Abstract
This review explores recent advancements and applications of 3D printing in healthcare, with a focus on personalized medicine, tissue engineering, and medical device production. It also assesses economic, environmental, and ethical considerations. In our review of the literature, we employed a comprehensive search strategy, utilizing well-known databases like PubMed and Google Scholar. Our chosen keywords encompassed essential topics, including 3D printing, personalized medicine, nanotechnology, and related areas. We first screened article titles and abstracts and then conducted a detailed examination of selected articles without imposing any date limitations. The articles selected for inclusion, comprising research studies, clinical investigations, and expert opinions, underwent a meticulous quality assessment. This methodology ensured the incorporation of high-quality sources, contributing to a robust exploration of the role of 3D printing in the realm of healthcare. The review highlights 3D printing's potential in healthcare, including customized drug delivery systems, patient-specific implants, prosthetics, and biofabrication of organs. These innovations have significantly improved patient outcomes. Integration of nanotechnology has enhanced drug delivery precision and biocompatibility. 3D printing also demonstrates cost-effectiveness and sustainability through optimized material usage and recycling. The healthcare sector has witnessed remarkable progress through 3D printing, promoting a patient-centric approach. From personalized implants to radiation shielding and drug delivery systems, 3D printing offers tailored solutions. Its transformative applications, coupled with economic viability and sustainability, have the potential to revolutionize healthcare. Addressing material biocompatibility, standardization, and ethical concerns is essential for responsible adoption.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
3D printing has initiated a revolution across various industries, and its impact on therapeutic delivery is undoubtedly no exception. Through its unique ability to fabricate intricate 3D structures with high precision and customization, 3D printing has garnered considerable attention as a promising approach for targeted and efficient therapeutic agent delivery. Within this review, we will delve into the noteworthy progress, utilizations, and forthcoming outlook of 3D printing concerning the domain of therapeutic delivery [1, 2]. 3D printing, also known as additive manufacturing, is an innovative technology that enables the creation of 3D objects through the layer-by-layer deposition of material. It has gained significant recognition in various fields, including engineering, manufacturing, and healthcare. Within the realm of therapeutic delivery, 3D printing is a versatile tool that has garnered attention due to its capacity to fabricate intricate structures with precision and customization [1, 3]. Advancements in 3D printing technology have undeniably reshaped therapeutic delivery as shown in Table I, offering unprecedented opportunities for personalized medicine and targeted drug release. The capability to fabricate intricate three-dimensional structures with precision has paved the way for developing delivery systems that can effectively surmount the limitations of traditional methods as shown in Fig. 1. Notably, researchers have achieved success in utilizing 3D printing to create personalized drug delivery systems tailored to individual patients, thereby enabling precise dosing, controlled release, and targeted drug delivery [4,5,6]. These noteworthy advancements possess the potential to revolutionize healthcare by optimizing therapeutic efficacy while simultaneously minimizing adverse side effects.
Contrasting Cellular Environments—An Analytical Comparison of 2D Monolayer Culture Versus 3D ECM-Based Growth. This graphical representation delineates the inherent distinctions in cellular morphology, conduct, and interactions between conventional 2D cell culture and the progressive 3D cell culture grown upon an Extracellular Matrix (ECM) scaffold. While 2D cultivation lacks the intricacies found in the native tissue microenvironment, 3D ECM-based cultivation provides a more physiologically pertinent framework for the investigation of cellular behavior and responses. This comparative analysis delves into the ramifications of culture dimensionality on cellular physiology and their potential implications in the realms of biomedical research and tissue engineering. Image was generated by Biorender.com
Over the past decade, 3D printing, also recognized as additive manufacturing, has garnered significant attention across diverse fields, including engineering, manufacturing, and healthcare. The capacity to create complex structures layer by layer using various biocompatible materials has paved the way for fabricating personalized drug delivery systems adapted to suit individual patients [7, 8]. This transformative potential can redefine healthcare by facilitating precise dosing, controlled release, and targeted delivery of therapeutic agents. A fundamental benefit of employing 3D printing for therapeutic delivery is its capability to construct drug delivery systems featuring intricate geometries and internal structures. These achievements would pose exceptional challenges if pursued through traditional manufacturing methods. For instance, researchers have successfully printed scaffolds with intricate porosity and interconnected pore networks, thereby promoting tissue growth and regeneration [9, 10]. This, in turn, holds tremendous promise in the realm of regenerative medicine, where 3D-printed scaffolds can serve as vehicles to deliver bioactive molecules, stem cells, or growth factors to damaged tissues, thereby facilitating healing and tissue regeneration. Furthermore, 3D printing empowers the fabrication of drug delivery systems capable of controlled and sustained drug release. Through the incorporation of drug-loaded polymeric matrices or encapsulation of drugs within 3D-printed micro/nanoparticles, researchers have achieved precise release kinetics, leading to improved therapeutic efficacy and reduced side effects [10]. This capability holds remarkable value in the treatment of chronic diseases, wherein long-term drug release is often necessary.
Another significant advantage of 3D printing lies in its potential to enable personalized medicine. Each patient possesses unique physiological characteristics, and their response to drugs can vary significantly. Through 3D printing, the creation of customized drug delivery systems becomes feasible, accounting for individual variations in anatomy, pathology, and drug metabolism. By integrating patient-specific data, such as medical imaging and pharmacokinetic profiles, into the design process, 3D-printed therapeutic delivery systems can be tailored to deliver precise drug doses to specific target sites, thereby maximizing therapeutic outcomes while minimizing systemic toxicity [11, 12].
Despite the remarkable potential of 3D printing for therapeutic delivery, several challenges and limitations necessitate attention as shown in Table I. The crucial factor of utmost significance revolves around the careful choice of materials possessing biocompatibility, mechanical attributes, and controlled degradation characteristics [13, 14]. Ongoing research is diligently focused on developing new biocompatible materials that can be printed with high resolution and exhibit optimal drug release profiles. Additionally, the regulatory landscape for 3D-printed therapeutic delivery systems is still evolving, demanding the standardization of manufacturing processes and quality control measures to ensure the safety and efficacy of these groundbreaking products [15]. In summary, 3D printing has emerged as a potent instrument for therapeutic delivery, facilitating the production of intricate structures while maintaining precise control over drug release. The capability to craft personalized drug delivery systems harbors the potential to transform healthcare, offering individualized treatments that optimize therapeutic effectiveness while minimizing adverse effects. Nevertheless, it is imperative to address challenges concerning material selection, manufacturing processes, and regulatory frameworks to fully unlock the potential of 3D printing in therapeutic delivery. In the upcoming sections of this review, we will delve deeper into the most recent scientific discoveries and explore the specific applications, advancements, and prospects of 3D printing in therapeutic delivery [16,17,18].
This comprehensive review holds a prominent position in contemporary research as it investigates the profound impact of 3D printing in healthcare, with a specific emphasis on therapeutic delivery. In addition to exploring recent advancements, it offers a thorough evaluation of the economic, environmental, and ethical dimensions associated with the widespread adoption of 3D printing in healthcare. What distinguishes this manuscript is its comprehensive examination of key elements, including the development of bioinks, the integration of nanotechnology, and the establishment of personalized drug delivery systems, all from diverse perspectives. Furthermore, it rigorously examines the intricate relationship between 3D printing technology and the continually evolving healthcare landscape, making it an invaluable resource for scholars, practitioners, and policymakers alike. In addition to its comprehensive analysis of the current state of 3D printing in healthcare, this review makes significant contributions to existing literature by bridging substantial gaps. It introduces novel perspectives on the multifaceted applications of 3D printing, illuminating innovative approaches to therapeutic delivery and personalized medicine. By emphasizing economic, environmental, and ethical considerations, it addresses evident gaps in the literature and provides a comprehensive framework for decision-makers. Moreover, the review's extensive analysis of bioink development, nanotechnology integration, and personalized drug delivery systems advances our understanding of these pivotal areas. It also serves as a model for scientific publications, adhering meticulously to the rigorous standards of academic research through its comprehensive research methodology, exhaustive analysis, and multifaceted perspectives.
Background on 3D Printing Technology
3D printing, often referred to as additive manufacturing, has emerged as a disruptive technology with far-reaching implications across a multitude of industries. Initially employed for rapid prototyping in the manufacturing sector, 3D printing has transformed the process of object design and production by meticulously constructing them layer by layer from digital blueprints. This construction is achieved using a wide range of materials, including polymers, metals, and even living cells. Over time, 3D printing has experienced substantial advancements in both technology and materials, making it progressively more accessible and versatile. As a result, it has found applications in a diverse array of fields, including architecture, fashion, art, and notably, healthcare [19]. The remarkable advancements in 3D printing technologies have extended to the field of therapeutic delivery, particularly in the fabrication of micro- and nano-robots. These developments have been driven by using smart materials, improved actuation techniques, and the integration of physical intelligence (PI) and artificial intelligence (AI) into the design process as shown in Table II. Consequently, 3D-printed microrobots hold immense potential as game-changers in minimally invasive medicine [20]. A key factor behind the success of 3D printing in therapeutic delivery lies in the layer-by-layer production process facilitated by computer-aided design (CAD). This approach allows for precise fabrication, overcoming challenges associated with conventional microrobot fabrication methods, such as lithography, deposition techniques, and assembly. When compared to conventional manufacturing techniques, 3D printing stands out for its cost-effectiveness, swift adaptability for design alterations, and its capacity to utilize an extensive array of materials, spanning from metals and polymers to bioinks and composites. The accessibility and consistency of 3D printing have firmly established it as the emerging method of choice for microrobot fabrication, even for individuals with limited expertise in micromanufacturing [21].
Looking ahead, the integration of AI and PI holds immense potential in enhancing the capabilities of 3D-printed microrobots for therapeutic delivery. AI can optimize parameters like dimensions and material selection based on the specific chemical properties of the target site, accelerating the design process. Furthermore, AI can predict the printability of designs and fine-tune 3D printing parameters for optimal outcomes. Once produced, AI can enable precise control of microrobots in vitro and in vivo, adjusting actuation parameters to navigate unpredictable changes in the surrounding environment, such as variations in blood flow rates within vessels [20]. PI empowers microrobots to autonomously sense and adapt to their operating environment. By utilizing stimuli-responsive materials, microrobots can navigate biological fluids with improved efficiency and precision, releasing drugs at specific pH levels, for instance. The convergence of AI and PI opens new avenues for developing intelligent microrobots capable of performing complex tasks with minimal external guidance [22]. While the potential of 3D-printed microrobots for therapeutic delivery is indeed promising, it is vital to acknowledge the challenges and limitations that may hinder their widespread adoption. For instance, ensuring the safety and biocompatibility of these microrobots remains a paramount concern. Rigorous testing and evaluation in preclinical and clinical settings will be essential to establish their efficacy and safety profiles [23]. Moreover, the complexity of integrating AI and PI into microrobot designs necessitates interdisciplinary collaboration among experts in robotics, materials science, biology, and medicine. Such cross-disciplinary efforts will be crucial in advancing the development of intelligent and efficient microrobots that can truly revolutionize therapeutic delivery [23, 24]. In conclusion, the transformative potential of 3D printing in therapeutic delivery is evident through the fabrication of micro- and nano-robots. The marriage of AI and PI in this context offers exciting prospects for enhanced microrobot capabilities and performance. However, comprehensive research, rigorous testing, and interdisciplinary collaboration are imperative to overcome existing challenges and fully exploit the benefits of 3D-printed microrobots in revolutionizing targeted therapeutic delivery. In the subsequent sections of this review, we will delve deeper into the latest scientific findings, explore specific applications, and discuss the future directions of 3D printing in therapeutic delivery [25].
Despite the impressive advancements in creating and controlling 3D-printed microrobots, the transition from laboratory experimentation to clinical deployment is met with substantial challenges. Foremost among these challenges is the economical large-scale production of microscale robotic devices, a hurdle that currently looms large. Additionally, microrobots face a series of obstacles, commencing with their introduction into the human body and the subsequent navigation to reach the intended destination. These hurdles encompass potential immune system reactions and clearance issues, demanding meticulous deliberation and the implementation of strategic mitigation measures. Furthermore, the current standard test procedures for ensuring safety and functionality impose cumbersome and costly barriers, resulting in delays that hinder the rapid translation of these groundbreaking technologies into commercially viable clinical applications. To facilitate the successful integration of 3D-printed microrobots into real-world medical practice, it is essential to address these challenges head-on [20]. Addressing these challenges requires a multifaceted approach involving the development of new materials, fabrication methods, and actuation modalities. Additionally, there is a pressing need for streamlined and efficient test procedures that maintain safety without incurring unnecessary delays. A collaborative, multidisciplinary effort involving engineers, clinicians, and regulatory bodies is necessary to establish comprehensive guidelines and standards. By fostering seamless communication and cooperation among these stakeholders, we can expedite the translation process from the laboratory bench to the patient's bedside. Besides, the field of 3D printing for therapeutic delivery has witnessed significant advancements, holding immense promise for revolutionizing minimally invasive medicine as shown in the SWOT analysis in Table III. The precision fabrication capabilities of 3D printing, combined with the integration of AI, PI, and smart materials, have propelled the development of intelligent microrobots capable of targeted drug delivery, microsurgeries, imaging, and other transformative biomedical applications [20, 26]. Nevertheless, to fully harness the potential of 3D-printed microrobots, it is imperative to address challenges associated with mass production, immune system responses, and test procedures. Through proactive engagement in future research initiatives and the cultivation of collaborative partnerships, we have the potential to surmount these challenges. This collective effort will enable a smooth transition from laboratory settings to the realization of commercially viable and broadly accessible clinical applications. With unwavering commitment and persistence, the fusion of state-of-the-art technology and medical expertise holds the promise of genuinely transforming the landscape of therapeutic delivery and patient care [16].
Importance of Therapeutic Delivery in Healthcare
Therapeutic delivery assumes a pivotal role in modern healthcare, aiming to administer medications, therapies, and medical interventions with unparalleled precision and efficacy. As the healthcare landscape shifts towards personalized medicine and patient-centered care, innovative solutions are imperative for tailored therapeutic delivery, accounting for each patient's unique characteristics and medical history. By customizing drug formulations and precisely delivering therapies to the affected site, therapeutic delivery optimizes treatment outcomes, enhances efficacy, reduces toxicity, and improves patient compliance [17]. In healthcare, the delivery of therapeutic agents plays a critical role in achieving desired treatment outcomes as depicted in Fig. 2. The primary challenge in drug delivery revolves around the precise targeting of specific sites within the body while simultaneously minimizing systemic side effects. Conventional drug delivery approaches like oral ingestion or intravenous infusion inherently possess limitations concerning accuracy, effectiveness, and patient adherence. Nonetheless, the emergence of 3D printing technology has ushered in a paradigm shift within the realm of therapeutic delivery, offering innovative solutions to tackle these challenges. This section delves into the significance of therapeutic delivery in healthcare and elucidates the advantages of 3D printing over traditional drug delivery methods [18]. One of the key advantages of 3D printing in therapeutic delivery resides in its capacity to fabricate complex structures with remarkable precision and customization. Traditional drug delivery methods often lack the ability to precisely target specific sites within the body, leading to broader distribution of therapeutic agents and potential off-target effects. In contrast, 3D printing empowers the design and production of drug delivery systems with intricate geometries, facilitating site-specific delivery and localized drug release. This heightened level of precision not only enhances therapeutic efficacy but also mitigates the risk of adverse effects associated with systemic drug distribution [8, 18, 27]. Another significant advantage of 3D printing in therapeutic delivery is the ability to create drug delivery systems with tailored release profiles. Conventional drug delivery methods frequently result in rapid drug release, leading to suboptimal drug concentrations at the target site or a short duration of action. With 3D printing, it becomes feasible to engineer drug delivery systems with controlled release kinetics, facilitating sustained and controlled drug release over an extended period. This capability proves particularly valuable in the treatment of chronic conditions where maintaining therapeutic drug levels is pivotal for long-term efficacy [28].
