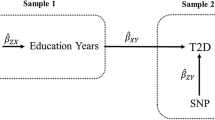
Abstract
Aims/hypothesis
Type 2 diabetes mellitus is a major health burden disproportionately affecting those with lower educational attainment (EA). We aimed to obtain causal estimates of the association between EA and type 2 diabetes and to quantify mediating effects of known modifiable risk factors.
Methods
We applied two-step, two-sample multivariable Mendelian randomisation (MR) techniques using SNPs as genetic instruments for exposure and mediators, thereby minimising bias due to confounding and reverse causation. We leveraged summary data on genome-wide association studies for EA, proposed mediators (i.e. BMI, blood pressure, smoking, television watching) and type 2 diabetes. The total effect of EA on type 2 diabetes was decomposed into a direct effect and indirect effects through multiple mediators. Additionally, traditional mediation analysis was performed in a subset of the National Health and Nutrition Examination Survey 2013–2014.
Results
EA was inversely associated with type 2 diabetes (OR 0.53 for each 4.2 years of schooling; 95% CI 0.49, 0.56). Individually, the largest contributors were BMI (51.18% mediation; 95% CI 46.39%, 55.98%) and television watching (50.79% mediation; 95% CI 19.42%, 82.15%). Combined, the mediators explained 83.93% (95% CI 70.51%, 96.78%) of the EA–type 2 diabetes association. Traditional analysis yielded smaller effects but showed consistent direction and priority ranking of mediators.
Conclusions/interpretation
These results support a potentially causal protective effect of EA against type 2 diabetes, with considerable mediation by a number of modifiable risk factors. Interventions on these factors thus have the potential of substantially reducing the burden of type 2 diabetes attributable to low EA.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Type 2 diabetes is a multifactorial group of disorders in which impaired insulin secretion and/or insulin resistance results in dysregulated carbohydrate, lipid and protein metabolism [1]. It confers an increase in risk of cardiovascular disease and all-cause mortality [2, 3]. Global prevalence has been continuously rising over the past few decades, with type 2 diabetes projected to affect 9.9% of the world population by the year 2045 [4,5,6,7,8], thus posing an increasingly unsustainable global health burden [9].
A large and consistent body of epidemiological data suggests that those with lower educational attainment (EA) are disproportionately affected by type 2 diabetes [10]. This association is likely mediated by modifiable risk factors, such as obesity, sedentary behaviour, physical activity (PA), smoking and blood pressure [11,12,13,14,15]. Knowledge of mediation in the EA–type 2 diabetes association will inform public health policies, e.g. by prioritising targets for intervention to reduce the excess risk of type 2 diabetes due to low EA. Our current knowledge of mediating pathways is predominantly based on traditional observational studies that are sensitive to confounding and reverse causation. Therefore, it is uncertain to what extent the associations between EA and type 2 diabetes, and their intermediates, are confounded or affected by reverse causation.
A well-acknowledged method to support causal inference in observational data is Mendelian randomisation (MR). This method uses SNPs, identified in genome-wide association studies (GWAS) to be strongly associated with an exposure, as instrumental variables [16]. Under a number of assumptions, MR yields estimates of an exposure–outcome relation that are less likely to be biased due to unobserved confounding. Recent advances in MR methodology include multivariable MR (MVMR), which can be applied to investigate mediation [17].
Previous MR studies have provided support for a potential causal effect of EA (measured by years of schooling) on coronary artery disease [18], with evidence of mediation through risk factors such as BMI, smoking and blood pressure [19]. For type 2 diabetes risk, recent MR studies have also provided evidence of such a causal effect of EA [20,21,22]. However, these studies did not assess mediation by modifiable factors [21, 22], while one recent study only examined mediation by BMI and smoking [23]. Furthermore, some MR studies relied on genetic associations leveraged from less recent, less precise GWAS data [20, 21]. Recent GWAS on EA [24] and type 2 diabetes [25] have yielded more precise estimates of SNP effects due to their larger sample size compared with less recent GWAS. Updating the results from previous MR studies on EA and type 2 diabetes, as well as assessing potential mediation, using the most recent GWAS data would result in more precise insights into the causal structure underlying the EA–type 2 diabetes association.
We therefore aimed to obtain causal estimates of the association between EA and type 2 diabetes and to characterise the causal structure by assessing mediation effects of BMI, sedentary behaviour, PA, smoking and blood pressure in an MVMR framework. In addition, we aimed to obtain observational mediation estimates from the 2013–2014 National Health and Nutrition Examination Survey (NHANES).
