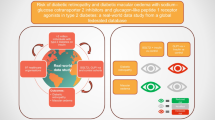
Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RAs, incretin mimetics) and dipeptidyl peptidase-4 inhibitors (DPP-4is, incretin enhancers) are glucose-lowering therapies with proven cardiovascular safety, but their effect on microvascular disease is not fully understood. Both therapies increase GLP-1 receptor agonism, which is associated with attenuation of numerous pathological processes that may lead to microvascular benefits, including decreased reactive oxygen species (ROS) production, decreased inflammation and improved vascular function. DPP-4is also increase stromal cell-derived factor-1 (SDF-1), which is associated with neovascularisation and tissue repair. Rodent studies demonstrate several benefits of these agents in the prevention or reversal of nephropathy, retinopathy and neuropathy, but evidence from human populations is less clear. For nephropathy risk in human clinical trials, meta-analyses demonstrate that GLP-1RAs reduce the risk of a composite renal outcome (doubling of serum creatinine, eGFR reduction of 30%, end-stage renal disease or renal death), whereas the benefits of DPP-4is appear to be limited to reductions in the risk of albuminuria. The relationship between GLP-1RAs and retinopathy is less clear. Many large trials and meta-analyses show no effect, but an observed increase in the risk of retinopathy complications with semaglutide therapy (a GLP-1RA) in the SUSTAIN-6 trial warrants caution, particularly in individuals with baseline retinopathy. Similarly, DPP-4is are associated with increased retinopathy risk in both trials and meta-analysis. The association between GLP-1RAs and peripheral neuropathy is unclear due to little trial evidence. For DPP-4is, one trial and several observational studies show a reduced risk of peripheral neuropathy, with others reporting no effect. Evidence in other less-established microvascular outcomes, such as microvascular angina, cerebral small vessel disease, skeletal muscle microvascular disease and autonomic neuropathies (e.g. cardiac autonomic neuropathy, gastroparesis, erectile dysfunction), is sparse. In conclusion, GLP-1RAs are protective against nephropathy, whereas DPP-4is are protective against albuminuria and potentially peripheral neuropathy. Caution is advised with DPP-4is and semaglutide, particularly for patients with background retinopathy, due to increased risk of retinopathy. Well-designed trials powered for microvascular outcomes are needed to clarify associations of incretin therapies and microvascular diseases.
Graphical Abstract

Similar content being viewed by others
Introduction
In the last 20 years, several incretin-based therapies, namely glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs, incretin mimetics) and dipeptidyl peptidase-4 inhibitors (DPP-4is, incretin enhancers), have been developed for people with type 2 diabetes [1]. Although DPP-4is have proven cardiovascular safety [2, 3], only GLP-1RAs have demonstrated a reduction in major adverse cardiac events (MACE) [4, 5]. These findings have come from the need for new glucose-lowering therapies (GLTs) to demonstrate cardiovascular safety prior to approval by the Food and Drug Administration. Whether these therapies reduce the risk of microvascular diseases is less clear, as many of these cardiovascular outcome trials (CVOTs) do not report extensive microvascular outcomes. Furthermore, these trials were not designed to investigate microvascular outcomes, meaning greater statistical uncertainty to detect an impact. This review summarises evidence on whether incretin therapies could reduce microvascular disease, summarising observational studies and clinical trials, and exploring potential mechanisms.
Microvascular disease and the potential of incretin therapies
Incretin therapies could play an important role in the prevention of microvascular disease via an increase in GLP-1 agonism. This was initially suggested by bariatric surgery, which increases GLP-1 and is highly efficacious in the primary prevention of microvascular disease (RR 0.37, 95% CI 0.30, 0.46) [6, 7]. Furthermore, GLP-1 agonism increases beta cell preservation and insulin secretion and decreases glucagon secretion, leading to a reduction in plasma glucose [8, 9]. GLP-1 agonism also delays gastric emptying, with subsequent slower digestion of carbohydrate and a reduction in the peak concentration of postprandial glucose [10]. The reduction in hyperglycaemia is likely to attenuate all pathophysiological processes that lead to microvascular disease in diabetes, as this is the ultimate cause of complications, however, GLP-1 agonism may also attenuate specific pathophysiological processes on top of the glucose-lowering effect [11]. These are summarised in Fig. 1, based on the processes summarised by Madonna et al [12].
Pathophysiology of microvascular disease in diabetes and protective actions of incretin-based therapies beyond glucose-lowering effects. Based on the processes as summarised by Madonna et al 2017 [1]. AQP, aquaporin; CACs, circulating angiogenic cells; COX, cycloxygenase; ECFC, endothelial colony forming cells; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; PAI, plasminogen activator inhibitor; PKA, protein kinase A; SMPCs, smooth muscle progenitor cells; TLR, toll-like receptor; TonEBP/NFAT5, tonicity-responsive enhancer-binding protein/nuclear factor of activated T cells 5. This figure is available as part of a downloadable slideset
Hyperglycaemia results in activation of the polyol pathway, which leads to loss of NADPH and increased production of reactive oxygen species (ROS) [13]. ROS production is further increased via generation of AGEs and their interaction with receptors (RAGE); the protein kinase C (PKC) pathway; and the hexosamine pathway. Interestingly, GLP-1 agonism may attenuate some of these processes as its administration is associated with decreased ROS [14, 15]. For example, GLP-1 agonism is associated with increased cellular cAMP and activity of protein kinase A, which potentially offset effects of the PKC pathway [11, 16], and is also associated with decreased RAGE expression [11, 17], which may additionally offset further pathological sequalae, such as the crosslinking of collagen (basement membrane thickening) [18], alteration of transcription factors [19] and inflammation [20].
The microcirculation in type 2 diabetes is associated with an abnormal profile of vascular progenitor cells [21,22,23], with decreased endothelial colony forming cells (CD34+KDR+ cells identified on flow cytometry) and decreased circulating angiogenic cells, which may adversely impact cardiovascular tissue repair/regeneration and susceptibility to atherogenesis [22, 23]. DPP-4is, in particular, may attenuate this pathway because as well as inhibiting the breakdown of GLP-1, they also inhibit the breakdown of stromal cell-derived factor-1 (SDF-1) (or its commonly measured isoform, SDF-1α) [24]. In vitro research supports a role of SDF-1 in promoting angiogenesis as it is associated with an increase in progenitor cells (identified by their uptake of acetylated LDL [AcLDL]) [25]. Furthermore, this is supported by evidence in human participants as sitagliptin (a DPP-4i) was associated with an increase in circulating CD34+KDR+ cells identified by flow cytometry, compared with control participants [26]. GLP-1 agonism, shown to improve angiogenesis in vitro [27, 28], may also improve vascular repair/regeneration.