The Evolution from 3D Printing to Clinical Utility: This caption highlights the ongoing advancement of 3D printing technology, showcasing its crucial contribution to the restructuring of clinical methodologies and the initiation of a novel era characterized by tailored healthcare solutions. Image was generated by Biorender.com
3D printing introduces a distinctive advantage when it comes to the advancement of combination therapies. This approach allows for the integration of multiple drugs or therapeutic agents into a single delivery system. In contrast, traditional drug delivery methods frequently necessitate the separate administration of individual drugs, which can pose challenges in terms of coordinating drug schedules, realizing synergistic effects, and ensuring patient adherence to complex treatment regimens. By harnessing 3D printing, it is possible to create sophisticated drug delivery systems that combine multiple therapeutic agents, enabling simultaneous and targeted delivery of different drugs. This approach augments treatment efficacy and unlocks new possibilities in personalized medicine [29]. Every patient possesses unique attributes, and their responses to therapeutic treatments can vary significantly. Conventional drug delivery techniques often employ a standardized approach, overlooking individual patient distinctions and requirements. Conversely, 3D printing enables the creation of patient-tailored drug delivery systems designed to precisely match the distinct needs of each individual. By integrating patient-specific data, such as anatomical scans or genetic information, into the design process, personalized drug delivery systems can be created, optimizing treatment outcomes and fostering patient adherence [30]. Therapeutic delivery stands as a crucial aspect of healthcare, and the advancements in 3D printing technology have revolutionized the landscape of drug delivery as shown in Table IV. The precision, customization, tailored release profiles, capacity to develop combination therapies, and patient-specific solutions offered by 3D printing have truly transformed therapeutic delivery. In contrast to conventional drug delivery approaches, 3D printing offers superior targeting precision, heightened treatment effectiveness, and increased patient contentment. As the field advances, ongoing research and innovation in 3D printing for therapeutic delivery are poised to open doors to even more personalized and efficacious healthcare interventions [31].
Significance of 3D Printing for Therapeutic Delivery
In recent years, 3D printing has emerged as a revolutionary technology in the field of therapeutic delivery, propelling the healthcare landscape towards personalized medicine. Its remarkable capacity to create intricate, patient-specific structures with precise control has opened unprecedented possibilities in healthcare interventions. Leveraging the capabilities of 3D printing, healthcare experts can presently conceive and produce personalized drug delivery mechanisms, implants, tissue support structures, and medical equipment, all meticulously crafted to cater to the specific requirements of individual patients [32]. A defining advantage of 3D printing in therapeutic delivery lies in its capability to fabricate complex geometries that prove challenging or even unattainable with traditional manufacturing methods. As an illustration, 3D-printed implants can be meticulously engineered to mirror the precise dimensions and contours of a patient's anatomical features, guaranteeing an ideal fit and functionality. Such a high degree of personalization markedly elevates patient comfort, diminishes the likelihood of complications, and augments the overall efficacy of the treatment [1]. Moreover, 3D printing offers a significant edge in achieving complex drug release profiles. While traditional drug delivery methods often entail simple drug release kinetics characterized by rapid initial release followed by a decline in drug concentration, certain therapeutic applications necessitate more sophisticated release patterns, such as pulsatile, sustained, or delayed release. Through 3D printing, researchers can engineer drug delivery systems with finely controlled release profiles, allowing for customized drug release kinetics tailored to the specific requirements of the therapeutic agent and the condition being treated [33]. Moreover, the utilization of various materials within 3D printing facilitates the direct integration of therapeutic substances, including drugs or growth factors, into the fabricated constructs. This groundbreaking capacity ushers in thrilling prospects for precise and regulated drug delivery, allowing for the release of medications at designated locations and in tailored dosages. Consequently, this approach minimizes systemic side effects, contributing to more effective therapeutic outcomes. The versatility of 3D printing also extends to the realm of combination therapies and multi-drug delivery, where the synergistic effects of multiple therapeutic agents can significantly enhance treatment outcomes. By incorporating different drugs within a single dosage form, researchers can harness the benefits of synergistic drug interactions, improve treatment efficacy, and simplify the drug administration process for patients [34]. Beyond its influence on individualized drug delivery, 3D printing assumes a central role in tissue engineering and regenerative medicine. The production of intricate scaffolds and biocompatible frameworks via 3D printing empowers the generation of intricate tissues and organs, thereby offering invaluable utility in transplantation, disease modeling, and drug experimentation. This domain exhibits considerable potential for tackling the organ shortage predicament and propelling the boundaries of regenerative medicine forward [35,36,37].
3D printing has the potential to revolutionize the field of advanced drug formulations, particularly for poorly soluble or highly potent drugs. By leveraging the versatility of 3D printing techniques, researchers can develop novel drug formulations that enhance drug solubility, improve bioavailability, or enable targeted drug delivery. For instance, the incorporation of nanoscale drug carriers or encapsulating drugs within biocompatible polymers through 3D printing can enhance drug stability, control drug release kinetics, and facilitate targeted drug delivery to specific tissues or cells. Such advancements in drug formulation have the potential to address longstanding challenges in drug delivery and unlock new avenues for therapeutic interventions [35]. The significance of 3D printing in therapeutic delivery cannot be overstated. This transformative technology offers unparalleled precision, personalization, and customization in drug delivery, thereby facilitating patient-centric treatment approaches. With the ability to fabricate customized dosage forms, achieve complex drug release profiles, facilitate combination therapies, and develop advanced drug formulations, 3D printing holds tremendous promise in revolutionizing healthcare. As researchers continue to explore and optimize 3D printing techniques, further advancements in therapeutic delivery are expected, leading to improved treatment outcomes, enhanced patient satisfaction, and ultimately, a more personalized and effective healthcare landscape [29, 35]. 3D printing technology has emerged as a powerful tool in the realm of therapeutic delivery, reshaping the landscape of healthcare interventions. Its capacity to customize drug delivery systems, create patient-specific implants, and facilitate tissue engineering has unlocked new possibilities in personalized medicine and patient care. As we delve further into this article, we will explore the various applications, advancements, challenges, and future directions of 3D printing in therapeutic delivery, providing a comprehensive understanding of this transformative technology in healthcare [38].
Overview of 3D Printing in Therapeutic Delivery
One of the key advantages that sets 3D printing apart in therapeutic delivery is its unparalleled ability to create intricate and customized drug delivery systems. By leveraging a diverse range of biocompatible materials, 3D printers can fabricate structures with precise control over their architectures, geometries, and internal features. This remarkable capability empowers the generation of patient-specific drug delivery devices tailored to accommodate individual physiological variations, ultimately leading to heightened therapeutic efficacy and minimized side effects [36]. Moreover, 3D printing unlocks the potential for controlled and sustained drug release. Through adeptly incorporating drugs into biodegradable polymeric matrices or encapsulating them within 3D-printed micro/nanoparticles, researchers have achieved remarkable control over drug release kinetics. Such a capacity proves particularly invaluable for treating chronic diseases and conditions requiring long-term therapy, where maintaining consistent therapeutic levels and minimizing fluctuations are pivotal [39, 40]. Another noteworthy advantage of 3D printing in therapeutic delivery lies in its capacity to seamlessly integrate multiple therapeutic agents into a single dosage form. By expertly orchestrating the spatial distribution of drugs within a 3D-printed structure, multi-drug delivery systems can be tailored to target complex diseases or address multiple pathological factors simultaneously. This innovative approach holds the promise of synergistic effects, personalized combination therapies, and significantly enhanced treatment outcomes [41]. Notwithstanding these promising advancements, the realm of 3D printing for therapeutic delivery is not without challenges and limitations. One such hurdle lies in the meticulous selection of materials boasting requisite biocompatibility, mechanical properties, and controlled degradation characteristics. Research efforts remain ongoing to develop novel biomaterials that seamlessly align with 3D printing processes while offering optimal drug release profiles. Additionally, the regulatory landscape encompassing 3D-printed therapeutic delivery systems is still evolving, necessitating standardized manufacturing processes and the implementation of rigorous quality control measures to ensure utmost safety and efficacy [42]. In conclusion, 3D printing stands as a commanding force in the field of therapeutic delivery, presenting unparalleled opportunities for personalized medicine and precise drug administration as highlighted in Table V. The capacity to fabricate customized drug delivery systems, exercise precise control over drug release kinetics, and integrate multiple therapeutic agents offers immense potential for elevating treatment outcomes to new heights. However, it is imperative to address challenges related to material selection and regulatory considerations to fully harness the capabilities of 3D printing in therapeutic delivery. In the subsequent sections of this review, we shall delve deeper into specific applications, recent advancements, and the promising future prospects of 3D printing in therapeutic delivery [43].
Definition and Principles of 3D Printing
3D printinghas surfaced as a game-changing technology across various sectors. It boasts the capacity to construct three-dimensional objects incrementally, guided by CAD. Within the domain of therapeutic delivery, 3D printing has attracted considerable interest owing to its extraordinary adaptability and precision. This state-of-the-art technology enables meticulous deposition and solidification of materials, resulting in the formation of intricate structures with exceptional precision and complex geometries [44].
Advantages of 3D Printing in Therapeutic Delivery
Within the realm of therapeutic delivery, 3D printing presents a striking advantage by enabling the construction of personalized drug delivery systems and medical equipment customized to meet the unique requirements of individual patients. This capability is made possible through the utilization of diverse bioactive materials, including polymers, hydrogels, and even living cells, allowing for the direct integration of therapeutic substances into the printed constructs. Consequently, this empowers precise and regulated drug release, enhancing targeted therapeutic outcomes. This unprecedented capability opens up new frontiers in personalized medicine, where treatment strategies can be precisely tailored to specific patient requirements [45].
Types of 3D Printing Technologies Applicable to Therapeutic Delivery
Several 3D printing technologies find application in therapeutic delivery, each endowed with distinct advantages and limitations. The selection of a specific technology hinges upon the desired application, the materials involved, and the required resolution. In this context, we highlight some prominent types of 3D printing technologies commonly employed in therapeutic delivery:
Fused Deposition Modeling (FDM): FDM, a widely utilized 3D printing technique, employs thermoplastic filaments. The material is heated and then extruded through a nozzle, layer by layer, to craft the desired object. FDM bestows versatility in material selection and is particularly well-suited for fabricating drug-loaded matrices and scaffolds in tissue engineering application [46, 47].
Stereolithography (SLA): SLA employs a liquid resin that undergoes solidification upon exposure to a particular wavelength of light. The process involves incremental movements of a build platform, with a laser or projector selectively curing the resin in a layer-by-layer fashion, ultimately shaping the desired structure. SLA boasts high-resolution printing capabilities, making it suitable for intricate drug delivery systems and microfluidic devices [48, 49].
Selective Laser Sintering (SLS): SLS entails the use of a laser to selectively fuse powdered materials, such as polymers or ceramics, layer by layer. This technique allows for the fabrication of porous structures with precise control over pore size and interconnectivity. SLS proves particularly useful for creating drug-loaded implants and scaffolds for tissue regeneration [50].
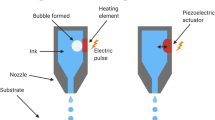
Inkjet-based 3D Printing: This technology utilizes inkjet printheads to deposit droplets of bioinks containing cells or therapeutic agents onto a substrate as shown in Fig. 3. By meticulously controlling the deposition process, complex structures can be created, enabling the fabrication of tissue constructs and drug-loaded microcapsules [51].
Divergent Strategies in Tissue Engineering; In this visual representation, we underscore two divergent strategies within the realm of tissue engineering. To the left, the bioink methodology entails the integration of cells, polymers, and assorted materials into a formable bioink blend. This bioink is then administered into a scaffold, followed by subsequent in vitro incubation to stimulate tissue growth. On the right, the biomaterial ink approach centers on the utilization of biomaterials such as biopolymers, DNA, and nanomaterials for the direct fabrication of the scaffold. Subsequently, cells of interest are introduced onto the scaffold, with subsequent in vitro nurturing. These methodologies provide distinct avenues for tissue engineering, each endowed with unique merits and considerations. Image was generated by Biorender.com
To sum up, 3D printing has risen as a potent instrument within the sphere of therapeutic delivery, affording the capability to craft personalized drug delivery systems and medical equipment precisely attuned to the unique requirements of individual patients. Various 3D printing technologies, including FDM, SLA, SLS, and inkjet-based printing, provide unique advantages in terms of material versatility, resolution, and fabrication capabilities. As we explore the subsequent sections, we will delve deeper into the specific applications, recent advancements, and future prospects of these 3D printing technologies in the context of therapeutic delivery [8, 52].
Advantages and Limitations of 3D Printing in Therapeutic Delivery
The integration of 3D printing in therapeutic delivery offers numerous advantages that have the potential to revolutionize healthcare. These advantages include personalization, the ability to fabricate complex geometries, precise control over drug release, and rapid prototyping and manufacturing. However, several limitations such as material selection, resolution and scalability, and regulatory considerations need to be addressed to fully realize the potential of 3D printing in therapeutic applications [53, 54].
Personalization: 3D printing enables the customization of drug delivery systems and medical devices, catering to individual patient requirements. This personalized approach leads to more effective and targeted treatments, improving patient outcomes [55].
Complex Geometries: Through the application of 3D printing, it becomes feasible to create structures with complex geometries that prove difficult to achieve through traditional manufacturing techniques. This capacity opens the door to designing drug delivery systems capable of adapting to precise anatomical locations, thereby enhancing the effectiveness of therapeutic interventions [52, 56].
Controlled Drug Release: Incorporating therapeutic agents directly into printed structures enables precise control over drug release kinetics. This control allows for sustained or triggered release profiles, improving the efficacy and safety of therapeutic interventions [57].
Rapid Prototyping and Manufacturing: 3D printing expedites the processes of rapid prototyping and manufacturing, thereby reducing the time necessary for design refinements and expediting the transition from conceptualization to practical clinical application. This advantage is particularly valuable in emergency situations or for patients with urgent medical needs [58, 59].
