Abstract
Diabetes is one of the most challenging health concerns facing society. Available drugs treat the symptoms but there is no cure. This presents an urgent need to better understand human diabetes in order to develop improved treatments or target remission. New disease models need to be developed that more accurately describe the pathology of diabetes. Organoid technology provides an opportunity to fill this knowledge gap. Organoids are 3D structures, established from pluripotent stem cells or adult stem/progenitor cells, that recapitulate key aspects of the in vivo tissues they mimic. In this review we briefly introduce organoids and their benefits; we focus on organoids generated from tissues important for glucose homeostasis and tissues associated with diabetic complications. We hope this review serves as a touchstone to demonstrate how organoid technology extends the research toolbox and can deliver a step change of discovery in the field of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.


What are organoids?
The past decade has born witness to the meteoric rise of organoid technology, a long-term heterogenous stem cell-based culture technique, chosen as Nature method of the year 2017 [1]. Organoids have transformative potential; they will help us to better model human diseases, leading to the development of novel treatments, the revolutionising of personalised medicine and the acceleration of regenerative medicine. But what exactly are organoids? The definition of an organoid has been nebulous, owing to its use in describing the many different types of 3D culture systems developed in the last 50 years [2]. Since the development of intestinal stem cell-derived organoid cultures in 2009 (discussed in the Text box: ‘Genesis of the modern organoid field’), the term now refers to a specific set of working criteria. The current definition requires an organoid to be established from pluripotent stem cells or adult stem/progenitor cells, to demonstrate a 3D structure resembling the in vivo organ landscape, exhibit an array of cell types found in vivo and demonstrate some aspects of the specialised functions of the tissues [3,4,5].
A step change in disease modelling
A staple tool of the medical research community is the modelling of cellular function and disease in vitro. In a dish, experimental variables can be accurately controlled, cells can easily be manipulated, and outputs measured by standard and high-throughput technologies. Cell lines and explant cultures have traditionally been used, but both have limitations. 2D-cultured cells may not act in vitro as they would in vivo, because they are not grown in conditions that adequately mimic the in vivo microenvironment. In addition, cell lines are transformed, making these cells far from ‘normal’, calling into question how representative of physiology they really are. In contrast, explant cultures are a more physiologically relevant model as they contain a heterogenous population of primary cells representative of the tissue of origin. However, these cultures cannot be maintained for long periods, which increases the need for multiple tissue donors, and they are generally difficult to genetically manipulate. Organoid cultures address many of these shortcomings as they are complex 3D, multicellular, self-renewing primary tissue structures that can be cultured over months to years without losing their faithful near physiological representation of the tissues they mimic [6]. These properties provide the research community with a step change in our ability to model diseases on the bench.
What can organoids do for you?
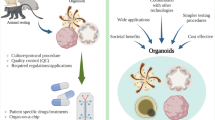
The power of organoids lies in their ability to be cultured from human patient-derived adult tissue-resident stem cells (ASCs) or pluripotent stem cells (PSCs), paving the way for personalised medicine and primary tissue disease modelling [18, 19]. Although, as with any model, organoids are not without limitations (See Text box: ‘Don’t believe the hype: organoid limitations’). Currently organoids have been used to investigate gene function, cell development, tissue and cellular level physiology and model host-microbiome interactions. They are also useful for modelling infectious and genetic diseases, investigating primary tumour growth, and have applications in drug screening and regenerative medicine [20, 21]. A further key driver of their utility, in addition to being a more physiologically relevant model, is their amenability to both standard and high-tech laboratory techniques and their genetic and molecular tractability. For example, organoids can easily be manipulated using viral and non-viral mediated delivery of CRISPR-Cas9 gene editing [22], they can be investigated using mass spectrometry [23], flow cytometry [24], multiple single cell ‘omics’ technologies [25] and naturally their 3D structure lends themselves to all manner of imaging technologies [26] (Fig. 1). This flexibility provides exciting opportunities for the generation of organoids from multiple organs and disease sources, coupled with their manipulation and phenotypic investigation.
Organoid generation and applications. Organoids can be generated from multiple species and multiple tissues. There are two general sources: adult tissue-resident stem cells (ASCs) and pluripotent stem cells (PSCs). Generation of PSC-derived organoids requires directed differentiation towards the tissue of interest, whereas those derived from ASCs do not. Both sources require specific niche factors and an extracellular matrix (ECM) in which they form 3D multicellular organoids mimicking the tissue of interest. The schematic shows archetypal intestinal organoid development; the different colours indicate different cell types. Both types of organoid can be utilised in many downstream methods, as shown (this list is not exhaustive). BME, Basement Membrane Extract; diff., differentiation; FACS, fluorescence-activated cell sorting; HTS, high-throughput screening. This figure is available as part of a downloadable slideset
Mini-me: Modelling diabetes in a dish
Organoid technology has the power to accelerate diabetes research, particularly cell replacement therapies. The technology perfectly lends itself to the generation of novel sources of beta cells or the production of new cell-based delivery systems for insulin. These opportunities and the current state of the field are well described elsewhere [40,41,42]. There are, however, exciting opportunities beyond cell-based treatments of insulin-dependent diabetes. Organoids allow the modelling of primary disease tissues, insulin-sensitive tissues and peripheral tissues associated with diabetic complication, from primary sources (Fig. 2) without the need for transformed cells or large numbers of donors. We now have the tools to generate organoids from individuals with identified diabetes-associated genetic variants or to introduce these traits with CRISPR/Cas9 and use these organoids to provide a more precise evaluation of their contribution to diabetes pathogenesis. Results from these types of experiment may be informative in stratifying patients for particular interventions. These opportunities suggest that organoids may eclipse current in vitro models, transformed cell lines and short-term culture of primary tissues and provide a new understanding of diabetes pathophysiology.