Methods
Overall study design
This study used a two-step MR analysis of genetic summary data to investigate to what extent BMI, blood pressure, smoking, sedentary behaviour (daily hours of television watching) and PA explain the protective effect of EA on type 2 diabetes risk. In addition, we estimated mediation in data from the NHANES 2013–2014 using traditional observational mediation analysis techniques.
Outcome definitions
In the original GWAS [24], educational level was categorised according to the International Standard Classification of Education (ISCED) 2011 [26], converted to US years of schooling and standardised, with each unit representing 4.2 years of schooling. Type 2 diabetes in the original GWAS was defined by diagnostic fasting glucose, casual glucose, 2 h plasma glucose or HbA1c levels; use of glucose-lowering medication (by Anatomical Therapeutic Chemical code or self-report); or type 2 diabetes history from electronic medical records, self-report and varying combinations of each, depending on the contributing cohort [25]. Mediator selection was based on potential for modification, observational epidemiological evidence of mediation in the EA–type 2 diabetes relation and availability of comprehensive GWAS data. BMI was calculated by dividing weight (kg) by height squared (m2) [27]. Sedentary behaviour was measured by daily hours of television watching [28]. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) values were the mean of two automated or two manual blood pressure measurements [29]. Smoking behaviour was a binary trait: participants who reported ever being a regular smoker in their life were defined as smokers; the remaining participants were defined as non-smokers [30]. PA was measured by accelerometry and reported as SDs of metabolic equivalent of task [31].
MR methods
For a brief discussion of assumptions underlying MR, see electronic supplementary material (ESM) Methods 1. We used two-sample MR (2SMR) methods using GWAS summary level data [32]. Two-step 2SMR (ESM Fig. 1) was used to assess whether an intermediate trait has a mediation effect between exposure and outcome [33]. The first step was to estimate the causal effect of EA on potential mediators using SNPs to genetically predict years of schooling. In the second step, SNPs for the potential mediating risk factors were used to genetically predict these mediators and to estimate their causal effect on the outcome, adjusting for EA using MVMR. The total effect of EA was then decomposed into a direct effect (i.e. the effect of EA on type 2 diabetes independent of the mediator) and an indirect effect (i.e. the effect of EA on type 2 diabetes via the mediator). This approach is currently being widely applied [19, 34].
For these analyses, we obtained summary statistics of the genetic associations from the most recent GWAS for each respective phenotype. Table 1 summarises the GWAS data used in this study.
Instrument selection
All selected SNPs and their associations with EA, mediators and type 2 diabetes were extracted from the GWAS studies in Table 1. For EA, genetic instruments were selected from the Social Science Genetic Association Consortium GWAS meta-analysis of years of schooling in 1,131,881 individuals of European ancestry [24]. A total of 1271 independent (r2 < 0.1) genome-wide significant (p < 5×10−8) SNPs were used as the primary genetic instruments.
For each mediator, genetic instruments were selected from the most recent large-scale GWAS data. We then selected genome-wide significant SNPs for each trait (p < 5×10−8 for SBP/DBP, television watching, smoking and PA; p < 1×10−8 for BMI). We applied pairwise linkage disequilibrium (LD) thresholds from the original GWAS for each mediating trait, with SNPs for each trait adhering to an LD cut-off of r2 < 0.1 within a window of 1 MB, except for television watching (LD r2 < 0.005 within 5 MB) and smoking (LD r2 < 0.1 within 500 kB). Then, for all SNPs, we harmonised coding and non-coding alleles in the summary statistics of each GWAS. In case of palindromic SNPs, we inferred strand based on allele frequency. Palindromic SNPs with ambiguous allele frequency (frequency 0.3–0.7) were removed.
Effect of EA on type 2 diabetes
We obtained estimates of the total effect of EA on type 2 diabetes using straightforward 2SMR. Here, single SNP estimates of the effect of EA on type 2 diabetes were investigated by calculating Wald ratios with standard errors derived using the delta method. We used inverse variance-weighted meta-analysis to pool Wald ratios as our main method [32].
Mediation analysis
We used inverse variance weighting (IVW) as our main approach to estimate the effect of EA on each mediator. We used regression-based MVMR to estimate the effect of each mediator on type 2 diabetes risk while adjusting for the genetic effect of the instruments on EA [35]. For the individual mediation effect of each risk factor (BMI, SBP, DBP, smoking, PA and television watching), we used the product of coefficients method as our main method to estimate the indirect effect (that is, the effect of EA on type 2 diabetes through the mediator) [36]. This involved first estimating the effect of EA on each mediator individually, then multiplying the EA–mediator effect with the EA-adjusted effect of the mediator on the outcome [34]. The proportion of the total effect of EA on type 2 diabetes that was mediated by each risk factor separately was estimated by dividing the indirect effect by the total effect. Standard errors were derived by using the delta method, using effect estimates obtained from 2SMR analysis.
