Abstract
Aims/hypothesis
To provide a systematic overview of the current body of evidence on high-risk phenotypes of diabetes associated with COVID-19 severity and death.
Methods
This is the first update of our recently published living systematic review and meta-analysis. Observational studies investigating phenotypes in individuals with diabetes and confirmed SARS-CoV-2 infection with regard to COVID-19-related death and severity were included. The literature search was conducted from inception up to 14 February 2022 in PubMed, Epistemonikos, Web of Science and the COVID-19 Research Database and updated using PubMed alert to 1 December 2022. A random-effects meta-analysis was used to calculate summary relative risks (SRRs) with 95% CIs. The risk of bias was evaluated using the Quality in Prognosis Studies (QUIPS) tool and the certainty of evidence using the GRADE approach.
Results
A total of 169 articles (147 new studies) based on approximately 900,000 individuals were included. We conducted 177 meta-analyses (83 on COVID-19-related death and 94 on COVID-19 severity). Certainty of evidence was strengthened for associations between male sex, older age, blood glucose level at admission, chronic insulin use, chronic metformin use (inversely) and pre-existing comorbidities (CVD, chronic kidney disease, chronic obstructive pulmonary disease) and COVID-19-related death. New evidence with moderate to high certainty emerged for the association between obesity (SRR [95% CI] 1.18 [1.04, 1.34], n=21 studies), HbA1c (53–75 mmol/mol [7–9%]: 1.18 [1.06, 1.32], n=8), chronic glucagon-like peptide-1 receptor agonist use (0.83 [0.71, 0.97], n=9), pre-existing heart failure (1.33 [1.21, 1.47], n=14), pre-existing liver disease (1.40 [1.17, 1.67], n=6), the Charlson index (per 1 unit increase: 1.33 [1.13, 1.57], n=2), high levels of C-reactive protein (per 5 mg/l increase: 1.07 [1.02, 1.12], n=10), aspartate aminotransferase level (per 5 U/l increase: 1.28 [1.06, 1.54], n=5), eGFR (per 10 ml/min per 1.73 m2 increase: 0.80 [0.71, 0.90], n=6), lactate dehydrogenase level (per 10 U/l increase: 1.03 [1.01, 1.04], n=7) and lymphocyte count (per 1×109/l increase: 0.59 [0.40, 0.86], n=6) and COVID-19-related death. Similar associations were observed between risk phenotypes of diabetes and severity of COVID-19, with some new evidence on existing COVID-19 vaccination status (0.32 [0.26, 0.38], n=3), pre-existing hypertension (1.23 [1.14, 1.33], n=49), neuropathy and cancer, and high IL-6 levels. A limitation of this study is that the included studies are observational in nature and residual or unmeasured confounding cannot be ruled out.
Conclusions/interpretation
Individuals with a more severe course of diabetes and pre-existing comorbidities had a poorer prognosis of COVID-19 than individuals with a milder course of the disease.
Registration
PROSPERO registration no. CRD42020193692.
Previous version
This is a living systematic review and meta-analysis. The previous version can be found at https://link.springer.com/article/10.1007/s00125-021-05458-8
Funding
The German Diabetes Center (DDZ) is funded by the German Federal Ministry of Health and the Ministry of Culture and Science of the State North Rhine-Westphalia. This study was supported in part by a grant from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD).
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
In our recent living systematic review and meta-analysis, we identified several risk phenotypes for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in individuals with diabetes, based on 22 studies [1]. There was moderate to high certainty of evidence that male sex, older age (≥65 years), pre-existing CVD, chronic kidney disease (CKD) and chronic obstructive pulmonary disease (COPD), diabetes treatment (insulin and [inverse association] metformin) and high blood glucose level at admission were associated with COVID-19-related death or disease severity. Since then, numerous studies on this topic have been published and thus new evidence is available. To provide the best current body of evidence, our aim was to update the living systematic review and meta-analysis on associations between risk phenotypes of diabetes and confirmed SARS-CoV-2 infection associated with COVID-19-related death and severity.
Methods
This is the first update of our living systematic review and meta-analysis and the methods are described in detail in our previous study [1]. The update was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [2].
Search strategy and selection criteria
The systematic literature search was updated to 14 February 2022, using the same search terms and databases (PubMed, Epistemonikos, Web of Science and the COVID-19 Research Database) as in the original study (see electronic supplementary material [ESM] Table 1). From 15 February 2022 until 1 December 2022, we used only the PubMed alert based on our search terms because 96% of the relevant studies up to 14 February 2022 were identified in PubMed and thus the inclusion of the further databases did not justify the additional work and expense.
We included studies of any design that reported risk estimates (HRs, RRs or ORs with 95% CIs) for associations between phenotypes (general characteristics of participants, diabetes-specific characteristics, presence of diabetes-related complications and underlying comorbidities, chronic medication use and laboratory variables) and COVID-19-related death and severity of COVID-19 in individuals with diabetes and WHO-defined confirmed SARS-CoV-2 infection (https://apps.who.int/iris/handle/10665/337834). We excluded studies without primary clinical data (e.g. modelling studies), editorials, letters without primary data, commentaries, reviews, articles not in English and guidelines. Studies that focused on mixed populations, including individuals without diabetes or without COVID-19, were also excluded. If articles were based on the same cohort/data, we selected the study with the largest number of cases. If the studies were based on the same number of cases, we selected the study with the more favourable adjustment set. We contacted study authors for missing data, to query implausible data or for further information if needed [3,4,5,6,7,8,9,10].
Data extraction and risk of bias assessment
Study selection, data extraction (ESM Table 2), assessment of risk of bias using the Quality in Prognosis Studies (QUIPS) tool [11] (ESM Methods; ESM Table 3) and assessment of certainty of evidence using the GRADEpro approach [12] were conducted independently by two investigators and, if necessary, a third investigator was consulted and consensus was reached through discussion.
Statistical analysis
Summary relative risks (SRRs) and 95% CIs were calculated by random-effects meta-analysis using the DerSimonian and Laird method. The data from the original systematic review and meta-analysis [1] were combined with the findings from the new studies. We followed our original analysis plan and calculated I2 as a measure of heterogeneity, assessed publication bias by generating funnel plots and applying Egger’s test, and stratified our meta-analyses by risk of bias due to confounding (low/moderate risk vs high risk of bias). All meta-analyses were conducted for COVID-19-related death and severity (defined as a composite endpoint including death, tracheal intubation for mechanical ventilation, acute respiratory distress syndrome, septic shock, intensive care unit admission, multiple organ dysfunction or failure, or hospital admission). We conducted sensitivity analyses by calculating 95% CIs using the Hartung–Knapp–Sidik–Jonkman method, which provides more adequate error rates than the DerSimonian and Laird method, particularly for meta-analyses based on small numbers of studies. All statistical analyses were conducted with Stata software version 15.1 (Stata Corporation, USA).
Results
Literature search and characteristics of included studies
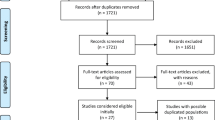
In total, 32,325 records (28,175 new) were identified from the database searches. After exclusion of duplicates, the titles and abstracts of 16,789 articles (14,243 new) were screened, of which 2598 articles (2385 new) were read in full. Excluded studies with corresponding reasons are shown in ESM Table 4. Finally, 169 publications were included, of which 147 were new publications (Fig. 1) [3,4,5,6,7,8,9,10, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173].
Flow chart of the literature search. Based on Page et al [2]. For more information, see http://www.prisma-statement.org/
We conducted 177 meta-analyses (83 on COVID-19-related death and 94 on COVID-19 severity), compared with 77 meta-analyses in our original systematic review and meta-analysis [1]. The number of included individuals per study ranged from 24 (smallest study) to 235,248 (largest study). In total, our meta-analyses included 859,262 individuals with diabetes and confirmed SARS-CoV-2 infection for COVID-19-related death and 927,975 for COVID-19 severity (compared with 15,063 individuals with diabetes and confirmed SARS-CoV-2 infection for COVID-19-related death and 17,687 for COVID-19 severity in the original meta-analysis [1]). Most of the publications (n=76) were from Asia (China, n=28; Iran, n=13 ; South Korea, n=11; Turkey, n=7; India, n=5; Hong Kong, n=3; Saudi Arabia, n=2; Israel, n=2; Japan, n=2; Singapore, n=1; Philippines; n=1, Kuwait, n=1), with 46 from Europe (Italy, n=12; UK, n=9; France, n=8; Spain, n=8; Sweden, n=2; Russia, n=2; Belgium, n=1; Romania, n=1; Denmark, n=1; the Netherlands, n=1; Greece, n=1), 35 from North America (USA, n=31; Mexico, n=4), four from Africa (Egypt, n=3; South Africa, n=1) and three from South America (Brazil, n=2; Peru, n=1). Five studies were performed in an international setting. The majority of the studies were conducted in a hospital setting and used data from hospital-based records (n=136); 33 studies used registry or insurance data. Regarding diabetes type, 78 publications included individuals with only type 2 diabetes, three included individuals with only type 1 diabetes and 38 focused on both type 1 and type 2 diabetes; in 50 publications diabetes type was not specified. The characteristics of the studies are shown in detail in ESM Table 5.
Risk of bias was low in 35 studies, moderate in 67 studies, high in 66 studies and unclear in one study (ESM Fig. 1). The main reason for a high risk of bias was insufficient adjustment for confounding factors and/or inappropriate statistical analysis and reporting of the findings (ESM Fig. 2).
The results of the meta-analyses can be found in ESM Figs. 3–97. Details of the papers included in the meta-analyses are provided in ESM Table 5.
General risk factors and COVID-19-related death and COVID-19 severity in individuals with diabetes and COVID-19
Updated meta-analyses (Fig. 2; ESM Table 6) confirmed a high certainty of evidence for the association between male sex and increased risk of COVID-19-related death (SRR 1.40 [95% CI 1.31, 1.50], n=39 studies [ESM Fig. 5]). For older age the certainty of evidence was now also rated as high (age ≥65 years: SRR 3.45 [95% CI 2.44, 4.87], n=20 studies [ESM Fig. 6]; age per 5 year increase: SRR 1.28 [95% CI 1.21, 1.36], n=30 studies [ESM Fig. 7]). New evidence emerged that obesity in patients with diabetes is related to an increased risk of COVID-19-related death (SRR 1.18 [95% CI 1.04, 1.34], n=21 studies, moderate certainty of evidence [ESM Fig. 9]). Similar associations were observed for COVID-19 severity (Fig. 3; ESM Table 7). There were no clear and consistent associations between being overweight, smoking status, area of residence and ethnicity and risk of COVID-19-related death and COVID-19 severity (certainty of evidence ranged from very low to moderate). For COVID-19 severity, new evidence became available for an association between vaccination against COVID-19 and lower risk of severe disease (SRR 0.32 [95% CI 0.26, 0.38], n=3 studies, high certainty of evidence [ESM Fig. 4]).
