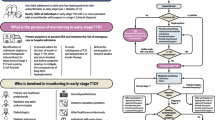
Abstract
Aims/hypothesis
We aimed to determine whether disease severity was reduced at onset of clinical (stage 3) type 1 diabetes in children previously diagnosed with presymptomatic type 1 diabetes in a population-based screening programme for islet autoantibodies.
Methods
Clinical data obtained at diagnosis of stage 3 type 1 diabetes were evaluated in 128 children previously diagnosed with presymptomatic early-stage type 1 diabetes between 2015 and 2022 in the Fr1da study and compared with data from 736 children diagnosed with incident type 1 diabetes between 2009 and 2018 at a similar age in the DiMelli study without prior screening.
Results
At the diagnosis of stage 3 type 1 diabetes, children with a prior early-stage diagnosis had lower median HbA1c (51 mmol/mol vs 91 mmol/mol [6.8% vs 10.5%], p<0.001), lower median fasting glucose (5.3 mmol/l vs 7.2 mmol/l, p<0.05) and higher median fasting C-peptide (0.21 nmol/l vs 0.10 nmol/l, p<0.001) compared with children without previous early-stage diagnosis. Fewer participants with prior early-stage diagnosis had ketonuria (22.2% vs 78.4%, p<0.001) or required insulin treatment (72.3% vs 98.1%, p<0.05) and only 2.5% presented with diabetic ketoacidosis at diagnosis of stage 3 type 1 diabetes. Outcomes in children with a prior early-stage diagnosis were not associated with a family history of type 1 diabetes or diagnosis during the COVID-19 pandemic. A milder clinical presentation was observed in children who participated in education and monitoring after early-stage diagnosis.
Conclusions/interpretation
Diagnosis of presymptomatic type 1 diabetes in children followed by education and monitoring improved clinical presentation at the onset of stage 3 type 1 diabetes.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Type 1 diabetes results from an autoimmune destruction of the insulin-producing beta cells [1]. Early presymptomatic stages of the disease are diagnosed by the detection of multiple islet autoantibodies, including autoantibodies against insulin (IAA), GAD (GADA), IA-2 (IA-2A) and ZnT8 (ZnT8A) [2], and are defined as stage 1 (normoglycaemia) or stage 2 (dysglycaemia) type 1 diabetes [3,4,5]. Several studies in individuals at familial or genetic risk for type 1 diabetes have shown that early-stage diagnosis may have benefits to individuals at clinical onset (stage 3) of type 1 diabetes [6,7,8,9]. In particular, diabetic ketoacidosis (DKA), which is observed in 20.3% to 48.4% of individuals at clinical disease onset [10], is reported in less than 10% of individuals who have a prior diagnosis of stage 1 or stage 2 type 1 diabetes [7,8,9, 11]. The presence of DKA at the time of clinical diagnosis is associated with poorer long-term glycaemic control and increases the risk of diabetic secondary diseases and cognitive impairment [12,13,14,15]. In addition, the acute and severe onset of the child’s disease causes psychological distress in parents and children [16].
In 2015, we initiated the Fr1da study in Bavaria, Germany, designed as a model project to evaluate population-based screening for multiple islet autoantibodies for early detection of type 1 diabetes in children [17, 18]. The study is conducted in collaboration with primary care paediatricians, and over 165,000 children have participated to date. The main purpose of the screening is to diagnose presymptomatic type 1 diabetes so that affected children and their families can be educated and monitored, and so that patients can begin insulin therapy early enough to prevent serious metabolic derangements at the onset of clinical disease. The aim of the current study was to determine whether disease severity at the onset of stage 3 type 1 diabetes is reduced in children previously diagnosed with presymptomatic type 1 diabetes in the Fr1da study. We compared the clinical presentation of Fr1da children at stage 3 diagnosis with that of children participating in the DiMelli study, a paediatric diabetes registry that enrolled children with incident stage 3 type 1 diabetes diagnosed through the same clinical centres in Bavaria as in the Fr1da study but without prior screening for islet autoantibodies.
Methods
Study design
The present study was conducted in children diagnosed with presymptomatic type 1 diabetes in the Fr1da public health screening programme [17,18,19] who subsequently developed stage 3 type 1 diabetes. Between February 2015 and 6 December 2022, 169,446 children participated in screening for islet autoantibodies offered by primary care paediatricians in the context of medical check-ups in Bavaria. Children without known diabetes aged 1.75–5.99 years (February 2015 to March 2019) or 1.75–10.99 years (since April 2019) were eligible for screening. Presymptomatic type 1 diabetes was diagnosed when multiple islet autoantibodies tested positive in an initial capillary blood screening sample and in a second venous blood confirmatory sample. Screening samples were tested for GADA, IA-2A and ZnT8A using the 3Screen ELISA (RSR, Cardiff, UK) [20, 21]. Samples above a threshold of 25 U in the ELISA (98th percentile of child samples) and confirmatory samples were tested for GADA, IA-2A, ZnT8A and IAA using radio-binding assays as previously described [19, 22,23,24].
