Abstract
Purpose
Pleural and abdominal pressure are clinically estimated by measuring the esophageal and bladder or intragastric pressure (IGP), respectively. A new nasogastric polyfunctional catheter is now commercially available, equipped with two balloons in the lower and distal part; this catheter allows simultaneous esophageal pressure (Pes) and IGP measurements and can be also used to feed the patient. We compared the Pes and IGP measured using this new device with those obtained with a standard balloon catheter taken as gold standard.
Methods
Twenty-four intubated patients requiring ventilator support (mean age 64.3 ± 16.8 years, body mass index 25.3 ± 3.0 kg/m2, and PaO2/FiO2 280.8 ± 123.4 mmHg) were enrolled. Esophageal pressure and IGP were measured with the new nasogastric polyfunctional catheter (Nutrivent, Sidam, Italy) and with a standard balloon catheter (Smart Cath Viasys, USA). The Smart Cath was first inserted in the stomach and then retracted to the esophagus to measure IGP and Pes, respectively. In each patient two paired measurements were averaged.
Results
In the Bland–Altman analysis, the bias and agreement bands for Pes, ΔPes (computed as the difference of esophageal pressure between end-inspiration and expiration), and IGP were −0.25 (−2.65 to +2.15), 0.0 (−0.9 to +0.9), and −0.45 (−2.85 to + 1.95) cmH2O, respectively. No side effects or complications were recorded.
Conclusions
The new polyfunctional catheter showed a clinically acceptable validity in recording esophageal and intragastric pressure. This device should help physicians to better individualize the clinical patient management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) is a syndrome which, despite recent therapeutic advances, still results in high mortality [1, 2]. Although mechanical ventilation is a life-saving support, it may induce several types of lung injuries collectively named as ventilator-induced lung injury (VILI) [3]. The lung inflation depends on transpulmonary pressure (airway pressure minus pleural pressure), which in turn depends on the characteristics of the chest wall and the lung [4, 5]. In addition, an abnormal increase in the intra-abdominal pressure, defined as intra-abdominal hypertension, is frequently reported (from 18 to 81%) in critically ill patients [6, 7].
The absence of a customized lung protective strategy based on the transpulmonary pressure could lead to an under- or over-application of positive end-expiratory pressure (PEEP) as well as misinterpretation of the airway pressure [8]. The esophageal pressure measured in the lower third of the esophagus is an adequate surrogate of the pleural pressure, because the esophagus behaves as a passive structure [9–11]. The intra-abdominal pressure can be estimated by measuring the bladder or the intragastric pressure [12].
There is currently no nasogastric catheter available which allows a continuous and simultaneous monitoring of the esophageal and intragastric pressure while also providing the possibility of feeding the patient. The aim of this study was to evaluate the accuracy of the esophageal and intragastric pressures measured with a new nasogastric polyfunctional catheter compared to those obtained by a standard balloon catheter.
Materials and methods
Subjects
Twenty-four intubated, mechanically ventilated, sedated patients, with or without paralysis, admitted to our intensive care unit (ICU) from January to June 2010 were enrolled. Baseline characteristics were mean age 64.3 ± 16.8 years, body mass index 25.3 ± 3.0 kg/m2, new simplified acute physiology (SAP) II score 39.3 ± 12.2, PEEP 6.3 ± 4.5 cmH2O, PaO2/FiO2 280.8 ± 123.3, and tidal volume 492 ± 116 ml. Inclusion criterion was the presence of mechanical ventilation in patients who required a catheter. Exclusion criteria were patients younger than 16 years, documented barotrauma, high grade of esophageal varices, a recent history of esophageal or gastric surgery, and severe coagulopathy. The study was approved by the institutional review board of our hospital, and informed consent was obtained according to the Italian national regulations.
Measurement
Esophageal and intragastric pressure were simultaneously recorded by using the new nasogastric polyfunctional catheter (Nutrivent, Sidam, Mirandola, Italy) and by a standard balloon catheter (Smart Cath, Viasys, Palm Springs, USA).
