Abstract
Purpose
There is growing interest in the use of both variable and pressure-controlled ventilation (PCV). The combination of these approaches as “noisy PCV” requires adaptation of the mechanical ventilator to the respiratory system mechanics. Thus, we developed and evaluated a new control system based on the least-mean-squares adaptive approach, which automatically and continuously adjusts the driving pressure during PCV to achieve the desired variability pattern of tidal volume (V T).
Methods
The controller was tested during numerical simulations and with a physical model reproducing the mechanical properties of the respiratory system. We applied step changes in respiratory system mechanics and mechanical ventilation settings. The time needed to converge to the desired V T variability pattern after each change (t c) and the difference in minute ventilation between the measured and target pattern of V T (ΔMV) were determined.
Results
During numerical simulations, the control system for noisy PCV achieved the desired variable V T pattern in less than 30 respiratory cycles, with limited influence of the dynamic elastance (E*) on t c, except when E* was underestimated by >25%. We also found that, during tests in the physical model, the control system converged in <60 respiratory cycles and was not influenced by airways resistance. In all measurements, the absolute value of ΔMV was <25%.
Conclusion
The new control system for noisy PCV can prove useful for controlled mechanical ventilation in the intensive care unit.
Similar content being viewed by others
Introduction
Variable mechanical ventilation improves lung function [1, 2] and reduces lung damage in experimental acute lung injury (ALI) [3], as compared to conventional controlled ventilation. Currently, variable ventilation is performed as volume-controlled ventilation, with the tidal volume (V T) of every respiratory cycle varying according to a pre-established pattern with desired mean (V T,m) and standard deviation (SD) (V T,s) [4].
Since pressure-controlled ventilation (PCV) reduces peak airway pressures and can homogenize the distribution of ventilation across the lungs compared to volume-controlled ventilation [5, 6], there is a growing interest in the use of PCV in patients with ALI. Theoretically, the combination of variable ventilation with PCV (noisy PCV) could be interesting for clinical practice. However, the respiratory system mechanics may vary over time [7], modifying the relationship between the driving pressure (Ps) and V T during PCV.
The aim of this work is to evaluate a novel adaptive control system for noisy PCV capable of tuning Ps breath-to-breath to obtain the desired variable V T pattern.
Materials and methods
Control system
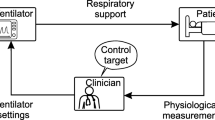
The control system (Fig. 1) is based on the least-mean-squares adaptive approach [8] (online supplement). Before each inspiration i, the control system transmits to the ventilator the value of Ps(i) for the next respiratory cycle, computed according to a normal distribution with given mean and standard deviation (SD) (Psm and Pss, respectively). When inspiration ends, the tidal volume V T(i) is measured and compared with the desired V T,d(i), computed according to a normal distribution with given mean and SD (V T,m and V T,s). Their difference [ΔV T(i) = V T(i) − V T,d(i)] is used to tune Psm and Pss, aimed at minimizing ΔV T(i) in future cycles. If ΔV T(i) is positive (negative), Psm is decreased (increased) by X cmH2O, where X is proportional to ΔV T(i). Similarly, if ΔV T(i) has the same (opposite) sign of the difference between V T,d(i) and V T,m, the Pss is decreased (increased). Psm and Pss changes are modulated by step factors that depend on the dynamic elastance (E*) and minimize over/undershooting. The maximum Ps value is limited to 40 cmH2O by the controller (even if in this study such value was never reached). Inspiration and expiration lengths are fixed as in conventional PCV mode.
Schematic illustration of the sequence of actions performed by the control system for noisy pressure-controlled ventilation (noisy PCV) starting at the onset of each inspiration i. V T, V T,d , ΔV T : measured and desired tidal volume, as well as the difference between the two, respectively; V T,m , V T,s : target (desired) mean and standard deviation (SD) of tidal volume, respectively; Ps, Ps m , Ps s : driving pressure, its mean and SD, respectively
The control system was tested during numerical simulations and at the bench.