Limitations of 3D Printing in Therapeutic Delivery
Material Selection: The availability of suitable materials for 3D printing, especially biocompatible and bioresorbable materials, remains a challenge. Further research and development are necessary to expand the range of materials compatible with 3D printing techniques [58].
Resolution and Scalability: Achieving high resolution and scalability simultaneously can be challenging. Printing small-scale intricate structures with high precision may require more time and resources, limiting the scalability of the process [60, 61].
Regulatory Considerations: The regulatory approval process for 3D-printed medical devices and drug delivery systems poses unique challenges. Ensuring the safety, efficacy, and quality control of these products requires compliance with rigorous regulatory standards [62, 63].
Applications of 3D Printing in Therapeutic Delivery
Fabrication of Patient-Specific Oral Dosage Forms: One significant advantage of 3D printing in therapeutic delivery is the ability to create patient-specific oral dosage forms. Traditional manufacturing methods often limit the production of oral medications to standardized formulations and sizes. However, with 3D printing, pharmaceutical companies can design and fabricate personalized dosage forms tailored to an individual's specific needs [64].
Utilizing 3D printing technologies such as FDM or SLS, drug-loaded matrices or tablets with precise geometries and drug release profiles can be produced. This customization allows for personalized dosing, optimizing therapeutic outcomes, and improving patient adherence to medication regimens. Furthermore, 3D printing enables the incorporation of multiple drugs or different dosages within a single tablet, facilitating combination therapy and simplifying treatment for patients with complex medication regimens [36, 65].
Personalized Drug Delivery Implants and Devices: In addition to oral dosage forms, 3D printing enables the fabrication of personalized drug delivery implants and devices. By combining biocompatible materials with therapeutic agents, 3D-printed implants can provide targeted and sustained drug release directly at the site of action. This localized drug delivery approach minimizes systemic side effects and maximizes therapeutic efficacy [66, 67]. For example, 3D-printed drug-eluting stents have been developed to treat cardiovascular diseases. These stents can be customized to match the patient's vascular anatomy and incorporate drugs that promote tissue healing and prevent restenosis. Similarly, personalized drug delivery devices, such as transdermal patches or inhalation devices, can be designed using 3D printing techniques to precisely control the release of drugs through the skin or respiratory system [68, 69]. In conclusion, the integration of 3D printing in therapeutic delivery offers significant advantages, including personalization, complex geometries, controlled drug release, and rapid prototyping and manufacturing. However, addressing limitations such as material selection, resolution and scalability, and regulatory considerations is crucial for fully harnessing the potential of 3D printing in therapeutic applications. As we delve further into this article, we will explore specific applications, recent advancements, and future prospects of 3D printing in therapeutic delivery, providing a comprehensive understanding of this transformative technology in healthcare [70, 71].
Tissue Engineering and Regenerative Medicine
Biofabrication of Complex Tissue Structures
Within the domain of tissue engineering and regenerative medicine, 3D printing assumes a central role by facilitating the biofabrication of intricate tissue structures. The fusion of biomaterials with living cells empowers researchers to generate 3D-printed constructs that replicate the structure and function of native tissues. This innovative methodology holds immense potential for applications in tissue repair and organ transplantation [36, 72]. Techniques like bioprinting, which involve the layer-by-layer deposition of bioinks containing cells and supporting materials, have successfully produced functional tissues such as skin, cartilage, and blood vessels. These biofabricated tissues can be utilized for drug testing, disease modeling, and, eventually, patient-specific tissue replacements [73, 74].
Scaffold-Based Approaches for Tissue Regeneration
Another notable application of 3D printing in tissue engineering pertains to the production of scaffolds. Scaffolds serve as transient supportive structures that oversee tissue regeneration by furnishing a platform for cell adherence, proliferation, and vascularization. 3D-printed scaffolds bring forth precise management of their physical and architectural attributes, allowing for customization of parameters such as pore size, porosity, and mechanical resilience [73, 75]. By incorporating bioactive factors and growth factors into the scaffold materials, researchers can promote cellular proliferation, differentiation, and tissue regeneration. These biofunctionalized scaffolds have demonstrated promise in various tissue engineering applications, including bone regeneration, wound healing, and organ-on-a-chip platforms for drug screening [76, 77].
Medical Devices and Implants: 3D-Printed Prosthetics and Orthotics
The field of prosthetics and orthotics has been revolutionized by 3D printing, allowing for the fabrication of customized devices with improved fit, functionality, and aesthetics. Traditional methods often involve labor-intensive and time-consuming processes to create individualized devices. However, 3D printing streamlines the production process, enabling rapid prototyping and customization [78, 79]. By leveraging 3D scanning technologies, patient-specific measurements can be obtained and used to design prosthetic limbs or orthotic braces that precisely match the patient's anatomy. Incorporating lightweight and durable materials, such as carbon fiber-reinforced polymers, 3D-printed prosthetics and orthotics offer enhanced comfort, mobility, and quality of life for individuals with limb loss or musculoskeletal conditions [80, 81].
Customized Implants and Surgical Instruments
Another significant application of 3D printing in therapeutic delivery is the creation of customized implants and surgical instruments. Traditional implants and instruments are often limited to standardized sizes and designs, which may not always meet the unique anatomical requirements of individual patients. 3D printing overcomes these limitations by allowing the fabrication of patient-specific implants and instruments based on medical imaging data [82]. In orthopedic surgery, for instance, 3D-printed implants can be tailored to fit precisely within a patient's bone defect, enhancing stability and promoting better osseointegration. Likewise, surgical guides and instruments can be custom-designed and 3D printed to assist surgeons in performing complex procedures with greater accuracy and efficiency [24, 83]. To sum up, 3D printing has brought about a revolution in therapeutic delivery, as depicted in Table VI. It has achieved this by facilitating the creation of tailored drug delivery systems, tissue-engineered constructs, and individualized medical equipment. In doing so, 3D printing has paved the way for personalized medicine and regenerative therapies. While there remains a need for additional research and development to address challenges related to material selection, resolution, scalability, and regulatory considerations, the potential of 3D printing in therapeutic applications is substantial. As technology continues to progress, we can anticipate further breakthroughs and enhancements within the realm of therapeutic delivery, ultimately enhancing patient outcomes and reshaping the healthcare landscape [7, 59].
Advancements and Innovations in 3D Printing for Therapeutic Delivery
Material Selection and Biocompatibility
One significant advancement in 3D printing for therapeutic delivery is the development of specialized materials called bioinks for bioprinting applications. Bioinks are biocompatible materials that contain living cells and provide support for cell growth and tissue formation. They serve as the building blocks for creating complex biological structures with precise spatial control [84]. Bioinks are meticulously designed to replicate the characteristics of the native extracellular matrix (ECM) and foster a conducive environment for cell viability and growth. Typically, these materials comprise a blend of biomaterials, including natural polymers like collagen and gelatin, or synthetic polymers such as polycaprolactone and polyethylene glycol. They may also incorporate cell-adhesive molecules. Researchers have explored diverse approaches to enhance bioink formulations, including the integration of growth factors, signaling molecules, and other bioactive agents aimed at stimulating cell differentiation and facilitating tissue regeneration [85]. The incorporation of bioinks into bioprinting applications has surfaced as a highly promising path within tissue engineering and regenerative medicine. In this section, we present a thorough examination and discerning assessment of the current body of literature concerning bioinks. Our aim is to spotlight noteworthy discoveries, progressions, and constraints. Moreover, we acknowledge contrasting viewpoints and alternative stances to offer a well-rounded analysis of this swiftly evolving domain [86]. One significant finding in the literature is the successful incorporation of various therapeutic agents into 3D-printed structures for controlled drug release. Studies have demonstrated the integration of small molecules, proteins, growth factors, and nucleic acids within 3D-printed matrices or scaffolds. This approach offers spatial and temporal control over drug release, enabling targeted therapy and enhanced treatment outcomes [87]. For instance, there are reports of a 3D-printed scaffold that incorporates bone morphogenetic protein-2 (BMP-2) for the purpose of bone tissue engineering, leading to enhanced bone regeneration [88, 89].
While bioinks have shown tremendous progress, it is crucial to acknowledge limitations and alternative perspectives in the existing literature as shown in Table VII. One key challenge lies in achieving spatial control over multiple cell types, their organization, and the formation of complex tissue architectures. Furthermore, the lack of standardized protocols and characterization techniques for assessing the quality and functionality of printed constructs hinders the reproducibility and comparability of results across different studies. Efforts should be made to establish standardized protocols and characterization methods to facilitate the advancement and translation of bioprinting technologies. Another viewpoint to consider is the ethical and regulatory aspects associated with bioprinting and the use of bioinks. The translation of bioprinting technologies to clinical applications raises concerns regarding safety, long-term stability, and the ethical implications of printing functional human organs or tissues. Addressing these concerns requires interdisciplinary collaboration involving scientists, ethicists, and regulatory bodies to establish guidelines and frameworks that ensure responsible and ethical use of bioprinting technologies [90, 91]. In summary, bioinks represent a remarkable advancement in 3D printing for therapeutic delivery, offering the potential for creating complex biological structures with controlled drug release capabilities. Although significant progress have been taken, there remains a need for deeper exploration into challenges associated with spatial precision, standardization, and ethical dimensions. As researchers persist in their efforts to investigate and enhance these technologies, the prospects for bioprinting in revolutionizing tissue engineering and regenerative medicine remain highly promising. Ultimately, such advancements stand to benefit patients on a global scale [15, 92].
Integration of Therapeutic Agents into 3D-Printed Structures: Another pivotal advancement in the field of 3D printing for therapeutic delivery lies in the seamless integration of therapeutic agents into 3D-printed structures. By incorporating drugs, growth factors, or other therapeutic molecules directly into the printing process, researchers can engineer functional structures with localized and controlled drug release capabilities [93, 94]. Through meticulous material selection and innovative design, 3D-printed structures can effectively act as sophisticated drug delivery systems, enabling sustained release of therapeutics at specific sites of action. For instance, researchers have successfully embedded antibiotics into 3D-printed bone scaffolds, effectively preventing post-surgical infections. The precise control over release kinetics allows 3D-printed structures to offer targeted therapy, minimize systemic side effects, and significantly enhance overall treatment outcomes [33]. The integration of therapeutic agents into 3D-printed structures has garnered substantial attention as a promising approach for advanced drug delivery systems. In this section, we present a thorough analysis and critical evaluation of the existing literature on this groundbreaking topic, meticulously highlighting key findings, advancements, and limitations. Our analysis also takes into account opposing viewpoints and alternative perspectives, providing a balanced outlook that acknowledges differing opinions and limitations in the field [34]. A remarkable finding in the literature is the successful incorporation of various therapeutic agents into 3D-printed structures, empowering precise control over drug release. Among the therapeutic agents that have been incorporated are small molecules, proteins, growth factors, and nucleic acids, each serving a specific therapeutic purpose within the 3D-printed matrices or scaffolds. This approach offers not only spatial but also temporal control over drug release, presenting an exciting opportunity for targeted therapy and substantially improved treatment outcomes [95]. For instance, Kawai et al. (2021) conducted pioneering work by developing a 3D-printed scaffold loaded with bone morphogenetic protein-2 (BMP-2), leading to remarkable advancements in bone tissue engineering and enhanced bone regeneration [96].
Furthermore, the utilization of 3D printing techniques enables precise distribution of therapeutic agents within the printed structures. This spatial control enables the creation of highly sophisticated drug delivery systems, such as multi-compartment devices or gradient release profiles. Through meticulous design and architectural planning, researchers can achieve differential drug release rates, effectively mimicking physiological conditions and optimizing therapeutic efficacy. Nonetheless, it is vital to be cognizant of potential challenges associated with achieving uniform distribution and homogeneity of the incorporated agents throughout the printed structures [29]. While the integration of therapeutic agents into 3D-printed structures shows immense promise, it is equally important to acknowledge limitations and alternative perspectives within the existing literature. A key challenge lies in the selection and compatibility of materials used for 3D printing with therapeutic agents. Different drugs or biologics may possess varying chemical properties and stability requirements, necessitating meticulous consideration during the formulation of bioinks or printable materials [97]. Moreover, the potential interactions between drug molecules and the printing process itself, such as exposure to high temperatures or shear forces, may impact the stability or activity of the therapeutic agents. Thus, further research is imperative to optimize the formulation and printing parameters to ensure the integrity and efficacy of the incorporated agents [98]. Moreover, the regulatory considerations and approval processes for 3D-printed drug delivery systems present formidable challenges. The complexity associated with integrating therapeutic agents within 3D-printed structures may necessitate additional scrutiny in terms of safety, quality control, and long-term stability. Regulatory agencies may demand compelling evidence regarding the compatibility, stability, and controlled release profile of the integrated agents before approving their clinical use. To overcome these challenges, it is of utmost importance to address regulatory considerations and engage in close collaboration with regulatory bodies to facilitate the seamless translation of 3D-printed drug delivery systems into clinical practice [99]. In conclusion, the integration of therapeutic agents into 3D-printed structures signifies an exciting and promising avenue for advanced drug delivery systems. The ability to achieve controlled drug release and spatial control within the printed structures holds great potential for personalized medicine and targeted therapies. However, it is crucial to confront challenges related to material compatibility, formulation optimization, and regulatory considerations. Future research endeavors should prioritize overcoming these limitations and establish comprehensive guidelines to support the safe and effective translation of 3D-printed drug delivery systems [9].
Multi-material and Multi-functional Printing
Advancements in 3D printing technology have ushered in a new era of multi-material printing, revolutionizing various applications by integrating distinct materials within a single 3D-printed device or construct. This innovation has resulted in enhanced functionality and performance, as different materials like polymers, metals, ceramics, and composites offer tailored mechanical, electrical, thermal, or biological properties that can be precisely engineered to meet specific requirements [100]. For example, in the field of prosthetics, the utilization of multi-material printing has facilitated the fabrication of personalized sockets featuring both sturdy structural elements and adaptable interfaces. This innovation has resulted in heightened comfort and functionality for users. Likewise, within drug delivery systems, the amalgamation of biocompatible polymers with hydrogels or porous materials enhances the precision of drug release kinetics, consequently bolstering therapeutic effectiveness [7]. In this section, we undertake a comprehensive analysis and critical evaluation of the existing literature on multi-material and multi-functional printing, with a focus on findings, advancements, and limitations of this cutting-edge technology. By acknowledging opposing viewpoints and alternative perspectives, we strive to provide a balanced analysis that encompasses differing opinions and limitations within the field. One key finding in the literature is the successful combination of different materials in 3D printing to create objects with enhanced functionality and performance. Researchers have delved into diverse methodologies for the integration of materials possessing distinct properties, thereby enabling the creation of intricate structures customized to specific characteristics. For instance, it has been demonstrated the integration of conductive materials into 3D-printed structures, enabling the creation of functional electronic devices [101]. Moreover, the use of multi-material printing has facilitated the development of objects with gradient properties. This design approach offers unique opportunities, particularly in tissue engineering, where the seamless integration of different materials can mimic the complex gradients found in natural tissues. However, precise control and optimization of material transitions within printed objects pose challenges that warrant consideration [102].