Families of children diagnosed with presymptomatic type 1 diabetes were invited to participate in metabolic staging and an educational programme at a clinical referral centre near their home, where they received training in urine and blood glucose monitoring, and information on normal and pathological blood glucose levels and on symptoms of hyperglycaemia and DKA [17]. Families were provided with a guidebook (‘Fr1da book’) specifically designed for children with presymptomatic type 1 diabetes and assigned a contact person from the local diabetes centre to whom they could turn at any time if they had questions. Metabolic staging into stage 1 (normoglycaemia), stage 2 (dysglycaemia) or stage 3 (hyperglycaemia) of type 1 diabetes was based on the OGTT and HbA1c levels, according to the 2015 JDRF, Endocrine Society and ADA consensus criteria, as previously described [5]. Dependent on the staging result, children were monitored at intervals of 3 months (stage 2) to 6 months (stage 1) for development of stage 3 type 1 diabetes, which was diagnosed based on ADA criteria [3]. Clinical referral centres were provided with recommendations regarding the timing of initiation of insulin therapy in children with presymptomatic type 1 diabetes (HbA1c >47.5 mmol/mol [>6.5%] or fasting glucose >8.0 mmol/l [>145 mg/dl] or postprandial blood glucose >11.1 mmol/l [>200 mg/dl] [25]).
Families of children who declined staging and/or monitoring were contacted by telephone and asked if the child had developed stage 3 type 1 diabetes. All children diagnosed with stage 3 type 1 diabetes by 6 December 2022 were included in the current analysis. The study was approved by the institutional review board at the Technical University of Munich. Written, informed consent was obtained from the children’s parents or legal guardians.
Clinical outcome variables at diagnosis of stage 3 type 1 diabetes
At diagnosis of stage 3 type 1 diabetes, local physicians at the clinical referral centre, hospital or primary care centre were asked to complete a structured questionnaire including information on HbA1c, fasting glucose, insulin treatment (yes/no), ketonuria (moderate or large), venous pH and/or serum bicarbonate, clinical symptoms (yes/no), hospitalisation (yes/no) and duration of hospitalisation (days), and whether the child was admitted to the intensive care unit (ICU) (yes/no).
DKA was defined according to International Society for Pediatric and Adolescent Diabetes (ISPAD) Clinical Practice Consensus Guidelines 2022 for the diagnosis of DKA [26], including the following biochemical criteria: hyperglycaemia (blood glucose >11 mmol/l [200 mg/dl]), venous pH <7.3 or serum bicarbonate <18 mmol/l, and ketonaemia (blood ß-hydroxybutyrate ≥3 mmol/l) or moderate or large ketonuria. For children with incomplete information on these variables, information on DKA (yes/no) was retrieved from medical records provided by physicians.
Additionally, the questionnaire included information on weight and height, which was used to calculate BMI and convert it into sex- and age-adjusted BMI-SD scores (SDSs) using national reference data [27]. At the time of stage 3 type 1 diabetes diagnosis, a fasting aprotinin-stabilised EDTA venous blood sample was requested to measure C-peptide levels on an automated immunoassay analyser (AIA-360, Tosoh, San Francisco, CA, USA).
Genotyping for 46 SNPs, which have been used to calculate a genetic risk score (GRS) as described previously [28], was performed if consent for ancillary research was provided.
Demographic variables
Demographic data of the participating children (date of birth, sex, date of blood collection, first-degree family history of type 1 diabetes) were collected using a questionnaire at the time of screening for islet autoantibodies by primary care paediatricians.
Comparison cohort
The clinical presentation at diagnosis of stage 3 type 1 diabetes in children in the Fr1da study was compared with that of children diagnosed with incident type 1 diabetes without prior screening for islet autoantibodies from the DiMelli study. This is a cohort and biobank study in Bavaria that enrolled incident cases of childhood and adolescent diabetes between 2009 and 2018. The design of the DiMelli study has been described previously [29]. Children who participated in both the Fr1da and DiMelli studies were assigned to the Fr1da cohort for our analysis and were not considered in the DiMelli cohort. At the time of enrolment into the DiMelli study, a fasting blood sample was requested and a structured questionnaire was completed by the local hospital or primary care centre physician, which contained information on patient status, including sex, the date of diagnosis of diabetes, first-degree family history and current glucose-lowering medications. The blood sample was used to determine C-peptide, HbA1c, islet autoantibodies, HLA genotyping and SNP analysis [30]. The current analysis included 736 children who were diagnosed before the age of 10.99 years and tested positive for ≥1 islet autoantibody within a median of 1.3 weeks (IQR 0.9–1.9 weeks) of diagnosis. Twenty-nine of the 736 children were enrolled >2 months after diagnosis. The study was approved by the ethical committee of Bavaria, Germany (Bayerische Landesaerztekammer, no. 08043).