Prognostic factors and COVID-19-associated death in individuals with diabetes and COVID-19: general risk factors, diabetes-specific risk factors and laboratory variables. See ESM Figs. 3–97 for full details of the meta-analyses. Poorly controlled blood glucose was defined as a lowest fasting blood glucose of ≥3.9 mmol/l and a highest 2 h plasma glucose level >10.0 mmol/l during the observation window. ESR, erythrocyte sedimentation rate
Prognostic factors and severity of COVID-19 in individuals with diabetes and COVID-19: general risk factors, diabetes-specific risk factors and laboratory variables. See ESM Figs. 3–97 for full details of the meta-analyses. Severity was defined as a composite endpoint including death, tracheal intubation for mechanical ventilation, acute respiratory distress syndrome, septic shock, intensive care unit admission, multiple organ dysfunction or failure, or hospital admission. See Fig. 2 for the definition of poorly controlled blood glucose. ESR, erythrocyte sedimentation rate
Diabetes-specific risk factors and COVID-19-related death and COVID-19 severity in individuals with diabetes and COVID-19
Since the initial review [1], several new studies have been published on diabetes type and duration and COVID-19-related death and COVID-19 severity, but the estimates remain imprecise and the certainty of evidence for these associations is low or very low (Figs. 2 and 3; ESM Tables 6 and 7). HbA1c level was not linearly related to COVID-19-related death (per 20 mmol/mol [per 4%] increase: SRR 0.99 [95% CI 0.81, 1.21], n=10 studies, very low certainty of evidence), but was linearly related to COVID-19 severity (per 20 mmol/mol [per 4%] increase: SRR 1.51 [95% CI 1.25, 1.80], n=28 studies, high certainty of evidence [ESM Fig. 22]). Using a cut-off of 53−75 mmol/mol (7–9%) vs <53 mmol/mol (<7%), high certainty of evidence was found for an increased risk of both outcomes (SRR for death: 1.18 [95% CI 1.06, 1.32], n=8 studies; SRR for severity: 1.21 [95% CI 1.09, 1.35], n=16 studies [ESM Fig. 20]). High blood glucose levels at admission were also related to an increased risk of both outcomes (per 5 mmol/l increase: SRR for death 1.38 [95% CI 1.15, 1.65], n=11 studies, moderate certainty of evidence; SRR for severity 1.10 [95% CI 1.05, 1.18], n=14 studies, high certainty of evidence [ESM Fig. 25]). Study findings on blood glucose thresholds (especially ≥10 mmol/l at admission) also indicated a higher risk of both outcomes, with high certainty of evidence (≥10 mmol/l: SRR for death 2.01 [95% CI 1.54, 2.63], n=19 studies; SRR for severity 1.81 [95% CI 1.42, 2.31], n=19 studies [ESM Fig. 24]).
Several new studies were available on diabetes treatment (Figs. 2 and 3; ESM Tables 6 and 7). There was high certainty of evidence that insulin use was related to a 33% increased risk of COVID-19-related death (SRR 1.33 [95% CI 1.18, 1.49], n=26 studies [ESM Fig. 27]), while metformin use was associated with a 31% decreased risk (SRR 0.69 [95% CI 0.60, 0.79], n=23 studies [ESM Fig. 28]). New evidence with high certainty emerged that glucagon-like peptide-1 receptor agonist (GLP-1RA) use was also associated with a lower risk of COVID-19-related death (SRR 0.83 [95% CI 0.71, 0.97], n=9 studies [ESM Fig. 31]). There was also evidence for a reduced risk of COVID-19-related death with use of dipeptidyl peptidase 4 (DPP-4) inhibitors (SRR 0.91 [95% CI 0.80, 1.03], n=22 studies, high certainty of evidence [ESM Fig. 29]) and use of sodium–glucose cotransporter 2 (SGLT2) inhibitors (SRR 0.88 [95% CI 0.73, 1.04], n=9 studies, moderate certainty of evidence [ESM Fig. 32]). For the other diabetes medications, there were no clear associations with risk of COVID-19-related death (Fig. 2; ESM Table 6). Similar findings were observed for COVID-19 severity (Fig. 3; ESM Table 7).
Laboratory variables on admission and COVID-19-related death and COVID-19 severity in individuals with diabetes and COVID-19
The results for laboratory markers are shown in Figs. 2 and 3 and ESM Tables 6 and 7. There was new evidence with high certainty that C-reactive protein (CRP) level at admission was related to an increased risk of COVID-19-related death and COVID severity (per 5 mg/l increase: SRR for death 1.07 [95% CI 1.02, 1.12], n=10 studies; SRR for severity 1.06 [95% CI 1.03, 1.10], n=14 studies [ESM Fig. 73]). IL-6 level was also associated with severity of COVID-19 (per 5 pg/ml increase: SRR 1.13 [95% CI 1.03, 1.25], n=6 studies, moderate certainty of evidence [ESM Fig. 74]).
There was new evidence that higher aspartate aminotransferase (AST) levels at admission were associated with a higher risk of COVID-19-related death (per 5 U/l increase: SRR 1.28 [95% CI 1.06, 1.54], n=5 studies, high certainty of evidence; similar findings for severity [ESM Fig. 79]). For alanine aminotransferase (ALT), no clear associations were observed (ESM Fig. 78). New evidence was also found for an association of higher eGFR with decreased risk of COVID-19-related death (per 10 ml/min per 1.73m2 increase: SRR 0.80 [95% CI 0.71, 0.90], n=6 studies, high certainty of evidence; similar findings for severity [ESM Fig. 80]).
Lymphocyte count was also inversely associated with COVID-19-related death and COVID-19 severity (per 1 × 109/l increase: SRR for death 0.59 [95% CI 0.40, 0.86], n=6 studies, moderate certainty of evidence; SRR for severity 0.62 [95% CI 0.48, 0.80] n=11 studies, low certainty of evidence [ESM Fig. 87]). Lactate dehydrogenase (LDH) level was also related to an increased risk of COVID-19-related death and COVID-19 severity, with high certainty of evidence for both (per 10 U/l increase: SRR for death 1.03 [95% CI 1.01, 1.04], n=7 studies; SRR for severity 1.04 [95% CI 1.01, 1.07], n=9 studies [ESM Fig. 91]).
Comorbidities, complications and medication use and COVID-19-related death and COVID-19 severity in individuals with diabetes and COVID-19
In the updated meta-analyses, it was confirmed that the certainty of evidence was high for an association of pre-existing CVD with COVID-19-related death (SRR 1.35 [95% CI 1.23, 1.50], n=23 studies [Fig. 4; ESM Table 6; ESM Fig. 37]). New evidence with high certainty was also found for an association of heart failure (SRR 1.33 [95% CI 1.21, 1.47], n=14 studies [ESM Fig. 40]), CKD (SRR 1.54 [95% CI 1.39, 1.70], n=28 studies [ESM Fig 46]), liver disease (SRR 1.40 [95% CI 1.17, 1.67], n=6 studies [ESM Fig. 50]) and COPD (SRR 1.38 [95% CI 1.24, 1.54], n=19 studies [ESM Fig. 51]) with COVID-19-related death. New evidence with moderate certainty was identified for an association between coronary artery disease (CAD) (SRR 1.30 [95% CI 1.11, 1.53], n=14 studies [ESM Fig. 38]) and a comorbidity index (Charlson index) (per 1 unit increase: SRR 1.33 [95% CI 1.13, 1.57], n=2 studies [ESM Fig. 61]) and COVID-19-related death (Fig. 4; ESM Table 6). Similar associations were seen for COVID-19 severity (Fig. 5; ESM Table 7). While no clear association with COVID-19-related death was found for pre-existing hypertension, neuropathy and cancer, there was evidence with moderate certainty of an association of all three comorbidities with COVID-19 severity (SRR 1.23 [95% CI 1.14, 1.33], n=49 studies; 1.17 [95% CI 1.07, 1.28], n=5 studies; and 1.37 [95% CI 1.07, 1.75], n=24 studies, respectively [ESM Figs. 35, 48 and 55, respectively; ESM Tables 6 and 7]).
Prognostic factors and COVID-19-associated death in individuals with diabetes and COVID-19: comorbidities and complications and other medication use. See ESM Figs. 3–97 for full details of the meta-analyses. Renin inhibitors included ACE inhibitors, ARBs and non-specified RAS inhibitors. n.s., not specified
Prognostic factors and severity of COVID-19 in individuals with diabetes and COVID-19: comorbidities and complications and other medication use. See ESM Figs. 3–97 for full details of the meta-analyses and Fig. 3 for the definition of severity. Renin inhibitors included ACE inhibitors, ARBs and non-specified RAS inhibitors. n.s., not specified
For medication use (other than diabetes medications), the certainty of evidence was moderate for use of antithrombotic drugs associated with an increased risk of COVID-19-related death but not with COVID-19 severity (SRR for death 1.14 [95% CI 1.02, 1.27], n=6 studies; SRR for severity 1.02 [0.89, 1.16], n=9 studies [Figs. 4 and 5; ESM Fig. 68]). New evidence emerged on the use of acetylsalicylic acid, also pointing to an increased risk, especially for COVID-19 severity, but the certainty of evidence was very low (ESM Fig. 67; ESM Tables 6 and 7).
Subgroup analysis, heterogeneity, publication bias and sensitivity analysis
For each association, meta-analyses were stratified by risk of bias due to confounding (ESM Figs. 3–97). For associations that showed apparently different findings in the stratified meta-analysis, we conducted meta-regression adjusted by risk of bias due to confounding (ESM Table 8). Effect modification by adjustment status was observed for HbA1c ≥75 mmol/mol (≥9%), use of statins and use of renin inhibitors with regard to COVID-19-related death and COVID-19 severity. For HbA1c ≥75 mmol/mol (≥9%), a clear increased risk was observed for both outcomes for studies with a low/moderate risk of bias due to confounding (SRR for death 1.31 [95% CI 1.18, 1.44]; SRR for severity 1.47 [95% CI 1.31, 1.66]), but imprecisely estimated inverse associations were found for studies with a high risk of bias due to confounding (SRR for death 0.89 [95% CI: 0.75, 1.04]; SRR for severity 0.91 [95% CI 0.68, 1.21] [ESM Fig. 21]). For chronic use of statins and renin inhibitors, inverse associations for studies with a low/moderate risk of bias due to confounding were observed for COVID-19-related death and COVID-19 severity, but there was an increased risk of both outcomes in studies with a high risk of bias due to confounding (ESM Figs. 62 and 63).