Endothelial dysfunction is also observed in diabetes, with impaired nitric oxide (NO) synthesis [21]. Contributing to this is the activation of poly-ADP-ribose polymerase (PARP) in response to diabetic microvascular damage, which triggers an inflammatory cascade via NF-κB activation [12, 29]. Decreased Notch-1 (which inhibits PARP) additionally augments this [29]. Potentially countering this, GLP-1 agonism is associated with improved NO production in vitro and flow-mediated vasodilation in human study participants [30, 31]. Through improved vascular angiogenesis and endothelial function, leading to increased blood flow, insulin sensitivity may also be improved as greater perfusion leads to greater glucose and insulin delivery and greater cellular glucose uptake [32].
Diabetes is also associated with a proinflammatory state, with increased circulating IL-8 and TNF-α [12, 13]. Toll-like receptors-2 and -4 play a central role by activating NF-κB and triggering an inflammatory cascade [33]. GLP-1 agonism ameliorates ROS, which may drive these processes [14, 15]. An anti-inflammatory effect of GLP-1 agonism is further suggested by an associated decrease in TNF-α, monocyte chemoattractant protein-1 and IL-6 [34,35,36].
Further microvascular damage occurs via hyperosmolarity, driven by hyperglycaemia and the highly osmotic sorbitol (polyol) pathway [12, 13]. Hyperosmolarity induces the expression of Aquaporin-1 channels, which are permeable to water, and consequently result in an adverse loss of intracellular volume [37]. Cyclooxygenase-2 is also triggered by hyperosmolarity, and has pathological pro-angiogenic, and potentially pro-atherogenic, effects [38,39,40]. It is unknown whether incretins have specific mechanisms to counteract hyperosmolarity-driven damage, but, as with other pathways, they will likely ameliorate this via glucose-lowering effects.
An appreciation that the vasculature differs across end-organs is also required when considering pathology, as the effects of incretins may similarly differ. For example, whilst insulin resistance is important in determining capillary rarefaction and vascular dysfunction in skeletal muscle [32], loss of retinal perfusion is more related to loss of autoregulation and early pericyte and endothelial cell death, amongst other factors [41]. Furthermore, end-stage proliferative retinopathy is driven by neovascularisation [41], which may be less detrimental elsewhere. Converse to reduced blood flow, vasodilation of renal afferent arterioles is seen in early diabetes, which increases glomerular BP and may drive pathological hyperfiltration [42]. Similar differences in both the pathology of complications and mechanisms of impact with incretin therapies may occur within different microvasculatures of relevance to common microvascular complications in diabetes, for example between the blood–brain- and blood–retinal barriers.
In addition to the pathways described in Fig. 1, concomitant obesity, hypertension and dyslipidaemia also contribute to vascular and end-organ damage. GLP-1RAs cause, via an anorexigenic mechanism, a reduction in mean body weight compared with placebo, which varies from −3.80 kg (95% CI −4.46, −3.14) for subcutaneous semaglutide to −0.80 kg (−1.41, −0.19) for dulaglutide [43]. Similarly, GLP-1RAs result in mean systolic BP reduction, from −1.76 mmHg (95% CI −2.82, −0.70) for exenatide extended release to −3.06 mmHg (−4.21, −1.91) for oral semaglutide, and, although to a lesser extent, several GLP-1RAs result in a diastolic BP reduction [43]. GLP-1RAs also decrease mean LDL-cholesterol (ranging from −0.08 to −0.16 mmol/l) and some show a modest decrease in triacylglycerol (for liraglutide: −0.30 mmol/l [95% CI −0.49, −0.11]) compared with placebo [44].
In addition to vascular effects, GLP-1 receptors have also been identified in the peripheral nervous system and kidneys, where they may have direct actions on organ-specific pathophysiological processes [45,46,47]. Similarly, SDF-1 may have beneficial end-organ effects, particularly for neuropathy, via enhancing tissue repair [48]. However, these benefits are debated [24, 46], with potential harms to the retina reported [49], and conflicting nephropathy findings [24, 46]. DPP-4 may also regulate other hormones, including granulocyte-macrophage colony-stimulating factor and IL-3 [50], but the significance of this for microvascular disease is unknown.
Nephropathy
GLP-1RAs
Evidence of a nephroprotective effect of GLP-1RAs comes from several rodent studies demonstrating reduced albuminuria, oxidative stress and inflammation; improved BP; and fewer pathological histology findings (glomerular hypertrophy, mesangial matrix expansion and glomerular lipid accumulation) [11, 51].
Data from CVOTs for GLP-1RAs are harder to interpret as they were not designed for renal outcomes. Hard renal outcomes (e.g. end-stage renal failure, dialysis or renal death) have a much lower incidence than MACE, for which these trials are powered to detect a difference; this could mean that even a very large difference may not be detected due to the lower absolute risk and subsequent larger statistical uncertainty. Therefore, unsurprisingly, individual CVOTs do not find a reduction in the risk of hard renal endpoints. However, trials do show a consistent reduction in albuminuria compared with placebo (RR 0.77, 95% CI 0.70, 0.84 for new macroalbuminuria in a meta-analysis) [52]. Similarly, meta-analysis demonstrated a reduction in a composite renal outcome that included albuminuria [53], but it remained unclear whether this simply reflected a large risk reduction in albuminuria rather than a reduction in other renal outcomes. However, meta-analysis has now confirmed a risk reduction for a renal composite outcome that excluded albuminuria with GLP-1RAs (RR 0.92, 95% CI 0.84, 0.99) [52], although of a lesser magnitude compared with albuminuria. The FLOW trial (semaglutide vs placebo), an RCT powered for a composite renal outcome that excludes albuminuria, is underway to clarify this [54].
The mechanisms behind the renoprotective effects of GLP-1 therapy are likely to be multifactorial, and GLP-1 receptors within the kidney may play a crucial role. One meta-analysis by Chalmoukou et al showed that the reduction in nephropathy was strongly explained by systolic BP reduction [52], whereas another found that the risk reduction was explained by HbA1c reduction (not found in the meta-analysis by Chalmoukou et al) [53]. Interestingly, despite the impressive reduction in BMI with GLP-1RA use, this was not a mediating factor. Analysis of individual-level data is needed alongside further mechanistic work to clarify these findings.