While multi-material and multi-functional printing show great promise, it is essential to acknowledge limitations and alternative perspectives in the existing literature. One significant challenge lies in achieving strong interfacial adhesion between different materials. Ensuring compatibility and bonding between dissimilar materials is crucial to avoid delamination or weak interfaces within the printed objects. Additionally, the compatibility of materials with the 3D printing process itself, such as their melt or cure temperatures, viscosity, or curing kinetics, can pose challenges for successful multi-material printing. Further research is needed to develop innovative approaches or surface treatments that promote better material compatibility and interfacial adhesion [103].
Moreover, the scalability and efficiency of multi-material printing techniques are areas of concern. Additional steps, such as material switching or nozzle changes, may increase printing time and complexity. Scaling up the process to larger volumes or industrial applications may pose challenges in terms of process reliability, speed, and cost-effectiveness. Future research should focus on developing efficient and high-throughput multi-material printing techniques to overcome these limitations [104, 105]. In conclusion, the integration of multiple materials in 3D printing has opened exciting avenues for enhanced functionality and performance of printed objects. The ability to tailor material properties allows for the creation of complex structures with unique characteristics as summarize in Table VIII. However, challenges related to material compatibility, bonding, scalability, and efficiency need to be addressed. By addressing these limitations, we can unlock the full potential of multi-material printing for diverse applications, ultimately advancing the field of 3D printing for therapeutic delivery [106]. Integration of Nanoparticles for Targeted Drug Delivery: The convergence of nanotechnology and 3D printing has given rise to a new era of targeted drug delivery systems. By incorporating nanoparticles within 3D-printed structures, researchers can augment the therapeutic efficacy and specificity of drug delivery. Nanoparticles serve as carriers that encapsulate drugs, shielding them from degradation and facilitating their precise delivery to specific cells or tissues [107]. An exemplary illustration of this synergy is the integration of magnetic nanoparticles into 3D-printed scaffolds or implants, enabling precise drug targeting through the application of external magnetic fields. Similarly, nanoparticles functionalized with specific ligands can selectively bind to particular cell receptors, offering targeted therapies for diseases such as cancer. The amalgamation of nanotechnology and 3D printing expands the therapeutic options available and lays the groundwork for the advancement of personalized medicine [108].
Surface Modification of 3D-Printed Structures for Enhanced Interactions
Surface modification assumes a pivotal role in enhancing the interactions between 3D-printed structures and biological entities. By introducing nanoscale attributes, coatings, or biomolecules onto the surface of 3D-printed constructs, researchers can stimulate cell adhesion, regulate immune reactions, and enhance the biocompatibility of the printed materials [109]. Various surface modification techniques, such as plasma treatment, electrospinning, or layer-by-layer assembly, can be employed to tailor the surface properties of 3D-printed structures. These modifications effectively promote cell attachment and proliferation, facilitate tissue integration, and minimize adverse reactions. The precise control over surface characteristics empowers researchers to optimize the performance and biocompatibility of 3D-printed devices and implants [110]. To summarize, the progress and innovations in 3D printing for therapeutic delivery have significantly expanded the frontiers of personalized medicine, tissue engineering, and precise drug delivery. Key developments such as bioinks, the incorporation of therapeutic substances, and the capacity to fabricate complex multi-material and multi-functional structures have brought about a revolution in the field. Additionally, the synergy between nanotechnology and 3D printing has facilitated accurate drug delivery and enhanced interactions with biological systems. As ongoing research and development in this domain advance, we can anticipate further breakthroughs that will reshape the landscape of therapeutic delivery, ultimately leading to improved patient outcomes and the continued advancement of healthcare [111, 112].
Challenges and Future Directions
The domain of 3D printing has experienced notable progress in a multitude of sectors, encompassing healthcare, aerospace, and manufacturing. Nonetheless, it is marked by a host of challenges and constraints that are instrumental in shaping the future trajectory of this technology. In this section, we offer a thorough examination and astute assessment of these challenges and potential future pathways in 3D printing. Our approach includes the acknowledgment of contrasting viewpoints and alternative perspectives to provide a comprehensive and balanced analysis of diverse opinions and limitations within the field [113]. One of the key challenges in 3D printing is the limited range of printable materials. Although 3D printing encompasses polymers, metals, ceramics, and composites, the selection is still relatively restricted compared to traditional manufacturing methods. Expanding the range of printable materials is crucial to meet diverse requirements and address limitations in material properties, such as strength, flexibility, or thermal stability. The development of multi-functional materials, such as self-healing polymers or shape-memory alloys, can introduce new capabilities and enhance the performance of 3D-printed objects [114]. Another challenge lies in the scalability and production efficiency of 3D printing. Current printing processes may suffer from limited production speed and capacity, making it challenging to meet the demands of large-scale manufacturing. Improving printing speed, enhancing throughput, and optimizing printing parameters are crucial aspects for the widespread adoption of 3D printing in industrial applications. Additionally, developing hybrid approaches that combine 3D printing with other manufacturing techniques, such as injection molding or machining, can potentially overcome the limitations in production efficiency [115]. Furthermore, the lack of standardization in 3D printing processes, materials, and design files poses challenges for interoperability and quality control. The absence of standardized file formats, printing protocols, and post-processing techniques can hinder the exchangeability and reproducibility of 3D-printed objects. Standardization efforts are required to establish guidelines and best practices that ensure compatibility, consistency, and quality across different printers, materials, and applications. Collaborative initiatives involving industry, academia, and regulatory bodies are necessary to develop comprehensive standards for the field [61, 116]. Despite these challenges, several future directions can shape the evolution of 3D printing. One direction involves advancements in multi-material printing and the integration of different functionalities. Exploring novel materials, such as conductive inks or biocompatible polymers, and developing techniques for precise material placement and transition can open up new possibilities for diverse applications. Additionally, the integration of 3D printing with other emerging technologies, such as nanotechnology or artificial intelligence, holds promise for further innovation and advancement in the field [61, 117]. Moreover, the development of sustainable and environmentally friendly approaches in 3D printing is gaining importance. The reduction of waste, the use of recycled materials, and the exploration of bio-based or biodegradable materials are essential considerations for the future of 3D printing. Implementing circular economy principles and optimizing energy consumption and material usage can contribute to a more sustainable and responsible practice of 3D printing [118]. In conclusion, while 3D printing has made significant strides, challenges and limitations persist in the field. The expansion of printable materials, improvements in scalability and production efficiency, and standardization efforts are crucial for the future development and adoption of 3D printing. Additionally, exploring advancements in multi-material printing, integration with other technologies, and sustainable practices can further enhance the capabilities and impact of 3D printing. Future research and collaborative efforts are necessary to address these challenges and shape the future direction of 3D printing [119]. Some of the major challenges in 3D printing are discussed below.
Evaluation of the Economic and Cost-Effectiveness of 3D-Printed Therapeutic Delivery Systems Compared to Traditional Approaches
The economic and cost-effectiveness evaluation of 3D-printed therapeutic delivery systems compared to traditional approaches is a pivotal aspect in determining the viability and widespread adoption of this technology in healthcare settings. While the apparent advantages of 3D printing in therapeutic delivery highlight its importance, it remains crucial to conduct a comprehensive evaluation of the economic ramifications, cost-effectiveness, and enduring value associated with the adoption of 3D-printed solutions as opposed to conventional methodologies [120]. At the forefront of evaluating the economic impact of 3D printing is the consideration of the initial investment required for setting up the infrastructure, encompassing the cost of 3D printers, materials, and supporting equipment. Although the upfront costs associated with acquiring 3D printing technology can be substantial, it is crucial to take into account the potential long-term benefits and cost savings that can be achieved through the utilization of 3D-printed therapeutic delivery systems [121]. One area where 3D printing can offer economic benefits is in the manufacturing of medical devices and implants customized for individual patients. Traditional manufacturing techniques frequently involve intricate and labor-intensive procedures, which result in higher costs and extended production schedules. In contrast, 3D printing enables the direct fabrication of customized devices based on patient-specific anatomical data, mitigating the need for manual customization and streamlining the production workflow. This can lead to cost savings in terms of reduced labor, material waste, and inventory management [122]. Furthermore, 3D printing facilitates the consolidation of multiple components into a single printed structure, thereby obviating the need for assembly and reducing associated costs. This aspect can be particularly advantageous in the production of complex medical devices or drug delivery systems necessitating intricate designs and precise integration of various functionalities. The capability to create multi-material and multi-functional objects in a single printing process enhances efficiency and potentially reduces overall production costs [123]. Integral to evaluating the economic viability of 3D-printed therapeutic delivery systems is cost-effectiveness analysis. Such studies compare the costs and outcomes of 3D-printed interventions with those of conventional approaches, taking into account factors like treatment efficacy, patient outcomes, quality of life, and long-term cost implications [30]. In certain cases, the use of 3D-printed therapeutic delivery systems has demonstrated cost-effectiveness when juxtaposed with traditional methods. For example, in orthopedics, the production of customized orthotic devices or implants using 3D printing has shown potential cost savings by diminishing the need for repeated fittings, adjustments, and revisions. Additionally, the enhanced patient comfort, functionality, and long-term outcomes associated with personalized 3D-printed solutions can contribute to overall cost-effectiveness by minimizing the need for subsequent interventions or rehabilitative care [124]. In the context of drug delivery systems, 3D printing's precise control over drug release profiles and optimized dosing can potentially improve treatment efficacy while reducing medication waste and associated costs. By tailoring drug delivery systems to individual patient needs, 3D printing offers the potential for personalized medicine and targeted therapies, maximizing therapeutic outcomes while minimizing adverse effects [125, 126]. However, it is crucial to acknowledge that the cost-effectiveness of 3D printing in therapeutic delivery is influenced by various factors, including the specific medical condition, the complexity of the intervention, the availability of alternative treatment options, and the healthcare system in which it is implemented. Economic evaluations should consider the entire patient care pathway, encompassing preoperative planning, surgical procedures, postoperative care, and long-term follow-up, to comprehensively assess the value proposition of 3D-printed therapeutic delivery systems [127]. Moreover, the long-term cost implications and sustainability of 3D printing in healthcare must be evaluated. While 3D printing can offer cost savings in certain aspects of care delivery, factors such as material costs, maintenance and upgrade expenses, regulatory compliance, and ongoing research and development efforts should be considered in assessing the overall economic impact [128]. In conclusion, the evaluation of the economic and cost-effectiveness of 3D-printed therapeutic delivery systems compared to traditional approaches is critical in determining the value proposition and feasibility of implementing this technology in healthcare settings. While initial investments in 3D printing infrastructure can be substantial, potential long-term cost savings, increased efficiency, and improved patient outcomes may justify the adoption of 3D-printed solutions. Cost-effectiveness analysis, taking into account treatment efficacy, patient outcomes, and long-term cost implications, can provide insights into the economic viability of 3D printing in therapeutic delivery. Sustained research efforts, exhaustive economic assessments, as demonstrated in Table IX, and collaborative endeavors involving various stakeholders are indispensable to gain a comprehensive understanding of the economic consequences and to unlock the potential advantages of integrating 3D printing into healthcare [129].
Integration of 3D-Printed Therapeutic Interventions: Understanding Patient Perspectives and Acceptance
The successful implementation and widespread adoption of personalized 3D-printed therapeutic interventions in healthcare depends significantly on understanding patient perspectives and acceptance of this innovative technology. Patient-centered care places significant emphasis on engaging patients in decision-making processes, taking into account their values, preferences, and experiences. Consequently, it is essential to delve into how patients perceive and embrace these personalized treatments, examining their expectations, apprehensions, and the overall influence on their healthcare journey. This section presents a comprehensive review of existing literature on patient perspectives and acceptance of personalized 3D-printed therapeutic interventions, highlighting key findings and offering insights into the implications for clinical practice [130, 131].
Patient Perceptions and Expectations
Patient perceptions and expectations of personalized 3D-printed therapeutic interventions play a pivotal role in influencing their acceptance and satisfaction with these treatments. Studies have identified several key factors that shape patient attitudes towards 3D printing in healthcare [132]. An essential factor is the perception of personalization and its impact on treatment outcomes. Patients often view personalized 3D-printed therapeutic interventions as innovative and tailored to their specific needs. The ability to customize medical devices, implants, or drug delivery systems based on individual anatomical data instills a sense of confidence and reassurance in patients. This personalized approach is associated with improved treatment efficacy, better functional outcomes, and enhanced quality of life, which positively influences patient acceptance [12, 133,134,135]. Additionally, patients appreciate the potential for reduced treatment complexity and improved surgical outcomes through the use of 3D-printed interventions. The ability of 3D printing to streamline surgical procedures, reduce surgical time, and minimize invasiveness is valued by patients as it may result in shorter hospital stays, faster recovery, and reduced postoperative complications. Patient perceptions of the potential benefits, such as improved treatment outcomes, reduced pain, and enhanced functionality, contribute to their acceptance and willingness to undergo personalized interventions [136, 137].
Challenges and Concerns
Despite the potential advantages, patients may also harbor concerns and reservations regarding personalized 3D-printed therapeutic interventions. One common concern is the safety and long-term durability of 3D-printed medical devices and implants. Patients may worry about the reliability and performance of 3D-printed interventions, particularly when compared to established, conventional treatments. Addressing these concerns requires clear communication regarding the safety standards, regulatory compliance, and rigorous testing protocols associated with 3D-printed therapeutic interventions [63, 138]. Another challenge is the accessibility and affordability of personalized 3D-printed treatments. While 3D printing holds the promise of customization, the availability and cost-effectiveness of these interventions can vary. Patients may express concerns about the accessibility of 3D printing technology, especially in regions with limited resources or underdeveloped healthcare infrastructure. Furthermore, the financial implications of personalized 3D-printed treatments, including potential costs not covered by insurance, can be significant barriers for some patients. Addressing these challenges involves considering the cost-effectiveness of 3D printing, expanding access to the technology, and exploring reimbursement options to ensure equitable availability [63, 139].
Ethical Considerations
Ethical considerations surrounding personalized 3D-printed therapeutic interventions also influence patient perspectives and acceptance. Patients value transparent communication regarding the use of their personal data, including medical imaging data used for 3D printing. Informed consent and privacy protection are essential to build patient trust and ensure ethical practice. Engaging patients in shared decision-making processes, involving them in discussions about the benefits, risks, and alternatives of personalized 3D-printed interventions, is essential to respect patient autonomy and promote ethical healthcare practices [140, 141]. Moreover, patients may have ethical concerns related to the potential overutilization of 3D-printed interventions. The allure of customization and innovation may lead to unwarranted utilization of 3D printing technology. Clinicians and researchers should carefully consider the appropriateness and necessity of personalized 3D-printed interventions, ensuring that they are used judiciously and when they offer clear advantages over conventional treatments [142, 143].