Heterogeneity was particularly high for the laboratory markers, probably because of the different analytical methods and reference ranges used (Figs. 2 and 3; ESM Tables 6 and 7).
Findings on potential publication bias and small study effects are shown in ESM Figs. 98–132. According to Egger’s test, there was a suggestion of publication bias for the association of obesity, blood glucose per unit increase at admission and unspecified chronic obstructive diseases with COVID-19-related death, as well as for the association of overweight, obesity, blood glucose per unit increase at admission, use of thiazolidinediones, CKD, unspecified chronic pulmonary diseases, CRP level and lymphocyte count with COVID-19 severity, and the funnel plots show that studies with null or negative findings were missing (ESM Figs. 101, 102, 110, 117, 124, 125, 130 and 132). For insulin use and severity (ESM Fig. 111), hypertension and death/severity (ESM Fig. 118) and CVD and death (ESM Fig. 120), Egger’s tests also suggested publication bias; however, the funnel plots did not show specific patterns, only that small studies tended to be absent. In a sensitivity analysis, we calculated the 95% CIs by applying the Hartung–Knapp–Sidik–Jonkman method. In general, the findings were comparable to the results using the DerSimonian and Laird method. The few discrepancies were mainly observed for meta-analyses based on low numbers of primary studies (ESM Tables 9 and 10).
Discussion
This updated systematic review and meta-analysis included 169 studies, of which 147 were new studies, with data from more than 910,000 new participants. In total, 177 meta-analyses were conducted to provide the best available evidence on risk phenotypes in diabetes regarding COVID-19-related death and COVID-19 severity. The evidence was strengthened that male sex, older age, blood glucose level at admission, use of insulin, use of metformin (inversely), lymphocyte count at admission (inversely) and pre-existing comorbidities such as CVD, CKD and COPD are associated with worse COVID-19-related outcomes. New robust evidence emerged that COVID-19 vaccination status, obesity, higher HbA1c levels, chronic GLP-1RA use (inversely), pre-existing hypertension, heart failure, liver disease, neuropathy, cancer, the Charlson index, higher levels of CRP, IL-6, AST and LDH, and higher eGFR (inversely) are related to COVID-19-related death and/or COVID-19 severity in people with diabetes.
In this updated systematic review and meta-analysis, obesity was now identified as a risk factor for severe COVID-19 among patients with diabetes and confirmed SARS-CoV-2 infection. This is in line with findings among the general population [174] and has been confirmed in Mendelian randomisation analyses [175]. Interestingly, smoking, which has been identified as a causal risk factor for COVID-19 in the general population [176], was not clearly associated with COVID-19-related death and COVID-19 severity in populations with diabetes. We speculate that the low number of smokers among people with diabetes might explain our findings.
For diabetes-specific risk factors, such as diabetes type and duration, only a few studies are available that met our inclusion criteria. Thus, the certainty of evidence was low or very low and the estimates were very imprecise. Findings from population-based studies, including total populations of people with diabetes (but not all with confirmed SARS-CoV-2 infection), were inconsistent. For example, one study found an increased risk of COVID-19-related death for participants with type 2 diabetes compared with those with type 1 diabetes [177], whereas another study found no differences in COVID-19-related death or COVID-19 severity by type of diabetes [178]. Another study showed that both type 1 and type 2 diabetes were associated with COVID-19 severity and that the RR was similar (about threefold) for both types compared with people without diabetes [4]. For HbA1c, the association was clearer for COVID-19 severity than for COVID-19-related death, with a non-linear association for death. Population-based studies (also including people without SARS-CoV-2 infection and/or individuals without diabetes) also reported positive associations between higher HbA1c levels and COVID-19 severity [178,179,180]. In addition, among the general population, a dose–response meta-analysis showed a linear increase in risk of COVID-19 severity for blood glucose levels [181], which was also observed in our meta-analysis including only people with diabetes. High blood glucose levels could be an indicator for poorly controlled diabetes, although it is also possible that blood glucose levels at admission were high because of COVID-19 infection, reflecting stress hyperglycaemia. A recent Mendelian randomisation analysis suggested that glycaemic traits and type 2 diabetes per se do not seem to increase the risk of COVID-19 severity [182]. Beyond this, it has been speculated that there is a bidirectional association between diabetes/blood glucose levels and COVID-19 [183, 184], and long-term studies exploring this relationship are warranted.
In this update we also identified several studies on the chronic use of glucose-lowering drugs, including insulin, metformin, DPP-4 inhibitors, sulfonylurea/glinides, GLP-1RAs, SGLT2 inhibitors, thiazolidinedione and alpha-glucosidase inhibitors. There was moderate to high certainty of evidence that insulin use was associated with an increased risk and use of metformin and GLP-1RA use were associated with a decreased risk of COVID-19-related death. Use of SGLT2 inhibitors and DPP-4 inhibitors was also associated with less severe illness. As discussed in our original review, we speculate that chronic insulin use can be seen as an indicator of more severe diabetes. For the other glucose-lowering medications, the certainty of evidence was low or very low, mainly because of a serious or even very serious risk of bias, inconsistency between studies and imprecise estimates. Another meta-analysis and a nationwide population study from England (including a population with diabetes but not all with SARS-CoV-2 infection) found similar associations between the use of glucose-lowering drugs and COVID-19 severity to those found in this study [185, 186]. These studies also reported a decreased risk for use of SGLT2 inhibitors but an increased risk for DPP-4 inhibitors, which was not seen in our meta-analyses.
In accordance with findings from the general population, we identified pre-existing CVD, CKD and COPD as clear risk factors for COVID-19 severity in people with diabetes [187,188,189,190]. New evidence emerged that heart failure, liver disease and pre-existing hypertension, neuropathy and cancer are also related to a worse course of COVID-19, which was also observed among the general population [174, 191,192,193,194].
With regard to other medications (not glucose-lowering drugs), the certainty of evidence was moderate for an association between the chronic use of antithrombotic drugs and increased risk of COVID-19-related death but not COVID-19 severity. This treatment is used for CVD prevention and therefore it can be seen as an indicator of early CVD. The findings on chronic use of statins and renin inhibitors merit further discussion. Interestingly, in meta-analyses stratified by risk of bias due to confounding, we observed inverse associations between statin and renin inhibitor use and COVID-19-related death and severity for studies with a low/moderate risk of bias due to confounding and an increased risk for studies with a high risk of bias due to confounding. Effect modification by adjustment for confounding was present. Systematic reviews and meta-analyses as well as Mendelian randomisation analyses among the general population also indicated a lower risk of severe COVID-19 with chronic use of statins and renin inhibitors, supporting our findings from meta-analyses adjusted for important confounders [195,196,197,198].
We also found robust new evidence that higher levels of inflammatory biomarkers (CRP, IL-6) at admission are associated with COVID-19-related death and disease severity. In addition, markers of liver disease (AST) and kidney disease (eGFR) were also related to worse outcomes. As these markers were measured at admission, the direction of the associations is not clear, and it has also been shown that COVID-19 causes systemic inflammation and leads to liver injury [199, 200].
Overall, the findings of our updated systematic review and meta-analysis support our hypothesis that it is not diabetes alone that influences the course of COVID-19, but rather the severity of diabetes and a person’s general health status that are important predictors of COVID-19 severity.
The following study limitations need to be taken into account. First, 39% of the studies were at high risk of bias, mainly because of inadequate adjustment and selection of important confounders. However, we stratified all meta-analyses by adjustment status and the findings were robust, with some exceptions as discussed above. Second, most of the included studies did not account for treatment of COVID-19 in the hospital setting and, thus, we could not consider this aspect in our meta-analyses. Third, the findings cannot be translated to all individuals with diabetes and SARS-CoV-2 infection, as most of the studies were conducted in the hospital setting and thus included people with a more severe form of COVID-19 and not those with a mild course of the disease. Fourth, we detected high levels of heterogeneity in some of the meta-analyses. We explored the influence of risk of bias due to confounding in stratified meta-analyses and meta-regression and heterogeneity could be partly explained. However, further aspects, for example geographic location or sex, were not investigated.