To estimate the combined proportion mediated, we used the difference in regression coefficient MVMR approach to adjust the genetic effect for several mediators simultaneously (such as BMI + SBP) to obtain the direct effect of EA on type 2 diabetes. Then, the combined indirect effect of considered mediators was the residual of the total effect. We explored all possible meaningful combinations of mediators to find the combination of mediation with the highest proportion mediated and to assess potential overlap between mediators.
Although PA was initially included in our selection of potential mediating risk factors, GWAS data on this variable were limited with regard to power and number of available genetic instruments: only two SNPs were available, precluding meaningful mediation analysis using the product of coefficients method. We therefore decided to abandon further investigation of PA as an intermediate trait in both our main MR and observational analyses, although we were able to examine mediation by PA using the difference method.
MR sensitivity analyses
We conducted several sensitivity analyses to evaluate the robustness of the MR results. First, in addition to our primary IVW meta-analysis, we applied 2SMR methods that are robust to violations of the assumptions regarding horizontal pleiotropy of SNPs (ESM Methods 1). Second, to validate estimates of the product of coefficients method, we used the MVMR approach to estimate the individual mediation effect of each mediator using the difference method. In this approach, the indirect effect of each mediator was estimated by subtracting the direct effect of education from the total effect. Third, we used the MVMR-Egger method to examine the robustness of the MVMR-IVW results. Fourth, to assess the validity of our mediation model we conducted reverse MR using genetic instruments for each purported mediator to explore bidirectionality between EA and potential mediators.
All MR analyses were conducted using R (version 4.0.2) [37] and the TwoSampleMR R package version 0.5.6 [38].
Observational mediation analysis in NHANES
Details on the sample and methods of the observational mediation analysis can be found in ESM Methods 2. Briefly, we applied standard regression-based mediation analysis methods to explore mediation through pathways presented in ESM Fig. 2.
Results
MR analysis
Genetic instruments
Detailed information on SNPs and their associations with EA, mediators and type 2 diabetes can be found in the ESM Data of SNPs.
Effect of EA on type 2 diabetes
Each SD (4.2 years of schooling) higher genetically predicted EA was associated with 0.53 times lower odds of type 2 diabetes (OR 0.53; 95% CI 0.49, 0.56).
Effect of EA on mediators
Effects of genetically predicted EA on each mediator are shown in Fig. 1a. Each SD (4.2 years of schooling) higher genetically predicted EA was associated with lower BMI (β = −0.34 kg/m2; 95% CI −0.37, −0.31); less television watching (β = −0.61 SD of television watching; 95% CI −0.63, −0.59, translating to 0.92 h less television watching per 4.2 years of schooling); lower odds of smoking (OR 0.63; 95% CI 0.61, 0.66); lower SBP (−1.83 mmHg; 95% CI −2.24, −1.41); lower DBP (β = −0.81 mmHg; 95% CI −1.06, −0.57); and more PA (β = 0.08 SD; 95% CI 0.05, 0.12).
(a) MR-estimated effects of EA (per 4.2 years of schooling) on each mediator separately, presented as β/OR with 95% CI. (b) MR-estimated effects of each mediator separately on type 2 diabetes after MVMR adjustment for education, presented as β/OR with 95% CI. (c) MR-estimated effects of indirect effects of each mediator separately, by product of coefficients method with delta method-estimated 95% CIs. MR-estimated proportions mediated (%) are presented with 95% CIs. T2D, type 2 diabetes; TV, television
Effect of mediators on type 2 diabetes with adjustment for EA
Figure 1b shows that each mediator was significantly associated with type 2 diabetes after adjusting for EA. We excluded PA from our analysis as only two out of five PA SNPs could be used in MVMR, precluding meaningful analysis using the product of coefficients method. A 1 kg/m2 higher genetically predicted BMI was associated with 2.60 times higher odds of type 2 diabetes (95% CI 2.38, 2.84). A 1 mmHg higher genetically predicted blood pressure (SBP/DBP) was associated with higher odds of type 2 diabetes (OR 1.02; 95% CI 1.01, 1.03). One SD (1.5 h) longer genetically predicted television watching was associated with 1.70 times higher odds of type 2 diabetes (95% CI 1.22, 2.37). Compared with never-smokers, the odds of type 2 diabetes among genetically predicted ever-smokers were 1.20 times higher (95% CI 1.10, 1.31).