Numerical simulations
Simulations were performed using an one-compartment linear model of the respiratory system [9] (online supplement). Two extreme tasks were considered: (1) transition from “conventional” PCV to noisy PCV, i.e., 0 to 30% coefficient of variation in V T,d. Such variability is found in healthy humans [10] and can optimize gas exchange and respiratory system mechanics in experimental ALI [2]; (2) step increase in E* of 30 cmH2O/l. For each task, 100 simulations, comprehending each 1,000 respiratory cycles, were performed using different V T,d patterns, but fixed mean and SD. Simulations were repeated for E* in the range 15 to 150 cmH2O/l (5 cmH2O/l steps) [11]. All simulations were repeated introducing constant errors in the estimates of E* (errE* = 25, −25, 50, and −50%).
Physical model tests
Bench tests used the lung model LS4000 (Dräger Medical, Lübeck, Germany) and a ventilator Evita XL (Dräger Medical) remotely operated by a personal computer running the noisy PCV control system. Due to safety constraints of the ventilator, the control system could modify Ps every other cycle only. Tests included transition from conventional to noisy PCV, followed by a sequence of tasks consisting in step changes in elastance, resistance, V T,m and V T,s (online supplement). Each task lasted 100 respiratory cycles after the change and was repeated five times using different V T,d patterns, but the same mean and SD.
Performance indexes
Convergence time (t c) was estimated as the last respiratory cycle for which the root mean square error between V T,d and V T along the ten preceding respiratory cycles was >10 ml, together with the deviation from target minute ventilation within t c (ΔMV).
Statistics
Values are given as mean, SD and/or ranges. In numerical simulations, the effect of errE* on t c and ΔMV were determined with Spearman’s correlation and Wilcoxon’s tests. Data from the physical model tests were assessed with two-way ANOVA. Statistical significance was accepted at p < 0.05.
Results
Figure 2 shows representative patterns of convergence of the control system during a test with the physical lung model when changing from conventional to noisy PCV, as well as after step changes in elastance, V T,d and V T,s. Values of t c were <25 cycles, and absolute values of ΔMV were <20% in all situations.
Representative recordings of convergence of the novel control system during transition from conventional to noisy pressure-controlled ventilation (PCV and noisy PCV, respectively), as well as during step changes in different parameters. Measurements were obtained in a physical lung model mimicking the mechanical properties of the respiratory system. Dashed vertical lines represent the moment of the step change (cycle no. 0), while vertical dotted lines represent the cycle at which convergence is reached (t c). E Elastance, V T tidal volume, V T,m , V T,s : mean and standard deviation of the target (desired) V T series, respectively, which is determined a priori. ΔMV difference between measured and desired minute ventilation until t c
Numerical simulations
The effects of E* on t c and ΔMV are shown in Fig. S4 (online supplement). When switching from conventional to noisy PCV, mean t c was 12.2 cycles [range = 1 to 37, SD = 6.7, Fig. S4a], while mean ΔMV was −2% [range = −17 to 8%, SD = 6%, Fig. S4c]. ΔMV and t c showed no correlation with E* (Fig. S4a/c, absolute value of r < 0.09). Also, E* underestimation resulted in slower convergence than overestimation (p < 0.001), and larger errE* resulted in larger t c and ΔMV (p < 0.001). A sudden increase of E* by 30 cmH2O/l led to mean t c of 13.5 cycles [range = 1 to 74), SD = 6, Fig. S4b], with t c > 30 found only when errE* = −50%, and was associated with a mean ΔMV of −5% [range = −1 to −22%, SD = 3%, Fig. S4d]. ΔMV and t c were inversely correlated with E* (p < 0.001) and with the magnitude of errE* (p < 0.001).