In conclusion, the update of our systematic review and meta-analysis provides new evidence on risk phenotypes of diabetes and COVID-19-related death and severity of COVID-19. There is robust evidence that vaccination against COVID-19, male sex, older age, obesity, higher HbA1c levels, high blood glucose level at admission, chronic use of insulin, metformin (inversely) and GLP-1RAs (inversely), pre-existing comorbidities, including CVD, hypertension, heart failure, liver disease, CKD, neuropathy, COPD and cancer, a high comorbidity index, and high levels of CRP, IL-6, AST and LDH, a low eGFR and a low lymphocyte count at admission are all related to COVID-19-related death and COVID-19 severity among individuals with diabetes and confirmed SARS-CoV-2 infection.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ALT:
-
Alanine aminotransferase
- ARB:
-
Angiotensin II receptor blockers
- AST:
-
Aspartate aminotransferase
- CAD:
-
Coronary artery disease
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- DPP-4:
-
Dipeptidyl peptidase 4
- GLP-1RA:
-
Glucagon-like peptide-1 receptor agonist
- LDH:
-
Lactate dehydrogenase
- RAS:
-
Renin–angiotensin system
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- SGLT2:
-
Sodium–glucose cotransporter 2
- SRR:
-
Summary relative risk
References
Schlesinger S, Neuenschwander M, Lang A et al (2021) Risk phenotypes of diabetes and association with COVID-19 severity and death: a living systematic review and meta-analysis. Diabetologia 64(7):1480–1491. https://doi.org/10.1007/s00125-021-05458-8
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Abe T, Egbuche O, Igwe J et al (2021) Cardiovascular complications in COVID-19 patients with or without diabetes mellitus. Endocrinol Diabetes Metab 4(2):e00218. https://doi.org/10.1002/edm2.218
Gregory JM, Slaughter JC, Duffus SH et al (2021) COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care 44(2):526–532. https://doi.org/10.2337/dc20-2260
Mondal S, DasGupta R, Lodh M et al (2021) Predictors of new-onset diabetic ketoacidosis in patients with moderate to severe COVID-19 receiving parenteral glucocorticoids: a prospective single-centre study among Indian type 2 diabetes patients. Diabetes Metab Syndr 15(3):795–801. https://doi.org/10.1016/j.dsx.2021.03.022
Assaad M, Hekmat-Joo N, Hosry J et al (2022) Insulin use in type 2 diabetic patients: a predictive of mortality in covid-19 infection. Diabetol Metab Syndr 14(1):85. https://doi.org/10.1186/s13098-022-00857-2
Demirci I, Haymana C, Tasci I et al (2022) Higher rate of COVID-19 mortality in patients with type 1 than type 2 diabetes: a nationwide study. Endokrynol Pol 73(1):87–95. https://doi.org/10.5603/EP.a2022.0008
Myers AK, Kim TS, Zhu X, Liu Y, Qiu M, Pekmezaris R (2021) Predictors of mortality in a multiracial urban cohort of persons with type 2 diabetes and novel coronavirus 19. J Diabetes 13(5):430–438. https://doi.org/10.1111/1753-0407.13158
Strollo R, Maddaloni E, Dauriz M, Pedone C, Buzzetti R, Pozzilli P (2021) Use of DPP4 inhibitors in Italy does not correlate with diabetes prevalence among COVID-19 deaths. Diabetes Res Clin Pract 171:108444. https://doi.org/10.1016/j.diabres.2020.108444
Vasbinder A, Anderson E, Shadid H et al (2022) Inflammation, hyperglycemia, and adverse outcomes in individuals with diabetes mellitus hospitalized for COVID-19. Diabetes Care 45(3):692–700. https://doi.org/10.2337/dc21-2102
Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158(4):280–286. https://doi.org/10.7326/0003-4819-158-4-201302190-00009
Schunemann HJ, Cuello C, Akl EA et al (2019) GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol 111:105–114. https://doi.org/10.1016/j.jclinepi.2018.01.012
Acharya D, Lee K, Lee DS, Lee YS, Moon SS (2020) Mortality rate and predictors of mortality in hospitalized COVID-19 Patients with diabetes. Healthcare (Basel) 8(3):338. https://doi.org/10.3390/healthcare8030338
Agarwal S, Schechter C, Southern W, Crandall JP, Tomer Y (2020) Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care 43(10):2339–2344. https://doi.org/10.2337/dc20-1543
Aghaaliakbari F, Abbasi MA, Ranjbar M et al (2020) Angiotensin converting enzyme inhibitors, a risk factor of poor outcome in diabetic patients with COVID-19 infection. Iran J Kidney Dis 14(6):482–487
Ahmed FW, Kirresh OZ, Robinson AV et al (2021) A retrospective study assessing the effect of diabetes on mortality in patients with COVID-19 at a teaching hospital in the United Kingdom. Cureus 13(3):e13902. https://doi.org/10.7759/cureus.13902
Al Hayek AA, Robert AA, Matar AB et al (2020) Risk factors for hospital admission among COVID-19 patients with diabetes. A study from Saudi Arabia. Saudi Med J 41(10):1090–1097. https://doi.org/10.15537/smj.2020.10.25419
Alhakak A, Butt JH, Gerds TA et al (2022) Glycated haemoglobin levels among 3295 hospitalized COVID-19 patients, with and without diabetes, and risk of severe infection, admission to an intensive care unit and all-cause mortality. Diabetes Obes Metab 24(3):499–510. https://doi.org/10.1111/dom.14604
Alrashed AA, Khan TM, Alhusseini NK et al (2021) Severity of COVID-19 infection in ACEI/ARB users in specialty hospitals: a retrospective cohort study. J Infect Public Health 14(6):726–733. https://doi.org/10.1016/j.jiph.2021.03.004
Aon M, Alsaeedi A, Alzafiri A et al (2022) Stress hyperglycemia ratio as a prognostic marker in diabetic patients hospitalized with COVID-19. Infect Dis Rep 14(5):675–685. https://doi.org/10.3390/idr14050073
Barrett CE, Park J, Kompaniyets L et al (2021) Intensive care unit admission, mechanical ventilation, and mortality among patients with type 1 diabetes hospitalized for COVID-19 in the U.S. Diabetes Care 44(8):1788–1796. https://doi.org/10.2337/dc21-060410.2337/dc21-0604
Bello-Chavolla OY, Bahena-Lopez JP, Antonio-Villa NE et al (2020) Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab 105(8):dgaa346. https://doi.org/10.1210/clinem/dgaa346
Borzouei S, Mohammadian-Khoshnoud M, Omidi T et al (2021) Predictors of COVID-19 related death in diabetes patients: a case-control study in Iran. Diabetes Metab Syndr 15(4):102149. https://doi.org/10.1016/j.dsx.2021.05.022
Boye KS, Tokar Erdemir E, Zimmerman N et al (2021) Risk factors associated with COVID-19 hospitalization and mortality: a large claims-based analysis among people with type 2 diabetes mellitus in the United States. Diabetes Ther 12(8):2223–2239. https://doi.org/10.1007/s13300-021-01110-1
Calapod OP, Marin AM, Onisai M, Tribus LC, Pop CS, Fierbinteanu-Braticevici C (2021) The impact of increased Fib-4 score in patients with type II diabetes mellitus on Covid-19 disease prognosis. Medicina (Kaunas) 57(5):434. https://doi.org/10.3390/medicina57050434
Cao P, Song Y, Zhuang Z et al (2021) Obesity and COVID-19 in adult patients with diabetes. Diabetes 70(5):1061–1069. https://doi.org/10.2337/db20-0671
Cariou B, Goronflot T, Rimbert A et al (2021) Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab 47(2):101202. https://doi.org/10.1016/j.diabet.2020.10.001
Cariou B, Hadjadj S, Wargny M et al (2020) Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 63(8):1500–1515. https://doi.org/10.1007/s00125-020-05180-x
Chai C, Chen K, Li S et al (2022) Effect of elevated fasting blood glucose level on the 1-year mortality and sequelae in hospitalized COVID-19 patients: a bidirectional cohort study. J Med Virol 94(7):3240–3250. https://doi.org/10.1002/jmv.27737
Charoenngam N, Alexanian SM, Apovian CM, Holick MF (2021) Association between hyperglycemia at hospital presentation and hospital outcomes in COVID-19 patients with and without type 2 diabetes: a retrospective cohort study of hospitalized inner-city COVID-19 patients. Nutrients 13(7):2199. https://doi.org/10.3390/nu13072199
Chen J, Zhao C, Huang Y et al (2021) Malnutrition predicts poor outcomes in diabetic COVID-19 patients in Huangshi, Hubei. J Biomed Res 36(1):32–38. https://doi.org/10.7555/JBR.35.20210083
Chen X, Chen Y, Wu C et al (2020) Coagulopathy is a major extrapulmonary risk factor for mortality in hospitalized patients with COVID-19 with type 2 diabetes. BMJ Open Diabetes Res Care 8(2):e001851. https://doi.org/10.1136/bmjdrc-2020-001851
Chen Y, Yang D, Cheng B et al (2020) Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care 43(7):1399–1407. https://doi.org/10.2337/dc20-0660
Cheng Y, Yue L, Wang Z, Zhang J, Xiang G (2021) Hyperglycemia associated with lymphopenia and disease severity of COVID-19 in type 2 diabetes mellitus. J Diabetes Complications 35(2):107809. https://doi.org/10.1016/j.jdiacomp.2020.107809
Choi HK, Koo H-J, Seok H et al (2020) ARB/ACEI use and severe COVID-19: a nationwide case-control study. medRxiv: 2020.2006.2012.20129916. https://doi.org/10.1101/2020.06.12.20129916
Chung SM, Lee YY, Ha E et al (2020) The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab J 44(3):405–413. https://doi.org/10.4093/dmj.2020.0105
Corcillo A, Cohen S, Li A, Crane J, Kariyawasam D, Karalliedde J (2021) Diabetic retinopathy is independently associated with increased risk of intubation: a single centre cohort study of patients with diabetes hospitalised with COVID-19. Diabetes Res Clin Pract 171:108529. https://doi.org/10.1016/j.diabres.2020.108529
Crouse AB, Grimes T, Li P, Might M, Ovalle F, Shalev A (2020) Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front Endocrinol (Lausanne) 11:600439. https://doi.org/10.3389/fendo.2020.600439
Dalan R, Ang LW, Tan WYT et al (2021) The association of hypertension and diabetes pharmacotherapy with COVID-19 severity and immune signatures: an observational study. Eur Heart J Cardiovasc Pharmacother 7(3):e48–e51. https://doi.org/10.1093/ehjcvp/pvaa098
de Abajo FJ, Rodriguez-Martin S, Lerma V et al (2020) Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 395(10238):1705–1714. https://doi.org/10.1016/S0140-6736(20)31030-8