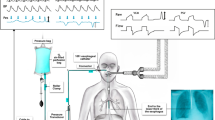
The Nutrivent consists of a polyurethane tube (110-cm long with an external diameter of 4.7 mm) with multiple small holes and two thin-walled polyethylene balloons (10-cm long and 15-mm diameter) incorporated in the lower and in the distal portion of the tube (Fig. 1). The intragastric position of the distal catheter was confirmed by aspiration of gastric juice, auscultation of air insufflations into the stomach, and by a rise in intra-abdominal pressure following external manual epigastric pressure. The balloon position in the lower third of the esophagus was confirmed in patients with paralysis by concordant positive changes in airway, esophageal and intragastric pressures during an inspiratory occlusion [13], and in patients able to perform an inspiratory effort by performing the Baydur test [14].
For an optimal reading of the pressures, the balloon should be inflated with an adequate volume of air so as to transmit the pressure without artifacts. Previous in vitro evaluation showed that the optimal volume both for the esophageal and gastric balloons was 4 ml of air.
The Smart Cath consists of a tube 103 cm long with an external diameter of 3 mm and a thin-walled balloon (10 cm long) located in the distal part. To measure the intragastric pressure, the catheter was first positioned in the stomach at a depth of 45–55 cm and then inflated with a volume of 2 ml of air. Subsequently, to measure the esophageal pressure, the catheter was retracted until it reached the upper third of the esophagus at a depth of 30–35 cm from the mouth. The Smart Cath does not allow one to aspirate the liquid from the stomach or to feed the patient.
Pressure signals were measured by pressure transducers (Bentley Trantec; Bentley Laboratories, Irvine, USA) and recorded on a personal computer for subsequent analysis (Colligo, Elekton, Milan, Italy). Two-min recording sections were performed. In each patient two paired measurements were averaged for the analysis. The two catheters were positioned at the same time in the patients and the measurements were obtained at the same time.
Protocol
Patients were in the supine position on mechanical ventilation with pre-study ventilator settings. The esophageal pressure was measured during an end-inspiratory and an end-expiratory occlusion of the airway (PesEIO and PesEEO). The variation of esophageal pressure during tidal inflation (ΔPes) was computed as PesEIO minus PesEEO. The intragastric pressure was measured only during an end-expiratory occlusion of the airway (IGPEEO).
Statistical analysis
Data are presented as mean ± standard deviation (SD). In addition, 95% confidence intervals (CI) were reported when necessary. Esophageal and intragastric pressures measured with the new nasogastric polyfunctional catheter and the standard balloon catheter were compared according to Bland–Altman analysis [15] together with the Passing and Bablok non-parametric regression [16] and concordance correlation coefficient [17]. Statistical analysis was performed with SAS(c) 9.1.
Results
Esophageal pressure
The mean PesEEO and ΔPes were 12.8 ± 3.1 and 3.2 ± 1.4 cmH2O, respectively, for the new nasogastric polyfunctional catheter and 12.9 ± 2.8 and 3.3 ± 1.5 cmH2O for the standard balloon catheter. The regression equations, calculated according to the Passing and Bablok method, had an intercept of −0.6909 (95% CI −3.1554 to 0.9022) and a slope of 1.0349 (95% CI 0.9130–1.2162) for the PesEEO and an intercept of −0.1500 (95% CI −0.6500 to 0.4071) and a slope of 1.000 (95% CI 0.8571–1.2000) for ΔPes.
In the Bland–Altman analysis, the bias and agreement bands (between brackets) for PesEEO (Fig. 2a) and ΔPes (computed as the difference of esophageal pressure between end-inspiration and expiration, Fig. 2b) were −0.25 (−2.65 to +2.15) and 0.0 (−0.9 to +0.9) cmH2O, respectively. There was no evidence of an increase of bias at the extremes of the pressure range (p = 0.1936 and 0.3593, respectively). The concordance correlation coefficient (CCC) was 0.91 (95% CI 0.80–0.96) for the PesEEO and 0.95 (95% CI 0.90–0.98) for ΔPes; these values translate into a within-sample variability of about 30% and 22% of the total variability, respectively.