Mini-me: modelling diabetes in 3D. Diabetes is a multi-organ disease and organoids present an opportunity to generate models that more closely recapitulate its pathology. (a) Organoids can be generated from multiple tissues to allow the modelling of disease progression, investigation of genetic associations, screening of drugs and probing of mechanisms. (b) In the future, combining organoid technology and bioengineering may make it possible to model inter-organ communication in diabetes pathogenesis, creating a virtual diabetic patient on a chip. The image in (b) is adapted from [107], with permission from Elsevier. This figure is available as part of a downloadable slideset
Tissues controlling glucose homeostasis
Pancreas
The first pancreatic organoids were generated from mouse and human embryonic pancreatic cells by Anne Grapin-Botton’s group [43, 44]. These organoids produced progenitor-biased hollow spheres that could be differentiated into branched structures containing acinar, ductal and endocrine lineages. Pancreatic organoids have also been generated from cells derived from the adult pancreas; CD133+ cells isolated from the mouse pancreas can be expanded in vitro and differentiated towards all pancreatic lineages [45, 46]. However, organoids derived from human CD133+ cells require transgenic overexpression of NGN3, MAFA and PDX1 to produce endocrine lineages [47].
Generation of islet organoids by turning 2D-directed differentiation of PSCs into 3D structures, has met with mixed success. Introduction of suspension cultures and an air–liquid interface produced immature beta cells that did not secrete insulin [48, 49]. In contrast, 3D islet-like organoids generated from either human embryonic stem cells [50] or from spontaneously formed endocrine cell clusters produced by stepwise differentiation of human PSCs [51] released insulin in response to glucose in vitro and in vivo. More recent efforts have introduced synthetic hydrogels [52] or co-cultures with HUVECs and mesenchymal stem cells (MSCs) combined with a self-condensation system [53]. These more advanced systems produced islet organoids with increased complexity and maturity, including endothelial cells and vascularisation.
Islet organoids provide potential improvements over the traditional pancreatic beta cell lines as they better mimic islet architecture and morphology. However, their apparent immaturity remains their major drawback. Islet organoids often do not recapitulate nutrient-stimulated insulin secretion adequately. As such, the current technology is not ready for prime-time functional exploration, leaving primary islets as the gold standard tool for assessing islet hormone secretion.
Pancreatic organoids are currently better suited to understanding pancreatic morphogenesis and differentiation. For example, a functional genetic screen in organoids derived from SOX9+ progenitors identified Prdm16 as a novel regulator of islet development [54]. Pancreatic organoids could also provide a platform for drug screening and personalised medicine. PSC-derived organoids have been used to model pancreatic facets of cystic fibrosis and to screen a set of cystic fibrosis transmembrane conductance regulator (CFTR) activators [34]. It is also easy to envisage how organoids could help deepen our understanding of the processes that lead to immune destruction of beta cells or provide more precise details as to how genetic susceptibility loci may interact with the immune system to drive disease initiation and progression. Modelling these immune interactions could range from the simple addition of cytokines or conditioned media from cultured immune cells [55], to the co-culture of sorted and activated immune cells [56], or to more complex techniques developed for mimicking tumour immune microenvironments [57]. To date, few of these types of experiment have been leveraged for diabetes research but the potential is self-evident.
Gut
The gut is a key player in the integration of luminal signals and the control of metabolism. It houses enteroendocrine cells (EECs) that produce over 20 different bioactive peptides implicated in the local control of absorption and motility, the signalling of satiety and augmentation of beta cell function [58, 59].
Organoids provide new opportunities to explore EEC differentiation, how this might be altered in diabetes and obesity or by dietary nutrients and how it could be targeted to manipulate the density of specific cell types for treating metabolic and other diseases.
The limited availability of donor tissue for islet transplantations has initiated an interest in identifying alternative sources. Several studies have explored using the gut as a potential source of insulin-producing cells for the treatment of diabetes. Both PSC- and ASC- derived organoids have been used to examine how EECs can be converted into insulin-secreting cells [60, 61].