Statistical analysis
As most of the continuous variables were not symmetrically distributed, descriptive values were expressed as median and IQR for continuous variables and as total number (n) and percentage (%) for frequencies. For the comparison of characteristics between Fr1da and DiMelli study cohorts and the comparison of clinical outcome variables between Fr1da subgroups, Fisher’s exact test or non-parametric Mann–Whitney U test was applied. For the comparison of clinical outcome variables between Fr1da and DiMelli study participants, multivariate linear or logistic regression analysis was applied, adjusting for sex, age and calendar year at diagnosis of stage 3 type 1 diabetes and having a first-degree relative with type 1 diabetes. Results were expressed as estimated difference in means or OR and 95% CI from the multivariate analysis. Numbers for each specific outcome measure are included in the results. Complete-case sensitivity analyses for the comparison of clinical outcome variables between Fr1da and DiMelli study cohorts were performed including only children from both cohorts who had complete information on the following variables: age at stage 3 diagnosis, sex, family history of type 1 diabetes, HbA1c, fasting glucose, fasting C-peptide, insulin treatment and ketonuria. All statistical analyses were performed with IBM SPSS Statistics version 28 (IBM, Armonk, NY, USA). Figures were created with GraphPad Prism version 9.5.0 (Graphstats Technologies, USA). The significance level was set at 5% (two-tailed) for all analyses.
Results
Of 169,446 children enrolled in the Fr1da study, 473 (0.3%) children tested positive for multiple islet autoantibodies in the screening and confirmatory samples and were diagnosed with presymptomatic type 1 diabetes; they were offered participation in metabolic staging, diabetes education and follow-up at clinical referral centres (electronic supplementary material [ESM] Fig. 1). Of these 473 children, metabolic staging and education were refused or pending for 103 children (22%). A total of 128 of the 473 children (59 girls) progressed to stage 3 type 1 diabetes within a median of 2.3 years (IQR 1.1–4.3 years). Among the children with valid data, the median pH at diagnosis of stage 3 type 1 diabetes was 7.40 (IQR 7.38–7.42) and three (2.5%) of 118 children had a laboratory diagnosis of DKA (Table 1). Clinical symptoms were reported in 53 (43.8%) of 121 children, and children were hospitalised for a median of 8 days (IQR 0–11 days). Two (1.9%) of 108 children were admitted to the ICU.
The 128 children of the Fr1da study who developed stage 3 type 1 diabetes with a prior early-stage diagnosis were compared with the 736 children with incident type 1 diabetes of the DiMelli study with respect to their clinical presentation at stage 3 diagnosis (Table 1). The children from the two studies did not differ significantly in age at diagnosis of stage 3 type 1 diabetes (median [IQR], 6.7 years [5.0–9.1] vs 7.2 years [4.5–9.2]; p=0.8), sex (girls, 46.1% vs 49.2%; p=0.6), frequency of type 1 diabetes risk genes (HLA risk genotypes, 37.1% vs 36.5%; p=0.8) or GRS (median [IQR], 13.1 [11.8–14.2] vs 13.0 [11.9–13.9]; p=0.7). Children from the Fr1da study more frequently had a first-degree family history of type 1 diabetes (14.1% vs 6.7% in DiMelli; p<0.01).