Improving Patient Education and Engagement
To enhance patient acceptance and engagement with personalized 3D-printed therapeutic interventions, effective patient education is essential. Clear and comprehensive communication about the benefits, limitations, and potential risks associated with 3D printing should be provided to patients. Educational materials, visual aids, and interactive platforms can be utilized to help patients understand the technology, its applications, and their role in decision-making processes [144, 145]. Additionally, healthcare professionals should actively engage patients in discussions about personalized 3D-printed interventions. Understanding patient preferences, values, and concerns can help align treatment decisions with patient goals and improve shared decision-making. Patient support groups, educational workshops, and access to expert opinions can also contribute to patient empowerment, facilitating their acceptance and engagement with personalized 3D-printed therapeutic interventions [146]. Exploring patient perspectives and acceptance of personalized 3D-printed therapeutic interventions is vital for their successful implementation and utilization in clinical practice. Patient perceptions of personalization, expectations of improved treatment outcomes, and the potential for reduced treatment complexity are factors that positively influence patient acceptance. However, concerns regarding safety, accessibility, affordability, and ethical considerations should be addressed to ensure patient trust and facilitate informed decision-making. Improving patient education, engagement, and shared decision-making processes can enhance patient acceptance and foster a patient-centered approach in the integration of personalized 3D-printed therapeutic interventions in healthcare [147].
Environmental Impact and Sustainability Assessment of 3D Printing in Healthcare
As the field of healthcare embraces 3D printing technology, it becomes imperative to assess the environmental impact and sustainability considerations associated with its implementation. While 3D printing offers numerous advantages in terms of customization, rapid prototyping, and on-demand manufacturing, it is crucial to evaluate its implications for resource consumption, waste generation, energy usage, and overall environmental sustainability. This section reviews the existing literature on the environmental impact of 3D printing in healthcare, highlighting key findings and providing insights into the sustainability considerations that need to be addressed [148, 149].
Resource Consumption and Waste Generation
The utilization of 3D printing in healthcare demands various raw materials, including polymers, metals, ceramics, and bioinks. The extraction, production, and transportation of these materials contribute to environmental impact, particularly concerning energy consumption and greenhouse gas emissions. Additionally, the disposal of unused or failed prints and post-processing waste add to the overall waste generation [150]. To mitigate resource consumption and waste generation, several strategies can be implemented. One approach is optimizing the design of 3D-printed objects to minimize material usage while maintaining structural integrity. Techniques such as topology optimization and lattice structures can reduce material waste and enhance the sustainability of 3D printing. Moreover, recycling and reusing materials can help minimize waste and decrease the environmental footprint of 3D printing processes [44].
Energy Consumption
Energy consumption is another important consideration when assessing the environmental impact of 3D printing on healthcare. 3D printers, particularly those used for large-scale production, consume significant amounts of energy during the printing process. The energy requirements for heating, melting, and curing materials can contribute to greenhouse gas emissions and resource depletion. To mitigate energy consumption, it is prudent to explore energy-efficient printing methods and equipment. Progress in printer technology, including low-energy consumption printers, energy recovery systems, and fine-tuned printing parameters, can contribute to a reduction in environmental footprint. Furthermore, the adoption of renewable energy sources like solar or wind power to fulfill the energy demands of 3D printing facilities can bolster sustainability efforts [151, 152].
Life Cycle Assessment
The use of life cycle assessments (LCAs) stands as an effective approach to gauge the environmental repercussions of 3D printing in healthcare. LCAs involve a comprehensive analysis of the complete life cycle of a product or process, encompassing everything from raw material extraction to disposal, thereby enabling a thorough evaluation of the environmental impact. By conducting LCAs, researchers can identify hotspots of environmental impact and develop strategies to mitigate them [153]. LCAs of 3D printing within the healthcare sector have yielded significant insights. Notably, research has illuminated that the environmental consequences of 3D printing are subject to variation contingent on the particular application, choice of materials, and printing methodologies employed. Acquiring a nuanced comprehension of these distinctions is paramount for identifying opportunities to enhance sustainability in these processes. LCAs can guide the selection of materials, printing methods, and post-processing techniques that minimize environmental impact and promote sustainability [154].
Material Selection and Biodegradability
Choosing the right materials for 3D printing in healthcare is critical for environmental sustainability. Researchers are exploring biodegradable and bio-based materials as alternatives to traditional petroleum-based polymers. Biodegradable materials have the advantage of reducing long-term environmental impact by decomposing naturally over time [155]. Additionally, the development of bioinks and biomaterials derived from renewable resources, such as cellulose or algae-based materials, can contribute to the sustainability of 3D printing in healthcare. These materials can minimize the reliance on fossil fuels and reduce the carbon footprint associated with 3D printing processes [156].
Regulatory Framework and Standards
To ensure the environmental sustainability of 3D printing in healthcare, the establishment of regulatory frameworks and standards is essential. Regulatory bodies can play a crucial role in setting guidelines and requirements for environmentally conscious 3D printing practices. This includes encouraging the use of eco-friendly materials, promoting energy-efficient printing processes, and enforcing responsible waste management [61]. Moreover, industry collaborations, certification programs, and eco-labeling initiatives can facilitate the adoption of sustainable practices in 3D printing. By creating incentives and recognition for environmentally friendly approaches, these efforts can drive the implementation of sustainable 3D printing processes in healthcare [157]. Assessing the environmental impact and sustainability considerations associated with 3D printing in healthcare is crucial for responsible implementation and long-term viability. Strategies to minimize resource consumption, reduce waste generation, and optimize energy usage should be implemented. Conducting life cycle assessments can provide valuable insights into the environmental impact of 3D printing processes and guide sustainability improvements. Material selection, biodegradability, and the establishment of regulatory frameworks and standards also contribute to the sustainability of 3D printing in healthcare as shown in Tables X, XI and XII. By considering these environmental considerations and promoting sustainable practices, the healthcare industry can embrace 3D printing technology while minimizing its ecological footprint [158, 159].
The transformative potential of 3D printing in healthcare is evident in a wide range of applications, where it enables personalized and patient-specific therapeutic interventions. This section highlights several examples that showcase the revolutionary impact of 3D printing in improving medical treatments and patient outcomes. In severe burn injuries, traditional skin grafting involves harvesting skin from another part of the body, leading to additional pain and scarring. 3D bioprinting presents a solution by utilizing the patient's own cells and a bioink to craft personalized skin grafts that closely mimic the characteristics of natural skin. This method diminishes the requirement for invasive treatments and delivers a result that is both more natural and aesthetically appealing to the patient [1, 27]. Furthermore, Cancer patients undergoing radiation therapy often require shielding devices to protect healthy tissues. 3D printing enables the creation and manufacturing of personalized shielding devices tailored to the individual patient's unique anatomy. This approach guarantees accurate coverage and mitigates the potential for complications associated with radiation exposure [38]. 3D printing has transformed the field of prosthetics by enabling the customization of prosthetic limbs to match the patient's unique specifications. This results in improved comfort, mobility, and quality of life for individuals with limb loss [160]. Besides, 3D printing has revolutionized dentistry by enabling the fabrication of precise and custom-fit dental crowns, bridges, and aligners in a single visit. This approach saves time, improves patient comfort, and enhances aesthetics [161]. 3D printing facilitates the creation of complex drug delivery systems capable of controlled medication release or precise targeting within the body. This capability enhances treatment effectiveness and paves the way for personalized medicine [55, 107]. In additions, Surgeons can benefit from patient-specific surgical guides produced through 3D printing. These guides assist in accurately placing implants or performing complex surgeries, improving surgical precision and patient safety [143]. Moreover, Medical education and surgical planning have been revolutionized by 3D printing, as it allows for the creation of realistic anatomical models based on patient-specific imaging data. These models enable surgeons to visualize complex structures and plan surgeries with greater precision and confidence [63]. 3D printing provides affordable and customized assistive devices for individuals with disabilities. From prosthetic limbs to adaptive equipment like wheelchair accessories, 3D printing improves the quality of life for people with mobility limitations [31]. 3D printing empowers the fabrication of surgical implants customized to the patient's unique anatomy, such as hip or knee replacements. The outcome is a superior fit, reduced surgical duration, and improved post-operative results [83]. One of the most promising applications of 3D printing is in the field of regenerative medicine. Researchers are exploring the use of 3D printing to create functional organs and tissues by layering cells and biomaterials. This breakthrough technology has the potential to revolutionize transplantation medicine and address the critical shortage of organ donors [57]. These examples highlight the incredible versatility and potential of 3D printing in healthcare. By enabling personalized and patient-specific therapeutic interventions, 3D printing is transforming medical treatments, improving patient outcomes, and paving the way for a more patient-centered approach to healthcare. As research and development in 3D printing continue, we can expect even more innovative applications that will further revolutionize the field of healthcare.
Conclusion
This comprehensive overview delves into the definition, principles, and various types of 3D printing technologies applicable to therapeutic delivery. We have explored the advantages and limitations of 3D printing in this field, highlighting its potential to revolutionize healthcare through customized drug delivery systems, advances in tissue engineering and regenerative medicine, and the production of medical devices and implants. Applications of 3D printing in therapeutic delivery encompass patient-specific oral dosage forms, personalized drug delivery implants and devices, biofabrication of complex tissue structures, scaffold-based approaches for tissue regeneration, and the production of 3D-printed prosthetics, orthotics, customized implants, and surgical instruments. These applications have the potential to enhance treatment outcomes, improve patient comfort and compliance, and enable personalized medicine. The implications of 3D printing in therapeutic delivery are profound and far-reaching. Customized drug delivery systems can enable precise dosing and targeted therapy, minimizing side effects and maximizing treatment efficacy. Applications in tissue engineering and regenerative medicine hold the potential to generate functional organs and tissues for transplantation, addressing the pressing issue of organ donor shortages. Additionally, 3D-printed medical devices and implants offer enhanced functionality, better patient fit, and improved surgical outcomes. The integration of therapeutic agents into 3D-printed structures opens up possibilities for controlled drug release, prolonged drug delivery, and personalized treatment approaches. This has the capacity to bring about a revolution in medication administration and the care of chronic conditions, ultimately leading to better patient outcomes and an enhanced quality of life. Nevertheless, despite notable advancements in the field of 3D printing in therapeutic delivery, there remain several areas that necessitate continued research and development. Key areas include:
-
Material Selection and Biocompatibility: Continued research focused on the development of biocompatible materials and bioinks for 3D printing holds the potential to broaden the spectrum of therapeutic agents that can be incorporated into printed constructs. Enhancing our comprehension of material characteristics and their interplay with biological systems assumes pivotal importance in driving advancements in the field.
-
Multi-material and Multi-functional Printing: Exploring new techniques for combining different materials within a single 3D-printed structure can enhance functionality and enable the creation of complex, multi-component devices. Integration of sensors, electronics, and smart functionalities into 3D-printed systems is an exciting avenue for exploration.
-
Nanotechnology in 3D Printing: Investigating the integration of nanoparticles into 3D-printed structures for targeted drug delivery and surface modification is an emerging field. Further research is needed to optimize the incorporation of nanoparticles and understand their impact on therapeutic efficacy and biocompatibility.
-
Regulatory Considerations and Standardization: Establishing clear regulatory guidelines and standards for 3D-printed therapeutic delivery systems is essential. Collaborative efforts among researchers, industry, and regulatory bodies are needed to ensure safety, efficacy, and quality control.
-
Ethical and Legal Implications: The ethical and legal implications of 3D printing in therapeutic delivery must be carefully considered. Ongoing discussions and collaborations are necessary to address concerns related to patient privacy, equitable access, intellectual property, and liability. In conclusion, 3D printing has the potential to revolutionize therapeutic delivery through customized drug delivery systems, tissue engineering, regenerative medicine, and the production of medical devices and implants. While challenges exist, ongoing research and development efforts in material science, multi-material printing, nanotechnology, and regulatory considerations will drive the field forward. The future of therapeutic delivery is promising, and with continued innovation and collaboration, we can unlock the full potential of 3D printing to transform healthcare and improve patient outcomes.
Data Availability
This paper does not incorporate any original data generated by the author. Instead, all data utilized in this research is drawn from previous publications, and we have diligently included the relevant citations to acknowledge their sources.
References
Javaid M, Haleem A, Singh RP, Suman R. 3D printing applications for healthcare research and development. Global Health Journal. 2022;6(4):217–26. https://doi.org/10.1016/j.glohj.2022.11.001.
Lin L, Fang Y, Liao Y, Chen G, Gao C, Zhu P. 3D Printing and Digital Processing Techniques in Dentistry: A Review of Literature. Adv Eng Mater. 2019;21(6):1801013. https://doi.org/10.1002/adem.201801013.
Mishra Y, Mishra V, Aljabali AAA, El-Tanani M, Naikoo GA, Charbe N, et al. 3D printed personalized colon-targeted tablets: a novel approach in ulcerative colitis management. Curr Drug Deliv. 2023. https://doi.org/10.2174/1567201821666230915150544.
Jacob S, Nair AB, Patel V, Shah J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech. 2020;21(6):220. https://doi.org/10.1208/s12249-020-01771-4.
Sommonte, F., Denora, N. and Lamprou, D.A. Combining 3D printing and microfluidic techniques: A powerful synergy for nanomedicine. Pharmaceuticals. 2023;16(1):69. https://doi.org/10.3390/ph16010069.
Tenreiro MF, Louro AF, Alves PM, Serra M. Next generation of heart regenerative therapies: progress and promise of cardiac tissue engineering. NPJ Regen Med. 2021;6(1):30. https://doi.org/10.1038/s41536-021-00140-4.
Lakkala P, Munnangi SR, Bandari S, Repka M. Additive manufacturing technologies with emphasis on stereolithography 3D printing in pharmaceutical and medical applications: A review. Int J Pharm: X. 2023;5:100159. https://doi.org/10.1016/j.ijpx.2023.100159.
Mahmood MA. 3D printing in drug delivery and biomedical applications: a state-of-the-art review. Compounds. 2021.1(3):94–115. https://doi.org/10.3390/compounds1030009.
Al-Nimry SS, Daghmash RM. Three dimensional printing and its applications focusing on microneedles for drug delivery. Pharmaceutics. 2023;15(6):1597 https://doi.org/10.3390/pharmaceutics15061597.
Mohapatra S, Kar RK, Biswal PK, Bindhani S. Approaches of 3D printing in current drug delivery. Sens Int. 2022;3:100146. https://doi.org/10.1016/j.sintl.2021.100146.
Englezos K, Wang L, Tan ECK, Kang L. 3D printing for personalised medicines: implications for policy and practice. Int J Pharm. 2023;635:122785. https://doi.org/10.1016/j.ijpharm.2023.122785.