Intestinal organoids also allow the investigation of the basic physiology of the gut epithelium and the function of EECs under physiological or metabolic disease conditions. For example, organoids have been used to investigate nutrient sensing, transport and absorption, lipid transport, hormone secretion and intracellular signalling processes [62]. The organoid platform will also help the microbiome field explore mechanisms of action. Microbial diversity is reduced in obesity, but we lack an understanding of the pathological implications. Protocols that give access to the apical (luminal) side of the organoid allow the controlled investigation of the microbiome and its metabolites [31, 39]. As yet, the generation of organoids derived from obese and/or diabetic individuals or those pre- and post-bariatric surgery, for example, have not been leveraged, but the platform offers a unique opportunity to ask critical questions of the role of the gut in the pathogenesis of metabolic disease and in the identification of mechanisms of metabolic surgeries or interventions. Finally, intestinal organoids may provide a finer understanding of the metabolic impact of nutrients on epithelial function at single cell and tissue resolution.
Liver
The liver plays a central role in glucose homeostasis. Insulin resistance in the liver directly leads to hyperglycaemia and is also implicated in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the subsequent development of non-alcoholic steatohepatitis (NASH). Current animal models of NAFLD and NASH do not perfectly mimic natural disease progression. Human liver organoids could provide a flexible tool for modelling the development of hepatic insulin resistance, studying glucose metabolism and hormonal responsiveness in the liver and identifying the underlying mechanisms driving NAFLD and its progression to NASH.
The first human liver organoids were generated from human induced PSC (iPSC)-derived hepatocytes, co-cultured with MSCs and endothelial cells, embedded in Matrigel. These original structures consisted mainly of proliferating hepatoblasts [63, 64]. More recently protocols have emerged to generate organoids from either human adult bile duct-derived bipotent progenitor cells [65] or primary hepatocytes [66, 67], these approaches are reviewed in [68, 69]. Steps to model NAFLD and NASH are under way. Exposing liver organoids from multiple species to fatty acids causes lipid accumulation, demonstrating proof of principle that organoids can be used to model aspects of NAFLD [70]. More recently, a comprehensive human model of steatohepatitis has been described by Takanori Takebe. Using healthy and diseased iPSCs, multicellular human liver organoids were derived, which, when exposed to NEFA, exhibited lipid accumulation, inflammation and fibrosis in a successive manner—key features of human steatohepatitis. This phenotype could be reversed with farnesoid X receptor (FXR)-treatment [71]. It is hoped that such a platform could be used to understand the mechanisms behind the progression of NAFLD to NASH and identify novel treatments for NAFLD/NASH, which currently have no approved pharmacological options.
Muscle
The use of 3D primary tissue-derived cultures to model skeletal muscle in vitro pre-dates the modern organoid era. Vandenburgh et al. pioneered the development of bioartificial muscles (BAMs) generated by suspending myoblasts (muscle progenitor cells), isolated from muscle biopsies, in collagen/Matrigel and then casting them in a silicone mould containing two end attachment sites [72,73,74]. Following differentiation, parallel arrays of myofibres aligned in the direction of the attachment points and contracted when stimulated, but their size was limited because of the lack of a vasculature. This was addressed using a co-culture system containing HUVECs, which produced organoids consisting of aligned fibres with an integrated endothelial network [75]. However, human myoblasts have a limited expansion potential and an unstable differentiated state. To overcome these issues, several protocols have emerged for PSC-derived muscle organoids (reviewed in [76]). These organoids develop myobundles, exhibit contractility following electrical stimulation and can be engineered to include a vasculature and a nervous system [77].
These cultures have the potential to model insulin resistance in 3D but have only been studied in 2D. Iovino et al. generated myotubes from healthy volunteers and individuals with Donohue syndrome, a genetic disorder associated with mutations in the insulin receptor [78]. The myotubes derived from individuals with Donohue syndrome exhibited defects in insulin signalling, glucose uptake and glycogen accumulation, as well as insulin-regulated gene expression. In the future the challenge will be to model insulin resistance in human skeletal muscle organoids generated from diabetic primary or human PSC-derived myoblasts to better understand the development of insulin resistance and its consequences.
Adipose tissue
Adipose tissue is a prominent site of insulin resistance in type 2 diabetic patients and is associated with increased chronic inflammation. Development of a human in vitro model to study the pathogenesis of adipose tissue in metabolic disease would be advantageous. There have been various attempts to generate 3D adipose cultures. Early protocols co-cultured human adipose stromal cells with HUVECS or used lipoaspirates and embedded them in either silk scaffolds or hydrogels [79, 80]. Differentiation of these cultures allowed lipid accumulation and these cultures secreted leptin, the archetypal adipokine. Other groups used adipose progenitors derived from the stromal-vascular fraction of human white adipose tissue and self-organised them into spheroids in hanging drops [81] or by first stirring and then embedding them in Matrigel they generated self-organised vascularised organoids [82]. These novel protocols open the door to using long-term patient-derived adipose cultures to explore the pathology of adipose tissue in metabolic disease.