Clinical presentation at diagnosis of stage 3 type 1 diabetes
There were substantial differences in metabolic variables at onset of stage 3 type 1 diabetes between children with and without a prior early-stage diagnosis (Table 2). Children in the Fr1da study had lower HbA1c levels (median [IQR], 51 mmol/mol [45–67] vs 91 mmol/mol [75–107]; 6.8% [6.2–8.3] vs 10.5% [9.0–11.9]; p<0.001), lower fasting glucose levels (median [IQR], 5.3 mmol/l [4.6–6.4] vs 7.2 mmol/l [5.7–9.1]; 95 mg/dl [82–116] vs 129 mg/dl [103–164]; p<0.05) and higher fasting C-peptide levels (median [IQR], 0.21 nmol/l [0.15–0.33] vs 0.10 nmol/l [0–0.17]; p<0.001) at diagnosis of stage 3 type 1 diabetes (Fig. 1a–d). The proportion of children with fasting C-peptide levels ≥0.2 nmol/l was higher in the Fr1da study (58.4%) as compared with DiMelli (18.8%; p<0.001). This was accompanied by a decreased frequency of participants requiring insulin treatment at the diagnosis of stage 3 type 1 diabetes (72.3% vs 98.1% in children from the DiMelli study; p<0.05) and a decreased frequency of participants with moderate or large ketonuria (22.2% vs 78.4%; p<0.001) in children with a prior early-stage diagnosis (Table 2). Children in the DiMelli study had lower BMI-SDS (median [IQR], −0.63 [−1.47 to 0.23] vs 0.05 [−0.77 to 0.77]; p<0.01).
HbA1c (a), fasting glucose (b), fasting C-peptide (c) and BMI-SDS (d) at diagnosis of stage 3 type 1 diabetes are shown for children previously diagnosed with presymptomatic type 1 diabetes in the Fr1da study compared with children diagnosed with incident type 1 diabetes in the DiMelli study who had not participated in prior screening. *p<0.05, **p<0.01, ***p<0.001
Similar findings were obtained when comparing clinical outcome variables between Fr1da and DiMelli children who were diagnosed with stage 3 type 1 diabetes at a younger age (<6 years) and at an older age (≥6 years) (ESM Table 1). Similar findings were also obtained when restricting the analysis to children with fasting C-peptide levels ≥0.2 nmol/l (ESM Table 2) and in a complete-case multivariate regression analysis (ESM Table 3).
Determinants of clinical presentation at diagnosis of stage 3 type 1 diabetes
Fourteen of the 128 children with a prior early-stage diagnosis from the Fr1da study did not participate in metabolic staging and education. As compared with the 114 children who did participate, these children had higher HbA1c levels at the time of stage 3 type 1 diabetes diagnosis and more hospitalisation days (ESM Table 4).
In the Fr1da study, the clinical presentation at diagnosis of stage 3 type 1 diabetes was not different between children who had or did not have a first-degree relative with type 1 diabetes (ESM Table 5). In contrast, children of the DiMelli study who had a first-degree relative with type 1 diabetes had lower HbA1c (median [IQR], 78 mmol/mol [67–89] vs 92 mmol/mol [77–108]; 9.3% [8.3–10.3] vs 10.6% [9.2–12.0]; p<0.001) and fasting glucose levels (median [IQR], 5.6 mmol/l [4.7–7.5] vs 7.2 mmol/l [5.8–9.2]; 100 mg/dl [84–135] vs 130 mg/dl [104–165]; p<0.001) and less frequent moderate or large ketonuria at diagnosis of stage 3 type 1 diabetes (51.2% vs 80.1%; p<0.001) than DiMelli children without a first-degree relative with type 1 diabetes, but HbA1c, fasting C-peptide and ketonuria did not reach levels or frequencies observed in the children with a first-degree relative with type 1 diabetes from the Fr1da study (ESM Fig. 2).
Since the COVID-19 pandemic is reported to affect diagnosis, we assessed whether the clinical presentation at diagnosis of stage 3 type 1 diabetes in children from the Fr1da study differed between children diagnosed before and during the COVID-19 pandemic. No difference in clinical presentation was observed between children who developed stage 3 type 1 diabetes from August 2015 to February 2020 and children who developed stage 3 type 1 diabetes from March 2020 to December 2022 (ESM Table 6).
Discussion
This study found that children who had been previously diagnosed with presymptomatic type 1 diabetes in a public health screening programme had a milder clinical presentation at diagnosis of stage 3 type 1 diabetes than children without prior screening for islet autoantibodies. This included lower HbA1c and fasting blood glucose levels, higher fasting C-peptide levels, fewer children with ketonuria, fewer children requiring insulin at diagnosis, a low prevalence of DKA and normal BMI-SDS, possibly reflecting less weight loss.
Strengths of our study are that participants with a prior early-stage diagnosis were not a priori selected on a genetic susceptibility criterion, but population-based, and that the comparison group was recruited from the same geographic region. Both cohorts were islet autoantibody-positive at diagnosis of stage 3 type 1 diabetes and were comparable in age, sex, GRS and frequency of HLA genotypes previously associated with type 1 diabetes risk. A limitation of this study was that the Fr1da screening was conducted between February 2015 and December 2022 and included the COVID-19 pandemic period, whereas the recruitment of children in DiMelli occurred between 2009 and 2018. However, no differences were observed in the clinical variables examined between children in Fr1da who developed stage 3 type 1 diabetes before or during the pandemic. It is possible that the DiMelli cohort includes a minority of children who had islet autoantibody screening performed without our knowledge. No information on socioeconomic status was available and, for some variables, the proportion of missing data was high. However, similar findings were observed when performing a complete-case analysis, suggesting that bias through missing data is limited. Nevertheless, confirmation of our results with a larger sample size is warranted.