DPP-4is
As with GLP-1RAs, early rodent studies were promising for nephroprotective effects of DPP-4is, with observed improvements in albuminuria, filtration barrier remodelling, glomerular oxidative stress, creatine clearance and histological markers [11, 51]. CVOTs similarly showed a reduction in albuminuria, although to a lesser extent than with GLP-1RAs, with less albuminuria progression (HR=0.86 [95% CI 0.78, 0.95]) in the CARMELINA trial (linagliptin) [55], and similar benefits in the SAVOR-TIMI 53 trial (saxagliptin) [56]. One observational study showed a further beneficial association of DPP-4is compared with sulfonylureas, with a composite renal outcome that did not include albuminuria (HR=0.91 [95% CI 0.85, 0.97]) [57]. However, this has not translated to a decrease in the composite renal outcome in a meta-analysis of RCTs [52]. Given the reasonably narrow confidence intervals (RR=1.03 [95% CI 0.93, 1.15]), it is unlikely that this is related to lack of power and suggests a true lack of substantial benefit.
Why GLP-1RAs may be more reno-protective than DPP-4is
Collectively, whilst both GLP-1RAs and DPP-4is appear to improve albuminuria, only GLP-1RAs result in an improvement in the more clinically meaningful composite renal outcome. There are two hypotheses for why this difference exists. First, GLP-1 agonism has numerous positive physiological effects (Fig. 1) and GLP-1RAs, as exogenous incretin mimetics, result in a far higher degree of GLP-1 agonism compared with DPP-4is, which are incretin enhancers and limited to increasing endogenous production of GLP-1 [58]. Furthermore, some of the benefits of GLP-1 agonism could be offset with DPP-4is through the increase in SDF-1, with potential associations with pathological processes such as natriuresis and renal hyperfiltration, atherogenesis, podocyte injury and glomerulosclerosis [24], although this is disputed [46]. Future RCTs involving DPP-4is could measure changes in plasma SDF-1 concentrations to investigate if this is associated with outcomes.
Retinopathy
GLP-1RAs
As with nephropathy, rodent studies suggested protective effects of GLP-1 agonism for retinopathy, including decreased glial activation, neural apoptosis and electroretinographical abnormalities; protection of the blood–retinal barrier; downregulation of growth factors; and prevention of cell loss in the inner and outer nuclear layers (albeit transiently) [11, 59]. Furthermore, these beneficial processes may occur through glucose-independent pathways, including reduced retinal glutamate production and increased prosurvival signalling pathways [11, 59].
Unfortunately, RCTs suggest a different picture to pre-clinical studies, and concern has arisen because semaglutide was associated with an increased risk of retinopathy complications in the SUSTAIN-6 RCT (HR 1.76, 95% CI 1.11, 2.78) [4]. Other CVOTs have not found significant differences in ocular outcomes (although they were not powered for this). Furthermore, most meta-analyses of RCTs do not find significant associations between GLP-1RAs and retinopathy outcomes [60,61,62], however, there were wide confidence intervals, contributed to by heterogeneity in findings. In two meta-analyses, GLP-1RAs were associated with retinopathy progression, but both had flaws. In one, authors only included trials with cardiovascular benefit, but excluded studies utilising off-market drugs and included a trial (PIONEER-6) where superiority of the primary cardiovascular outcome was not shown [63, 64]. After changing the included studies accordingly, there was no longer an association with retinopathy [64]. The other positive meta-analysis was contributed to by a mistaken input for the LEADER trial [65]. One meta-analysis looking at semaglutide use only, found an increased risk of retinopathy compared with placebo [66].
There has been much debate as to why retinopathy risk was higher in the SUSTAIN-6 trial. First, participants in SUSTAIN-6 had a high prevalence of background retinopathy, and post hoc analysis suggested that in individuals without pre-existing retinopathy, ocular events were no different [67]. As such, it may be that these adverse outcomes are limited to those at high baseline risk. Furthermore, compared with other CVOTs, participants in SUSTAIN-6 had a high baseline HbA1c, owing to no upper limit for HbA1c in the inclusion criteria, and a subsequent large reduction in HbA1c (larger still in the subgroup with pre-existing retinopathy) [60, 67]. The increased risk may be related to the rapid decrease in HbA1c that occurs with GLP-1RA use; this is supported by meta-analysis showing that the magnitude of HbA1c reduction is associated with the risk of retinopathy outcomes with GLP-1RA use (and not with systolic BP or weight) [60]. This phenomenon has been noted before, such as within the DCCT where intensive treatment was associated with both a larger HbA1c reduction from baseline and an increased risk of worsening of retinopathy at 6 and/or 12 months [68]. Interestingly, over a mean 6.5 years follow-up, the overall risk of retinopathy and progression of retinopathy was reduced with intensive therapy compared to conventional therapy [69]. Given that SUSTAIN-6 had an observation period of approximately 2 years, it is unclear whether a longer follow-up may have seen a reversal of the negative relationship between semaglutide and retinopathy complications. The FOCUS trial, an ongoing RCT comparing semaglutide and placebo, will investigate this further and will report retinopathy progression at 5 years as the primary outcome [70].
DPP-4is
Pre-clinical studies showed similar promise that DPP-4is may reduce the risk of retinopathy. In rodent studies, DPP-4 inhibition has been shown to prevent blood–retinal barrier breakdown, decrease retinal inflammation and neuronal apoptosis, reduce gene expression responsible for increased levels of growth factors and decrease neovascularisation [11, 59]. Moreover, there is supportive observational evidence in humans. In a retrospective cohort study of German electronic medical records (N=630 after propensity score matching), vildagliptin was associated with a lower incidence of retinopathy (OR 0.55, 95% CI 0.39, 0.77) [71]. This was supported by another smaller retrospective observational study (N=82) in South Korea finding that DPP-4is were associated with reduced progression of retinopathy [72].
Despite these positive findings, interventional studies suggest a different picture: DPP-4is may increase risk of diabetic retinopathy. In the TECOS trial, there was a higher crude prevalence of diabetic eye disease (3.1% in the sitagliptin group vs 2.5% in placebo) and retinopathy (2.8% with sitagliptin vs 2.2% with placebo) [73]. Although not reported, these differences correspond to an OR of 1.26 (95% CI 1.04, 1.54) for diabetic eye disease and 1.31 (1.06, 1.61) for retinopathy. Meta-analysis of RCTs supports that DPP-4i use is associated with increased risk of diabetic retinopathy in pairwise meta-analysis (OR 1.27, 95% CI 1.05, 1.53) [74]. Similar to the increased retinopathy risk with GLP-1RA use, this may be related to the reduction in HbA1c with DPP-4is (mean reduction: 0.30% to 0.80%), as across GLTs retinopathy risk is related to the magnitude of HbA1c decrease [74].