de Miguel-Yanes JM, Jimenez-Garcia R, de Miguel-Diez J et al (2022) Impact of type 2 diabetes mellitus on the incidence and outcomes of COVID-19 needing hospital admission according to sex: retrospective cohort study using hospital discharge data in Spain, year 2020. J Clin Med 11(9):2654. https://doi.org/10.3390/jcm11092654
Djuric O, Ottone M, Vicentini M et al (2022) Diabetes and COVID-19 testing, positivity, and mortality: a population-wide study in Northern Italy. Diabetes Res Clin Pract 191:110051. https://doi.org/10.1016/j.diabres.2022.110051
Do JY, Kim SW, Park JW, Cho KH, Kang SH (2021) Is there an association between metformin use and clinical outcomes in diabetes patients with COVID-19? Diabetes Metab 47(4):101208. https://doi.org/10.1016/j.diabet.2020.10.006
Duan W, Li L, Li X et al (2022) Association of blood glucose level and prognosis of inpatients with coexistent diabetes and COVID-19. Endocrine 75(1):1–9. https://doi.org/10.1007/s12020-021-02923-7
Elibol A, Eren D, Erdogan MD et al (2021) Factors influencing on development of COVID-19 pneumonia and association with oral anti-diabetic drugs in hospitalized patients with diabetes mellitus. Prim Care Diabetes 15(5):806–812. https://doi.org/10.1016/j.pcd.2021.08.001
Emami A, Akbari A, Basirat A et al (2021) The role of comorbidities on mortality of COVID-19 in patients with diabetes. Obes Med 25:100352. https://doi.org/10.1016/j.obmed.2021.100352
Emral R, Haymana C, Demirci I et al (2021) Lower COVID-19 mortality in patients with type 2 diabetes mellitus taking dipeptidyl peptidase-4 inhibitors: results from a Turkish nationwide study. Diabetes Ther 12(11):2857–2870. https://doi.org/10.1007/s13300-021-01133-8
Erol RS, Sen EC, Ozturk FY et al (2022) Does DPP-4 inhibitor treatment affect the clinical outcomes of COVID-19 in type 2 diabetes mellitus patients? North Clin Istanb 9(3):207–214. https://doi.org/10.14744/nci.2022.34341
Fernández-Pombo A, Santamaria-Nieto A, Rodríguez-Carnero G et al (2021) Factores predictores de formas graves de COVID-19 que requieren hospitalización en pacientes con diabetes mellitus. Galicia Clínica 82(3) [article in Spanish]. https://doi.org/10.22546/62/2561
Ferrannini G, Lund LH, Benson L et al (2022) Association between use of novel glucose-lowering drugs and COVID-19 hospitalization and death in patients with type 2 diabetes: a nationwide registry analysis. Eur Heart J Cardiovasc Pharmacother 9(1):10–17. https://doi.org/10.1093/ehjcvp/pvac044
Fox T, Ruddiman K, Lo KB et al (2021) The relationship between diabetes and clinical outcomes in COVID-19: a single-center retrospective analysis. Acta Diabetol 58(1):33–38. https://doi.org/10.1007/s00592-020-01592-8
Fu Y, Hu L, Ren HW et al (2022) Prognostic factors for COVID-19 hospitalized patients with preexisting type 2 diabetes. Int J Endocrinol 2022:9322332. https://doi.org/10.1155/2022/9322332
Fukushima T, Chubachi S, Namkoong H et al (2023) Clinical significance of prediabetes, undiagnosed diabetes and diagnosed diabetes on critical outcomes in COVID-19: integrative analysis from the Japan COVID-19 task force. Diabetes Obes Metab 25(1):144–155. https://doi.org/10.1111/dom.14857
Ghany R, Palacio A, Dawkins E et al (2021) Metformin is associated with lower hospitalizations, mortality and severe coronavirus infection among elderly medicare minority patients in 8 states in USA. Diabetes Metab Syndr 15(2):513–518. https://doi.org/10.1016/j.dsx.2021.02.022
Giorda CB, Picariello R, Tartaglino B et al (2021) From swab testing to health outcomes within the T2DM population: impact of diabetes background on COVID19 progression. Diabetes Res Clin Pract 180:109021. https://doi.org/10.1016/j.diabres.2021.109021
Hadjadj S, Saulnier PJ, Ruan Y et al (2023) Associations of microvascular complications with all-cause death in patients with diabetes and COVID-19: the CORONADO, ABCD COVID-19 UK national audit and AMERICADO study groups. Diabetes Obes Metab 25(1):78–88. https://doi.org/10.1111/dom.14845
Hammad MO, Alseoudy MM (2021) The sex-related discrepancy in laboratory parameters of severe COVID-19 patients with diabetes: a retrospective cohort study. Prim Care Diabetes 15(4):713–718. https://doi.org/10.1016/j.pcd.2021.05.002
Harris S, Ruan Y, Wild SH et al (2022) Association of statin and/or renin-angiotensin-aldosterone system modulating therapy with mortality in adults with diabetes admitted to hospital with COVID-19: a retrospective multicentre European study. Diabetes Metab Syndr 16(5):102484. https://doi.org/10.1016/j.dsx.2022.102484
Heald AH, Jenkins DA, Williams R et al (2022) The risk factors potentially influencing hospital admission in people with diabetes, following SARS-CoV-2 infection: a population-level analysis. Diabetes Ther 13(5):1007–1021. https://doi.org/10.1007/s13300-022-01230-2
Huang J, Zhu L, Bai X et al (2020) Multidimensional analysis of risk factors for the severity and mortality of patients with COVID-19 and diabetes. Infect Dis Ther 9(4):981–1002. https://doi.org/10.1007/s40121-020-00359-6
Hui Y, Li Y, Tong X et al (2020) The risk factors for mortality of diabetic patients with severe COVID-19: a retrospective study of 167 severe COVID-19 cases in Wuhan. PLoS One 15(12):e0243602. https://doi.org/10.1371/journal.pone.0243602
Ikram AS, Pillay S (2021) Hyperglycaemia, diabetes mellitus and COVID-19 in a tertiary hospital in KwaZulu-Natal. J Endocrinol Metab Diabetes South Africa 27(1):32–41. https://doi.org/10.1080/16089677.2021.1997427
Iqbal A, Greig M, Arshad MF, Julian TH, Ee Tan S, Elliott J (2021) Higher admission activated partial thromboplastin time, neutrophil-lymphocyte ratio, serum sodium, and anticoagulant use predict in-hospital COVID-19 mortality in people with diabetes: findings from two university hospitals in the U.K. Diabetes Res Clin Pract 178:108955. https://doi.org/10.1016/j.diabres.2021.108955
Izzi-Engbeaya C, Distaso W, Amin A et al (2021) Adverse outcomes in COVID-19 and diabetes: a retrospective cohort study from three London teaching hospitals. BMJ Open Diabetes Res Care 9(1):e001858. https://doi.org/10.1136/bmjdrc-2020-001858
Jayaswal SK, Singh S, Malik PS et al (2021) Detrimental effect of diabetes and hypertension on the severity and mortality of COVID-19 infection: a multi-center case-control study from India. Diabetes Metab Syndr 15(5):102248. https://doi.org/10.1016/j.dsx.2021.102248
Kabootari M, Habibi Tirtashi R, Hasheminia M et al (2022) Clinical features, risk factors and a prediction model for in-hospital mortality among diabetic patients infected with COVID-19: data from a referral centre in Iran. Public Health 202:84–92. https://doi.org/10.1016/j.puhe.2021.11.007
Kang IS, Kong KA (2021) Body mass index and severity/fatality from coronavirus disease 2019: a nationwide epidemiological study in Korea. PLoS One 16(6):e0253640. https://doi.org/10.1371/journal.pone.0253640
Khalili S, Moradi O, Kharazmi AB, Raoufi M, Sistanizad M, Shariat M (2021) Comparison of mortality rate and severity of pulmonary involvement in coronavirus disease-2019 adult patients with and without type 2 diabetes: a cohort study. Can J Diabetes 45(6):524–530. https://doi.org/10.1016/j.jcjd.2020.10.014
Khalili S, Sabaghian T, Sedaghat M, Soroureddin Z, Askari E, Khalili N (2021) Prevalence, risk factors and outcomes associated with acute kidney injury in patients hospitalized for COVID-19: a comparative study between diabetic and nondiabetic patients. J Diabetes Res 2021:6666086. https://doi.org/10.1155/2021/6666086
Khunti K, Ruan Y, Davies J et al (2022) Association between SGLT2 inhibitor treatment and diabetic ketoacidosis and mortality in people with type 2 diabetes admitted to hospital with COVID-19. Diabetes Care. https://doi.org/10.2337/dc22-0357
Kim MK, Jeon JH, Kim SW et al (2020) The clinical characteristics and outcomes of patients with moderate-to-severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab J 44(4):602–613. https://doi.org/10.4093/dmj.2020.0146
Kristan MM, Kim YK, Nelson T et al (2021) Predictors of severe COVID-19 in patients with diabetes: a multicenter review. Endocr Pract 27(8):842–849. https://doi.org/10.1016/j.eprac.2021.05.011
Lalau JD, Al-Salameh A, Hadjadj S et al (2021) Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19. Diabetes Metab 47(5):101216. https://doi.org/10.1016/j.diabet.2020.101216
Lampasona V, Secchi M, Scavini M et al (2020) Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia 63(12):2548–2558. https://doi.org/10.1007/s00125-020-05284-4
Laurenzi A, Caretto A, Molinari C et al (2021) Pre-existing diabetes and COVID-associated hyperglycaemia in patients with COVID-19 pneumonia. Biology (Basel) 10(8):754. https://doi.org/10.3390/biology10080754
Lee HY, Ahn J, Park J et al (2021) Beneficial effect of statins in COVID-19-related outcomes-brief report: a national population-based cohort study. Arterioscler Thromb Vasc Biol 41(3):e175–e182. https://doi.org/10.1161/ATVBAHA.120.315551
Lei M, Lin K, Pi Y et al (2020) Clinical features and risk factors of ICU admission for COVID-19 patients with diabetes. J Diabetes Res 2020:5237840. https://doi.org/10.1155/2020/5237840
Leon-Pedroza JI, Rodriguez-Cortes O, Flores-Mejia R, Gaona-Aguas CV, Gonzalez-Chavez A (2021) Impact of metabolic syndrome in the clinical outcome of disease by SARS-COV-2. Arch Med Res 52(7):738–745. https://doi.org/10.1016/j.arcmed.2021.04.001
Li J, Wei Q, Li WX et al (2020) Metformin use in diabetes prior to hospitalization: effects on mortality in Covid-19. Endocr Pract 26(10):1166–1172. https://doi.org/10.4158/EP-2020-0466
Li Y, Han X, Alwalid O et al (2020) Baseline characteristics and risk factors for short-term outcomes in 132 COVID-19 patients with diabetes in Wuhan China: a retrospective study. Diabetes Res Clin Pract 166:108299. https://doi.org/10.1016/j.diabres.2020.108299
Liu G, Zhang S, Hu H, Liu T, Huang J (2020) The role of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio in the prognosis of type 2 diabetics with COVID-19. Scott Med J 65(4):154–160. https://doi.org/10.1177/0036933020953516