a Bland–Altman analysis of the esophageal pressure measured with the new nasogastric polyfunctional catheter and the standard balloon catheter. The x-axis shows the mean of the two measurements and the y-axis the difference between the esophageal pressure measured with the new nasogastric polyfunctional catheter and the standard balloon catheter. b Bland–Altman analysis of the delta esophageal pressure measured with the new nasogastric polyfunctional catheter and the standard balloon catheter. The x-axis shows the mean of the two measurements and the y-axis the difference between the delta esophageal pressure measured with the new nasogastric polyfunctional catheter and the standard balloon catheter. c Bland–Altman analysis of the intragastric pressure measured with the new nasogastric polyfunctional catheter and the standard balloon catheter. The x-axis shows the mean of the two measurements and the y-axis the difference between the intragastric pressure measured with the new nasogastric polyfunctional catheter and the standard balloon catheter
Intragastric pressure
The mean IGPEEO was 9.7 ± 2.7 and 10.2 ± 2.7 cmH2O for the new nasogastric polyfunctional catheter and standard balloon catheter, respectively. The regression equation calculated according to the Passing and Bablok method, considering the IGPEEO between the two methods, had an intercept of −0.1493 (95% CI −3.0595 to 1.7500) and a slope of 0.9841 (95% CI 0.7500–1.2619).
The Bland–Altman analysis showed a bias of −0.45 (−2.85 to 1.95) cmH2O (Fig. 2c). The CCC was 0.89 (95% CI 0.75–0.95); this value translates into a within-sample variability of about 33% of the total variability.
The new nasogastric polyfunctional catheter was successfully inserted in all the subjects, and it was left in some patients after the study depending on their clinical requirements. No side effects or complications were recorded.
Discussion
The primary finding of this study is that the new nasogastric polyfunctional catheter is able to record both the esophageal and intragastric pressure similarly to a standard balloon catheter.
The respiratory system includes the lung and the chest wall, and consequently the mechanical behavior depends on the mechanical properties of these two components [4]. The distending force of the lung is not the airway pressure, which is commonly used in clinical practice, but the transpulmonary pressure [8].
The measurement of esophageal pressure, obtained by an esophageal balloon, has been used as an estimate of pleural pressure since Buytendijk [18] pioneered the technique. The most widely used method employs an air-containing balloon sealed over a catheter, which transmits balloon pressure to manometers or, more recently, to monitoring transducers [10, 11]. When simultaneously recorded in spontaneous breathing subjects, the esophageal pressure changed at the same rate as the pleural pressure, but it was consistently more positive [18, 19]. In an experimental study, the esophageal pressure only reflected the pleural pressure measured in the middle regions, whereas it overestimated the pleural pressure in the non-dependent lung regions and underestimated it in the dependent lung regions [20].
In the present study the new nasogastric polyfunctional catheter showed a good clinical agreement compared to a standard balloon catheter in estimating either the absolute or the changes of esophageal pressure in mechanically ventilated patients. The level of agreement was slightly lower when recording the absolute esophageal pressure compared with the variations of esophageal pressure during the tidal inflation. This was probably due to the difference in location of the balloon of the new nasogastric polyfunctional catheter in the esophagus compared with the standard balloon catheter. To minimize the pressure artifacts due to the catheter itself, we employed a balloon 10-cm long with a very thin wall, inflated with a minimum amount of air volume, to not increase the pressure inside the balloon, and positioned in the lower part of the esophagus [9–11].
Several techniques have been proposed for the measurement of intra-abdominal pressure [12]. The bladder technique was originally described by Kron et al. [21] in which the bladder was infused with 50–100 ml of saline via the patients’ Foley catheter and the pressure was recorded by a pressure transducer at the symphysis pubis. The bladder technique, due to its simplicity, low cost, and excellent correlation with direct measurement of intra-abdominal pressure, still remains the most common technique. However, it is time consuming, it requires instillation of saline into the bladder, it is intermittent and, moreover, gives a trend of intra-abdominal pressure depending on the interval between separate measurements [12]. Previous studies showed that the intra-abdominal pressure can be also accurately measured by the intragastric pressure [22]. In our experimental setting, the bias in recording the intragastric pressure was clinically acceptable. Even though the width of the agreement bands may be considered numerically high, the agreement was judged to be acceptable because the variations of a few units are not relevant.