Modelling diabetic complications
Many of the long-term complications associated with diabetes are caused by microvascular damage, which leads to nephropathy, retinopathy and diabetic neuropathy. A paucity of accurate models that mimic the functional and molecular pathology of these complications has hampered our understanding of the disease mechanism involved and how the complications could be managed or prevented. Organoids may offer an opportunity to address this.
Exposure to hyperglycaemia causes abnormal thickening of the basement membrane of the vasculature, impairing the delivery of oxygen and nutrients to tissues, causing inflammation and damage. Generation of iPSC-derived human blood vessel organoids recapitulates the abnormal thickening of the basement membrane when they are exposed to hyperglycaemia. A subsequent drug screen using this model has identified a novel pathway for drug targeting, underscoring the potential of organoid disease modelling [83].
Human and mouse PSC-derived kidney organoids have been generated using a number of different protocols [84,85,86]. However, these protocols either failed to recapitulate the necessary cell types or produced disconnected nephrons and collecting ducts. Using optimised stepwise differentiation to separately generate nephron and ureteric bud progenitors before mixing in culture with embryo-derived stromal cells produced more refined kidney organoids [87]. In depth reviews of the protocols used for generation of kidney organoids and their uses are available [88,89,90,91]. Kidney organoids have yet to be leveraged for investigating diabetic nephropathy but there are several groups working in this area. The European Commission and the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) have awarded funding for projects to establish in vitro models of diabetic nephropathy and kidney damage using PSC-derived organoids [92, 93]. The Diabetic Complications Consortium have also funded a project to model kidney fibrosis [94]. It will be exciting to see the outcomes of these innovative projects.
The human retina is a complex organ with no regenerative capacity, making it particularly sensitive to damage. Retinal organoids can be generated from both mouse and human ESCs and human iPSCs, which, when embedded in Matrigel, spontaneously form hemispherical epithelial optic vesicle, that invaginate and form the optic cup [95,96,97]. Further protocol refinement has enabled the generation of 3D retinal cups containing mature photoreceptors, an outer-segment-disc and demonstrable photosensitivity [98]. In depth information about retinal organoids is well described in other reviews [99,100,101]. Patient-derived retinal organoids offer an opportunity to more precisely understand the pathophysiology of diabetic retinopathy provide a platform for drug screening and will enable the exploration of genomic variants that render some diabetic patients more susceptible to retinal damage.
The future in 3D
The arguments for applying organoid technology to diabetes research are persuasive. This technology has facilitated the generation of high-fidelity models of virtually any tissue in the body. They offer unprecedented predictive power over traditional 2D models, promise to bring speed and reliability to drug discovery and enable the discovery of novel disease mechanisms. However, organs do not exist in isolation; inter-organ crosstalk is highly relevant to pathophysiology, particularly for diseases like diabetes, which affect multiple organ systems. As such, organoids cannot replace whole body studies, but the field of bioengineering may provide opportunities to move towards a virtual diabetic individual. Organ-on-a-chip technologies have rapidly developed in a short space of time [102]. The technology allows the simultaneous culture of cells from different organs on a microfluidic chip, allowing the precise control of flow between compartments, nutrient supply, shear stress and local mechanical and electrical properties [103]. These systems have often relied on 2D cultures; the challenge now will be to combine organ-on-a-chip technology and 3D organoid technology. Human islet organoids derived from human iPSCs have been generated on an organ-on-a-chip platform [104]. PSCs were initially differentiated into embryonic bodies followed by endoderm differentiation, islet differentiation and maturation. Theses organoids contained heterogeneous islet-like components and functionalities and may resemble their native tissue more closely than static organoid cultures. There are several commercial companies who have developed specialised organ-on-a-chip equipment, ranging from simple multi-well plate systems using gravity to drive flow [105], to complete chip-based pump perfused technologies [106]. It is easy to envisage a future where we will be able to link key human organoid tissue models using chip-based technology to investigate organ-level communication in the pathogenesis of diabetes. Organoid technology is poised to enable researchers to transform our understanding and treatment of diabetes.