Serrano DR, Kara A, Yuste I, Luciano FC, Ongoren B, Anaya BJ, et al. 3D printing technologies in personalized medicine, nanomedicines, and biopharmaceuticals. Pharmaceutics. 2023;15(2):313. https://doi.org/10.3390/pharmaceutics15020313.
Bao Y, Paunović N, Leroux J-C. Challenges and Opportunities in 3D Printing of Biodegradable Medical Devices by Emerging Photopolymerization Techniques. Adv Func Mater. 2022;32(15):2109864. https://doi.org/10.1002/adfm.202109864.
Moroni S, Khorshid S, Aluigi A, Tiboni M, Casettari L. Poly(3-hydroxybutyrate): A potential biodegradable excipient for direct 3D printing of pharmaceuticals. Int J Pharm. 2022;623:121960. https://doi.org/10.1016/j.ijpharm.2022.121960.
Agrawal R, Garg A, Deshmukh R. A Snapshot of Current Updates and Future Prospects of 3D Printing in Medical and Pharmaceutical Science. Curr Pharm Des. 2023;29(8):604–19. https://doi.org/10.2174/1381612829666230228115442.
Alqahtani AA, Ahmed MM, Mohammed AA, Ahmad J. 3D printed pharmaceutical systems for personalized treatment in metabolic syndrome. Pharmaceutics. 2023;15(4):1152. https://doi.org/10.3390/pharmaceutics15041152.
Shao Y, Liao Z, Gao B, He B. Emerging 3D Printing Strategies for Enzyme Immobilization: Materials, Methods, and Applications. ACS Omega. 2022;7(14):11530–43. https://doi.org/10.1021/acsomega.2c00357.
Vaz VM, Kumar L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech. 2021;22(1):49. https://doi.org/10.1208/s12249-020-01905-8.
Sandeep B, Kannan TTM, Chandradass J, Ganesan M, John RA. Scope of 3D printing in manufacturing industries-A review. Materials Today: Proceedings. 2021;45:6941–5. https://doi.org/10.1016/j.matpr.2021.01.394.
Dabbagh SR, Sarabi MR, Birtek MT, Seyfi S, Sitti M, Tasoglu S. 3D-printed microrobots from design to translation. Nat Commun. 2022;13(1):5875. https://doi.org/10.1038/s41467-022-33409-3.
Sarabi MR, Karagoz AA, Yetisen AK, Tasoglu S. 3D-printed microrobots: translational challenges. Micromachines (Basel), 2023;14(6):1099. https://doi.org/10.3390/mi14061099.
Shah ZH, Wu B, Das S. Multistimuli-responsive microrobots: a comprehensive review. Front Robot AI. 2022;9:1027415. https://doi.org/10.3389/frobt.2022.1027415.
Lee JG, Raj RR, Day NB, Shields CWt. Microrobots for biomedicine: unsolved challenges and opportunities for translation. ACS Nano. 2023;17(15):14196–204. https://doi.org/10.1021/acsnano.3c03723.
Betz UA, Arora L, Assal RA, Azevedo H, Baldwin J, Becker MS, et al. Game changers in science and technology-now and beyond. Technol Forecast Soc Chang. 2023;193:122588. https://doi.org/10.1016/j.techfore.2023.122588.
Wang H, Pumera M. Fabrication of Micro/Nanoscale Motors. Chem Rev. 2015;115(16):8704–35. https://doi.org/10.1021/acs.chemrev.5b00047.
Kim J, Park H, Yoon C. Advances in biodegradable soft robots. Polymers. 2022. https://doi.org/10.3390/polym14214574.
Eshkalak SK, Erfan RG, Yunqian D, Deepak C, Seeram R. The role of three-dimensional printing in healthcare and medicine. Mater Des. 2020;194:108940. https://doi.org/10.1016/j.matdes.2020.108940.
Prasad LK, Smyth H. 3D Printing technologies for drug delivery: a review. Drug Dev Ind Pharm. 2016;42(7):1019–31. https://doi.org/10.3109/03639045.2015.1120743.
Pavan Kalyan BG, Kumar L. 3D Printing: Applications in Tissue Engineering, Medical Devices, and Drug Delivery. AAPS PharmSciTech. 2022;23(4):92. https://doi.org/10.1208/s12249-022-02242-8.
Amekyeh H, Tarlochan F, Billa N. Practicality of 3D Printed Personalized Medicines in Therapeutics. Front Pharmacol. 2021;12:646836. https://doi.org/10.3389/fphar.2021.646836.
Khan FA, Narasimhan K, Swathi CSV, Mustak S, Mustafa G, Ahmad MZ, et al. 3D Printing Technology in Customized Drug Delivery System: Current State of the Art, Prospective and the Challenges. Curr Pharm Des. 2018;24(42):5049–61. https://doi.org/10.2174/1381612825666190110153742.
Trenfield SJ, Awad A, Madla CM, Hatton GB, Firth J, Goyanes A, et al. Shaping the future: recent advances of 3D printing in drug delivery and healthcare. Expert Opin Drug Deliv. 2019;16(10):1081–94.
Bácskay I, Ujhelyi Z, Fehér P, Arany P. The evolution of the 3D-printed drug delivery systems: a review. Pharmaceutics. 2022;14(7):1312. https://doi.org/10.3390/pharmaceutics14071312.
Gao G, Ahn M, Cho WW, Kim BS, Cho DW. 3D printing of pharmaceutical application: drug screening and drug delivery. Pharmaceutics. 2021;13(9):1373. https://doi.org/10.3390/pharmaceutics13091373
Tracy T, Wu L, Liu X, Cheng S, Li X. 3D printing: Innovative solutions for patients and pharmaceutical industry. Int J Pharm. 2023;631:122480. https://doi.org/10.1016/j.ijpharm.2022.122480.
Jain P, Kathuria H, Dubey N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials. 2022;287:121639. https://doi.org/10.1016/j.biomaterials.2022.121639.
Mani MP, Sadia M, Jaganathan SK, Khudzari AZ, Supriyanto E, Saidin S, et al. A review on 3D printing in tissue engineering applications. J Polym Eng. 2022;42(3):243–65.
Safhi AY. Three-dimensional (3D) printing in cancer therapy and diagnostics: current status and future perspectives. Pharmaceuticals. 2022. https://doi.org/10.3390/ph15060678.
Borandeh S, van Bochove B, Teotia A, Seppälä J. Polymeric drug delivery systems by additive manufacturing. Adv Drug Deliv Rev. 2021;173:349–73.
Zamboulis A, Michailidou G, Koumentakou I, Bikiaris DN. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics. 2022. https://doi.org/10.3390/pharmaceutics14010145.
Basit AW, Trenfield SJ. 3D printing of pharmaceuticals and the role of pharmacy. Pharm J. 2022;308:7959.
Osouli-Bostanabad K, Masalehdan T, Kapsa RMI, Quigley A, Lalatsa A, Bruggeman KF, et al. Traction of 3D and 4D Printing in the Healthcare Industry: From Drug Delivery and Analysis to Regenerative Medicine. ACS Biomater Sci Eng. 2022;8(7):2764–97. https://doi.org/10.1021/acsbiomaterials.2c00094.
Mathew E, Pitzanti G, Larrañeta E, Lamprou DA. 3D printing of pharmaceuticals and drug delivery devices. Pharmaceutics. 2020;12(3):266.
Nazir A, Gokcekaya O, Billah KMM, Ertugrul O, Jiang J, Sun J, et al. Multi-material additive manufacturing: A systematic review of design, properties, applications, challenges, and 3D Printing of materials and cellular metamaterials. Mater Des. 2023:111661. https://doi.org/10.1016/j.matdes.2023.111661.
Ahmad J, Garg A, Mustafa G, Mohammed AA, Ahmad MZ. 3D printing technology as a promising tool to design nanomedicine-based solid dosage forms: contemporary research and future scope. Pharmaceutics. 2023. https://doi.org/10.3390/pharmaceutics15051448.
Mwema FM, Akinlabi ET, Mwema FM, Akinlabi ET. Basics of fused deposition modelling (FDM). Fused deposition modeling: strategies for quality enhancement. 2020:1–15.
Sharma A, Rai A. Fused deposition modelling (FDM) based 3D & 4D Printing: A state of art review. Mater Today Proc. 2022;62:367–72.
Alsaadi M, Hinchy EP, McCarthy CT, Moritz VF, Zhuo S, Fuenmayor E, et al. Liquid-based 4D printing of shape memory nanocomposites: a review. J Manuf Mater Process. 2023;7(1):35.
Bhanvadia AA, Farley RT, Noh Y, Nishida T. High-resolution stereolithography using a static liquid constrained interface. Commun Mater. 2021;2(1):41.
Milazzo M, Libonati F. The synergistic role of additive manufacturing and artificial intelligence for the design of new advanced intelligent systems. Adv Intell Syst. 2022;4(6):2100278. https://doi.org/10.1002/aisy.202100278.
Yi H-G, Kim H, Kwon J, Choi Y-J, Jang J, Cho D-W. Application of 3D bioprinting in the prevention and the therapy for human diseases. Signal Transduct Target Ther. 2021;6(1):177. https://doi.org/10.1038/s41392-021-00566-8.
Wang J, Zhang Y, Aghda NH, Pillai AR, Thakkar R, Nokhodchi A, et al. Emerging 3D printing technologies for drug delivery devices: Current status and future perspective. Adv Drug Deliv Rev. 2021;174:294–316. https://doi.org/10.1016/j.addr.2021.04.019.
Lim SH, Kathuria H, Tan JJY, Kang L. 3D printed drug delivery and testing systems—a passing fad or the future? Adv Drug Deliv Rev. 2018;132:139–68.
Patel SK, Khoder M, Peak M, Alhnan MA. Controlling drug release with additive manufacturing-based solutions. Adv Drug Deliv Rev. 2021;174:369–86. https://doi.org/10.1016/j.addr.2021.04.020.
Jain V, Haider N, Jain K. 3D printing in personalized drug delivery. Curr Pharm Des. 2018;24(42):5062–71. https://doi.org/10.2174/1381612825666190215122208.
Jamróz W, Szafraniec J, Kurek M, Jachowicz R. 3D Printing in Pharmaceutical and Medical Applications - Recent Achievements and Challenges. Pharm Res. 2018;35(9):176. https://doi.org/10.1007/s11095-018-2454-x.
Geraili A, Xing M, Mequanint K. Design and fabrication of drug-delivery systems toward adjustable release profiles for personalized treatment. VIEW. 2021;2(5):20200126. .
Iftekar SF, Aabid A, Amir A, Baig M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers. 2023;15(11):2519.
Jandyal A, Chaturvedi I, Wazir I, Raina A, Haq MIU. 3D printing–A review of processes, materials and applications in industry 4.0. Sustain Oper Comput. 2022;3:33–42.
Puzatova A, Shakor P, Laghi V, Dmitrieva M. Large-scale 3D printing for construction application by means of robotic arm and gantry 3d printer: a review. Buildings. 2022. https://doi.org/10.3390/buildings12112023.
Shahrubudin N, Koshy P, Alipal J, Kadir MHA, Lee TC. Challenges of 3D printing technology for manufacturing biomedical products: A case study of Malaysian manufacturing firms. Heliyon. 2020;6(4):e03734. https://doi.org/10.1016/j.heliyon.2020.e03734.
Jin Z, He C, Fu J, Han Q, He Y. Balancing the customization and standardization: exploration and layout surrounding the regulation of the growing field of 3D-printed medical devices in China. Biodes Manuf. 2022;5(3):580–606. https://doi.org/10.1007/s42242-022-00187-2.
Mamo HB, Adamiak M, Kunwar A. 3D printed biomedical devices and their applications: A review on state-of-the-art technologies, existing challenges, and future perspectives. J Mech Behav Biomed Mater. 2023;143:105930. https://doi.org/10.1016/j.jmbbm.2023.105930.
Karalia D, Siamidi A, Karalis V, Vlachou M. 3D-printed oral dosage forms: mechanical properties, computational approaches and applications. Pharmaceutics. 2021;13(9). https://doi.org/10.3390/pharmaceutics13091401.
Dumpa N, Butreddy A, Wang H, Komanduri N, Bandari S, Repka MA. 3D printing in personalized drug delivery: an overview of hot-melt extrusion-based fused deposition modeling. Int J Pharm. 2021;600:120501.
Al-Litani K, Ali T, Robles Martinez P, Buanz A. 3D printed implantable drug delivery devices for women’s health: Formulation challenges and regulatory perspective. Adv Drug Deliv Rev. 2023;198:114859. https://doi.org/10.1016/j.addr.2023.114859.
Chakka LRJ, Chede S. 3D printing of pharmaceuticals for disease treatment. Front Med Technol. 2023;4. https://doi.org/10.3389/fmedt.2022.1040052.
Lu J, Hu X, Yuan T, Cao J, Zhao Y, Xiong C, et al. 3D-printed poly (P-Dioxanone) stent for endovascular application: in vitro evaluations. Polymers (Basel). 2022;14(9). https://doi.org/10.3390/polym14091755.
Shen Y, Cui J, Yu X, Song J, Cai P, Guo W, et al. Recent advances in three-dimensional printing in cardiovascular devices: bench and bedside applications. Smart Mater Med. 2024;5(1):36–51. https://doi.org/10.1016/j.smaim.2023.07.001.
Auriemma G, Tommasino C, Falcone G, Esposito T, Sardo C, Aquino RP. Additive manufacturing strategies for personalized drug delivery systems and medical devices: fused filament fabrication and semi solid extrusion. Molecules. 2022. https://doi.org/10.3390/molecules27092784.
Detamornrat U, McAlister E, Hutton ARJ, Larrañeta E, Donnelly RF. The role of 3D printing technology in microengineering of microneedles. Small. 2022;18(18):2106392. https://doi.org/10.1002/smll.202106392.
Ramadan Q, Zourob M. 3D bioprinting at the frontier of regenerative medicine, pharmaceutical, and food industries. Front Med Technol. 2020;2:607648. https://doi.org/10.3389/fmedt.2020.607648.
Di Piazza E, Pandolfi E, Cacciotti I, Del Fattore A, Tozzi AE, Secinaro A, et al. Bioprinting technology in skin, heart, pancreas and cartilage tissues: Progress and challenges in clinical practice. Int J Environ Res Public Health. 2021;18(20):10806. https://doi.org/10.3390/ijerph182010806.
Leberfinger AN, Dinda S, Wu Y, Koduru SV, Ozbolat V, Ravnic DJ, et al. Bioprinting functional tissues. Acta Biomater. 2019;95:32–49. https://doi.org/10.1016/j.actbio.2019.01.009.
Chung JJ, Im H, Kim SH, Park JW, Jung Y. Toward biomimetic scaffolds for tissue engineering: 3D printing techniques in regenerative medicine. Front Bioeng Biotechnol. 2020;8:586406. https://doi.org/10.3389/fbioe.2020.586406.
Han F, Wang J, Ding L, Hu Y, Li W, Yuan Z, et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front Bioeng Biotechnol. 2020;8:83. https://doi.org/10.3389/fbioe.2020.00083.