Physical model tests
Table S3 (online supplement) depicts the results from the physical model tests. Switching from conventional to noisy PCV resulted in t c < 39 cycles and ΔMV in the range of −5 to 25%. The other tasks resulted in t c < 56 and ΔMV in the range of −19 to 21%. The magnitude of the step change of V T,s influenced t c (larger changes resulted in larger t c, p < 0.01; increases associated with larger t c than decreases, p < 0.01), while that of elastance and V T,m affected ΔMV (larger changes resulted in larger ΔMV, p < 0.05; the sing of the change was always opposite to that of ΔMV). Changes in resistance did not influence the control system.
Discussion
One of the main findings of this work was that, during numerical simulations, the novel control system for noisy PCV achieved the desired variable V T pattern in less than 30 respiratory cycles, with limited influence of E* on t c, except when E* was underestimated by >25%. We also found that, during physical model tests, the control system converged within 56 respiratory cycles and was not influenced by resistance. In all measurements, the absolute value of ΔMV was <25%.
The use of automatic control algorithms to perform adjustments of mechanical ventilation parameters has been previously reported [12–15]. A time-adaptive control system similar to ours has been introduced [14], but did not deal with the variability of the respiratory pattern. To our knowledge, our control system represents the first solution to the problem of automatically maintaining a desired V T variability pattern during PCV.
The performance of the noisy PCV control system was satisfactory in both numerical simulations and physical model tests, guaranteeing a convergence of the measured V T pattern to the desired 1 in <60 cycles. In a fully embedded control system, i.e. when ventilator settings can be modified at the end of each respiratory cycle, and with a reasonably reliable method for E* estimation (i.e., error <25%), t c is expected to be always <30. It is worth noting that the conditions simulated in this work, including step changes in elastance and resistance as high as 60 cmH2O/l and 6 cmH2O/(l/s), respectively, posed more difficult challenges to the controller than what is expected in vivo. Romero et al. [16] showed namely that a methacholine challenge doubles the elastance and resistance after 60 and 10 s, respectively.
Compared to a conventional approach to adaptive control that used fixed values for the step factors [17], our system yielded faster convergence over a broad range of conditions (online supplement, supplementary discussion). Since the step factors are functions of E* estimates, errors in such estimates might result in slower convergence. However, errors as high as 50% had only a limited effect on t c.
The ΔMV was always less than 25%. Moreover, the higher the accuracy of E* estimation, the lesser ΔMV was. Theoretically, MV transients may result in alterations of mean airway pressure as well as gas exchange. However, due to its relatively short duration, which is equivalent to t c, derecruitment or overdistension of lung units, as well as deterioration of oxygenation and CO2 elimination are unlikely. It should be kept in mind that, under conventional PCV in clinical practice, such transients may last much longer, since manual adjustments of mechanical ventilator settings are performed after MV changes are detected by the device after several cycles.
Limitations
This study has two important limitations: (1) we used a linear model of lung mechanics. Nonlinearities in the relationship between Ps and V T may influence t c, but investigating such effects was beyond the scope of this work; (2) smooth changes in respiratory system mechanics across consecutive respiratory cycles were not investigated. Due to the adaptive nature of the control system and its relatively fast response during step changes, it is likely that the V T pattern would converge even faster during smoother changes.
Conclusion
The novel adaptive control system is able to accomplish noisy PCV with relatively low t c and ∆MV, as well as smooth transients upon the desired pattern of variable ventilation, during the simulations and bench tests considered in this study. This control system may prove useful for mechanical ventilation in the intensive care unit.