Lack of retinal protection with incretin therapies
Given the data, caution should be given to the use of semaglutide and DPP-4is with regards to risk of retinopathy. Debate exists as to why the protective effects of incretin therapies were never realised in CVOTs. This could be related to the physiological differences between rodents and humans, or to the lack of GLP-1 receptors within the retina, in contrast to the kidneys and nerves [30]. For DPP-4is, the additional increase in SDF-1 may be harmful, due to its neovascular effect that may cause proliferation and damage that may mimic the pathological processes that occur in the development of diabetic retinopathy [49].
Peripheral neuropathy
GLP-1RAs
Early pre-clinical diabetic rodent experiments demonstrated various improvements in nerve function following initiation of a GLP-1RA. These benefits included improvement of sensory and motor nerve electrophysiology and behavioural sensory loss, reduction of intraepidermal nerve fibre densities in the sole skins, restoration of myelin fibre size, prevention of Schwann cell apoptosis, reduction of myelinated nerve fibre density and reduction of neuropathic pain [75].
Whether GLP-1RAs result in a clinically significant decrease in neuropathy incidence or severity remains to be seen, with only a few studies in humans undertaken, most of which involved less than 100 participants (Table 1). In the two larger studies, an observational study from the United States national claims database OptumLabs Data Warehouse (N=8252) and the GRADE RCT (N=5047), no difference in incidence of neuropathy was seen compared with other GLTs [76, 77].
DPP-4is
Similar to rodent studies with GLP-1RAs, those with DPP-4is showed promise in reducing the incidence of neuropathy, with observations including decreased nerve fibre density and improved nerve conduction velocity [78]. Evidence from human studies is suggestive of a beneficial effect (Table 1). Two large observational studies of electronic medical records suggested a decreased incidence of neuropathy with both vildagliptin and sitagliptin compared to sulfonylureas [71, 76]. Findings from large RCTs are mixed, however, with only one trial suggesting a 19.5% (95% CI 10.1, 29.0) reduction in small fibre peripheral neuropathy with linagliptin compared to placebo [79]. It is unclear why these findings differ, but it may be related to different comparators (placebo or other GLTs), different DPP-4is, different measures of neuropathy and varying statistical power.
Comparing GLP-1RAs and DPP-4is for peripheral neuropathy
Although evidence is inconclusive, DPP-4is appear to be more promising than GLP-1RAs in reducing peripheral neuropathy incidence. This is further supported by an observational study showing that liraglutide appeared to be associated with a higher incidence of neuropathy compared with sitagliptin (HR 1.36 [95% CI 1.03, 1.80]). However, this was not significant when accounting for multiple testing (p=0.09) [76]. A potential reason for a theoretical superiority of DPP-4is over GLP-1RAs may be because of the additional increase in SDF-1 [48].
Autonomic neuropathy
Given that some evidence exists that incretin therapies may decrease peripheral neuropathy risk, particularly within rodent studies, it follows that they may additionally protect against disorders of the autonomic nervous system via the same mechanisms, including cardiac autonomic neuropathy (CAN), gastroparesis and erectile dysfunction.
Whilst cardiovascular safety for DPP-4is and GLP-1RAs is clear [2,3,4,5], measures of CAN are strong predictors of cardiovascular death and can also cause symptoms, most notably from orthostatic hypotension. It is unclear how GLP-1 agonism affects the autonomic nervous system and these may be more complex than the mechanisms related to peripheral neuropathy due to the many inputs that determine both sympathetic and parasympathetic autonomic activity [80]. Furthermore, the role of GLP-1RAs on sympathetic drive is particularly debated. For example, GLP-1 receptors have been observed in the carotid body and activation of these diminish the sympathetic response to high plasma glucose and/or insulin [81]. Conversely, GLP-1RAs cause an increased resting heart rate (increased by approximately 3 beats-per-min), which may suggest an increase in sympathetic drive [80]. Other studies have reported decreased vagal tone, decreased 30:15 value from the lying-to-standing test and a decreased heart rate variability with GLP-1 RAs [47, 80], However, there are inconsistencies in the literature. Although unclear at present, this evidence suggests that GLP-1RAs may lead to autonomic imbalance and CAN.
Determining whether incretins may be beneficial for reducing autonomic neuropathy that contributes to gastroparesis is difficult, namely because GLP-1 receptor activation in the stomach has a potent effect at delaying gastric emptying [82]. Despite this, glucose-lowering effects may protect against a neuropathy-induced gastroparesis, as long-term glucose control is associated with lower incidence of gastroparesis in the follow-up of the DCCT/EPIC trials [83], in addition to the other discussed mechanisms by which incretin therapies may protect nerves and the microvasculature.
Erectile function depends on healthy vascular and nervous function; disorders of either system can result in erectile dysfunction [84]. There are therefore two pathophysiological processes by which diabetes can result in erectile dysfunction and that incretin therapies may modify. Few data exist, although a positive effect was suggested in the REWIND RCT of dulaglutide, with a small reduction in erectile dysfunction incidence and severity vs placebo [85].
Incretins and other microvascular pathologies
Vascular damage may also have a direct causative effect in other microvascular pathologies, such as microvascular cardiac angina and cerebral small vessel disease (cSVD), and may have further clinical significance in determining microvascular flow reserve in skeletal muscle. The effect of incretin therapies on these outcomes has not been well studied in humans thus far.
Type 2 diabetes is a recognised risk factor for microvascular cardiac angina, which is principally considered a disorder of endothelial function and a subsequent impairment in the control of coronary blood flow, leading to hypoxia and chest pain [21]. Given that GLP-1 has been shown in several studies to improve endothelial function and flow-mediated vasodilation [27, 28, 30], research should investigate whether incretins may be beneficial for the prevention or management of microvascular angina.
Similarly, cSVD is associated with type 2 diabetes as well as cognitive decline, dementia and lacunar stroke [86]. However, the pathophysiological mechanisms may differ to microvascular cardiac angina, as it is related to inflammation, blood–brain barrier disruption and vascular remodelling. Again, as incretin therapies are associated with decreased inflammation and ROS production [11, 14, 15], these may also have a role in the prevention of cSVD.
Skeletal muscle microvascular rarefaction and subsequent reduction in capillary blood flow is also associated with type 2 diabetes [87, 88]. This is likely to have important implications for cardiovascular fitness and response to exercise, both features of health status. It may also impair the endocrine function of skeletal muscle in glucose regulation in a theoretical negative cycle: less skeletal muscle blood flow, less glucose uptake in response to insulin, higher plasma glucose, further microvascular damage [87, 88]. In rodent studies, GLP-1 agonism has already been demonstrated to increase capillary density and improves features of insulin resistance in skeletal muscle, with evidence of improved skeletal muscle blood flow in humans with diabetes [32]. Given the benefits of GLP-1 to endothelial function and angiogenesis [27, 28, 30], further research into skeletal muscle microvascular disease and incretin therapies is warranted in humans.
