Abstract
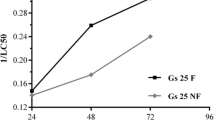
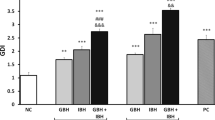
Effects of pure glyphosate and a glyphosate-based product were evaluated comparatively using two embryonic development stages of Xenopus laevis as model system. When pure glyphosate was applied in pH adjusted media, lethal or developmental effects were not observed at concentrations up to 500 mg L−1. The 96 h LC50 values for the commercial herbicide, in contrast, were 32.1 and 35.1 mg active ingredient L−1 for embryos and tadpoles, respectively. Since pure glyphosate has no effect on the selected biomarkers, it is thought that developmental toxic effects caused by glyphosate-based products are increased mainly due to formulation additives.
Similar content being viewed by others
References
ASTM (2003) American Society for Testing and Materials, Standard guide for conducting the Frog Embryo Teragonesis Assay-Xenopus (FETAX), E1439-98. In: ASTM standards on biological effects and environmental fate, Vol. 11.05. Philadelphia, PA, pp 447–457
Babalola OO, Truter JC, van Wyk JH (2019) Mortality, teratogenicity and growth inhibition of three glyphosate formulations using frog embryo teratogenesis Assay-Xenopus. J Appl Toxicol. https://doi.org/10.1002/jat.3811
Bach NC, Marino DJG, Natale GS, Somoza GM (2018) Effects of glyphosate and its commercial formulation, Roundup((R)) Ultramax, on liver histology of tadpoles of the neotropical frog, Leptodactylus latrans (amphibia: Anura). Chemosphere 202:289–297
Bantle JA (1995) FETAX-A developmental toxicity assay using frog embryos. In: Rand GM (ed) Fundamental aquatic toxicology. Taylor and Francis, Washington, DC, pp 207–230
Benbrook CM (2016) Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28:3. https://doi.org/10.1186/s12302-016-0070-0
Benbrook CM (2019) How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides? Environ Sci Eur 31:2. https://doi.org/10.1186/s12302-018-0184-7
Bonfanti P, Saibene M, Bacchetta R, Mantecca P, Colombo A (2018) A glyphosate micro-emulsion formulation displays teratogenicity in Xenopus laevis. Aquat Toxicol 195:103–113
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brausch JM, Smith PN (2007) Toxicity of three polyethoxylated tallowamine surfactant formulations to laboratory and field collected fairy shrimp, Thamnocephalus platyurus. Arch Environ Contam Toxicol 52:217–221
Cattani D, de Liz Oliveira Cavalli VL, Heinz Rieg CE, Domingues JT, Dal-Cim T, Tasca CI, Mena Barreto Silva FR, Zamoner A, (2014) Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: involvement of glutamate excitotoxicity. Toxicology 320:34–45
Carvalho WF, Ruiz de Arcaute C, Pérez-Iglesias JM, Laborde MRR, Soloneski S, Larramendy ML (2019) DNA damage exerted by mixtures of commercial formulations of glyphosate and imazethapyr herbicides in Rhinella arenarum (Anura, Bufonidae) tadpoles. Ecotoxicology 28(3):367–377
Edge C, Gahl M, Thompson D, Hao C, Houlahan J (2014) Variation in amphibian response to two formulations of glyphosate-based herbicides. Environ Toxicol Chem 33:2628–2632
Edginton AN, Sheridan PM, Stephenson GR, Thompson DG, Boermans HJ (2004) Comparative effects of pH and Vision (R) herbicide on two life stages of four anuran amphibian species. Environ Toxicol Chem 23:815–822
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fort DJ, Guiney PD, Weeks JA, Thomas JH, Rogers RL, Noll AM, Spaulding CD (2004) Effect of methoxychlor on various life stages of Xenopus laevis. Toxicol Sci 81:413–454
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for Roundup (R) Herbicide. Rev Environ Contam Toxicol 167(167):35–120
Gill JPK, Sethi N, Mohan A, Datta S, Girdhar M (2018) Glyphosate toxicity for animals. Environ Chem Lett 16:401–426
Güngördü A (2013) Comparative toxicity of methidathion and glyphosate on early life stages of three amphibian species: Pelophylax ridibundus, Pseudepidalea viridis, and Xenopus laevis. Aquat Toxicol 140–141:220–228