Liu Z, Bai X, Han X et al (2020) The association of diabetes and the prognosis of COVID-19 patients: a retrospective study. Diabetes Res Clin Pract 169:108386. https://doi.org/10.1016/j.diabres.2020.108386
Llanera DK, Wilmington R, Shoo H et al (2021) Clinical characteristics of COVID-19 patients in a regional population with diabetes mellitus: the ACCREDIT study. Front Endocrinol (Lausanne) 12:777130. https://doi.org/10.3389/fendo.2021.777130
Llaurado G, Vlacho B, Wargny M et al (2022) The association between macrovascular complications and intensive care admission, invasive mechanical ventilation, and mortality in people with diabetes hospitalized for coronavirus disease-2019 (COVID-19). Cardiovasc Diabetol 21(1):216. https://doi.org/10.1186/s12933-022-01657-8
Lombardi A, Agarwal S, Schechter C, Tomer Y (2022) In-hospital hyperglycemia is associated with worse outcomes in patients admitted with COVID-19. Diabetes Care 45(11):2683–2688. https://doi.org/10.2337/dc22-0708
Longmore DK, Miller JE, Bekkering S et al (2021) Diabetes and overweight/obesity are independent, nonadditive risk factors for in-hospital severity of COVID-19: an international, multicenter retrospective meta-analysis. Diabetes Care 44(6):1281–1290. https://doi.org/10.2337/dc20-2676
Lopez-Huamanrayme E, Garate-Chirinos DD, Espinoza-Morales F et al (2021) Association between hyperglycemia treatment and mortality in patients with diabetes and COVID-19 in a Peruvian hospital: a retrospective cohort study. J Clin Transl Endocrinol 26:100265. https://doi.org/10.1016/j.jcte.2021.100265
Luk AOY, Yip TCF, Zhang X et al (2021) Glucose-lowering drugs and outcome from COVID-19 among patients with type 2 diabetes mellitus: a population-wide analysis in Hong Kong. BMJ Open 11(10):e052310. https://doi.org/10.1136/bmjopen-2021-052310
Ma Z, Patel N, Vemparala P, Krishnamurthy M (2022) Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci Rep 12(1):5553. https://doi.org/10.1038/s41598-022-09639-2
Madaschi S, Resmini E, Bonfadini S et al (2022) Predictive markers for clinical outcomes in a cohort of diabetic patients hospitalized for COVID-19. Diabetol Metab Syndr 14(1):168. https://doi.org/10.1186/s13098-022-00941-7
Mannucci F, Vitturi G, Benacchio L et al (2022) Infection rates and impact of glucose lowering medications on the clinical course of COVID-19 in people with type 2 diabetes: a retrospective observational study. Diabetes Metab Syndr Obes 15:3093–3101. https://doi.org/10.2147/DMSO.S385646
Marimuthu Y, Kunnavil R, Satyanarayana N et al (2022) Clinical presentation and mortality risk factors for COVID-19 among diabetic patients in a tertiary care center in South India. Indian J Tuberc 69(4):496–502. https://doi.org/10.1016/j.ijtb.2021.08.015
Mehta PB, Kohn MA, Koliwad SK, Rushakoff RJ (2021) Lack of association between either outpatient or inpatient glycemic control and COVID-19 illness severity or mortality in patients with diabetes. BMJ Open Diabetes Res Care 9(1):e002203. https://doi.org/10.1136/bmjdrc-2021-002203
Meijer RI, Hoekstra T, van den Oever NCG et al (2021) Treatment with a DPP-4 inhibitor at time of hospital admission for COVID-19 is not associated with improved clinical outcomes: data from the COVID-PREDICT cohort study in The Netherlands. J Diabetes Metab Disord 20(2):1155–1160. https://doi.org/10.1007/s40200-021-00833-z
Merzon E, Green I, Shpigelman M et al (2021) Haemoglobin A1c is a predictor of COVID-19 severity in patients with diabetes. Diabetes Metab Res Rev 37(5):e3398. https://doi.org/10.1002/dmrr.3398
Mirani M, Favacchio G, Carrone F et al (2020) Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID-19: a case series from an academic hospital in Lombardy, Italy. Diabetes Care 43(12):3042–3049. https://doi.org/10.2337/dc20-1340
Mohamed M, Gouda TM, Hanafy AS et al (2021) Clinical, laboratory and radiological predictors of unfavorable hospital admission course for diabetic patients with COVID-19. Egypt J Hosp Med 83(1):1321–1329. https://doi.org/10.21608/ejhm.2021.165528
Mondal S, DasGupta R, Lodh M et al (2022) Stress hyperglycemia ratio, rather than admission blood glucose, predicts in-hospital mortality and adverse outcomes in moderate-to severe COVID-19 patients, irrespective of pre-existing glycemic status. Diabetes Res Clin Pract 190:109974. https://doi.org/10.1016/j.diabres.2022.109974
Morse J, Gay W, Korwek KM et al (2021) Hyperglycaemia increases mortality risk in non-diabetic patients with COVID-19 even more than in diabetic patients. Endocrinol Diabetes Metab 4(4):e00291. https://doi.org/10.1002/edm2.291
Nikniaz Z, Somi MH, Dinevari MF, Taghizadieh A, Mokhtari L (2021) Diabesity associates with poor COVID-19 outcomes among hospitalized patients. J Obes Metab Syndr 30(2):149–154. https://doi.org/10.7570/jomes20121
Numaguchi R, Kurajoh M, Hiura Y et al (2022) Glycated hemoglobin level on admission associated with progression to severe disease in hospitalized patients with non-severe coronavirus disease 2019. J Diabetes Investig 13(10):1779–1787. https://doi.org/10.1111/jdi.13845
Nyland JE, Raja-Khan NT, Bettermann K et al (2021) Diabetes, drug treatment, and mortality in COVID-19: a multinational retrospective cohort study. Diabetes 70(12):2903–2916. https://doi.org/10.2337/db21-0385
Oh TK, Song IA (2021) Metformin use and risk of COVID-19 among patients with type II diabetes mellitus: an NHIS-COVID-19 database cohort study. Acta Diabetol 58(6):771–778. https://doi.org/10.1007/s00592-020-01666-7
Ojeda-Fernandez L, Foresta A, Macaluso G et al (2022) Metformin use is associated with a decrease in the risk of hospitalization and mortality in COVID-19 patients with diabetes: a population-based study in Lombardy. Diabetes Obes Metab 24(5):891–898. https://doi.org/10.1111/dom.14648
O’Malley G, Ebekozien O, Desimone M et al (2021) COVID-19 Hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab 106(2):e936–e942. https://doi.org/10.1210/clinem/dgaa825
Orioli L, Servais T, Belkhir L et al (2021) Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: a retrospective study from an academic center in Belgium. Diabetes Metab Syndr 15(1):149–157. https://doi.org/10.1016/j.dsx.2020.12.020
Ortega E, Corcoy R, Gratacos M et al (2021) Risk factors for severe outcomes in people with diabetes hospitalised for COVID-19: a cross-sectional database study. BMJ Open 11(7):e051237. https://doi.org/10.1136/bmjopen-2021-051237
Ouchi D, Vilaplana-Carnerero C, de Dios V, Giner-Soriano M, Morros R (2022) Antidiabetic treatment and COVID-19 Outcomes: a population-based cohort study in primary health care in Catalonia during the first wave of the pandemic. Prim Care Diabetes 16(6):753–759. https://doi.org/10.1016/j.pcd.2022.10.001
Palazzuoli A, Mancone M, De Ferrari GM et al (2020) Antecedent administration of angiotensin-converting enzyme inhibitors or angiotensin II receptor antagonists and survival after hospitalization for COVID-19 syndrome. J Am Heart Assoc 9(22):e017364. https://doi.org/10.1161/JAHA.120.017364
Patel AJ, Klek SP, Peragallo-Dittko V et al (2021) Correlation of hemoglobin A1C and outcomes in patients hospitalized with COVID-19. Endocr Pract 27(10):1046–1051. https://doi.org/10.1016/j.eprac.2021.07.008
Pazoki M, Chichagi F, Hadadi A et al (2021) Association of clinical characteristics, antidiabetic and cardiovascular agents with diabetes mellitus and COVID-19: a 7-month follow-up cohort study. J Diabetes Metab Disord 20(2):1545–1555. https://doi.org/10.1007/s40200-021-00901-4
Pazoki M, Keykhaei M, Kafan S et al (2021) Risk indicators associated with in-hospital mortality and severity in patients with diabetes mellitus and confirmed or clinically suspected COVID-19. J Diabetes Metab Disord 20(1):59–69. https://doi.org/10.1007/s40200-020-00701-2
Perez-Belmonte LM, Torres-Pena JD, Lopez-Carmona MD et al (2020) Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med 18(1):359. https://doi.org/10.1186/s12916-020-01832-2
Petrakis V, Panagopoulos P, Trypsianis G, Papazoglou D, Papanas N (2021) Glucose on admission: unfavourable effects on hospitalisation and outcomes in type 2 diabetes mellitus patients with COVID-19 pneumonia. Exp Clin Endocrinol Diabetes 130(08):561–562. https://doi.org/10.1055/a-1686-8738
Pettrone K, Burnett E, Link-Gelles R et al (2021) Characteristics and risk factors of hospitalized and nonhospitalized COVID-19 patients, Atlanta, Georgia, USA, March-April 2020. Emerg Infect Dis 27(4):1164–1168. https://doi.org/10.3201/eid2704.204709
Phan F, Boussouar S, Lucidarme O et al (2021) Cardiac adipose tissue volume and IL-6 level at admission are complementary predictors of severity and short-term mortality in COVID-19 diabetic patients. Cardiovasc Diabetol 20(1):165. https://doi.org/10.1186/s12933-021-01327-1
Pulido-Perez P, Pondigo-de Los Angeles JA, Hernandez-Hernandez ME, Torres-Rasgado E, Romero JR (2021) Renal function, serum magnesium levels and mortality in COVID-19 patients with type 2 diabetes. Magnes Res 34(1):20–31. https://doi.org/10.1684/mrh.2021.0481
Ramesh J, Rajesh M, Varghese J, Reddy SLS (2021) Calculated plasma osmolality at hospital admission correlates well with eGFR and D-Dimer, a simple outcome predictor and guiding tool for management of severe COVID-19 patients. Diabetes Metab Syndr 15(5):102240. https://doi.org/10.1016/j.dsx.2021.102240
Ramos-Rincon JM, Perez-Belmonte LM, Carrasco-Sanchez FJ et al (2021) Cardiometabolic therapy and mortality in very old patients with diabetes hospitalized due to COVID-19. J Gerontol A Biol Sci Med Sci 76(8):e102–e109. https://doi.org/10.1093/gerona/glab124
Rastad H, Ejtahed HS, Mahdavi-Ghorabi A et al (2020) Factors associated with the poor outcomes in diabetic patients with COVID-19. J Diabetes Metab Disord 19(2):1293–1302. https://doi.org/10.1007/s40200-020-00646-6
Rastad H, Karim H, Ejtahed HS et al (2020) Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabetol Metab Syndr 12:57. https://doi.org/10.1186/s13098-020-00565-9