Conclusion
In conclusion, incretin therapies differ in their effect on microvascular disease by drug type (GLP-1RA vs DPP-4i) and by microvascular outcome (Fig. 2). High quality evidence demonstrates that GLP-1RAs reduce risk of adverse renal outcomes whereas DPP-4is do not. It remains unclear whether GLP-1RAs may cause adverse ocular events, but use in patients with background retinopathy, high HbA1c or the use of semaglutide specifically, may carry higher risk and caution is advised. Adequately powered RCTs of long duration are required to clarify this which should report and investigate known risk factors for retinopathy complications (e.g. background retinopathy, diabetes duration, beta cell function) as potential confounders/mediators. Clinical caution with DPP-4is and retinopathy is similarly warranted. There is little evidence that GLP-1RAs reduce the incidence of neuropathic pathologies (peripheral neuropathy, CAN, gastroparesis and erectile dysfunction) in interventional trials, despite promising pre-clinical research. Similarly, the relationship with DPP-4is and neuropathy remains unclear, although it appears to be more promising for peripheral neuropathy. Again, adequately powered trials and real-world monitoring are needed, and ideally all trials should report peripheral neuropathy incidence (alongside less studied microvascular outcomes) to enable meta-analysis and improve understanding. Clearly, the mechanisms by which incretins exert their protective/adverse effects on microvascular outcomes are likely to be multifactorial, but they remain poorly understood and further mechanistic work is needed.
A summary of the observational and trial evidence for incretin-based therapies and microvascular diseases. This figure is available as part of a downloadable slideset
Abbreviations
- CAN:
-
Cardiac autonomic neuropathy
- cSVD:
-
Cerebral small vessel disease
- CVOTs:
-
Cardiovascular outcome trials
- DPP-4i:
-
Dipeptidyl peptidase-4 inhibitor
- GLP-1:
-
Glucagon-like peptide-1
- GLP-1RA:
-
Glucagon-like peptide-1 receptor agonist
- GLTs:
-
Glucose-lowering therapies
- MACE:
-
Major adverse cardiac events
- NO:
-
Nitric oxide
- PARP:
-
Poly-ADP-ribose polymerase
- PKC:
-
Protein kinase C
- RAGE:
-
Receptors for advanced glycation end-products
- ROS:
-
Reactive oxygen species
- SDF-1:
-
Stromal cell-derived factor-1
References
Nauck MA (2020) The rollercoaster history of using physiological and pharmacological properties of incretin hormones to develop diabetes medications with a convincing benefit-risk relationship. Metabolism 103:154031. https://doi.org/10.1016/j.metabol.2019.154031
White WB, Cannon CP, Heller SR et al (2013) Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 369(14):1327–1335. https://doi.org/10.1056/NEJMoa1305889
Scirica BM, Bhatt DL, Braunwald E et al (2013) Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 369(14):1317–1326. https://doi.org/10.1056/NEJMoa1307684
Marso SP, Bain SC, Consoli A et al (2016) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375(19):1834–1844. https://doi.org/10.1056/NEJMoa1607141
Marso SP, Daniels GH, Brown-Frandsen K et al (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4):311–322. https://doi.org/10.1056/NEJMoa1603827
Sheng B, Truong K, Spitler H, Zhang L, Tong X, Chen L (2017) The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg 27(10):2724–2732. https://doi.org/10.1007/s11695-017-2866-4
Sista F, Abruzzese V, Clementi M, Carandina S, Cecilia M, Amicucci G (2017) The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surg Obes Relat Dis 13(1):7–14. https://doi.org/10.1016/j.soard.2016.08.004
Watanabe Y, Kawai K, Ohashi S, Yokota C, Suzuki S, Yamashita K (1994) Structure-activity relationships of glucagon-like peptide-1(7–36)amide: insulinotropic activities in perfused rat pancreases, and receptor binding and cyclic AMP production in RINm5F cells. J Endocrinol 140(1):45–52. https://doi.org/10.1677/joe.0.1400045
Nauck MA, Heimesaat MM, Behle K et al (2002) Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 87(3):1239–1246. https://doi.org/10.1210/jcem.87.3.8355
Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA (1996) Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab 81(1):327–332. https://doi.org/10.1210/jcem.81.1.8550773
Mima A (2016) Incretin-based therapy for prevention of diabetic vascular complications. J Diabetes Res 2016:1379274. https://doi.org/10.1155/2016/1379274
Madonna R, Balistreri CR, Geng Y-J, De Caterina R (2017) Diabetic microangiopathy: pathogenetic insights and novel therapeutic approaches. Vascul Pharmacol 90:1–7. https://doi.org/10.1016/j.vph.2017.01.004
Madonna R, De Caterina R (2011) Cellular and molecular mechanisms of vascular injury in diabetes — part I: pathways of vascular disease in diabetes. Vascul Pharmacol 54(3):68–74. https://doi.org/10.1016/j.vph.2011.03.005
Wang R, Lu L, Guo Y et al (2015) Effect of glucagon-like peptide-1 on high-glucose-induced oxidative stress and cell apoptosis in human endothelial cells and its underlying mechanism. J Cardiovasc Pharmacol 66(2):135–140. https://doi.org/10.1097/FJC.0000000000000255
Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HHW (2010) Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol 30(7):1407–1414. https://doi.org/10.1161/ATVBAHA.110.206425
Mima A, Hiraoka-Yamomoto J, Li Q et al (2012) Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes 61(11):2967–2979. https://doi.org/10.2337/db11-1824
Ishibashi Y, Nishino Y, Matsui T, Takeuchi M, Yamagishi S (2011) Glucagon-like peptide-1 suppresses advanced glycation end product-induced monocyte chemoattractant protein-1 expression in mesangial cells by reducing advanced glycation end product receptor level. Metabolism 60(9):1271–1277. https://doi.org/10.1016/j.metabol.2011.01.010
Sell DR, Lapolla A, Odetti P, Fogarty J, Monnier VM (1992) Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes 41(10):1286–1292. https://doi.org/10.2337/diab.41.10.1286
Pugliese G (2008) Do advanced glycation end products contribute to the development of long-term diabetic complications? Nutr Metab Cardiovasc Dis 18(7):457–460. https://doi.org/10.1016/j.numecd.2008.06.006