Abbreviations
- ASC:
-
Adult tissue-resident stem cell
- EEC:
-
Enteroendocrine cell
- HIO:
-
Human intestinal organoid
- iPSC:
-
Induced pluripotent stem cell
- MSC:
-
Mesenchymal stem cell
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- PSC:
-
Pluripotent stem cell
References
(2018) Method of the year 2017: organoids. Nat Methods 15(1):1. https://doi.org/10.1038/nmeth.4575
Simian M, Bissell MJ (2017) Organoids: a historical perspective of thinking in three dimensions. J Cell Biol 216(1):31–40. https://doi.org/10.1083/jcb.201610056
Clevers H (2016) Modeling development and disease with organoids. Cell 165(7):1586–1597. https://doi.org/10.1016/j.cell.2016.05.082
Fatehullah A, Tan SH, Barker N (2016) Organoids as an in vitro model of human development and disease. Nat Cell Biol 18(3):246–254. https://doi.org/10.1038/ncb3312
Schutgens F, Clevers H (2019) Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol 24:211–234
Lancaster MA, Knoblich JA (2014) Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345(6194):1247125. https://doi.org/10.1126/science.1247125
Lancaster MA, Huch M (2019) Disease modelling in human organoids. Dis Model Mech 12(7):dmm039347. https://doi.org/10.1242/dmm.039347
Sato T, Vries RG, Snippert HJ et al (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244):262–265. https://doi.org/10.1038/nature07935
Sugimoto S, Sato T (2017) Establishment of 3D intestinal organoid cultures from intestinal stem cells. Methods Mol Biol 1612:97–105. https://doi.org/10.1007/978-1-4939-7021-6_7
Sato T, van Es JH, Snippert HJ et al (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469(7330):415–418. https://doi.org/10.1038/nature09637
Sato T, Stange DE, Ferrante M et al (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141(5):1762–1772. https://doi.org/10.1053/j.gastro.2011.07.050
Drost J, van Jaarsveld RH, Ponsioen B et al (2015) Sequential cancer mutations in cultured human intestinal stem cells. Nature 521(7550):43–47. https://doi.org/10.1038/nature14415
McCracken KW, Howell JC, Wells JM, Spence JR (2011) Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc 6(12):1920–1928. https://doi.org/10.1038/nprot.2011.410
Spence JR, Mayhew CN, Rankin SA et al (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470(7332):105–109. https://doi.org/10.1038/nature09691
Wells JM, Spence JR (2014) How to make an intestine. Development 141(4):752–760. https://doi.org/10.1242/dev.097386
Munera JO, Wells JM (2017) Generation of gastrointestinal organoids from human pluripotent stem cells. Methods Mol Biol 1597:167–177
Takebe T, Wells JM (2019) Organoids by design. Science 364(6444):956–959. https://doi.org/10.1126/science.aaw7567
Sinagoga KL, Wells JM (2015) Generating human intestinal tissues from pluripotent stem cells to study development and disease. EMBO J 34(9):1149–1163. https://doi.org/10.15252/embj.201490686
Drost J, Clevers H (2017) Translational applications of adult stem cell-derived organoids. Development 144(6):968–975. https://doi.org/10.1242/dev.140566
Artegiani B, Clevers H (2018) Use and application of 3D-organoid technology. Hum Mol Genet 27(R2):R99–R107. https://doi.org/10.1093/hmg/ddy187
Takahashi T (2019) Organoids for drug discovery and personalized medicine. Annu Rev Pharmacol Toxicol 59:447–462. https://doi.org/10.1146/annurev-pharmtox-010818-021108
Driehuis E, Clevers H (2017) CRISPR/Cas 9 genome editing and its applications in organoids. Am J Physiol Gastrointest Liver Physiol 312(3):G257–G265. https://doi.org/10.1152/ajpgi.00410.2016
Gonneaud A, Asselin C, Boudreau F, Boisvert FM (2017) Phenotypic analysis of Organoids by proteomics. Proteomics 17(20):1700023. https://doi.org/10.1002/pmic.201700023
Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, Clevers H (2017) Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells. Cell Stem Cell 20(2):177–190 e174. https://doi.org/10.1016/j.stem.2016.11.001
Lindeboom RG, van Voorthuijsen L, Oost KC et al (2018) Integrative multi-omics analysis of intestinal organoid differentiation. Mol Syst Biol 14:e8227
Dekkers JF, Alieva M, Wellens LM et al (2019) High-resolution 3D imaging of fixed and cleared organoids. Nat Protoc 14(6):1756–1771. https://doi.org/10.1038/s41596-019-0160-8
Nozaki K, Mochizuki W, Matsumoto Y et al (2016) Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol 51(3):206–213. https://doi.org/10.1007/s00535-016-1170-8
Noel G, Baetz NW, Staab JF et al (2017) A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep 7(1):45270. https://doi.org/10.1038/srep45270
Workman MJ, Mahe MM, Trisno S et al (2017) Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 23(1):49–59. https://doi.org/10.1038/nm.4233
Engevik KA, Matthis AL, Montrose MH, Aihara E (2018) Organoids as a model to study infectious disease. Methods Mol Biol 1734:71–81. https://doi.org/10.1007/978-1-4939-7604-1_8