Rezaei N, Montazeri F, Malekpour MR et al (2021) COVID-19 in patients with diabetes: factors associated with worse outcomes. J Diabetes Metab Disord 20(2):1605–1614. https://doi.org/10.1007/s40200-021-00910-3
Rhee SY, Lee J, Nam H, Kyoung DS, Shin DW, Kim DJ (2021) Effects of a DPP-4 Inhibitor and RAS blockade on clinical outcomes of patients with diabetes and COVID-19. Diabetes Metab J 45(2):251–259. https://doi.org/10.4093/dmj.2020.0206
Riahi S, Sombra LRS, Lo KB et al (2021) Insulin use, diabetes control, and outcomes in patients with COVID-19. Endocr Res 46(2):45–50. https://doi.org/10.1080/07435800.2020.1856865
Roussel R, Darmon P, Pichelin M et al (2021) Use of dipeptidyl peptidase-4 inhibitors and prognosis of COVID-19 in hospitalized patients with type 2 diabetes: a propensity score analysis from the CORONADO study. Diabetes Obes Metab 23(5):1162–1172. https://doi.org/10.1111/dom.14324
Ruan Y, Ryder REJ, De P et al (2021) A UK nationwide study of people with type 1 diabetes admitted to hospital with COVID-19 infection. Diabetologia 64(8):1717–1724. https://doi.org/10.1007/s00125-021-05463-x
Sadidi M, Zare A, Nasrollahzadehsabet M, Dastan F, Mosadegh Khah A, Jafari Asheyani M (2022) The roles of dipeptidyl peptidase-4 inhibitors in prognosis of COVID-19 infection in patients with type 2 diabetes mellitus. J Res Med Sci 27:62. https://doi.org/10.4103/jrms.jrms_71_22
Sarigumba M, Aragon J, Kanapi MP (2021) Baseline glycemic status and outcome of persons with type 2 diabetes with COVID-19 infections: a single-center retrospective study. J ASEAN Fed Endocr Soc 36(1):45–49. https://doi.org/10.15605/jafes.036.01.06
Satman I, Demirci I, Haymana C et al (2021) Unexpectedly lower mortality rates in COVID-19 patients with and without type 2 diabetes in Istanbul. Diabetes Res Clin Pract 174:108753. https://doi.org/10.1016/j.diabres.2021.108753
Savarese G, Benson L, Sundstrom J, Lund LH (2021) Association between renin-angiotensin-aldosterone system inhibitor use and COVID-19 hospitalization and death: a 1.4 million patient nationwide registry analysis. Eur J Heart Fail 23(3):476–485. https://doi.org/10.1002/ejhf.206010.1002/ejhf.2060
Saygili ES, Karakilic E, Mert E, Sener A, Mirci A (2022) Preadmission usage of metformin and mortality in COVID-19 patients including the post-discharge period. Ir J Med Sci 191(2):569–575. https://doi.org/10.1007/s11845-021-02823-9
Seiglie J, Platt J, Cromer SJ et al (2020) Diabetes as a risk factor for poor early outcomes in patients hospitalized with COVID-19. Diabetes Care 43(12):2938–2944. https://doi.org/10.2337/dc20-1506
Shah P, Owens J, Franklin J, Jani Y, Kumar A, Doshi R (2020) Baseline use of angiotensin-converting enzyme inhibitor/AT1 blocker and outcomes in hospitalized coronavirus disease 2019 African-American patients. J Hypertens 38(12):2537–2541. https://doi.org/10.1097/HJH.0000000000002584
Shang J, Wang Q, Zhang H et al (2021) The relationship between diabetes mellitus and COVID-19 prognosis: a retrospective cohort study in Wuhan. China. Am J Med 134(1):e6–e14. https://doi.org/10.1016/j.amjmed.2020.05.033
Shang R, Liu H, Ni J (2021) Evaluation of prognostic indicators for COVID⁃19 patients with diabetes. Med J Wuhan Univ 42(5):694–697
Shauly-Aharonov M, Shafrir A, Paltiel O et al (2021) Both high and low pre-infection glucose levels associated with increased risk for severe COVID-19: new insights from a population-based study. PLoS One 16(7):e0254847. https://doi.org/10.1371/journal.pone.0254847
Shestakova MV, Vikulova OK, Elfimova AR, Deviatkin AA, Dedov II, Mokrysheva NG (2022) Risk factors for COVID-19 case fatality rate in people with type 1 and type 2 diabetes mellitus: a nationwide retrospective cohort study of 235,248 patients in the Russian Federation. Front Endocrinol (Lausanne) 13:909874. https://doi.org/10.3389/fendo.2022.909874
Shi Q, Zhang X, Jiang F et al (2020) Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care 43(7):1382–1391. https://doi.org/10.2337/dc20-0598
Shukla AP, Tchang BG, Lam T et al (2021) Preadmission predictors of severe COVID-19 in patients with diabetes mellitus. J Diabetes Complications 35(8):107967. https://doi.org/10.1016/j.jdiacomp.2021.107967
Silverii GA, Monami M, Cernigliaro A et al (2021) Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr Metab Cardiovasc Dis 31(2):396–398. https://doi.org/10.1016/j.numecd.2020.09.028
Smati S, Tramunt B, Wargny M et al (2021) Relationship between obesity and severe COVID-19 outcomes in patients with type 2 diabetes: results from the CORONADO study. Diabetes Obes Metab 23(2):391–403. https://doi.org/10.1111/dom.14228
Solerte SB, D’Addio F, Trevisan R et al (2020) Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care 43(12):2999–3006. https://doi.org/10.2337/dc20-1521
Soliman AR, Abdelaziz TS, Fathy A (2021) Impact of vitamin D therapy on the progress COVID-19: six weeks follow-up study of vitamin D deficient elderly diabetes patients. Proc Singapore Healthc. https://doi.org/10.1177/20101058211041405
Sonmez A, Demirci I, Haymana C et al (2021) Clinical characteristics and outcomes of COVID-19 in patients with type 2 diabetes in Turkey: a nationwide study (TurCoviDia). J Diabetes 13(7):585–595. https://doi.org/10.1111/1753-0407.13171
Souza SM, Quintao APC, Soares MCB et al (2022) Survival of patients with diabetes mellitus hospitalized for acute respiratory syndrome due to COVID-19. Rev Inst Med Trop Sao Paulo 64:e74. https://doi.org/10.1590/S1678-9946202264074
Stevens JS, Bogun MM, McMahon DJ et al (2021) Diabetic ketoacidosis and mortality in COVID-19 infection. Diabetes Metab 47(6):101267. https://doi.org/10.1016/j.diabet.2021.101267
Tallon EM, Ebekozien O, Sanchez J et al (2022) Impact of diabetes status and related factors on COVID-19-associated hospitalization: a nationwide retrospective cohort study of 116,370 adults with SARS-CoV-2 infection. Diabetes Res Clin Pract 194:110156. https://doi.org/10.1016/j.diabres.2022.110156
Tamura RE, Said SM, de Freitas LM, Rubio IGS (2021) Outcome and death risk of diabetes patients with Covid-19 receiving pre-hospital and in-hospital metformin therapies. Diabetol Metab Syndr 13(1):76. https://doi.org/10.1186/s13098-021-00695-8
Tian Z, Heald AH, Stedman M et al (2021) Age of people with type 2 diabetes and the risk of dying following SARS-CoV-2 infection. Int J Clin Pract 75(8):e14053. https://doi.org/10.1111/ijcp.14053
Tramunt B, Smati S, Coudol S et al (2021) Sex disparities in COVID-19 outcomes of inpatients with diabetes: insights from the CORONADO study. Eur J Endocrinol 185(2):299–311. https://doi.org/10.1530/EJE-21-0068
Tuan WJ, Lennon RP, Zhang A, Macherla A, Zgierska AE (2022) Risks of severe COVID-19 outcomes among patients with diabetic polyneuropathy in the United States. J Public Health Manag Pract 28(6):674–681. https://doi.org/10.1097/PHH.0000000000001587
Valle A, Rodriguez J, Camina F, Martinez-Olmos MA, Ortola JB, Rodriguez-Segade S (2022) At-admission HbA(1c) levels in hospitalized COVID-19 participants with and without known diabetes. Clin Chim Acta 532:188–192. https://doi.org/10.1016/j.cca.2022.05.027
Vargas-Vazquez A, Bello-Chavolla OY, Ortiz-Brizuela E et al (2021) Impact of undiagnosed type 2 diabetes and pre-diabetes on severity and mortality for SARS-CoV-2 infection. BMJ Open Diabetes Res Care 9(1):e002026. https://doi.org/10.1136/bmjdrc-2020-002026
Wander PL, Lowy E, Beste LA et al (2021) Prior glucose-lowering medication use and 30-day outcomes among 64,892 veterans with diabetes and COVID-19. Diabetes Care 44(12):2708–2713. https://doi.org/10.2337/dc21-1351
Wang B, Glicksberg BS, Nadkarni GN, Vashishth D (2021) Evaluation and management of COVID-19-related severity in people with type 2 diabetes. BMJ Open Diabetes Res Care 9(1):e002299. https://doi.org/10.1136/bmjdrc-2021-002299
Wang X, Liu Z, Li J et al (2020) Impacts of type 2 diabetes on disease severity, therapeutic effect, and mortality of patients with COVID-19. J Clin Endocrinol Metab 105(12):dgaa535. https://doi.org/10.1210/clinem/dgaa535
Wang Y, Zhang J, Li H et al (2021) Prognostic value of leucocyte to high-density lipoprotein-cholesterol ratios in COVID-19 patients and the diabetes subgroup. Front Endocrinol (Lausanne) 12:727419. https://doi.org/10.3389/fendo.2021.727419
Wargny M, Potier L, Gourdy P et al (2021) Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia 64(4):778–794. https://doi.org/10.1007/s00125-020-05351-w
Wong CKH, Lui DTW, Lui AYC et al (2022) Use of DPP4i reduced odds of clinical deterioration and hyperinflammatory syndrome in COVID-19 patients with type 2 diabetes: propensity score analysis of a territory-wide cohort in Hong Kong. Diabetes Metab 48(1):101307. https://doi.org/10.1016/j.diabet.2021.101307
Wong CKH, Lui DTW, Lui AYC et al (2022) Metformin use in relation to clinical outcomes and hyperinflammatory syndrome among COVID-19 patients with type 2 diabetes: a propensity score analysis of a territory-wide cohort. Front Endocrinol (Lausanne) 13:810914. https://doi.org/10.3389/fendo.2022.810914
Wong R, Hall M, Vaddavalli R et al (2022) Glycemic control and clinical outcomes in U.S. patients with COVID-19: data from the National COVID Cohort Collaborative (N3C) Database. Diabetes Care 45(5):1099–1106. https://doi.org/10.2337/dc21-218610.2337/dc21-2186
Wu B, Zhou JH, Wang WX et al (2021) Association analysis of hyperlipidemia with the 28-day all-cause mortality of COVID-19 in hospitalized patients. Chin Med Sci J 36(1):17–26. https://doi.org/10.24920/003866
Xiao YF, He JL, Xu Y et al (2021) Major characteristics of severity and mortality in diabetic patients with COVID-19 and establishment of severity risk score. Front Med (Lausanne) 8:655604. https://doi.org/10.3389/fmed.2021.655604
Xu Z, Wang Z, Wang S et al (2020) The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID-19. J Diabetes 12(12):909–918. https://doi.org/10.1111/1753-0407.13084