Piarulli F, Sartore G, Lapolla A (2013) Glyco-oxidation and cardiovascular complications in type 2 diabetes: a clinical update. Acta Diabetol 50(2):101–110. https://doi.org/10.1007/s00592-012-0412-3
Strain WD, Paldánius PM (2018) Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol 17(1):57. https://doi.org/10.1186/s12933-018-0703-2
De Pascale MR, Bruzzese G, Crimi E et al (2016) Severe type 2 diabetes induces reversible modifications of endothelial progenitor cells which are ameliorate by glycemic control. Int J Stem Cells 9(1):137–144. https://doi.org/10.15283/ijsc.2016.9.1.137
van Ark J, Moser J, Lexis CPH et al (2012) Type 2 diabetes mellitus is associated with an imbalance in circulating endothelial and smooth muscle progenitor cell numbers. Diabetologia 55(9):2501–2512. https://doi.org/10.1007/s00125-012-2590-5
Packer M (2018) Have dipeptidyl peptidase-4 inhibitors ameliorated the vascular complications of type 2 diabetes in large-scale trials? The potential confounding effect of stem-cell chemokines. Cardiovasc Diabetol 17(1):9. https://doi.org/10.1186/s12933-017-0648-x
De Falco E, Porcelli D, Torella AR et al (2004) SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 104(12):3472–3482. https://doi.org/10.1182/blood-2003-12-4423
Fadini GP, Boscaro E, Albiero M et al (2010) The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care 33(7):1607–1609. https://doi.org/10.2337/dc10-0187
Aronis KN, Chamberland JP, Mantzoros CS (2013) GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metabolism 62(9):1279–1286. https://doi.org/10.1016/j.metabol.2013.04.010
Erdogdu O, Nathanson D, Sjöholm A, Nyström T, Zhang Q (2010) Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 325(1–2):26–35. https://doi.org/10.1016/j.mce.2010.04.022
Qin X, Zhang Z, Xu H, Wu Y (2011) Notch signaling protects retina from nuclear factor-κB- and poly-ADP-ribose-polymerase-mediated apoptosis under high-glucose stimulation. Acta Biochim Biophys Sin 43(9):703–711. https://doi.org/10.1093/abbs/gmr069
Nauck MA, Quast DR, Wefers J, Pfeiffer AFH (2021) The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes Metab 23(S3):5–29. https://doi.org/10.1111/dom.14496
Lim D-M, Park K-Y, Hwang W-M, Kim J-Y, Kim B-J (2017) Difference in protective effects of GIP and GLP-1 on endothelial cells according to cyclic adenosine monophosphate response. Exp Ther Med 13(5):2558–2564. https://doi.org/10.3892/etm.2017.4279
Love KM, Liu J, Regensteiner JG, Reusch JEB, Liu Z (2020) GLP-1 and insulin regulation of skeletal and cardiac muscle microvascular perfusion in type 2 diabetes. J Diabetes 12(7):488–498. https://doi.org/10.1111/1753-0407.13045
Zhao M, Li CH, Liu YL (2016) Toll-like receptor (TLR)-2/4 expression in retinal ganglion cells in a high-glucose environment and its implications. Genet Mol Res GMR 15(2). https://doi.org/10.4238/gmr.15026998
Panjwani N, Mulvihill EE, Longuet C et al (2013) GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology 154(1):127–139. https://doi.org/10.1210/en.2012-1937
Daousi C, Pinkney JH, Cleator J, Wilding JP, Ranganath LR (2013) Acute peripheral administration of synthetic human GLP-1 (7–36 amide) decreases circulating IL-6 in obese patients with type 2 diabetes mellitus: a potential role for GLP-1 in modulation of the diabetic pro-inflammatory state? Regul Pept 183:54–61. https://doi.org/10.1016/j.regpep.2013.03.004
Arakawa M, Mita T, Azuma K et al (2010) Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 59(4):1030–1037. https://doi.org/10.2337/db09-1694
Verkman AS (2011) Aquaporins at a glance. J Cell Sci 124(13):2107–2112. https://doi.org/10.1242/jcs.079467
Amano H, Ito Y, Suzuki T et al (2009) Roles of a prostaglandin E-type receptor, EP3, in upregulation of matrix metalloproteinase-9 and vascular endothelial growth factor during enhancement of tumor metastasis. Cancer Sci 100(12):2318–2324. https://doi.org/10.1111/j.1349-7006.2009.01322.x
Masferrer JL, Leahy KM, Koki AT et al (2000) Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 60(5):1306–1311
Spektor G, Fuster V (2005) Drug insight: cyclo-oxygenase 2 inhibitors and cardiovascular risk–where are we now? Nat Clin Pract Cardiovasc Med 2(6):290–300. https://doi.org/10.1038/ncpcardio0214
Lechner J, O’Leary OE, Stitt AW (2017) The pathology associated with diabetic retinopathy. Vision Res 139:7–14. https://doi.org/10.1016/j.visres.2017.04.003
Carmines PK (2010) The renal vascular response to diabetes. Curr Opin Nephrol Hypertens 19(1):85–90. https://doi.org/10.1097/MNH.0b013e32833240fc
Tsapas A, Karagiannis T, Kakotrichi P et al (2021) Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab 23(9):2116–2124. https://doi.org/10.1111/dom.14451
Sun F, Wu S, Wang J et al (2015) Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clin Ther 37(1):225-241.e8. https://doi.org/10.1016/j.clinthera.2014.11.008
Jolivalt CG, Fineman M, Deacon CF, Carr RD, Calcutt NA (2011) GLP-1 signals via ERK in peripheral nerve and prevents nerve dysfunction in diabetic mice. Diabetes Obes Metab 13(11):990–1000. https://doi.org/10.1111/j.1463-1326.2011.01431.x
Fadini GP, Avogaro A (2018) How to interpret the role of SDF-1α on diabetic complications during therapy with DPP-4 inhibitors. Cardiovasc Diabetol 17(1):22. https://doi.org/10.1186/s12933-018-0668-1
Greco C, Santi D, Brigante G, Pacchioni C, Simoni M (2022) Effect of the glucagon-like peptide-1 receptor agonists on autonomic function in subjects with diabetes: a systematic review and meta-analysis. Diabetes Metab J 46(6):901–911. https://doi.org/10.4093/dmj.2021.0314
Kim BJ, Lee JK, Schuchman EH, Jin HK, Bae J (2013) Synergistic vasculogenic effects of AMD3100 and stromal-cell-derived factor-1α in vasa nervorum of the sciatic nerve of mice with diabetic peripheral neuropathy. Cell Tissue Res 354(2):395–407. https://doi.org/10.1007/s00441-013-1689-4
Butler JM, Guthrie SM, Koc M et al (2005) SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest 115(1):86–93. https://doi.org/10.1172/JCI22869
Broxmeyer HE, Hoggatt J, O’Leary HA et al (2012) Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med 18(12):1786–1796. https://doi.org/10.1038/nm.2991
