expensive than fentanyl, the absolute costs were quite low for both opioids.
Remifentanil and fentanyl provided comparable results regarding the mean Hartwig score. In both groups we found an optimum sedation score in more than two-thirds of the time, reflecting the efficacy of both regimens. We have no good explanation why analgesia and sedation was insufficient in a 44-day-old infant with RSV pneumonia despite the maximum allowed remifentanil (30 μg/kg/h) and midazolam (400 μg/kg/h) dosage. This infant was the only patient outside the neonatal age range, which might suggest that these slightly older infants require higher dosages. However, many infants of this age are effectively treated with much lower remifentanil doses during surgery [25–27].
At the time when study medication was terminated, both groups had a midazolam concentration of around 0.20 mg/l. This proves that the considerably shorter extubation time of the remifentanil group was caused by the favorable properties of remifentanil and not by midazolam effects. Our midazolam concentration of 0.20 mg/l was within the desired therapeutic range of 0.1–0.5 mg/l. However, we found a large range of midazolam concentrations, as its metabolism is associated with high interindividual variability [28].
The tendency for a longer need of mechanical ventilation and analgesic/sedative medication in the remifentanil group compared to the fentanyl group was probably caused by more pronounced initial respiratory distress (Table 1).
Overall remifentanil was well tolerated in our ICU infants. The hemodynamic and adverse events safety profiles in the remifentanil group were similar to those in the fentanyl group. Overall, the reported incidence of adverse events was in keeping with events that one would expect in a neonatal ICU population. There was no drug-related serious adverse event.
The results of our pilot study are limited by the small sample size. Therefore, future studies should specifically investigate safety aspects in a larger number of infants. Furthermore, the large difference of the extubation time might be explained in part by our opioid-based analgesia and sedation regimen with quite high opioid doses. Additionally, it is possible that our weaning strategy not to decrease study medication until improvement of the clinical condition has contributed to the huge difference of the extubation time. However, in this regard it has to be emphasized that it is an important advantage of remifentanil not to require early dose reduction, as it allows fast recovery with a short transition period from hypnosis to the development of regular spontaneous breathing, airway protective reflexes, and an appropriate level of alertness [29].
In conclusion, remifentanil is a promising option for analgesia and sedation of mechanically ventilated infants. Remifentanil allowed a more rapid emergence from sedation and much earlier extubation compared to fentanyl. Our remifentanil-based analgesia and sedation regimen was as effective as a fentanyl-based regimen, and the incidence of adverse events was comparably low across the two treatment groups.
References
Jenkins IA, Playfor SD, Bevan C, Davies G, Wolf AR (2007) Current United Kingdom sedation practice in pediatric intensive care. Paediatr Anaesth 17:675–683
Playfor S, Jenkins I, Boyles C, Choonara I, Davies G, Haywood T, Hinson G, Mayer A, Morton N, Ralph T, Wolf A (2006) Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Med 32:1125–1136
Katz R, Kelly HW (1993) Pharmacokinetics of continuous infusions of fentanyl in critically ill children. Crit Care Med 21:995–1000
Molina PE (2006) Opioids and opiates: analgesia with cardiovascular, haemodynamic and immune implications in critical illness. J Intern Med 259:138–154
Jackson DL, Proudfoot CW, Cann KF, Walsh T (2010) A systematic review of the impact of sedation practice in the ICU on resource use, costs and patient safety. Crit Care 14:R59
Egan TD, Lemmens HJ, Fiset P, Hermann DJ, Muir KT, Stanski DR, Shafer SL (1993) The pharmacokinetics of the new short-acting opioid remifentanil (GI87084B) in healthy adult male volunteers. Anesthesiology 79:881–892
Glass PS, Gan TJ, Howell S (1999) A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg 89:S7–S14
Hunseler C, Merkt V, Gerloff M, Eifinger F, Kribs A, Roth B (2011) Assessing pain in ventilated newborns and infants: validation of the Hartwig score. Eur J Pediatr 170:837–843