Individual proportion mediated
Figure 1c displays the proportion of the effect of EA on type 2 diabetes explained by each mediator separately. BMI explained 51.18% (95% CI 46.39%, 55.98%) of the total effect of EA on type 2 diabetes, while television watching explained 50.79% (95% CI 19.42%, 82.15%). Smoking explained 12.94% (95% CI 6.59%, 19.30%) of the total effect. DBP and SBP only subtly mediated the total effect of EA (2.47% for DBP, 6.41% for SBP).
Combined proportion mediated
We examined the proportion mediated of different combinations of mediating variables. This was done in an effort to find the combination that explained the most variation in the EA–type 2 diabetes association, as well as to investigate potential overlap in effects between mediators (Fig. 2).
Combining any one of the other mediators with BMI was observed to increase the mediating proportion to around 60–77%. The same trend could be observed when combining television watching with any of the other mediating variables.
Among the two-mediator combinations, the combination of BMI + SBP mediated 63.85% (95% CI 52.81%, 74.88%) of the effect of EA on type 2 diabetes. The BMI + television watching combination showed the highest proportion mediated (77.21%; 95% CI 59.82%, 94.61%). This relatively large combined mediation effect suggests little overlap between BMI and television watching.
Among the three-mediator combinations, the combination of BMI + television watching + smoking accounted for the largest mediation effect (80.18%; 95% CI 61.81%, 98.54%).
As expected, any combination of both SBP and DBP did not result in a meaningful increase in proportion mediated compared with a combination with either SBP or DBP.
The four-mediator combination with the largest proportion mediated was BMI + SBP + smoking + television watching, accounting for 83.93% (95% CI 70.51%, 96.78%) of the effect of EA.
No meaningful increase in proportion mediated was observed for five- and six-mediator combinations compared with that of the most effective four-mediator group.
MR sensitivity analyses
We assessed heterogeneity using Cochran’s Q statistic. We observed substantial heterogeneity from genetic instruments for EA to the outcome and mediators (ESM Table 1), indicating potential pleiotropy of SNP effects. To evaluate potential directional horizontal pleiotropy, we performed MR-Egger regression to assess whether the mean value of the Egger intercept was non-zero, in which case pleiotropy could be directional [39]. In our study, we found no significant directional pleiotropy for any of the 2SMR analyses (p > 0.05, ESM Table 2). In addition, MR-weighted median methods were generally consistent with MR-IVW with regard to magnitude and direction (ESM Tables 3, 4, ESM Fig. 3), suggesting that any horizontal pleiotropy did not greatly bias our results.
To assess the consistency of our main MR product of coefficients estimates of individual mediation, we performed additional MVMR mediation analysis using the difference in regression coefficient method; estimates were highly similar (ESM Fig. 4). In contrast to the product of coefficients method, we were able to examine mediation effects of PA using the difference method; here, PA explained 4.47% (95% CI 0.69%, 8.31%) of the total effect (ESM Fig. 4). The combined proportion mediated of PA and BMI was not larger than that of BMI alone, suggesting substantial overlap in mediation effects between the two (ESM Fig. 4, Fig. 2). Results from MVMR-Egger sensitivity analyses were highly consistent with those from MVMR-IVW analysis (ESM Table 5), suggesting low risk of bias due to horizontal pleiotropy. There was reasonable instrument strength (F > 10) of SNPs for EA, BMI, SBP and DBP in all MVMR analyses. However, conditional instrument strength for television watching, smoking initiation and PA was low.
In reverse MR analyses, higher BMI suggestively reduced EA, although this reverse effect was likely driven by horizontal pleiotropy (pEgger intercept = 0.0166). There was no evidence for a causal effect of SBP, DBP and PA on EA. Reverse MR suggested that television watching and smoking reduce EA level (ESM Table 6), indicating that some bidirectionality exists between these factors and EA, possibly affecting validity of our mediation model. Therefore, we constructed a final mediation model including only BMI, SBP and DBP, while excluding television watching and smoking. In this mediation model, the combined proportion explained by BMI, SBP and DBP was 64.35% (95% CI 53.28%, 75.41%) (ESM Figs. 5, 6).
Observational mediation results in NHANES
Descriptive statistics of 1912 participants from the NHANES 2013–2014 are presented in ESM Table 7. In observational analysis (ESM Table 8), each 4.2 year higher EA was associated with lower odds of type 2 diabetes (OR 0.64; 95% CI 0.56, 0.73), similar in magnitude and direction to the MR estimates. Higher EA was associated with lower BMI, less television watching, lower odds of smoking, lower SBP and lower DBP. Product of coefficients estimates of individual proportion mediated were 25%, 16%, 7%, 4% and 0%, respectively, while the total combined proportion mediated estimated by the difference method was 30%.