Bae EJ (2016) DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control. Arch Pharm Res 39(8):1114–1128. https://doi.org/10.1007/s12272-016-0813-x
Chalmoukou K, Polyzos D, Manta E et al (2022) Renal outcomes associated with glucose-lowering agents: systematic review and meta-analysis of randomized outcome trials. Eur J Intern Med 97:78–85. https://doi.org/10.1016/j.ejim.2021.12.018
Yoshiji S, Minamino H, Tanaka D, Yamane S, Harada N, Inagaki N (2022) Effects of glucagon-like peptide-1 receptor agonists on cardiovascular and renal outcomes: a meta-analysis and meta-regression analysis. Diabetes Obes Metab 24(6):1029–1037. https://doi.org/10.1111/dom.14666
Rossing P, Baeres FMM, Bakris G et al (2023) The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant gfad009. https://doi.org/10.1093/ndt/gfad009
Rosenstock J, Perkovic V, Johansen OE et al (2019) Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 321(1):69–79. https://doi.org/10.1001/jama.2018.18269
Mosenzon O, Leibowitz G, Bhatt DL et al (2017) Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care 40(1):69–76. https://doi.org/10.2337/dc16-0621
Xie Y, Bowe B, Gibson AK et al (2020) Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 43(11):2859–2869. https://doi.org/10.2337/dc20-1890
Gilbert MP, Pratley RE (2020) GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol 11:178. https://doi.org/10.3389/fendo.2020.00178
Saw M, Wong VW, Ho I-V, Liew G (2019) New anti-hyperglycaemic agents for type 2 diabetes and their effects on diabetic retinopathy. Eye Lond Engl 33(12):1842–1851. https://doi.org/10.1038/s41433-019-0494-z
Bethel MA, Diaz R, Castellana N, Bhattacharya I, Gerstein HC, Lakshmanan MC (2021) HbA1c change and diabetic retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: a meta-analysis and meta-regression. Diabetes Care 44(1):290–296. https://doi.org/10.2337/dc20-1815
Andreadis P, Karagiannis T, Malandris K et al (2018) Semaglutide for type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 20(9):2255–2263. https://doi.org/10.1111/dom.13361
Wei J, Yang B, Wang R et al (2022) Risk of stroke and retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: an eight RCTs meta-analysis. Front Endocrinol 13:1007980. https://doi.org/10.3389/fendo.2022.1007980
Yoshida Y, Joshi P, Barri S et al (2022) Progression of retinopathy with glucagon-like peptide-1 receptor agonists with cardiovascular benefits in type 2 diabetes - a systematic review and meta-analysis. J Diabetes Complications 36(8):108255. https://doi.org/10.1016/j.jdiacomp.2022.108255
Goldenberg RM (2022) Progression of retinopathy with glucagon-like peptide-1 receptor agonists with cardiovascular benefits in type 2 diabetes - a systematic review and meta-analysis. J Diabetes Complications 36(9):108285. https://doi.org/10.1016/j.jdiacomp.2022.108285
Qian W, Liu F, Yang Q (2021) Effect of glucagon-like peptide-1 receptor agonists in subjects with type 2 diabetes mellitus: a meta-analysis. J Clin Pharm Ther 46(6):1650–1658. https://doi.org/10.1111/jcpt.13502
Wang F, Mao Y, Wang H, Liu Y, Huang P (2022) Semaglutide and diabetic retinopathy risk in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Clin Drug Investig 42(1):17–28. https://doi.org/10.1007/s40261-021-01110-w
Vilsbøll T, Bain SC, Leiter LA et al (2018) Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab 20(4):889–897. https://doi.org/10.1111/dom.13172
The Diabetes Control and Complications Trial Research Group (1998) Early worsening of diabetic retinopathy in the diabetes control and complications trial. Arch Ophthalmol 116(7):874–886. https://doi.org/10.1001/archopht.116.7.874
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S et al (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329(14):977–986. https://doi.org/10.1056/NEJM199309303291401
ClinicalTrials.gov (2023) A research study to look at how semaglutide compared to placebo affects diabetic eye disease in people with type 2 diabetes (FOCUS). https://clinicaltrials.gov/ct2/show/NCT03811561. Accessed 23 May 2023
Kolaczynski WM, Hankins M, Ong SH, Richter H, Clemens A, Toussi M (2016) Microvascular outcomes in patients with type 2 diabetes treated with vildagliptin vs. sulfonylurea: a retrospective study using german electronic medical records. Diabetes Ther Res Treat Educ Diabetes Relat Disord 7(3):483–496. https://doi.org/10.1007/s13300-016-0177-8
Chung Y-R, Park SW, Kim JW, Kim JH, Lee K (2016) Protective effects of dipeptidyl peptidase-4 inhibitors on progression of diabetic retinopathy in patients with type 2 diabetes. Retina Phila Pa 36(12):2357–2363. https://doi.org/10.1097/IAE.0000000000001098
Green JB, Bethel MA, Armstrong PW et al (2015) Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 373(3):232–242. https://doi.org/10.1056/NEJMoa1501352
Tang H, Li G, Zhao Y et al (2018) Comparisons of diabetic retinopathy events associated with glucose-lowering drugs in patients with type 2 diabetes mellitus: a network meta-analysis. Diabetes Obes Metab 20(5):1262–1279. https://doi.org/10.1111/dom.13232
Sango K, Takaku S, Tsukamoto M, Niimi N, Yako H (2022) Glucagon-like peptide-1 receptor agonists as potential myelination-inducible and anti-demyelinating remedies. Front Cell Dev Biol 10:950623. https://doi.org/10.3389/fcell.2022.950623
Deng Y, Polley EC, Wallach JD et al (2022) Emulating the GRADE trial using real world data: retrospective comparative effectiveness study. BMJ 379:e070717. https://doi.org/10.1136/bmj-2022-070717
GRADE Study Research Group, Nathan DM, Lachin JM et al (2022) Glycemia reduction in type 2 diabetes - microvascular and cardiovascular outcomes. N Engl J Med 387(12):1075–1088. https://doi.org/10.1056/NEJMoa2200436
El Mouhayyar C, Riachy R, Khalil AB, Eid A, Azar S (2020) SGLT2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors in diabetes and microvascular complications: a review. Int J Endocrinol 2020:1762164. https://doi.org/10.1155/2020/1762164
Gabriel R, Boukichou-Abdelkader N, Gilis-Januszewska A et al (2023) Reduction in the risk of peripheral neuropathy and lower decrease in kidney function with metformin, linagliptin or their fixed-dose combination compared to placebo in prediabetes: A randomized controlled trial. J Clin Med 12(5):2035. https://doi.org/10.3390/jcm12052035