Nigro G, Hanson M, Fevre C, Lecuit M, Sansonetti PJ (2019) Intestinal organoids as a novel tool to study microbes-epithelium interactions. Methods Mol Biol 1576:183–194. https://doi.org/10.1007/7651_2016_12
Holloway EM, Capeling MM, Spence JR (2019) Biologically inspired approaches to enhance human organoid complexity. Development 146(8):dev166173. https://doi.org/10.1242/dev.166173
Watson CL, Mahe MM, Munera J et al (2014) An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20(11):1310–1314. https://doi.org/10.1038/nm.3737
Hohwieler M, Illing A, Hermann PC et al (2017) Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 66(3):473–486. https://doi.org/10.1136/gutjnl-2016-312423
Oksdath M, Perrin SL, Bardy C et al (2018) Review: synthetic scaffolds to control the biochemical, mechanical, and geometrical environment of stem cell-derived brain organoids. APL Bioeng 2(4):041501. https://doi.org/10.1063/1.5045124
Bartfeld S, Bayram T, van de Wetering M et al (2015) In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148(1):126–136 e126. https://doi.org/10.1053/j.gastro.2014.09.042
Moon C, VanDussen KL, Miyoshi H, Stappenbeck TS (2014) Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol 7(4):818–828. https://doi.org/10.1038/mi.2013.98
Wang Y, Chiang IL, Ohara TE et al (2019) Long-term culture captures injury-repair cycles of colonic stem cells. Cell 179(5):1144–1159 e1115. https://doi.org/10.1016/j.cell.2019.10.015
Co JY, Margalef-Catala M, Li X et al (2019) Controlling epithelial polarity: a human enteroid model for host-pathogen interactions. Cell Rep 26(9):2509–2520 e2504. https://doi.org/10.1016/j.celrep.2019.01.108
Dayem AA, Lee SB, Kim K, Lim KM, Jeon TI, Cho SG (2019) Recent advances in organoid culture for insulin production and diabetes therapy: methods and challenges. BMB Rep 52(5):295–303
Bakhti M, Bottcher A, Lickert H (2019) Modelling the endocrine pancreas in health and disease. Nat Rev Endocrinol 15(3):155–171. https://doi.org/10.1038/s41574-018-0132-z
Shahjalal HM, Abdal Dayem A, Lim KM, Jeon TI, Cho SG (2018) Generation of pancreatic beta cells for treatment of diabetes: advances and challenges. Stem Cell Res Ther 9(1):355. https://doi.org/10.1186/s13287-018-1099-3
Greggio C, De Franceschi F, Figueiredo-Larsen M et al (2013) Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140(21):4452–4462. https://doi.org/10.1242/dev.096628
Bonfanti P, Nobecourt E, Oshima M et al (2015) Ex vivo expansion and differentiation of human and mouse fetal pancreatic progenitors are modulated by epidermal growth factor. Stem Cells Dev 24(15):1766–1778. https://doi.org/10.1089/scd.2014.0550
Jin L, Feng T, Shih HP et al (2013) Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci U S A 110(10):3907–3912. https://doi.org/10.1073/pnas.1301889110
Jin L, Feng T, Zerda R, Chen CC, Riggs AD, Ku HT (2014) In vitro multilineage differentiation and self-renewal of single pancreatic colony-forming cells from adult C57BL/6 mice. Stem Cells Dev 23(8):899–909. https://doi.org/10.1089/scd.2013.0466
Lee J, Sugiyama T, Liu Y et al (2013) Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife 2:e00940. https://doi.org/10.7554/eLife.00940
Pagliuca FW, Millman JR, Gurtler M et al (2014) Generation of functional human pancreatic beta cells in vitro. Cell 159(2):428–439. https://doi.org/10.1016/j.cell.2014.09.040
Russ HA, Parent AV, Ringler JJ et al (2015) Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 34(13):1759–1772. https://doi.org/10.15252/embj.201591058
Shim JH, Kim J, Han J et al (2015) Pancreatic islet-like three-dimensional aggregates derived from human embryonic stem cells ameliorate hyperglycemia in Streptozotocin-induced diabetic mice. Cell Transplant 24(10):2155–2168. https://doi.org/10.3727/096368914X685438
Kim Y, Kim H, Ko UH et al (2016) Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo. Sci Rep 6(1):35145. https://doi.org/10.1038/srep35145
Candiello J, Grandhi TSP, Goh SK et al (2018) 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials 177:27–39. https://doi.org/10.1016/j.biomaterials.2018.05.031
Takahashi Y, Sekine K, Kin T, Takebe T, Taniguchi H (2018) Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation. Cell Rep 23(6):1620–1629. https://doi.org/10.1016/j.celrep.2018.03.123
Sugiyama T, Benitez CM, Ghodasara A et al (2013) Reconstituting pancreas development from purified progenitor cells reveals genes essential for islet differentiation. Proc Natl Acad Sci U S A 110(31):12691–12696. https://doi.org/10.1073/pnas.1304507110
Powell N, Pantazi E, Pavlidis P et al (2019) Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut 69(3):578–590. https://doi.org/10.1136/gutjnl-2019-318483
Ibiza S, Garcia-Cassani B, Ribeiro H et al (2016) Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535(7612):440–443. https://doi.org/10.1038/nature18644