Güngördü A, Uckun M, Yologlu E (2016) Integrated assessment of biochemical markers in premetamorphic tadpoles of three amphibian species exposed to glyphosate- and methidathion-based pesticides in single and combination forms. Chemosphere 144:2024–2035
Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K (2015) Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol 16:490–491
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hansen LR, Roslev P (2016) Behavioral responses of juvenile Daphnia magna after exposure to glyphosate and glyphosate-copper complexes. Aquat Toxicol 179:36–43
Hong YH, Yang XZ, Huang Y, Yan GW, Cheng YX (2018) Assessment of the oxidative and genotoxic effects of the glyphosate-based herbicide roundup on the freshwater shrimp, Macrobrachium nipponensis. Chemosphere 210:896–906
Howe CM, Berrill M, Pauli BD, Helbing CC, Werry K, Veldhoen N (2004) Toxicity of glyphosate-based pesticides to four North American frog species. Environ Toxicol Chem 23:1928–1938
IUCN (2019) www.iucnredlist.org. Accessed Nov 4, 2019
Iummato MM, Sabatini SE, Cacciatore LC, Cochón AC, Cataldo D, de Molina MDCR, Juárez ÁB (2018) Biochemical responses of the golden mussel Limnoperna fortunei under dietary glyphosate exposure. Ecotoxicol Environ Saf 15:69–75
Jacques MT, Bornhorst J, Soares MV, Schwerdtle T, Garcia S, Avila DS (2019) Reprotoxicity of glyphosate-based formulation in Caenorhabditis elegans is not due to the active ingredient only. Environ Pollut 252(Pt B):1854–1862
Lanzarin GAB, Felix LM, Santos D, Venancio CAS, Monteiro SM (2019) Dose-dependent effects of a glyphosate commercial formulation - Roundup® UltraMax - on the early zebrafish embryogenesis. Chemosphere 223:514–522
Lajmanovich RC, Attademo AM, Peltzer PM, Junges CM, Cabagna MC (2011) Toxicity of Four Herbicide Formulations with Glyphosate on Rhinella arenarum (Anura: Bufonidae) Tadpoles: B-esterases and Glutathione S-transferase Inhibitors. Arch Environ Contam Toxicol 60:681–689
Lajmanovich RC, Junges CM, Attademo AM, Peltzer PM, Cabagna-Zenklusen MC, Basso A (2013) Individual and Mixture Toxicity of Commercial Formulations Containing Glyphosate, Metsulfuron-Methyl, Bispyribac-Sodium, and Picloram on Rhinella arenarum Tadpoles. Water Air Soil Pollut 224:1404
Li J, Smeda RJ, Sellers BA, Johnson WG (2005) Influence of formulation and glyphosate salt on absorption and translocation in three annual weeds. Weed Sci 53:153–159
Mann RM, Bidwell JR (1999) The toxicity of glyphosate and several glyphosate formulations to four species of southwestern Australian frogs. Arch Environ Contam Toxicol 36:193–199
Miko Z, Ujszegi J, Gal Z, Hettyey A (2017) Effects of a glyphosate-based herbicide and predation threat on the behaviour of agile frog tadpoles. Ecotoxicol Environ Saf 140:96–102
Monsanto (2019) Monsanto product safety assistance website. https://www.sdslibrary.monsanto.com/Pages/Default.aspx. Accessed Dec 4, 2019
Moore LJ, Fuentes L, Rodgers JH Jr, Bowerman WW, Yarrow GK, Chao WY, Bridges WC Jr (2012) Relative toxicity of the components of the original formulation of Roundup to five North American anurans. Ecotoxicol Environ Saf 78:128–133
Navarro-Martin L, Lanctot C, Jackman P, Park BJ, Doe K, Pauli BD, Trudeau VL (2014) Effects of glyphosate-based herbicides on survival, development, growth and sex ratios of wood frogs (Lithobates sylvaticus) tadpoles. I: Chronic laboratory exposures to VisionMax (R). Aquat Toxicol 154:278–290
Pereira AG et al (2018) Low-concentration exposure to glyphosate-based herbicide modulates the complexes of the mitochondrial respiratory chain and induces mitochondrial hyperpolarization in the Danio rerio brain. Chemosphere 209:353–362
Perez GL, Vera MS, Miranda L (2011) Effects of herbicide glyphosate and glyphosate-based formulations on aquatic ecosystems. In: Kortekamp A (ed) Herbicides and environment. IntechOpen, Rijeka.