Spallone V (2019) Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J 43(1):3–30. https://doi.org/10.4093/dmj.2018.0259
Pauza AG, Thakkar P, Tasic T et al (2022) GLP1R attenuates sympathetic response to high glucose via carotid body inhibition. Circ Res 130(5):694–707. https://doi.org/10.1161/CIRCRESAHA.121.319874
Beti C, Stratmann B, Bokman G et al (2019) Exenatide delays gastric emptying in patients with type 2 diabetes mellitus but not in those with gastroparetic conditions. Horm Metab Res 51(4):267–273. https://doi.org/10.1055/a-0818-6374
Bharucha AE, Batey-Schaefer B, Cleary PA et al (2015) Delayed gastric emptying is associated with early and long-term hyperglycemia in type 1 diabetes mellitus. Gastroenterology 149(2):330–339. https://doi.org/10.1053/j.gastro.2015.05.007
MacDonald SM, Burnett AL (2021) Physiology of erection and pathophysiology of erectile dysfunction. Urol Clin North Am 48(4):513–525. https://doi.org/10.1016/j.ucl.2021.06.009
Bajaj HS, Gerstein HC, Rao-Melacini P et al (2021) Erectile function in men with type 2 diabetes treated with dulaglutide: an exploratory analysis of the REWIND placebo-controlled randomised trial. Lancet Diabetes Endocrinol 9(8):484–490. https://doi.org/10.1016/S2213-8587(21)00115-7
Evans LE, Taylor JL, Smith CJ, Pritchard HAT, Greenstein AS, Allan SM (2021) Cardiovascular comorbidities, inflammation, and cerebral small vessel disease. Cardiovasc Res 117(13):2575–2588. https://doi.org/10.1093/cvr/cvab284
Abushamat LA, McClatchey PM, Scalzo RL et al (2020) Mechanistic causes of reduced cardiorespiratory fitness in type 2 diabetes. J Endocr Soc 4(7):bvaa063. https://doi.org/10.1210/jendso/bvaa063
Yates T, Henson J, Sargeant J et al (2021) Exercise, pharmaceutical therapies and type 2 diabetes: looking beyond glycemic control to whole body health and function. Transl Med Exerc Prescr 1(1):33–42. https://doi.org/10.53941/tmep.v1i1.33
Hammes H-P, Lin J, Renner O et al (2002) Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51(10):3107–3112. https://doi.org/10.2337/diabetes.51.10.3107
Nyström T, Gutniak MK, Zhang Q et al (2004) Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 287(6):E1209–E1215. https://doi.org/10.1152/ajpendo.00237.2004
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116(11):3015–3025. https://doi.org/10.1172/JCI28898
Favale NO, Casali CI, Lepera LG, Pescio LG, Fernández-Tome MC (2009) Hypertonic induction of COX2 expression requires TonEBP/NFAT5 in renal epithelial cells. Biochem Biophys Res Commun 381(3):301–305. https://doi.org/10.1016/j.bbrc.2008.12.189
Issar T, Kwai NCG, Poynten AM, Arnold R, Milner K-L, Krishnan AV (2021) Effect of exenatide on peripheral nerve excitability in type 2 diabetes. Clin Neurophysiol 132(10):2532–2539. https://doi.org/10.1016/j.clinph.2021.05.033
Ponirakis G, Abdul-Ghani MA, Jayyousi A et al (2020) Effect of treatment with exenatide and pioglitazone or basal-bolus insulin on diabetic neuropathy: a substudy of the Qatar Study. BMJ Open Diabetes Res Care 8(1):e001420. https://doi.org/10.1136/bmjdrc-2020-001420
Jaiswal M, Martin CL, Brown MB et al (2015) Effects of exenatide on measures of diabetic neuropathy in subjects with type 2 diabetes: results from an 18-month proof-of-concept open-label randomized study. J Diabetes Complications 29(8):1287–1294. https://doi.org/10.1016/j.jdiacomp.2015.07.013
Brock C, Hansen CS, Karmisholt J et al (2019) Liraglutide treatment reduced interleukin-6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy. Br J Clin Pharmacol 85(11):2512–2523. https://doi.org/10.1111/bcp.14063
Sullivan SD, Alfonso-Cristancho R, Conner C, Hammer M, Blonde L (2009) A simulation of the comparative long-term effectiveness of liraglutide and glimepiride monotherapies in patients with type 2 diabetes mellitus. Pharmacotherapy 29(11):1280–1288. https://doi.org/10.1592/phco.29.11.1280
da Silva GM, Heise CO, Hirata MT et al (2015) Comparative effects of a dipeptidyl peptidase-4 inhibitor and of NPH insulin on peripheral nerve conduction of patients with type 2 diabetes. Diabetol Metab Syndr 7(Suppl 1):A59. https://doi.org/10.1186/1758-5996-7-S1-A59
Engel SS, Suryawanshi S, Stevens SR et al (2017) Safety of sitagliptin in patients with type 2 diabetes and chronic kidney disease: outcomes from TECOS. Diabetes Obes Metab 19(11):1587–1593. https://doi.org/10.1111/dom.12983
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
Mr Michael Bonar (Creative Director, Diabetes Research Centre, College of Life Sciences, University of Leicester, Leicester, UK) designed and created the figures.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. SAH and MJD are supported by the Leicester NHR Leicester Biomedical Research Centre. JG, NIHR Academic Clinical Fellow (ACF-2021-11-006), is funded by Health Education England (HEE)/NIHR for this research project. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS or the UK Department of Health and Social Care.
Authors’ relationships and activities
JG declares no relevant relationships or activities. JAS has received funding in the form of an investigator-initiated study grant from AstraZeneca UK. MJD has acted as consultant, advisory board member and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi, an advisory board member Lexicon, Pfizer, ShouTi Pharma Inc and Medtronic and as a speaker for AstraZeneca, Napp Pharmaceuticals, Novartis and Amgen. She has received grants from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Janssen and Sanofi-Aventis and Eli Lilly.
Contribution statement
JG, JAS and MJD contributed to conceptualisation and design of review. JG drafted the manuscript, JAS and MJD revised it critically for important intellectual content. All authors approved the final manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goldney, J., Sargeant, J.A. & Davies, M.J. Incretins and microvascular complications of diabetes: neuropathy, nephropathy, retinopathy and microangiopathy. Diabetologia 66, 1832–1845 (2023). https://doi.org/10.1007/s00125-023-05988-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-05988-3