The findings support the hypothesis that screening for presymptomatic early stages of type 1 diabetes reduces disease severity at clinical onset. Moreover, the observations are largely consistent with previous findings. The lower HbA1c levels observed at clinical manifestation in the Fr1da study were comparable to those of children in The Environmental Determinants of Diabetes in the Young (TEDDY) observational study who underwent regular metabolic monitoring after seroconversion of multiple islet autoantibodies [31]. Furthermore, HbA1c levels in the DiMelli comparison cohort were comparable to those of children followed in the Diabetes Prospective Follow-up Registry (DPV), which, similar to DiMelli, captures metabolic variables at the time of manifestation without prior screening [32]. The lower HbA1c and fasting blood glucose and increased fasting C-peptide concentrations indicate that a timely diagnosis was made in Fr1da cases. We would attribute this to both the diagnosis that came from the islet autoantibody results and the education and follow-up programme in which families were informed and reminded of the symptoms associated with the onset of stage 3 type 1 diabetes, as well as being monitored for worsening of metabolic variables. The low prevalence of DKA observed irrespective of whether families participated in education and monitoring or were followed during the lockdown period in the COVID-19 pandemic suggests that notification of islet autoantibody status is sufficient to raise awareness of the disease among families and paediatricians. The slightly worse metabolic status of individuals who declined education and follow-up indicates that early-stage diagnosis should include follow-up and monitoring to achieve optimal benefit for patients; however, this finding was limited by the small sample size and needs further confirmation.
An important observation was the higher fasting C-peptide concentrations and reduced need for insulin therapy in the Fr1da cases, indicating a higher beta cell reserve. These findings are also consistent with those in the TEDDY study [31]. Preservation of beta cell function was associated with a lower risk of hypoglycaemia and microvascular complications in the DCCT [33]. A threshold of 0.075 nmol/l for fasting C-peptide levels is proposed to define preserved beta cell function [33]. More than 90% of children from the Fr1da cohort had a fasting C-peptide ≥0.075 nmol/l and 58.4% ≥0.2 nmol/l. While it is expected that C-peptide will decline, the substantially higher C-peptide at diagnosis of stage 3 type 1 diabetes will increase eligibility for clinical trials aimed at preserving residual beta cell function and likely improve the response to intervention drugs used in these trials [34].
An objective of the Fr1da study was to prevent or significantly reduce the incidence of DKA and hospitalisation in children diagnosed with stage 3 type 1 diabetes. An association of DKA at the onset of clinical diabetes with increased mortality, prolonged hospital stays and increased ICU admission rate [35], as well as with lower residual beta cell function, poorer metabolic control and higher insulin requirements until several years after diagnosis, is well established [14, 31, 36, 37]. In addition to the individual burden of short- and long-term health consequences, the occurrence of DKA is also associated with increased costs to the healthcare system. Consistent with these associations, we observed a shorter median length of hospitalisation among children in the Fr1da study compared with data from the DPV cohort [35], and a lower incidence of children admitted to the ICU. It is expected that length of hospitalisation can be further reduced as soon as more highly qualified outpatient services are established for paediatric patients with newly diagnosed stage 3 type 1 diabetes.
Overall, the clinical presentation of children participating in the population-based Fr1da screening was comparable to that of children diagnosed with presymptomatic type 1 diabetes by islet autoantibody screening in natural history studies [7, 11], or clinical trials [38], and similarly with respect to stress and anxiety levels in parents in response to screening. Previous findings from the Fr1da study indicated that psychological stress scores were transiently elevated in mothers of children diagnosed with presymptomatic type 1 diabetes in the Fr1da screening and that the distress reported by the families was low or moderate in the majority of families [18], consistent with findings in children diagnosed with islet autoantibodies in natural history studies [39, 40] and in parents of children identified with an increased genetic risk for type 1 diabetes [41, 42]. With an appropriate education and care programme, islet autoantibody screening and a diagnosis of presymptomatic type 1 diabetes appear unlikely to lead to the level of parental stress observed in families of children diagnosed with stage 3 type 1 diabetes without prior screening [18]. A young age at stage 3 type 1 diabetes diagnosis and no family history of type 1 diabetes are associated with a more severe clinical presentation of diabetes, including a higher frequency of DKA [11]. This was also observed in the DiMelli cohort. Children in the Fr1da cohort had a milder diabetes onset regardless of age. No differences in clinical outcome variables were observed between Fr1da children with and without a first-degree relative with type 1 diabetes. It is therefore unlikely that the higher proportion of children with a first-degree relative in the Fr1da study resulted in a better clinical presentation; however, this finding needs to be confirmed with a larger sample size. Furthermore, in contrast to several previous reports [10, 43], in the Fr1da study no differences in clinical presentation were observed between children diagnosed before and during the COVID-19 pandemic. Overall, our results suggest that children both with and without first-degree relatives with type 1 diabetes benefit from screening, education and metabolic monitoring, and that clinical benefit was not affected by the COVID-19 pandemic.