Perkins PJ, Boermans HJ, Stephenson GR (2000) Toxicity of glyphosate and triclopyr using the frog embryo teratogenesis assay–Xenopus. Environ Toxicol Chem 19:940–945
Relyea RA, Jones DK (2009) The toxicity of Roundup Original Max to 13 species of larval amphibians. Environ Toxicol Chem 28:2004–2008
Relyea RA (2011) Amphibians Are Not Ready for Roundup®. In: Elliott JE, Bishop CA, Morrissey CA (eds) Wildlife ecotoxicology: forensic approaches. Springer, New York, pp 267–300
Samanta P, Pal S, Mukherjee AK, Ghosh AR (2014) Biochemical effects of glyphosate based herbicide, Excel Mera 71 on enzyme activities of acetylcholinesterase (AChE), lipid peroxidation (LPO), catalase (CAT), glutathione-S-transferase (GST) and protein content on teleostean fishes. Ecotoxicol Environ Saf 107:120–125
SERA (2011) Glyphosate—human health and ecological risk assessment—final Report. USDA, Forest Service, Forest Health Protection. SERA TR-052-22-03b. Atlanta, Georgia. https://www.fs.fed.us/foresthealth/pesticide/pdfs/Glyphosate_SERA_TR-052-22-03b.pdf
Silveira T et al (2019) Roundup (R) Herbicide decreases quality parameters of spermatozoa of silversides odontesthes humensis. Bull Environ Contam Toxicol 102:1–6
Smith GR (2001) Effects of acute exposure to a commercial formulation of glyphosate on the tadpoles of two species of anurans. Bull Environ Contam Toxicol 67:483–488
Stephensen E, Svavarsson J, Sturve J, Ericson G, Adolfsson-Erici M, Forlin L (2000) Biochemical indicators of pollution exposure in shorthorn sculpin (Myoxocephalus scorpius), caught in four harbours on the southwest coast of Iceland. Aquat Toxicol 48:431–442
Szekacs A, Darvas B (2018) Re-registration challenges of glyphosate in the European Union. Front Environ Sci 6:1–35
Tush D, Loftin KA, Meyer MT (2013) Characterization of polyoxyethylene tallowamine surfactants in technical mixtures and glyphosate formulations using ultra-high performance liquid chromatography and triple quadrupole mass spectrometryle. J Chromatogr A 1319:80–87
Tush D, Maksimowicz MM, Meyer MT (2018) Dissipation of polyoxyethylene tallowamine (POEA) and glyphosate in an agricultural field and their co-occurrence on streambed sediments. Sci Total Environ 636:212–219
Walsh PT, Downie JR, Monaghan P (2008) Plasticity of the duration of metamorphosis in the African clawed toad. J Zool 274:143–149
Wagner N, Reichenbecher W, Teichmann H, Tappeser B, Lotters S (2013) Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ Toxicol Chem 32:1688–1700
Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol 31:117–165
Yanniccari M, Istilart C, Gimenez DO, Castro AM (2012) Effects of glyphosate on the movement of assimilates of two Lolium perenne L. populations with differential herbicide sensitivity. Environ Exp Bot 82:14–19
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turhan, D.Ö., Güngördü, A. & Ozmen, M. Developmental and lethal effects of glyphosate and a glyphosate-based product on Xenopus laevis embryos and tadpoles. Bull Environ Contam Toxicol 104, 173–179 (2020). https://doi.org/10.1007/s00128-019-02774-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02774-z