Neal JT, Li X, Zhu J et al (2018) Organoid modeling of the tumor immune microenvironment. Cell 175(7):1972–1988. https://doi.org/10.1016/j.cell.2018.11.021
Tsakmaki A, FPPaBG (2017) 3D intestinal organoids in metabolic research: Virtual reality in a dish. Curr Opin Pharmacol 37:51–58. https://doi.org/10.1016/j.coph.2017.09.003
Chang-Graham AL, Danhof HA, Engevik MA et al (2019) Human intestinal Enteroids with inducible Neurogenin-3 expression as a novel model of gut hormone secretion. Cell Mol Gastroenterol Hepatol 8(2):209–229. https://doi.org/10.1016/j.jcmgh.2019.04.010
Chen YJ, Finkbeiner SR, Weinblatt D et al (2014) De novo formation of insulin-producing "neo-beta cell islets" from intestinal crypts. Cell Rep 6(6):1046–1058. https://doi.org/10.1016/j.celrep.2014.02.013
Bouchi R, Foo KS, Hua H et al (2014) FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun 5(1):4242. https://doi.org/10.1038/ncomms5242
Zietek T, Rath E, Haller D, Daniel H (2015) Intestinal organoids for assessing nutrient transport, sensing and incretin secretion. Sci Rep 5(1):16831. https://doi.org/10.1038/srep16831
Takebe T, Sekine K, Enomura M et al (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499(7459):481–484. https://doi.org/10.1038/nature12271
Takebe T, Sekine K, Kimura M et al (2017) Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep 21(10):2661–2670. https://doi.org/10.1016/j.celrep.2017.11.005
Huch M, Gehart H, van Boxtel R et al (2015) Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160(1-2):299–312. https://doi.org/10.1016/j.cell.2014.11.050
Peng WC, Logan CY, Fish M et al (2018) Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell 175(6):1607–1619. https://doi.org/10.1016/j.cell.2018.11.012
Hu H, Gehart H, Artegiani B et al (2018) Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175(6):1591–1606. https://doi.org/10.1016/j.cell.2018.11.013
Prior N, Inacio P, Huch M (2019) Liver organoids: from basic research to therapeutic applications. Gut 68(12):2228–2237. https://doi.org/10.1136/gutjnl-2019-319256
Akbari S, Arslan N, Senturk S, Erdal E (2019) Next-generation liver medicine using Organoid models. Front Cell Dev Biol 7:345. https://doi.org/10.3389/fcell.2019.00345
Kruitwagen HS, Oosterhoff LA, Vernooij I et al (2017) Long-term adult feline liver Organoid cultures for disease modeling of hepatic Steatosis. Stem Cell Rep 8(4):822–830. https://doi.org/10.1016/j.stemcr.2017.02.015
Ouchi R, Togo S, Kimura M et al (2019) Modeling Steatohepatitis in humans with pluripotent stem cell-derived Organoids. Cell Metab 30(2):374–384. https://doi.org/10.1016/j.cmet.2019.05.007
Vandenburgh H, DelTatto M, Shansky J et al (1996) Tissue-engineered skeletal muscle organoids for reversible gene therapy. Hum Gene Ther 7(17):2195–2200. https://doi.org/10.1089/hum.1996.7.17-2195
Vandenburgh H, Shansky J, Del Tatto M, Chromiak J (1999) Organogenesis of skeletal muscle in tissue culture. Methods Mol Med 18:217–225. https://doi.org/10.1385/0-89603-516-6:217
Powell CA, Smiley BL, Mills J, Vandenburgh HH (2002) Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol 283(5):C1557–C1565. https://doi.org/10.1152/ajpcell.00595.2001
Gholobova D, Decroix L, Van Muylder V et al (2015) Endothelial network formation within human tissue-engineered skeletal muscle. Tissue Eng Part A 21(19-20):2548–2558. https://doi.org/10.1089/ten.TEA.2015.0093
Chal J, Pourquie O (2017) Making muscle: skeletal myogenesis in vivo and in vitro. Development 144(12):2104–2122. https://doi.org/10.1242/dev.151035
Maffioletti SM, Sarcar S, Henderson ABH et al (2018) Three-dimensional human iPSC-derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep 23(3):899–908. https://doi.org/10.1016/j.celrep.2018.03.091
Iovino S, Burkart AM, Warren L, Patti ME, Kahn CR (2016) Myotubes derived from human-induced pluripotent stem cells mirror in vivo insulin resistance. Proc Natl Acad Sci U S A 113(7):1889–1894. https://doi.org/10.1073/pnas.1525665113
Pellegrinelli V, Rouault C, Veyrie N, Clement K, Lacasa D (2014) Endothelial cells from visceral adipose tissue disrupt adipocyte functions in a three-dimensional setting: partial rescue by angiopoietin-1. Diabetes 63(2):535–549. https://doi.org/10.2337/db13-0537
Abbott RD, Wang RY, Reagan MR et al (2016) The use of silk as a scaffold for mature, sustainable unilocular adipose 3D tissue engineered systems. Adv Healthc Mater 5(13):1667–1677. https://doi.org/10.1002/adhm.201600211
Klingelhutz AJ, Gourronc FA, Chaly A et al (2018) Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery. Sci Rep 8(1):523. https://doi.org/10.1038/s41598-017-19024-z
Muller S, Ader I, Creff J et al (2019) Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci Rep 9(1):7250. https://doi.org/10.1038/s41598-019-43624-6