In conclusion, this study suggests clinical benefit of a public health screening for type 1 diabetes. By identifying children with presymptomatic type 1 diabetes and offering them participation in education and metabolic follow-up, the clinical presentation at manifestation of stage 3 type 1 diabetes is improved. Of particular note are the low prevalence of DKA, lower rates of hospitalisation and ICU admissions, and better-preserved residual beta cell function. These results may inform considerations of screening children for presymptomatic type 1 diabetes at a population-based level.
Abbreviations
- DKA:
-
Diabetic ketoacidosis
- DPV:
-
Diabetes Prospective Follow-up Registry
- GRS:
-
Genetic risk score
- ICU:
-
Intensive care unit
- SDS:
-
SD score
- TEDDY:
-
The Environmental Determinants of Diabetes in the Young
References
Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383(9911):69–82. https://doi.org/10.1016/S0140-6736(13)60591-7
Bonifacio E, Achenbach P (2019) Birth and coming of age of islet autoantibodies. Clin Exp Immunol 198(3):294–305. https://doi.org/10.1111/cei.13360
American Diabetes Association Professional Practice Committee (2022) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care 45(Suppl 1):S17–S38. https://doi.org/10.2337/dc22-S002
Besser REJ, Bell KJ, Couper JJ et al (2022) ISPAD clinical practice consensus guidelines 2022: stages of type 1 diabetes in children and adolescents. Pediatr Diabetes 23(8):1175–1187. https://doi.org/10.1111/pedi.13410
Insel RA, Dunne JL, Atkinson MA et al (2015) Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 38(10):1964–1974. https://doi.org/10.2337/dc15-1419
Elding Larsson H, Vehik K, Bell R et al (2011) Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 34(11):2347–2352. https://doi.org/10.2337/dc11-1026
Winkler C, Schober E, Ziegler AG, Holl RW (2012) Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes 13(4):308–313. https://doi.org/10.1111/j.1399-5448.2011.00829.x
Hekkala AM, Ilonen J, Toppari J, Knip M, Veijola R (2018) Ketoacidosis at diagnosis of type 1 diabetes: effect of prospective studies with newborn genetic screening and follow up of risk children. Pediatr Diabetes 19(2):314–319. https://doi.org/10.1111/pedi.12541
Wentworth JM, Oakey H, Craig ME et al (2022) Decreased occurrence of ketoacidosis and preservation of beta cell function in relatives screened and monitored for type 1 diabetes in Australia and New Zealand. Pediatr Diabetes 23(8):1594–1601. https://doi.org/10.1111/pedi.13422
Birkebaek NH, Kamrath C, Grimsmann JM et al (2022) Impact of the COVID-19 pandemic on long-term trends in the prevalence of diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes: an international multicentre study based on data from 13 national diabetes registries. Lancet Diabetes Endocrinol 10(11):786–794. https://doi.org/10.1016/S2213-8587(22)00246-7
Jacobsen LM, Vehik K, Veijola R et al (2022) Heterogeneity of DKA incidence and age-specific clinical characteristics in children diagnosed with type 1 diabetes in the TEDDY study. Diabetes Care 45(3):624–633. https://doi.org/10.2337/dc21-0422
Fredheim S, Johannesen J, Johansen A et al (2013) Diabetic ketoacidosis at the onset of type 1 diabetes is associated with future HbA1c levels. Diabetologia 56(5):995–1003. https://doi.org/10.1007/s00125-013-2850-z
Duca LM, Wang B, Rewers M, Rewers A (2017) Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care 40(9):1249–1255. https://doi.org/10.2337/dc17-0558
Duca LM, Reboussin BA, Pihoker C et al (2019) Diabetic ketoacidosis at diagnosis of type 1 diabetes and glycemic control over time: the SEARCH for diabetes in youth study. Pediatr Diabetes 20(2):172–179. https://doi.org/10.1111/pedi.12809
Cameron FJ, Scratch SE, Nadebaum C et al (2014) Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care 37(6):1554–1562. https://doi.org/10.2337/dc13-1904
Whittemore R, Jaser S, Chao A, Jang M, Grey M (2012) Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. Diabetes Educ 38(4):562–579. https://doi.org/10.1177/0145721712445216
Raab J, Haupt F, Scholz M et al (2016) Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open 6(5):e011144. https://doi.org/10.1136/bmjopen-2016-011144