Thus, the intragastric pressure can be considered an alternative in patients without bladder catheter, in cases of bladder trauma, pelvic hematomas or fractures, and in all the clinical situations in which a continuous monitoring of intra-abdominal pressure is advisable.
Conclusions
Because the majority of patients admitted to ICU require a nasogastric catheter for nutrition during mechanical ventilation, the new nasogastric polyfunctional catheter can be a good alternative allowing the possibility to continuously monitor esophageal and intragastric pressure. The knowledge of esophageal and intragastric pressures should help in tailoring a more “physiologic” mechanical ventilation.
References
Detsky ME, Stewart TE (2010) Long-term outcomes of patients after acute respiratory distress syndrome: hard work for nothing? Minerva Anestesiol 76:641–644
Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, Gattas DJ, Hallett D, Tomlinson G, Stewart TE, Ferguson ND (2009) Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 179:220–227
Slutsky AS (1999) Lung injury caused by mechanical ventilation. Chest 116:9S–15S
Gattinoni L, Chiumello D, Carlesso E, Valenza F (2004) Bench-to-bedside review: chest wall elastance in acute lung injury/acute respiratory distress syndrome patients. Crit Care 8:350–355
Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH (2008) Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 359:2095–2104
Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L (2004) Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 30:822–829
Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V, Cohen J, Japiassu A, De Keulenaer BL, Daelemans R, Jacquet L, Laterre PF, Frank G, de Souza P, Cesana B, Gattinoni L (2005) Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med 33:315–322
Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, Tallarini F, Cozzi P, Cressoni M, Colombo A, Marini JJ, Gattinoni L (2008) Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 178:346–355
Higgs BD, Behrakis PK, Bevan DR, Milic-Emili J (1983) Measurement of pleural pressure with esophageal balloon in anesthetized humans. Anesthesiology 59:340–343
Milic-Emili J, Mead J, Turner JM (1964) Topography of esophageal pressure as a function of posture in man. J Appl Physiol 19:212–216
Milic-Emili J, Mead J, Turner JM, Glauser EM (1964) Improved technique for estimating pleural pressure from esophageal balloons. J Appl Physiol 19:207–211
Malbrain ML (2004) Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med 30:357–371
Chiumello D, Cressoni M, Racagni M, Landi L, Li Bassi G, Polli F, Carlesso E, Gattinoni L (2006) Effects of thoraco-pelvic supports during prone position in patients with acute lung injury/acute respiratory distress syndrome: a physiological study. Crit Care 10:R87
Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J (1982) A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126:788–791
Bland JM, Altman DG (1990) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Passing H, Bablok W (1983) A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem 21:709–720
Lin L, Hedavat AS, Yang M (2002) Statistical methods in assessing agreement: models, issues and tools. J Am Stat Assoc 97:257–270
Buytendijk HJ (1949) Oesophagusdruk en longelasticiteit. Electrische Drukkerij I. Oppenheim NV, Groningen
Mead J, McIlroy MB, Selverstone NJ, Kriete BC (1955) Measurement of intraesophageal pressure. J Appl Physiol 7:491–495
Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ (2001) Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 164:122–130
Kron IL, Harman PK, Nolan SP (1984) The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg 199:28–30
Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, D’Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A (2006) Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 32:1722–1732
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Chiumello and L. Gattinoni participated in the development of Nutrivent, but do not have any patent or financial agreement, and received only an honorarium as members of the advisory board.
Rights and permissions
About this article
Cite this article
Chiumello, D., Gallazzi, E., Marino, A. et al. A validation study of a new nasogastric polyfunctional catheter. Intensive Care Med 37, 791–795 (2011). https://doi.org/10.1007/s00134-011-2178-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2178-4