Discussion
In this two-step MVMR study, we found evidence suggestive of a causal, protective effect of EA on type 2 diabetes, with up to 84% mediation by a combination of the modifiable factors BMI, television watching, blood pressure and smoking. Observational mediation estimates in the NHANES 2013–2014 were consistent with the MR mediation estimates with regard to directionality and priority ranking of mediators, but overall suggested less pronounced mediation by the risk factors of interest.
In the present study, the MR-estimated causal effect of a 1 SD (4.2 years of schooling) increase in EA was a 47% reduction in odds of type 2 diabetes (OR 0.53, similar to previous MR studies, ORs ranging from 0.39 to 0.61) [20,21,22,23].
Previous observational studies of the EA–type 2 diabetes association reported 31–53% mediation by a range of risk factors [12, 13]. A previous MR study found that 64% of the association between EA and type 2 diabetes was mediated by BMI and smoking [23], similar to the combined mediation estimate of BMI and smoking in the present study (58%). In general, observational estimates of mediation are lower than those derived from MR. This could be due to underestimation of associations in observational studies due to confounding or measurement error. Although MR is less sensitive to confounding or measurement error, it has been suggested to yield higher associations given that SNP effects represent an estimate of lifetime exposure [19].
Up to ~84% of the EA–type 2 diabetes association was mediated by traditional (i.e. clinical) risk factors, while ~16% remains unexplained. Potential factors that may explain the remainder of the association include factors such as area deprivation, income, diet, health literacy, healthcare access and psychosocial factors. Many of these factors may be not heritable and therefore not suitable for GWAS and consequently unsuitable for 2SMR. However, these factors are expected to show high overlap with factors investigated in the present study, i.e. BMI, television watching, smoking and blood pressure; we therefore expect that these omitted factors would not have contributed substantially to explaining the EA–type 2 diabetes relation. They might however play a role in intervention strategies to reduce type 2 diabetes risk, e.g. reducing BMI through improving diet and health literacy.
Estimates generated from MR are generally insensitive to reverse causation due to the random assignment of alleles at conception. However, our results suggested a bidirectional negative relationship of television watching and smoking with EA, which may imply that EA could also be a mediator of these two traits, complicating the hypothesised model. Additionally, given the low instrument strength for these two traits, results for these two factors should be cautiously interpreted.
Directly intervening on EA by raising the school-leaving age has been shown to be effective in improving adult health (including type 2 diabetes) and reducing mortality in the UK [40]. Other interventions may involve improving access to education, and improving quality of (health) education. However, such interventions are impractical short-term solutions to reducing the burden of type 2 diabetes. In the present study, we provide evidence of substantial mediation of the EA–type 2 diabetes relation through several risk factors that are more easily modifiable than EA. Although population-wide intervention strategies on these modifiable mediators are expected to increase public health, such an approach may widen the inequality gap of type 2 diabetes risk [41]; a high-risk prevention approach (i.e. interventions that target mediators in those with low EA) may therefore be necessary to reduce socioeconomic disparities in type 2 diabetes risk. Our results ranked BMI and television watching to be the strongest contributing factors in the EA–type 2 diabetes association, interestingly, with relatively little overlap, thus suggesting partly independent effects. Interventions on BMI may involve addressing the obesogenic environment associated with low-socioeconomic status neighbourhoods [42, 43], e.g. by limiting fast food outlets in these neighbourhoods. Screen time interventions (television or otherwise) have previously been successfully implemented to improve diet, weight and PA in children [44]. Future studies may further investigate the feasibility and potential impact of such interventions on adult type 2 diabetes risk.
The present MR study yields population-averaged causal estimates of association and mediation. Given the sex differences in both EA [45] and type 2 diabetes [46], it is likely that associations and mediators are also different between sexes, as shown previously [11]. Future MR studies may investigate this using sex-stratified GWAS data.
The predominance of GWAS, including those used in the present study, were performed in white European ancestry populations from high-income countries; generalisation to other ethnicities and low- and medium-income countries is therefore uncertain. Furthermore, a strong relation exists among ethnicity/race, socioeconomic status and health [47] in multi-ethnic communities. Future studies (including GWAS and MR) should therefore be more inclusive with regard to non-white community dwellers.