Muzzio N, Moya S, Romero G. Multifunctional scaffolds and synergistic strategies in tissue engineering and regenerative medicine. Pharmaceutics. 2021;13(6):792. https://doi.org/10.3390/pharmaceutics13060792.
Ligon SC, Liska R, Stampfl J, Gurr M, Mülhaupt R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem Rev. 2017;117(15):10212–90. https://doi.org/10.1021/acs.chemrev.7b00074.
Nyberg EL, Farris AL, Hung BP, Dias M, Garcia JR, Dorafshar AH, et al. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann Biomed Eng. 2017;45(1):45–57. https://doi.org/10.1007/s10439-016-1668-5.
Arefin AME, Khatri NR, Kulkarni N, Egan PF. Polymer 3D printing review: materials, process, and design strategies for medical applications. Polymers. 2021. https://doi.org/10.3390/polym13091499.
Wong KC. 3D-printed patient-specific applications in orthopedics. Orthop Res Rev. 2016;8:57–66. https://doi.org/10.2147/orr.S99614.
Ballard DH, Trace AP, Ali S, Hodgdon T, Zygmont ME, DeBenedectis CM, et al. Clinical Applications of 3D Printing: Primer for Radiologists. Acad Radiol. 2018;25(1):52–65. https://doi.org/10.1016/j.acra.2017.08.004.
Fiani B, Newhouse A, Cathel A, Sarhadi K, Soula M. Implications of 3-Dimensional Printed Spinal Implants on the Outcomes in Spine Surgery. J Korean Neurosurg Soc. 2021;64(4):495–504. https://doi.org/10.3340/jkns.2020.0272.
Fang Y, Guo Y, Liu T, Xu R, Mao S, Mo X, et al. Advances in 3D Bioprinting. Chin J Mech Eng: Addit Manuf Front. 2022;1(1):100011. https://doi.org/10.1016/j.cjmeam.2022.100011.
Ashammakhi N, Ahadian S, Xu C, Montazerian H, Ko H, Nasiri R, et al. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Materials Today Bio. 2019;1:100008. https://doi.org/10.1016/j.mtbio.2019.100008.
Ghosh S, Yi HG. A Review on Bioinks and their Application in Plant Bioprinting. Int J Bioprint. 2022;8(4):612. https://doi.org/10.18063/ijb.v8i4.612.
Shende P, Trivedi R. 3D Printed Bioconstructs: Regenerative Modulation for Genetic Expression. Stem Cell Rev Rep. 2021;17(4):1239–50. https://doi.org/10.1007/s12015-021-10120-2.
Yazdanpanah Z, Johnston JD, Cooper DML, Chen X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front Bioeng Biotechnol. 2022;10:824156. https://doi.org/10.3389/fbioe.2022.824156.
Zhou X, Ren L, Song Z, Li G, Zhang J, Li B, et al. Advances in 3D/4D printing of mechanical metamaterials: From manufacturing to applications. Compos B Eng. 2023;254:110585. https://doi.org/10.1016/j.compositesb.2023.110585.
Potyondy T, Uquillas JA, Tebon PJ, Byambaa B, Hasan A, Tavafoghi M, et al. Recent advances in 3D bioprinting of musculoskeletal tissues. Biofabrication. 2021;13(2). https://doi.org/10.1088/1758-5090/abc8de.
Tripathi S, Mandal SS, Bauri S, Maiti P. 3D bioprinting and its innovative approach for biomedical applications. MedComm (2020). 2023;4(1):e194. https://doi.org/10.1002/mco2.194.
Freeman S, Calabro S, Williams R, Jin S, Ye K. Bioink formulation and machine learning-empowered bioprinting optimization. Front Bioeng Biotechnol. 2022;10. https://doi.org/10.3389/fbioe.2022.913579.
Ramezani M, Mohd Ripin Z. 4D printing in biomedical engineering: advancements, challenges, and future directions. J Funct Biomater. 2023;14(7). https://doi.org/10.3390/jfb14070347.
Safhi AY. Three-dimensional (3D) printing in cancer therapy and diagnostics: current status and future perspectives. Pharmaceuticals (Basel). 2022;15(6). https://doi.org/10.3390/ph15060678.
Zhao M, Wang R, Yang K, Jiang Y, Peng Y, Li Y, et al. Nucleic acid nanoassembly-enhanced RNA therapeutics and diagnosis. Acta Pharmaceutica Sinica B. 2023;13(3):916–41. https://doi.org/10.1016/j.apsb.2022.10.019.
Kawai T, Pan CC, Okuzu Y, Shimizu T, Stahl AM, Matsuda S, et al. Combining a Vascular Bundle and 3D Printed Scaffold with BMP-2 Improves Bone Repair and Angiogenesis. Tissue Eng Part A. 2021;27(23–24):1517–25. https://doi.org/10.1089/ten.TEA.2021.0049.
Singh S, Kumar M, Doolaanea AA, Mandal UK. A Recent Review On 3D-Printing: Scope and Challenges with Special Focus on Pharmaceutical Field. Curr Pharm Des. 2022;28(30):2488–507. https://doi.org/10.2174/1381612828666220623091629.
Park D, Lee SJ, Choi DK, Park JW. Therapeutic agent-loaded fibrous scaffolds for biomedical applications. Pharmaceutics. 2023;15(5). https://doi.org/10.3390/pharmaceutics15051522.
Adalbert L, Kanti SY, Jójárt-Laczkovich O, Akel H, Csóka I. Expanding quality by design principles to support 3D printed medical device development following the renewed regulatory framework in Europe. Biomedicines. 2022;10(11):2947. https://doi.org/10.3390/biomedicines10112947.
Liu Y, Xia X, Liu Z, Dong M. The next frontier of 3D bioprinting: bioactive materials functionalized by bacteria. Small. 2023;19(10):2205949.
Guamán-Rivera R, Martínez-Rocamora A, García-Alvarado R, Muñoz-Sanguinetti C, González-Böhme LF, Auat-Cheein F. Recent developments and challenges of 3D-printed construction: A review of research fronts. Buildings. 2022;12(2):229. https://doi.org/10.3390/buildings12020229.
Bittner SM, Guo JL, Melchiorri A, Mikos AG. Three-dimensional printing of multilayered tissue engineering scaffolds. Mater Today. 2018;21(8):861–74.
Mostafaei A, Elliott AM, Barnes JE, Li F, Tan W, Cramer CL, et al. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog Mater Sci. 2021;119:100707. https://doi.org/10.1016/j.pmatsci.2020.100707.
Schuldt SJ, Jagoda JA, Hoisington AJ, Delorit JD. A systematic review and analysis of the viability of 3D-printed construction in remote environments. Autom Constr. 2021;125:103642. https://doi.org/10.1016/j.autcon.2021.103642.
Rouf S, Malik A, Singh N, Raina A, Naveed N, Siddiqui MIH, et al. Additive manufacturing technologies: Industrial and medical applications. Sustain Oper Comput. 2022;3:258–74. https://doi.org/10.1016/j.susoc.2022.05.001.
Ngo TD, Kashani A, Imbalzano G, Nguyen KTQ, Hui D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos B Eng. 2018;143:172–96. https://doi.org/10.1016/j.compositesb.2018.02.012.
Afzal O, Altamimi AS, Nadeem MS, Alzarea SI, Almalki WH, Tariq A, et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials. 2022;12(24):4494. https://doi.org/10.3390/nano12244494.
Jain K, Shukla R, Yadav A, Ujjwal RR, Flora SJS. 3D printing in development of nanomedicines. Nanomaterials (Basel). 2021;11(2):420. https://doi.org/10.3390/nano11020420.
Tolabi H, Bakhtiary N, Sayadi S, Tamaddon M, Ghorbani F, Boccaccini AR, et al. A critical review on polydopamine surface-modified scaffolds in musculoskeletal regeneration. Frontiers in Bioengineering and Biotechnology. 2022;10:1008360. https://doi.org/10.3389/fbioe.2022.1008360.
Ilyas K, Akhtar MA, Ammar EB, Boccaccini AR. Surface modification of 3D-printed PCL/BG composite scaffolds via mussel-inspired polydopamine and effective antibacterial coatings for biomedical applications. Materials (Basel). 2022;15(23):8289. https://doi.org/10.3390/ma15238289.
Zhao W, Hu C, Xu T. In vivo bioprinting: Broadening the therapeutic horizon for tissue injuries. Bioact Mater. 2023;25:201–22. https://doi.org/10.1016/j.bioactmat.2023.01.018.
Chakraborty PK, Azadmanjiri J, Pavithra CLP, Wang X, Masood SH, Dey SR, et al. Advancements in Therapeutics via 3D Printed Multifunctional Architectures from Dispersed 2D Nanomaterial Inks. Small. 2020;16(49):2004900. https://doi.org/10.1002/smll.202004900.
Iftekar SF, Aabid A, Amir A, Baig M. Advancements and limitations in 3D printing materials and technologies: a critical review. Polymers. 2023. https://doi.org/10.3390/polym15112519.
Park S, Shou W, Makatura L, Matusik W, Fu K. 3D printing of polymer composites: Materials, processes, and applications. Matter. 2022;5(1):43–76. https://doi.org/10.1016/j.matt.2021.10.018.
Lee BJ, Hsiao K, Lipkowitz G, Samuelsen T, Tate L, DeSimone JM. Characterization of a 30 µm pixel size CLIP-based 3D printer and its enhancement through dynamic printing optimization. Addit Manuf. 2022;55:102800. https://doi.org/10.1016/j.addma.2022.102800.
Morrison RJ, Kashlan KN, Flanangan CL, Wright JK, Green GE, Hollister SJ, et al. Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin Transl Sci. 2015;8(5):594–600.
Dasgupta A, Dutta P. A comprehensive review on 3D printing technology: current applications and challenges. Jordan J Mech Ind Eng. 2022;16(4).
Ruiz LE, Pinho AC, Resende DN. 3D printing as a disruptive technology for the circular economy of plastic components of end-of-life vehicles: a systematic review. Sustainability. 2022;14(20):13256 https://doi.org/10.3390/su142013256.
Liu PYL, Liou JJH, Huang S-W. Exploring the Barriers to the Advancement of 3D Printing Technology. Mathematics. 2023;11(14):3068.
Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: A state of the art. J Healthc Eng. 2019;2019:5340616:5340616. https://doi.org/10.1155/2019/5340616.
Goh O, Goh WJ, Lim SH, Hoo GS, Liew R, Ng TM. Preferences of healthcare professionals on 3D-printed tablets: a pilot study. Pharmaceutics. 2022;14(7). https://doi.org/10.3390/pharmaceutics14071521.
Belhouideg S. Impact of 3D printed medical equipment on the management of the Covid19 pandemic. Int J Health Plann Manage. 2020;35(5):1014–22.
Cui M, Pan H, Su Y, Fang D, Qiao S, Ding P, et al. Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development. Acta Pharm Sin B. 2021;11(8):2488–504. https://doi.org/10.1016/j.apsb.2021.03.015.
Ballard DH, Mills P, Duszak R Jr, Weisman JA, Rybicki FJ, Woodard PK. Medical 3D printing cost-savings in orthopedic and maxillofacial surgery: Cost analysis of operating room time saved with 3D printed anatomic models and surgical guides. Acad Radiol. 2020;27(8):1103-13. https://doi.org/10.1016/j.acra.2019.08.011.
Mancilla-De-la-Cruz J, Rodriguezsalvador M, An J, Chua CK. Three-dimensional printing technologies for drug delivery applications: processes, materials, and effects. Int J Bioprint. 2022;8(4):622. https://doi.org/10.18063/ijb.v8i4.622.
Huanbutta K, Burapapadh K, Sriamornsak P, Sangnim T. Practical application of 3D printing for pharmaceuticals in hospitals and pharmacies. Pharmaceutics. 2023;15(7):1877 https://doi.org/10.3390/pharmaceutics15071877.
Domsta V, Seidlitz A. 3D-printing of drug-eluting implants: an overview of the current developments described in the literature. Molecules. 2021;26(13):4066. https://doi.org/10.3390/molecules26134066.
Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27–40. https://doi.org/10.1136/bmjstel-2017-000234.
Beer N, Kaae S, Genina N, Sporrong SK, Alves TL, Hoebert J, et al. Magistral Compounding with 3D Printing: A Promising Way to Achieve Personalized Medicine. Ther Innov Regul Sci. 2023;57(1):26–36. https://doi.org/10.1007/s43441-022-00436-7.
Amjad A, Kordel P, Fernandes G. A Review on Innovation in Healthcare Sector (Telehealth) through Artificial Intelligence. Sustainability. 2023. https://doi.org/10.3390/su15086655.
Nardini C, Osmani V, Cormio PG, Frosini A, Turrini M, Lionis C, et al. The evolution of personalized healthcare and the pivotal role of European regions in its implementation. Pers Med. 2021;18(3):283–94. https://doi.org/10.2217/pme-2020-0115.
Fastø MM, Genina N, Kaae S, Kälvemark SS. Perceptions, preferences and acceptability of patient designed 3D printed medicine by polypharmacy patients: a pilot study. Int J Clin Pharm. 2019;41(5):1290–8. https://doi.org/10.1007/s11096-019-00892-6.
Rojek I, Mikołajewski D, Dostatni E, Kopowski J. Specificity of 3D printing and AI-based optimization of medical devices using the example of a group of exoskeletons. Appl Sci. 2023.13(2):1060.https://doi.org/10.3390/app13021060.
Meyer-Szary J, Luis MS, Mikulski S, Patel A, Schulz F, Tretiakow D, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6). https://doi.org/10.3390/ijerph19063331.
Sandler N, Salmela I, Fallarero A, Rosling A, Khajeheian M, Kolakovic R, et al. Towards fabrication of 3D printed medical devices to prevent biofilm formation. Int J Pharm. 2014;459(1–2):62–4.
Cornejo J, Cornejo-Aguilar JA, Vargas M, Helguero CG, Milanezi de Andrade R, Torres-Montoya S, et al. Anatomical engineering and 3D printing for surgery and medical devices: International review and future exponential innovations. Biomed Res Int. 2022;6797745. https://doi.org/10.1155/2022/6797745.
Hoang D, Perrault D, Stevanovic M, Ghiassi A. Surgical applications of three-dimensional printing: a review of the current literature & how to get started. Ann Transl Med. 2016;4(23).
Christensen A, Rybicki FJ. Maintaining safety and efficacy for 3D printing in medicine. 3D Print Med. 2017;3(1):1. https://doi.org/10.1186/s41205-016-0009-5.
Bozkurt Y, Karayel E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J Market Res. 2021;14:1430–50. https://doi.org/10.1016/j.jmrt.2021.07.050.
Seok J, Yoon S, Ryu CH, Kim SK, Ryu J, Jung YS. A personalized 3D-printed model for obtaining informed consent process for thyroid surgery: a randomized clinical study using a deep learning approach with mesh-type 3D modeling. J Pers Med. 2021;11(6). https://doi.org/10.3390/jpm11060574.