Yan H, Valdes AM, Vijay A et al (2020) Role of drugs used for chronic disease management on susceptibility and severity of COVID-19: a large case-control study. Clin Pharmacol Ther 108(6):1185–1194. https://doi.org/10.1002/cpt.2047
Yeh HC, Kraschnewski JL, Kong L et al (2022) Hospitalization and mortality in patients with COVID-19 with or at risk of type 2 diabetes: data from five health systems in Pennsylvania and Maryland. BMJ Open Diabetes Res Care 10(3):e002774. https://doi.org/10.1136/bmjdrc-2022-002774
Yoo J, Choi Y, Park SA, Seo JY, Ahn CW, Han J (2022) Glycated albumin and glycated albumin/HbA1c predict the progression of coronavirus disease 2019 from mild to severe disease in Korean patients with type 2 diabetes. J Clin Med 11(9):2327. https://doi.org/10.3390/jcm11092327
You JH, Lee SA, Chun SY et al (2020) Clinical outcomes of COVID-19 patients with type 2 diabetes: a population-based study in Korea. Endocrinol Metab (Seoul) 35(4):901–908. https://doi.org/10.3803/EnM.2020.787
Zeltyn-Abramov EM, Lysenko MA, Frolova NF et al (2021) Risk factors of adverse outcome of COVID-19 and experience of tocilizumab administration in patients on maintenance hemodialysis due to diabetic kidney disease. Diabetes Mellitus 24(1):17–31. https://doi.org/10.14341/dm12688
Zhan K, Zhang X, Wang B et al (2022) Short- and long-term prognosis of glycemic control in COVID-19 patients with type 2 diabetes. QJM 115(3):131–139. https://doi.org/10.1093/qjmed/hcac020
Zhang N, Wang C, Zhu F et al (2020) Risk factors for poor outcomes of diabetes patients with COVID-19: a single-center, retrospective study in early outbreak in China. Front Endocrinol (Lausanne) 11:571037. https://doi.org/10.3389/fendo.2020.571037
Zhang Q, Wei Y, Chen M, Wan Q, Chen X (2020) Clinical analysis of risk factors for severe COVID-19 patients with type 2 diabetes. J Diabetes Complications 34(10):107666. https://doi.org/10.1016/j.jdiacomp.2020.107666
Zhu L, She ZG, Cheng X et al (2020) Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 31(6):1068–1077. https://doi.org/10.1016/j.cmet.2020.04.021
Mahamat-Saleh Y, Fiolet T, Rebeaud ME et al (2021) Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: a systematic review and meta-analysis of observational studies. BMJ Open 11(10):e052777. https://doi.org/10.1136/bmjopen-2021-052777
Leong A, Cole JB, Brenner LN, Meigs JB, Florez JC, Mercader JM (2021) Cardiometabolic risk factors for COVID-19 susceptibility and severity: a Mendelian randomization analysis. PLoS Med 18(3):e1003553. https://doi.org/10.1371/journal.pmed.1003553
Rosoff DB, Yoo J, Lohoff FW (2021) Smoking is significantly associated with increased risk of COVID-19 and other respiratory infections. Commun Biol 4(1):1230. https://doi.org/10.1038/s42003-021-02685-y
Barron E, Bakhai C, Kar P et al (2020) Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 8(10):813–822. https://doi.org/10.1016/S2213-8587(20)30272-2
McGurnaghan SJ, Weir A, Bishop J et al (2021) Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol 9(2):82–93. https://doi.org/10.1016/S2213-8587(20)30405-8
Williamson EJ, Walker AJ, Bhaskaran K et al (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821):430–436. https://doi.org/10.1038/s41586-020-2521-4
Holman N, Knighton P, Kar P et al (2020) Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. https://doi.org/10.1016/S2213-8587(20)30271-0
Lazarus G, Audrey J, Wangsaputra VK, Tamara A, Tahapary DL (2021) High admission blood glucose independently predicts poor prognosis in COVID-19 patients: a systematic review and dose-response meta-analysis. Diabetes Res Clin Pract 171:108561. https://doi.org/10.1016/j.diabres.2020.108561
Au Yeung SL, Zhao JV, Schooling CM (2021) Evaluation of glycemic traits in susceptibility to COVID-19 risk: a Mendelian randomization study. BMC Med 19(1):72. https://doi.org/10.1186/s12916-021-01944-3
Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P (2021) Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab 23(3):870–874. https://doi.org/10.1111/dom.14269
Harding JL, Oviedo SA, Ali MK et al (2023) The bidirectional association between diabetes and long-COVID-19 - a systematic review. Diabetes Res Clin Pract 195:110202. https://doi.org/10.1016/j.diabres.2022.110202
Khunti K, Knighton P, Zaccardi F et al (2021) Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endocrinol 9(5):293–303. https://doi.org/10.1016/S2213-8587(21)00050-4
Nguyen NN, Ho DS, Nguyen HS et al (2022) Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: a meta-analysis. Metabolism 131:155196. https://doi.org/10.1016/j.metabol.2022.155196
Singh AK, Gillies CL, Singh R et al (2020) Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab 22(10):1915–1924. https://doi.org/10.1111/dom.14124
Bae S, Kim SR, Kim MN, Shim WJ, Park SM (2021) Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart 107(5):373–380. https://doi.org/10.1136/heartjnl-2020-317901
Singh J, Malik P, Patel N et al (2022) Kidney disease and COVID-19 disease severity-systematic review and meta-analysis. Clin Exp Med 22(1):125–135. https://doi.org/10.1007/s10238-021-00715-x
Reyes FM, Hache-Marliere M, Karamanis D et al (2021) Assessment of the association of COPD and asthma with in-hospital mortality in patients with COVID-19. A Systematic review, meta-analysis, and meta-regression analysis. J Clin Med 10(10):2087. https://doi.org/10.3390/jcm10102087
Morsali S, Rezazadeh-Gavgani E, Oladghaffari M et al (2022) Effects of underlying heart failure on outcomes of COVID-19; a systematic review and meta-analysis. Rom J Intern Med. https://doi.org/10.2478/rjim-2022-0021
Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS (2022) COVID-19 severity and mortality among chronic liver disease patients: a systematic review and meta-analysis. Prev Chronic Dis 19:E53. https://doi.org/10.5888/pcd19.210228
Liu L, Ni SY, Yan W et al (2021) Mental and neurological disorders and risk of COVID-19 susceptibility, illness severity and mortality: a systematic review, meta-analysis and call for action. EClinicalMedicine 40:101111. https://doi.org/10.1016/j.eclinm.2021.101111
Venkatesulu BP, Chandrasekar VT, Girdhar P et al (2021) A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. JNCI Cancer Spectr 5(2):pkaa102. https://doi.org/10.1093/jncics/pkaa102
Baral R, Tsampasian V, Debski M et al (2021) Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA Netw Open 4(3):e213594. https://doi.org/10.1001/jamanetworkopen.2021.3594
Kollias A, Kyriakoulis KG, Kyriakoulis IG et al (2021) Statin use and mortality in COVID-19 patients: updated systematic review and meta-analysis. Atherosclerosis 330:114–121. https://doi.org/10.1016/j.atherosclerosis.2021.06.911
Huang W, Xiao J, Ji J, Chen L (2021) Association of lipid-lowering drugs with COVID-19 outcomes from a Mendelian randomization study. Elife 10:e73873. https://doi.org/10.7554/eLife.73873
Butler-Laporte G, Nakanishi T, Mooser V et al (2021) The effect of angiotensin-converting enzyme levels on COVID-19 susceptibility and severity: a Mendelian randomization study. Int J Epidemiol 50(1):75–86. https://doi.org/10.1093/ije/dyaa229
Junqueira C, Crespo A, Ranjbar S et al (2022) FγgammaR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 606(7914):576–584. https://doi.org/10.1038/s41586-022-04702-4
Wong YJ, Tan M, Zheng Q et al (2020) A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol 19(6):627–634. https://doi.org/10.1016/j.aohep.2020.08.064
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgement
We thank T. Schiemann from the German Diabetes Center (Institute for Biometric and Epidemiology) for assistance in contacting authors of studies with missing information and for data preparation.
Data availability
Data were extracted from published research papers, all of which are available and accessible. All datasets generated during the current study are available from the corresponding author on reasonable request. The study protocol has been published (PROSPERO registration no. CRD42020193692; www.crd.york.ac.uk/PROSPERO/) and is available without restrictions.
Funding
The German Diabetes Center (DDZ) is funded by the German Federal Ministry of Health and the Ministry of Culture and Science of the State of North Rhine-Westphalia. This study was supported in part by a grant from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD). The funders had no role in the study design or data collection, analysis and interpretation.
Open Access funding enabled and organised by Projekt DEAL.
Authors’ relationships and activities
CH is a member of the editorial board of Diabetologia. MR received personal fees from Boehringer Ingelheim Pharma, Eli Lilly, Fishawack Group, Novo Nordisk, Sanofi US, Target NASH and Terra Firma, and investigator-initiated research support from Boehringer Ingelheim, Nutricia/Danone and Sanofi-Aventis. All other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
MR and SS designed the study and wrote the first draft of the manuscript. AL, NC, PL, MN, JB and SS performed the literature search and literature screening. CH assisted in the selection of eligible studies. AL, NC, PL and MN extracted data and KP assisted with extraction of data on treatment. AL, NC, PL, MN and SS assessed the risk of bias of the studies and JB, MN and SS assessed the certainty of evidence of the associations. SS performed the statistical analysis and OK assisted with the statistical analysis. All authors contributed to data acquisition, data interpretation and revision of manuscript drafts and read and approved the final manuscript. SS is the guarantor of this work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlesinger, S., Lang, A., Christodoulou, N. et al. Risk phenotypes of diabetes and association with COVID-19 severity and death: an update of a living systematic review and meta-analysis. Diabetologia 66, 1395–1412 (2023). https://doi.org/10.1007/s00125-023-05928-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-05928-1