Strengths of the present study include that it uses SNPs as genetic instruments to minimise bias due to confounding and reverse causation. We used the most recent large-scale GWAS data to generate highly precise SNP effect estimates, facilitating precise MR analysis. The mediated effects estimated were consistent across the two MR mediation approaches and in the statistical sensitivity analyses. Furthermore, MR estimates were corroborated by observational mediation analyses in NHANES 2013–2014, allowing for triangulation [48] and thus improving the robustness of our findings.
Several limitations must be addressed. First, MR may be biased by pleiotropic effects of SNPs, i.e. genetic variants directly influencing both exposure and outcome: a violation of the exclusion restriction criterion. While sensitivity analyses (i.e. MR-Egger, weighted median) robust to pleiotropy [49, 50] showed consistent results, we did not adjust for cognitive ability, which is highly related to EA and a potential confounder in any EA–outcome relation. However, a recent study showed that MVMR adjustment of EA for cognitive ability did not meaningfully affect MR estimates [22]. Second, a potential limitation of using genetic data on social traits such as EA is that ‘population phenomena’ play a role. These phenomena include population stratification, dynastic effects (i.e. transgenerational effects of non-inherited parental SNP alleles) and assortative mating (e.g. non-random mating based on educational level). Whereas population stratification is usually accounted for in GWAS, dynastic effects and assortative mating are not; SNP–EA associations might thus be confounded and therefore may bias MR estimates [51,52,53,54]. Future MR studies might exploit within-family genetic data (e.g. parent–offspring trios, siblings) that have the potential of accounting for such phenomena [55]. Third, the present study assumes absence of exposure × mediator interaction, which currently cannot be modelled in the present 2SMR setting. Fourth, type 2 diabetes and smoking were binary traits, requiring the use of log-odds (as per the original GWAS) in MR analysis for estimating direct and indirect effects. This is non-ideal as ORs are non-collapsible, i.e. marginal ORs are not directly comparable with conditional ORs [56]. Fifth, SNP effects on blood pressure traits were adjusted for BMI in the original GWAS, which subjects MR estimates involving SBP or DBP to potential bias with unpredictable direction [57]. Sixth, sample overlap between GWAS studies may have biased MR estimates towards observational association estimates [58].
To conclude, these results support a potentially causal protective effect of higher EA against type 2 diabetes, with substantial mediation by the modifiable risk factors BMI, television watching and, to a lesser extent, smoking, SBP and DBP. Interventions on these factors thus have the potential of substantially reducing the burden of type 2 diabetes attributable to low EA.
Data availability
GWAS data are available through the MRC IEU Open GWAS database (https://gwas.mrcieu.ac.uk/). NHANES data are publicly available through the Center for Disease Control (https://wwwn.cdc.gov/nchs/nhanes/). Code can be shared upon request.
Abbreviations
- DBP:
-
Diastolic blood pressure
- EA:
-
Educational attainment
- GWAS:
-
Genome-wide association studies
- IVW:
-
Inverse variance weighting
- LD:
-
Linkage disequilibrium
- MR:
-
Mendelian randomisation
- MVMR:
-
Multivariable MR
- NHANES:
-
National Health and Nutrition Examination Survey
- PA:
-
Physical activity
- SBP:
-
Systolic blood pressure
- 2SMR:
-
Two-sample MR
References
DeFronzo RA, Ferrannini E, Groop L et al (2015) Type 2 diabetes mellitus. Nat Rev Dis Primers 1:15059
Emerging Risk Factors Collaboration (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375:2215–2222. https://doi.org/10.1016/S0140-6736(10)60484-9
Rawshani A, Rawshani A, Franzén S et al (2017) Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 376:1407–1418. https://doi.org/10.1056/NEJMoa1608664
Amos AF, McCarty DJ, Zimmet P (1997) The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 14:S7–S85. https://doi.org/10.1002/(SICI)1096-9136(199712)14:5+<S7::AID-DIA522>3.0.CO;2-R
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14. https://doi.org/10.1016/j.diabres.2009.10.007
Zimmet PZ, Magliano DJ, Herman WH, Shaw JE (2014) Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol 2:56–64. https://doi.org/10.1016/S2213-8587(13)70112-8
Zhou B, Lu Y, Hajifathalian K et al (2016) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387:1513–1530. https://doi.org/10.1016/S0140-6736(16)00618-8
Cho N, Shaw J, Karuranga S et al (2018) IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281. https://doi.org/10.1016/j.diabres.2018.02.023