References
Gama de Abreu M, Spieth PM, Pelosi P, Carvalho AR, Walter C, Schreiber-Ferstl A, Aikele P, Neykova B, Hubler M, Koch T (2008) Noisy pressure support ventilation: a pilot study on a new assisted ventilation mode in experimental lung injury. Crit Care Med 36:818–827
Spieth P, Carvalho AR, Güldner A, Pelosi P, Kirichuck O, Koch T, Gama de Abreu M (2009) Effects of different levels of pressure support variability in experimental lung injury. Anesthesiology 110:342–350
Spieth PM, Carvalho AR, Pelosi P, Hoehn C, Meissner C, Kasper M, Hubler M, von Neindorff M, Dassow C, Barrenschee M, Uhlig S, Koch T, Gama de Abreu M (2009) Variable tidal volumes improve lung protective ventilation strategies in experimental lung injury. Am J Respir Crit Care Med 179:684–693
Lefevre GR, Kowalski SE, Girling LG, Thiessen DB, Mutch WA (1996) Improved arterial oxygenation after oleic acid lung injury in the pig using a computer-controlled mechanical ventilator. Am J Respir Crit Care Med 154:1567–1572
Tugrul M, Camci E, Karadeniz H, Senturk M, Pembeci K, Akpir K (1997) Comparison of volume controlled with pressure controlled ventilation during one-lung anaesthesia. Br J Anaesth 79:306–310
Prella M, Feihl F, Domenighetti G (2002) Effects of short-term pressure-controlled ventilation on gas exchange, airway pressures, and gas distribution in patients with acute lung injury/ARDS. Chest 122:1382–1388
Putensen C, Zech S, Wrigge H, Zinserling J, Stuber F, von Spiegel T, Mutz N (2001) Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 164:43–49
Widrow B, Stearns SD (1985) Adaptive signal processing. Prentice-Hall, Englewood Cliffs
Khoo MC (1999) Physiological control systems: analysis simulation and estimation. Wiley-IEEE Press, New York
Tobin MJ, Mador MJ, Guenther SM, Lodato RF, Sackner MA (1988) Variability of resting respiratory drive and timing in healthy subjects. J Appl Physiol 65:309–317
Boker A, Graham MR, Walley KR, McManus BM, Girling LG, Walker E, Lefevre GR, Mutch WA (2002) Improved arterial oxygenation with biologically variable or fractal ventilation using low tidal volumes in a porcine model of acute respiratory distress syndrome. Am J Respir Crit Care Med 165:456–462
Dojat M, Brochard L, Lemaire F, Harf A (1992) A knowledge-based system for assisted ventilation of patients in intensive care units. Int J Clin Monit Comput 9:239–250
Jandre FC, Pino AV, Lacorte I, Neves JH, Giannella-Neto A (2004) A closed-loop mechanical ventilation controller with explicit objective functions. IEEE Trans Biomed Eng 51:823–831
Laubscher TP, Heinrichs W, Weiler N, Hartmann G, Brunner JX (1994) An adaptive lung ventilation controller. IEEE Trans Biomed Eng 41:51–59
Mersmann S, Dojat M (2004) SmartCare––automated clinical guidelines in critical care. In: Proceedings of 16th Eur Conf on artificial intelligence, pp 745–749
Romero PV, Rodriguez B, Lopez-Aguilar J, Manresa F (1998) Parallel airways inhomogeneity and lung tissue mechanics in transition to constricted state in rabbits. J Appl Physiol 84:1040–1047
Feuer A, Weinstein E (1985) Convergence analysis of LMS filters with uncorrelated Gaussian data. IEEE Trans Acoust Speech 33:222–230
Disclosures
A patent on the adaptive controller for noisy pressure-controlled ventilation has been submitted by A. Beda, A. Güldner, P.M. Spieth, and M.G. de Abreu, and is pending. T. Handzsuj works for Dräger Medical AG & Co. KG, which produces and develops devices that could make use of the funcionalities described in this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beda, A., Spieth, P.M., Handzsuj, T. et al. A novel adaptive control system for noisy pressure-controlled ventilation: a numerical simulation and bench test study. Intensive Care Med 36, 164–168 (2010). https://doi.org/10.1007/s00134-009-1665-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1665-3