Ziegler AG, Kick K, Bonifacio E et al (2020) Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA 323(4):339–351. https://doi.org/10.1001/jama.2019.21565
Weiss A, Zapardiel-Gonzalo J, Voss F et al (2022) Progression likelihood score identifies substages of presymptomatic type 1 diabetes in childhood public health screening. Diabetologia 65(12):2121–2131. https://doi.org/10.1007/s00125-022-05780-9
Amoroso M, Achenbach P, Powell M et al (2016) 3 Screen islet cell autoantibody ELISA: a sensitive and specific ELISA for the combined measurement of autoantibodies to GAD65, to IA-2 and to ZnT8. Clin Chim Acta 462:60–64. https://doi.org/10.1016/j.cca.2016.08.013
Ziegler AG, Haupt F, Scholz M et al (2016) 3 Screen ELISA for high-throughput detection of beta cell autoantibodies in capillary blood. Diabetes Technol Ther 18(11):687–693. https://doi.org/10.1089/dia.2016.0199
Bonifacio E, Yu L, Williams AK et al (2010) Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 95(7):3360–3367. https://doi.org/10.1210/jc.2010-0293
Achenbach P, Lampasona V, Landherr U et al (2009) Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 52(9):1881–1888. https://doi.org/10.1007/s00125-009-1438-0
Naserke HE, Dozio N, Ziegler AG, Bonifacio E (1998) Comparison of a novel micro-assay for insulin autoantibodies with the conventional radiobinding assay. Diabetologia 41(6):681–683. https://doi.org/10.1007/s001250050968
Insel RA, Dunne JL, Ziegler AG (2015) General population screening for type 1 diabetes: has its time come? Curr Opin Endocrinol Diabetes Obes 22(4):270–276. https://doi.org/10.1097/MED.0000000000000173
Glaser N, Fritsch M, Priyambada L et al (2022) ISPAD clinical practice consensus guidelines 2022: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes 23(7):835–856. https://doi.org/10.1111/pedi.13406
Kromeyer-Hauschild K, Moss A, Wabitsch M (2015) Referenzwerte für den Body-Mass-Index für Kinder, Jugendliche und Erwachsene in Deutschland. Anpassung der AGA-BMI-Referenz im Altersbereich von 15 bis 18 Jahren. Adipositas 9:123-127 [article in German]. https://doi.org/10.1055/s-0037-1618928
Bonifacio E, Beyerlein A, Hippich M et al (2018) Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLoS Med 15(4):e1002548. https://doi.org/10.1371/journal.pmed.1002548
Thumer L, Adler K, Bonifacio E et al (2010) German new onset diabetes in the young incident cohort study: DiMelli study design and first-year results. Rev Diabet Stud 7(3):202–208. https://doi.org/10.1900/RDS.2010.7.202
Achenbach P, Hippich M, Zapardiel-Gonzalo J et al (2022) A classification and regression tree analysis identifies subgroups of childhood type 1 diabetes. EBioMedicine 82:104118. https://doi.org/10.1016/j.ebiom.2022.104118
Steck AK, Larsson HE, Liu X et al (2017) Residual beta-cell function in diabetes children followed and diagnosed in the TEDDY study compared to community controls. Pediatr Diabetes 18(8):794–802. https://doi.org/10.1111/pedi.12485
Karges B, Prinz N, Placzek K et al (2021) A comparison of familial and sporadic type 1 diabetes among young patients. Diabetes Care 44(5):1116–1124. https://doi.org/10.2337/dc20-1829
Lachin JM, McGee P, Palmer JP, DCCT/EDIC Research Group (2014) Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 63(2):739–748. https://doi.org/10.2337/db13-0881
Hagopian W, Ferry RJ Jr, Sherry N et al (2013) Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protege trial. Diabetes 62(11):3901–3908. https://doi.org/10.2337/db13-0236
Nagl K, Rosenbauer J, Neu A et al (2020) Children with onset-ketoacidosis are admitted to the nearest hospital available, regardless of center size. J Pediatr Endocrinol Metab 33(6):751–759. https://doi.org/10.1515/jpem-2020-0038
Fernandez Castaner M, Montana E, Camps I et al (1996) Ketoacidosis at diagnosis is predictive of lower residual beta-cell function and poor metabolic control in type 1 diabetes. Diabetes Metab 22(5):349–355
Bowden SA, Duck MM, Hoffman RP (2008) Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes 9(3 Pt 1):197–201. https://doi.org/10.1111/j.1399-5448.2008.00376.x
Nakhla M, Cuthbertson D, Becker DJ et al (2021) Diabetic ketoacidosis at the time of diagnosis of type 1 diabetes in children: insights from TRIGR. JAMA Pediatr 175(5):518–520. https://doi.org/10.1001/jamapediatrics.2020.5512