Vos T, Lim SS, Abbafati C et al (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396:1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9
Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A (2011) Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol 40:804–818. https://doi.org/10.1093/ije/dyr029
Agardh EE, Ahlbom A, Andersson T et al (2004) Explanations of socioeconomic differences in excess risk of type 2 diabetes in Swedish men and women. Diabetes Care 27:716–721. https://doi.org/10.2337/diacare.27.3.716
Stringhini S, Tabak AG, Akbaraly TN et al (2012) Contribution of modifiable risk factors to social inequalities in type 2 diabetes: prospective Whitehall II cohort study. BMJ 345:e5452. https://doi.org/10.1136/bmj.e5452
Steele CJ, Schottker B, Marshall AH et al (2017) Education achievement and type 2 diabetes-what mediates the relationship in older adults? Data from the ESTHER study: a population-based cohort study. BMJ Open 7:e013569. https://doi.org/10.1136/bmjopen-2016-013569
Wemrell M, Bennet L, Merlo J (2019) Understanding the complexity of socioeconomic disparities in type 2 diabetes risk: a study of 4.3 million people in Sweden. BMJ Open Diabetes Res Care 7:e000749. https://doi.org/10.1136/bmjdrc-2019-000749
Mathisen J, Jensen AK, Andersen I, Andersen GS, Hvidtfeldt UA, Rod NH (2020) Education and incident type 2 diabetes: quantifying the impact of differential exposure and susceptibility to being overweight or obese. Diabetologia 63:1764–1774. https://doi.org/10.1007/s00125-020-05150-3
Davey Smith G, Ebrahim S (2003) ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32:1–22. https://doi.org/10.1093/ije/dyg070
Sanderson E (2021) Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med 11:a038984. https://doi.org/10.1101/cshperspect.a038984
Tillmann T, Vaucher J, Okbay A et al (2017) Education and coronary heart disease: mendelian randomisation study. BMJ 358:j3542. https://doi.org/10.1136/bmj.j3542
Carter AR, Gill D, Davies NM et al (2019) Understanding the consequences of education inequality on cardiovascular disease: mendelian randomisation study. BMJ 365:l1855. https://doi.org/10.1136/bmj.l1855
Adams CD, Boutwell BB (2020) Can increasing years of schooling reduce type 2 diabetes (T2D)?: evidence from a Mendelian randomization of T2D and 10 of its risk factors. Sci Rep 10:12908
Cao M, Cui B (2020) Association of Educational Attainment with Adiposity, type 2 diabetes, and coronary artery diseases: a Mendelian randomization study. Front Public Health 8:112
Liang J, Cai H, Liang G et al (2021) Educational attainment protects against type 2 diabetes independently of cognitive performance: a Mendelian randomization study. Acta Diabetol 58:567–574. https://doi.org/10.1007/s00592-020-01647-w
Yuan S, Xiong Y, Michaëlsson M, Michaëlsson K, Larsson SC (2021) Genetically predicted education attainment in relation to somatic and mental health. Sci Rep 11:4296
Lee JJ, Wedow R, Okbay A et al (2018) Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 50:1112–1121. https://doi.org/10.1038/s41588-018-0147-3
Mahajan A, Taliun D, Thurner M et al (2018) Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 50:1505. https://doi.org/10.1038/s41588-018-0241-6
UNESCO Institute for Statistics (2011) International Standard Classification of Education. Available from http://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf. Accessed 9 Sept 2021
Yengo L, Sidorenko J, Kemper KE et al (2018) Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet 27:3641–3649. https://doi.org/10.1093/hmg/ddy271
van de Vegte YJ, Said MA, Rienstra M, van der Harst P, Verweij N (2020) Genome-wide association studies and Mendelian randomization analyses for leisure sedentary behaviours. Nat Commun 11:1770
Evangelou E, Warren HR, Mosen-Ansorena D et al (2018) Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 50:1412. https://doi.org/10.1038/s41588-018-0205-x
Liu M, Jiang Y, Wedow R et al (2019) Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 51:237–244. https://doi.org/10.1038/s41588-018-0307-5
Doherty A, Smith-Byrne K, Ferreira T et al (2018) GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat Commun 9:5257
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37:658–665. https://doi.org/10.1002/gepi.21758
Relton CL, Davey Smith G (2012) Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol 41:161–176. https://doi.org/10.1093/ije/dyr233
Burgess S, Daniel RM, Butterworth AS, Thompson SG, EPIC-InterAct Consortium (2015) Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol 44:484–495. https://doi.org/10.1093/ije/dyu176
Burgess S, Thompson SG (2015) Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 181:251–260. https://doi.org/10.1093/aje/kwu283