Wimmer RA, Leopoldi A, Aichinger M et al (2019) Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565(7740):505–510. https://doi.org/10.1038/s41586-018-0858-8
Taguchi A, Kaku Y, Ohmori T et al (2014) Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14(1):53–67. https://doi.org/10.1016/j.stem.2013.11.010
Takasato M, Er PX, Chiu HS et al (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526(7574):564–568. https://doi.org/10.1038/nature15695
Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33(11):1193–1200. https://doi.org/10.1038/nbt.3392
Taguchi A, Nishinakamura R (2017) Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21(6):730–746 e736. https://doi.org/10.1016/j.stem.2017.10.011
Nishinakamura R (2019) Human kidney organoids: progress and remaining challenges. Nat Rev Nephrol 15(10):613–624. https://doi.org/10.1038/s41581-019-0176-x
Islam M, Nishinakamura R (2019) How to rebuild the kidney: recent advances in kidney organoids. J Biochem 166(1):7–12. https://doi.org/10.1093/jb/mvz021
Little MH, Combes AN (2019) Kidney organoids: accurate models or fortunate accidents. Genes Dev 33(19-20):1319–1345. https://doi.org/10.1101/gad.329573.119
Little MH, Hale LJ, Howden SE, Kumar SV (2019) Generating kidney from stem cells. Annu Rev Physiol 81:335–357. https://doi.org/10.1146/annurev-physiol-020518-114331
Dimensions (2019) Diabetic nephropathy modelling in hESC-derived 3D kidney organoids. Available from https://app.dimensions.ai/details/grant/grant.7926732. Accessed 1 September 2019
National Centre for the Replacement Refinement & Reduction of Animals in Research (2019) Development of novel models of kidney damage using induced human pluripotent stem cells. Available from www.nc3rs.org.uk/development-novel-models-kidney-damage-using-induced-human-pluripotent-stem-cells. Accessed 1 September 2019
DiaComp (2018) Modeling diabetic kidney fibrosis with kidney organoids derived from human pluripotent stem cells. Available from www.diacomp.org/shared/showSubContractAbstract.aspx?id=3799. Accessed 1 September 2019
Eiraku M, Takata N, Ishibashi H et al (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472(7341):51–56. https://doi.org/10.1038/nature09941
Nakano T, Ando S, Takata N et al (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10(6):771–785. https://doi.org/10.1016/j.stem.2012.05.009
Meyer JS, Howden SE, Wallace KA et al (2011) Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 29(8):1206–1218. https://doi.org/10.1002/stem.674
Zhong X, Gutierrez C, Xue T et al (2014) Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun 5(1):4047. https://doi.org/10.1038/ncomms5047
Achberger K, Haderspeck JC, Kleger A, Liebau S (2019) Stem cell-based retina models. Adv Drug Deliv Rev 140:33–50. https://doi.org/10.1016/j.addr.2018.05.005
Llonch S, Carido M, Ader M (2018) Organoid technology for retinal repair. Dev Biol 433(2):132–143. https://doi.org/10.1016/j.ydbio.2017.09.028
Jin ZB, Gao ML, Deng WL et al (2019) Stemming retinal regeneration with pluripotent stem cells. Prog Retin Eye Res 69:38–56. https://doi.org/10.1016/j.preteyeres.2018.11.003
Ingber DE (2018) Developmentally inspired human 'organs on chips'. Development 145(16):dev156125. https://doi.org/10.1242/dev.156125
Sosa-Hernandez JE, Villalba-Rodriguez AM, Romero-Castillo KD et al (2018) Organs-on-a-chip module: a review from the development and applications perspective. Micromachines 9(10):536. https://doi.org/10.3390/mi9100536
Tao T, Wang Y, Chen W et al (2019) Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip 19(6):948–958. https://doi.org/10.1039/c8lc01298a
van Duinen V, Trietsch SJ, Joore J, Vulto P, Hankemeier T (2015) Microfluidic 3D cell culture: from tools to tissue models. Curr Opin Biotechnol 35:118–126. https://doi.org/10.1016/j.copbio.2015.05.002
Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32(8):760–772. https://doi.org/10.1038/nbt.2989
Huh D, Hamilton GA, Ingber DE (2011) From 3D cell culture to organs-on-chips. Trends Cell Biol 21(12):745–754. https://doi.org/10.1016/j.tcb.2011.09.005
Funding
Work in the authors’ laboratories is supported by European Foundation for the Study of Diabetes (EFSD) and JDRF. PFP is funded as part of the MRC Doctoral Training Partnership programme.
Author information
Authors and Affiliations
Contributions
All authors were responsible for drafting the article and revising it critically for important intellectual content. All authors approved the version to be published.
Corresponding author
Ethics declarations
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Slideset of figures
(PPTX 642 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsakmaki, A., Fonseca Pedro, P. & Bewick, G.A. Diabetes through a 3D lens: organoid models. Diabetologia 63, 1093–1102 (2020). https://doi.org/10.1007/s00125-020-05126-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-020-05126-3