Bennett Johnson S, Tercyak KP Jr (1995) Psychological impact of islet cell antibody screening for IDDM on children, adults, and their family members. Diabetes Care 18(10):1370–1372. https://doi.org/10.2337/diacare.18.10.1370
Smith LB, Liu X, Johnson SB et al (2018) Family adjustment to diabetes diagnosis in children: can participation in a study on type 1 diabetes genetic risk be helpful? Pediatr Diabetes 19(5):1025–1033. https://doi.org/10.1111/pedi.12674
Swartling U, Lynch K, Smith L, Johnson SB, TEDDY Study Group (2016) Parental estimation of their child’s increased type 1 diabetes risk during the first 2 years of participation in an international observational study: results from the TEDDY study. J Empir Res Hum Res Ethics 11(2):106–114. https://doi.org/10.1177/1556264616648589
Houben J, Janssens M, Winkler C et al (2022) The emotional well-being of parents with children at genetic risk for type 1 diabetes before and during participation in the POInT-study. Pediatr Diabetes 23(8):1707–1716. https://doi.org/10.1111/pedi.13448
Rugg-Gunn CEM, Dixon E, Jorgensen AL et al (2022) Factors associated with diabetic ketoacidosis at onset of type 1 diabetes among pediatric patients: a systematic review. JAMA Pediatr 176(12):1248–1259. https://doi.org/10.1001/jamapediatrics.2022.3586
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Acknowledgements
We are grateful to the participating families and children and all members of the Fr1da study team at the Institute of Diabetes Research, Helmholtz Munich; the Forschergruppe Diabetes at Klinikum rechts der Isar; School of Medicine, Technical University Munich; and the Forschergruppe Diabetes e.V. at Helmholtz Munich, Germany. We also thank all primary care paediatricians and clinical centres participating in the Fr1da study group (https://www.typ1diabetes-frueherkennung.de/informationen-fuer-aerzte/the-fr1da-study-group.html).
Data availability
The de-identified individual participant data that underlie the results (text, tables, figures and ESM, excluding genetic data) reported in this article can be shared between 9 and 36 months after publication of the article. Requests will be honoured from researchers who provide a methodologically sound proposal and who complete a Data Use Agreement with Helmholtz Munich. Requests should be directed by email to the corresponding authors.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by a grant from the Federal Ministry of Education and Research (BMBF, grant FKZ01KX1818). The Fr1da study was supported by JDRF International (1-SRA-2014–310-M-R, 3-SRA-2015–72-M-R, 3-SRA-2019–718-Q-R), LifeScience Stiftung (HMGU 2014.01 and HMGU 2016.01), The Leona M. and Harry B. Helmsley Charitable Trust (G-1911–3274) and the German Center for Diabetes Research (DZD e.V.). The DiMelli cohort study was supported by funds from the German Federal Ministry of Education and Research to the Competence Network Diabetes Mellitus (FKZ 01GI0805), and to the German Center for Diabetes Research (DZD e.V.). The funding sources were not involved in the design or conduct of the study; the collection, management, analysis or interpretation of the data; the preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Authors’ relationships and activities
PA is a member of the Editorial Board of Diabetologia. All other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
SH, JC, NF and PA performed data analysis. JC, CW, KK, J. Stock, FR, CR, J. Schmidt, DL, S. Braig, DD, UE, E-MG, LW, NN-H, S. Brämswig, MS, ST and AL researched the data and reviewed and edited the manuscript. A-GZ and EB were responsible for study design. A-GZ and PA were responsible for study conduct. A-GZ is the principal investigator of the Fr1da study. SH, A-GZ, EB and PA drafted the manuscript. All authors reviewed and approved the final version of the manuscript. SH and PA are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hummel, S., Carl, J., Friedl, N. et al. Children diagnosed with presymptomatic type 1 diabetes through public health screening have milder diabetes at clinical manifestation. Diabetologia 66, 1633–1642 (2023). https://doi.org/10.1007/s00125-023-05953-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-05953-0