VanderWeele TJ (2016) Mediation analysis: a practitioner's guide. Annu Rev Public Health 37:17–32. https://doi.org/10.1146/annurev-publhealth-032315-021402
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Hemani G, Zheng J, Elsworth B et al (2018) The MR-base platform supports systematic causal inference across the human phenome. eLife 7:e34408
Hemani G, Bowden J, Davey Smith G (2018) Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet 27:R195–R208. https://doi.org/10.1093/hmg/ddy163
Davies NM, Dickson M, Smith GD, Van Den Berg GJ, Windmeijer F (2018) The causal effects of education on health outcomes in the UK biobank. Nat Hum Behav 2:117. https://doi.org/10.1038/s41562-017-0279-y
Lorenc T, Petticrew M, Welch V, Tugwell P (2013) What types of interventions generate inequalities? Evidence from systematic reviews. J Epidemiol Community Health 67:190–193. https://doi.org/10.1136/jech-2012-201257
Schneider S, Gruber J (2013) Neighbourhood deprivation and outlet density for tobacco, alcohol and fast food: first hints of obesogenic and addictive environments in Germany. Public Health Nutr 16:1168–1177. https://doi.org/10.1017/S1368980012003321
Mohammed SH, Habtewold TD, Birhanu MM et al (2019) Neighbourhood socioeconomic status and overweight/obesity: a systematic review and meta-analysis of epidemiological studies. BMJ Open 9:e028238. https://doi.org/10.1136/bmjopen-2018-028238
Buchanan LR, Rooks-Peck CR, Finnie RK et al (2016) Reducing recreational sedentary screen time: a community guide systematic review. Am J Prev Med 50:402–415. https://doi.org/10.1016/j.amepre.2015.09.030
Buchmann C, DiPrete TA, McDaniel A (2008) Gender inequalities in education. Annu Rev Sociol 34:319–337. https://doi.org/10.1146/annurev.soc.34.040507.134719
Kautzky-Willer A, Harreiter J, Pacini G (2016) Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 37:278–316. https://doi.org/10.1210/er.2015-1137
Williams DR, Mohammed SA, Leavell J, Collins C (2010) Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci 1186:69–101
Lawlor DA, Tilling K, Davey Smith G (2016) Triangulation in aetiological epidemiology. Int J Epidemiol 45:1866–1886. https://doi.org/10.1093/ije/dyw314
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525. https://doi.org/10.1093/ije/dyv080
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40:304–314. https://doi.org/10.1002/gepi.21965
Kong A, Thorleifsson G, Frigge ML et al (2018) The nature of nurture: effects of parental genotypes. Science 359:424–428. https://doi.org/10.1126/science.aan6877
Hartwig FP, Davies NM, Davey Smith G (2018) Bias in Mendelian randomization due to assortative mating. Genet Epidemiol 42:608–620. https://doi.org/10.1002/gepi.22138
Koellinger PD, de Vlaming R (2019) Mendelian randomization: the challenge of unobserved environmental confounds. Int J Epidemiol 48:665–671. https://doi.org/10.1093/ije/dyz138
Morris TT, Davies NM, Hemani G, Smith GD (2020) Population phenomena inflate genetic associations of complex social traits. Science. Advances 6:eaay0328. https://doi.org/10.1126/sciadv.aay0328
Brumpton B, Sanderson E, Heilbron K et al (2020) Avoiding dynastic, assortative mating, and population stratification biases in Mendelian randomization through within-family analyses. Nat Commun 11:3519
Greenland S, Pearl J, Robins JM (1999) Confounding and collapsibility in causal inference. Stat Sci 14:29–46
Hartwig FP, Tilling K, Smith GD, Lawlor DA, Borges MC (2021) Bias in two-sample Mendelian randomization when using heritable covariable-adjusted summary associations. Int J Epidemiol 50(5):1639–1650. https://doi.org/10.1093/ije/dyaa266
Burgess S, Davies NM, Thompson SG (2016) Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol 40:597–608. https://doi.org/10.1002/gepi.21998
Acknowledgements
The authors wish to acknowledge Alice R. Carter, University of Bristol, for kindly providing R code.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
JZ, CHLT and HS were responsible for study conception and design. JZ, CHLT and ZC performed the data analysis. JZ, CHLT, KP, ZC, SKRvZ and HS performed the data interpretation. CHLT, JZ and KP drafted the manuscript. JZ, CHLT, ZC, KP, SKRvZ and HS critically reviewed the manuscript. All authors approved the final version of this manuscript to be published. HS and CHLT are the guarantors of this work.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Chen, Z., Pärna, K. et al. Mediators of the association between educational attainment and type 2 diabetes mellitus: a two-step multivariable Mendelian randomisation study. Diabetologia 65, 1364–1374 (2022). https://doi.org/10.1007/s00125-022-05705-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-022-05705-6