Schmidt B, Adelmann C, Stutzer H, Welzing L, Hunseler C, Kribs A, Roth B (2010) Comparison of sufentanil versus fentanyl in ventilated term neonates. Klin Padiatr 222:62–66
Marsh DF, Hodkinson B (2009) Remifentanil in paediatric anaesthetic practice. Anaesthesia 64:301–308
Penido MG, Garra R, Sammartino M, Pereira ES (2010) Remifentanil in neonatal intensive care and anaesthesia practice. Acta Paediatr 99:1454–1463
Welzing L, Roth B (2006) Experience with remifentanil in neonates and infants. Drugs 66:1339–1350
Giannantonio C, Sammartino M, Valente E, Cota F, Fioretti M, Papacci P (2009) Remifentanil analgosedation in preterm newborns during mechanical ventilation. Acta Paediatr 98:1111–1115
Stoppa F, Perrotta D, Tomasello C, Cecchetti C, Marano M, Pasotti E, Barbieri MA, Conti G, Pirozzi N (2004) Low dose remifentanyl infusion for analgesia and sedation in ventilated newborns. Minerva Anestesiol 70:753–761
Pereira ES, Gomez RS, Barbosa RF, Silva AC (2005) Remifentanil for sedation and analgesia in a preterm neonate with respiratory distress syndrome. Paediatr Anaesth 15:993–996
Akinci SB, Kanbak M, Guler A, Aypar U (2005) Remifentanil versus fentanyl for short-term analgesia-based sedation in mechanically ventilated postoperative children. Paediatr Anaesth 15:870–878
Muellejans B, Lopez A, Cross MH, Bonome C, Morrison L, Kirkham AJ (2004) Remifentanil versus fentanyl for analgesia based sedation to provide patient comfort in the intensive care unit: a randomized, double-blind controlled trial [ISRCTN43755713]. Crit Care 8:R1–R11
Spies C, MacGuill M, Heymann A, Ganea C, Krahne D, Assman A, Kosiek HR, Scholtz K, Wernecke KD, Martin J (2011) A prospective, randomized, double-blind, multicenter study comparing remifentanil with fentanyl in mechanically ventilated patients. Intensive Care Med 37:469–476
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167
Welzing L, Ebenfeld S, Dlugay V, Wiesen MH, Roth B, Mueller C (2011) Remifentanil degradation in umbilical cord blood of preterm infants. Anesthesiology 114:570–577
Ross AK, Davis PJ, Gd Dear GL, Ginsberg B, McGowan FX, Stiller RD, Henson LG, Huffman C, Muir KT (2001) Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg 93:1393–1401
Breen D, Karabinis A, Malbrain M, Morais R, Albrecht S, Jarnvig IL, Parkinson P, Kirkham AJ (2005) Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497]. Crit Care 9:R200–R210
Dahaba AA, Grabner T, Rehak PH, List WF, Metzler H (2004) Remifentanil versus morphine analgesia and sedation for mechanically ventilated critically ill patients: a randomized double blind study. Anesthesiology 101:640–646
Notterman DA (1997) Sedation with intravenous midazolam in the pediatric intensive care unit. Clin Pediatr (Phila) 36:449–454
Michel F, Lando A, Aubry C, Arnaud S, Merrot T, Martin C (2008) Experience with remifentanil–sevoflurane balanced anesthesia for abdominal surgery in neonates and children less than 2 years. Paediatr Anaesth 18:532–538
Pietrini D, Ciano F, Forte E, Tosi F, Zanghi F, Velardi F, Di Rocco C, Chiaretti A, Caresta E, Piastra M (2005) Sevoflurane–remifentanil versus isoflurane–remifentanil for the surgical correction of craniosynostosis in infants. Paediatr Anaesth 15:653–662
Roulleau P, Gall O, Desjeux L, Dagher C, Murat I (2003) Remifentanil infusion for cleft palate surgery in young infants. Paediatr Anaesth 13:701–707
Burtin P, Jacqz-Aigrain E, Girard P, Lenclen R, Magny JF, Betremieux P, Tehiry C, Desplanques L, Mussat P (1994) Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther 56:615–625
Welzing L, Vierzig A, Junghaenel S, Eifinger F, Oberthuer A, Trieschmann U, Roth B (2011) Remifentanil and propofol for weaning of mechanically ventilated pediatric intensive care patients. Eur J Pediatr 170:477–481
Acknowledgments
This study was supported by a grant from GlaxoSmithKline (Munich, Germany) and by departmental sources. GlaxoSmithKline had no influence on study design, data analysis, or writing of the manuscript. The sponsor of the clinical trial, in accordance with Good Clinical Practice, was the University of Cologne.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov Identifier: NCT00419601.
Rights and permissions
About this article
Cite this article
Welzing, L., Oberthuer, A., Junghaenel, S. et al. Remifentanil/midazolam versus fentanyl/midazolam for analgesia and sedation of mechanically ventilated neonates and young infants: a randomized controlled trial. Intensive Care Med 38, 1017–1024 (2012). https://doi.org/10.1007/s00134-012-2532-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2532-1