Douglas DM, Lacey J, Howard D. Ethical risks of AI-designed products: bespoke surgical tools as a case study. AI Ethics. 2022. https://doi.org/10.1007/s43681-022-00219-8.
Dodds S. 3D printing raises ethical issues in medicine. ABC Sci. 2015;11. https://www.abc.net.au/science/articles/2015/02/11/4161675.htm
Fox M, Peregrin T. 3-D printing: Revolutionizing preoperative planning, resident training, and the future of surgical care. Bull Am Coll Surg. 2016;101(7):9–18.
Zhuang YD, Zhou MC, Liu SC, Wu JF, Wang R, Chen CM. Effectiveness of personalized 3D printed models for patient education in degenerative lumbar disease. Patient Educ Couns. 2019;102(10):1875–81. https://doi.org/10.1016/j.pec.2019.05.006.
Sun Z. Patient-specific 3D-printed models in pediatric congenital heart disease. Children. 2023. https://doi.org/10.3390/children10020319.
Légaré F, Adekpedjou R, Stacey D, Turcotte S, Kryworuchko J, Graham ID, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7(7):CD006732. https://doi.org/10.1002/14651858.CD006732.pub4.
Algahtani MS. Assessment of pharmacist's knowledge and perception toward 3D printing technology as a dispensing method for personalized medicine and the readiness for implementation. Pharmacy (Basel). 2021;9(1). https://doi.org/10.3390/pharmacy9010068.
Khosravani MR, Reinicke T. On the environmental impacts of 3D printing technology. Appl Mater Today. 2020;20:100689.
Ozyilmaz ED, Turan A, Comoglu T. An overview on the advantages and limitations of 3D printing of microneedles. Pharm Dev Technol. 2021;26(9):923–33.
Nadagouda MN, Ginn M, Rastogi V. A review of 3D printing techniques for environmental applications. Curr Opin Chem Eng. 2020;28:173–8. https://doi.org/10.1016/j.coche.2020.08.002.
Elbadawi M, Basit AW, Gaisford S. Energy consumption and carbon footprint of 3D printing in pharmaceutical manufacture. Int J Pharm. 2023;639:122926. https://doi.org/10.1016/j.ijpharm.2023.122926.
Shuaib M, Haleem A, Kumar S, Javaid M. Impact of 3D Printing on the environment: A literature-based study. Sustain Oper Comput. 2021;2:57–63. https://doi.org/10.1016/j.susoc.2021.04.001.
Kumar R, Sharma H, Saran C, Tripathy TS, Sangwan KS, Herrmann C. A Comparative Study on the Life Cycle Assessment of a 3D Printed Product with PLA ABS & PETG Materials. Procedia CIRP. 2022;107:15–20.
Ulkir O. Energy-consumption-based life cycle assessment of additive-manufactured product with different types of materials. Polymers (Basel). 2023;15(6):1466. https://doi.org/10.3390/polym15061466.
Joseph TM, Kallingal A, Suresh AM, Mahapatra DK, Hasanin MS, Haponiuk J, et al. 3D printing of polylactic acid: recent advances and opportunities. Int J Adv Manuf Technol. 2023;125(3):1015–35. https://doi.org/10.1007/s00170-022-10795-y.
Su C, Chen Y, Tian S, Lu C, Lv Q. Natural materials for 3D printing and their applications. Gels. 2022. https://doi.org/10.3390/gels8110748.
To TT, Al Mahmud A, Ranscombe C. Teaching sustainability using 3D printing in engineering education: an observational study. Sustainability. 2023. https://doi.org/10.3390/su15097470.
Gopal M, Lemu HG, Gutema EM. Sustainable additive manufacturing and environmental implications: literature review. Sustainability. 2023;15(1):504. https://doi.org/10.3390/su15010504.
Rejeski D, Zhao F, Huang Y. Research needs and recommendations on environmental implications of additive manufacturing. Addit Manuf. 2018;19:21–8.
Manero A, Smith P, Sparkman J, Dombrowski M, Courbin D, Kester A, et al. Implementation of 3D printing technology in the field of prosthetics: past, present, and future. Int J Environ Res Public Health. 2019;16(9):1641. https://doi.org/10.3390/ijerph16091641.
Pillai S, Upadhyay A, Khayambashi P, Farooq I, Sabri H, Tarar M, et al. Dental 3D-printing: transferring art from the laboratories to the clinics. Polymers (Basel). 2021;13(1). https://doi.org/10.3390/polym13010157.
Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules. 2021;26(19). https://doi.org/10.3390/molecules26195905.
Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as drug delivery systems: a review of the implication of nanoparticles’physicochemical properties on responses in biological systems. Polymers. 2023. https://doi.org/10.3390/polym15071596.
Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394. https://doi.org/10.1016/j.heliyon.2022.e09394.
Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27(4). https://doi.org/10.3390/molecules27041372.
Stewart SA, Domínguez-Robles J, Donnelly RF, Larrañeta E. Implantable polymeric drug delivery devices: classification, manufacture, materials, and clinical applications. Polymers (Basel). 2018;10(12):1379. https://doi.org/10.3390/polym10121379.
Alipour S, Mahmoudi L, Ahmadi F. Pulmonary drug delivery: an effective and convenient delivery route to combat COVID-19. Drug Deliv Transl Res. 2023;13(3):705–15. https://doi.org/10.1007/s13346-022-01251-1.
Ruiz F, Nunn A, Gill A, Clapham D, Fotaki N, Salunke S, et al. A review of paediatric injectable drug delivery to inform the study of product acceptability – An introduction. Eur J Pharm Biopharm. 2023;188:265–70. https://doi.org/10.1016/j.ejpb.2023.04.010.
Alqahtani MS, Kazi M, Alsenaidy MA, Ahmad MZ. Advances in Oral Drug Delivery. Front Pharmacol. 2021;12:618411. https://doi.org/10.3389/fphar.2021.618411.
Nikolova MP, Kumar EM, Chavali MS. Updates on responsive drug delivery based on liposome vehicles for cancer treatment. Pharmaceutics. 2022;14(10):2195. https://doi.org/10.3390/pharmaceutics14102195.
Alrbyawi H, Poudel I, Annaji M, Boddu SHS, Arnold RD, Tiwari AK, et al. pH-Sensitive liposomes for enhanced cellular uptake and cytotoxicity of daunorubicin in melanoma (B16-BL6) cell lines. Pharmaceutics. 2022;14(6). https://doi.org/10.3390/pharmaceutics14061128.
Smagina V, Yudaev P, Kuskov A, Chistyakov E. Polymeric gel systems cytotoxicity and drug release as key features for their effective application in various fields of addressed pharmaceuticals delivery. Pharmaceutics. 2023;15(3):830. https://doi.org/10.3390/pharmaceutics15030830.
Shen S, Wu Y, Liu Y, Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomedicine. 2017;12:4085-109 https://doi.org/10.2147/ijn.S132780.
Cheng X, Xie Q, Sun Y. Advances in nanomaterial-based targeted drug delivery systems. Front Bioeng Biotechnol. 2023;11:1177151. https://doi.org/10.3389/fbioe.2023.1177151.
Gagliardi A, Giuliano E, Venkateswararao E, Fresta M, Bulotta S, Awasthi V, et al. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front Pharmacol. 2021;12:601626. https://doi.org/10.3389/fphar.2021.601626.
Tripathi D, Hajra K, Maity D. Recent advancement of bio-inspired nanoparticles in cancer theragnostic. J Nanotheranostics. 2023. https://doi.org/10.3390/jnt4030014.
Barkat A, Beg S, Panda SK, Alharbi KS, Rahman M, Ahmed FJ. Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin Cancer Biol. 2021;69:365–75. https://doi.org/10.1016/j.semcancer.2019.08.022.
Santos A, Veiga F, Figueiras A. Dendrimers as pharmaceutical excipients: synthesis, properties, toxicity and biomedical applications. Materials (Basel). 2019;13(1). https://doi.org/10.3390/ma13010065.
Wang J, Li B, Qiu L, Qiao X, Yang H. Dendrimer-based drug delivery systems: history, challenges, and latest developments. J Biol Eng. 2022;16(1):18. https://doi.org/10.1186/s13036-022-00298-5.
Altuntaş E, Özkan B, Güngör S, Özsoy Y. Biopolymer-based nanogel approach in drug delivery: basic concept and current developments. Pharmaceutics. 2023;15(6). https://doi.org/10.3390/pharmaceutics15061644.
Soni KS, Desale SS, Bronich TK. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J Control Release. 2016;240:109–26. https://doi.org/10.1016/j.jconrel.2015.11.009.
Yin Y, Hu B, Yuan X, Cai L, Gao H, Yang Q. Nanogel: a versatile nano-delivery system for biomedical applications. Pharmaceutics. 2020;12(3). https://doi.org/10.3390/pharmaceutics12030290.
Pandey S, Shaikh F, Gupta A, Tripathi P, Yadav JS. A Recent Update: Solid Lipid Nanoparticles for Effective Drug Delivery. Adv Pharm Bull. 2022;12(1):17–33. https://doi.org/10.34172/apb.2022.007.
Anantaworasakul P, Anuchapreeda S, Yotsawimonwat S, Naksuriya O, Lekawanvijit S, Tovanabutra N, et al. Nanomaterial lipid-based carrier for non-invasive capsaicin delivery; manufacturing scale-up and human irritation assessment. Molecules. 2020;25(23). https://doi.org/10.3390/molecules25235575.
Debnath SK, Srivastava R. Drug delivery with carbon-based nanomaterials as versatile nanocarriers: progress and prospects. Front Nanotechnol. 2021;3. https://doi.org/10.3389/fnano.2021.644564.
Pivetta TP, Botteon CEA, Ribeiro PA, Marcato PD, Raposo M. Nanoparticle systems for cancer phototherapy: an overview. Nanomaterials (Basel). 2021;11(11):3132. https://doi.org/10.3390/nano11113132.
Xu JJ, Zhang WC, Guo YW, Chen XY, Zhang YN. Metal nanoparticles as a promising technology in targeted cancer treatment. Drug Deliv. 2022;29(1):664–78. https://doi.org/10.1080/10717544.2022.2039804.
Socoliuc V, Peddis D, Petrenko VI, Avdeev MV, Susan-Resiga D, Szabó T, et al. Magnetic nanoparticle systems for nanomedicine—a materials science perspective. Magnetochemistry. 2020;6(1):2 https://doi.org/10.3390/magnetochemistry6010002.
Jain R, Mohanty S, Sarode I, Biswas S, Singhvi G, Dubey SK. Multifunctional photoactive nanomaterials for photodynamic therapy against tumor: recent advancements and perspectives. Pharmaceutics. 2022;15(1):109. https://doi.org/10.3390/pharmaceutics15010109.
Zhou Y, Quan G, Wu Q, Zhang X, Niu B, Wu B, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm Sin B. 2018;8(2):165–77. https://doi.org/10.1016/j.apsb.2018.01.007.
Zhou Y, Quan G, Wu Q, Zhang X, Niu B, Wu B, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm Sin B. 2018;8(2):165–77. https://doi.org/10.1016/j.apsb.2018.01.007.
Li Y, Ren X, Zhu L, Li C. Biomass 3D printing: principles, materials, post-processing and applications. Polymers. 2023. https://doi.org/10.3390/polym15122692.
Khan SA, Jassim M, Ilcan H, Sahin O, Bayer İR, Sahmaran M, et al. 3D printing of circular materials: Comparative environmental analysis of materials and construction techniques. Case Stud Constr Mater. 2023;18:e02059. https://doi.org/10.1016/j.cscm.2023.e02059.
Song JH, Murphy RJ, Narayan R, Davies GB. Biodegradable and compostable alternatives to conventional plastics. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2127–39. https://doi.org/10.1098/rstb.2008.0289.
Wang F, Harindintwali JD, Yuan Z, Wang M, Wang F, Li S, et al. Technologies and perspectives for achieving carbon neutrality. Innovation. 2021;2(4):100180. https://doi.org/10.1016/j.xinn.2021.100180.
Beitler BG, Abraham PF, Glennon AR, Tommasini SM, Lattanza LL, Morris JM, et al. Interpretation of regulatory factors for 3D printing at hospitals and medical centers, or at the point of care. 3D Print Med. 2022;8(1):7. https://doi.org/10.1186/s41205-022-00134-y.
Gad MM, Fouda SM. Factors affecting flexural strength of 3D-printed resins: A systematic review. J Prosthodont. 2023;32(S1):96–110.
Mendaza-DeCal R, Peso-Fernandez S, Rodriguez-Quiros J. Orthotics and prosthetics by 3D-printing: Accelerating its fabrication flow. Res Vet Sci. 2023;162:104960. https://doi.org/10.1016/j.rvsc.2023.104960.
Meng M, Wang J, Sun T, Zhang W, Zhang J, Shu L, et al. Clinical applications and prospects of 3D printing guide templates in orthopaedics. J Orthop Translat. 2022;34:22–41. https://doi.org/10.1016/j.jot.2022.03.001.
Safali S, Berk T, Makelov B, Acar MA, Gueorguiev B, Pape HC. The possibilities of personalized 3D printed implants-a case series study. Medicina (Kaunas). 2023;59(2). https://doi.org/10.3390/medicina59020249.
Zhang Q, Zhou J, Zhi P, Liu L, Liu C, Fang A, et al. 3D printing method for bone tissue engineering scaffold. Med Nov Technol Devices. 2023;17:100205. https://doi.org/10.1016/j.medntd.2022.100205.
Zhang J, Wehrle E, Rubert M, Müller R. 3D bioprinting of human tissues: biofabrication, bioinks, and bioreactors. Int J Mol Sci. 2021;22(8). https://doi.org/10.3390/ijms22083971.
Sandström C. Adopting 3D printing for manufacturing—the case of the hearing aid industry. Ratio Working Papers. 2015;262(1):20.
Ayyildiz O. Customised spectacles using 3-D printing technology. Clin Exp Optom. 2018;101(6):747–51.
Yu D, Peng W, Mo X, Zhang Y, Zhang X, He J. Personalized 3D-Printed Bioresorbable Airway External Splint for Tracheomalacia Combined With Congenital Heart Disease. Front Bioeng Biotechnol. 2022;10:859777. https://doi.org/10.3389/fbioe.2022.859777.
Fang J-J, Lin C-L, Tsai J-Y, Lin R-M. Clinical assessment of customized 3D-printed wrist orthoses. Appl Sci. 2022;12(22):11538. https://doi.org/10.3390/app122211538.
Funding
No funding was received for the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The authors (LA, AAAA, MMT) collectively contributed to the manuscript, with each individual being involved in various aspects such as conceptualization, data acquisition, analysis, original draft writing, and reviewing, ensuring a comprehensive and collaborative effort across all stages of the research process.
Corresponding authors
Ethics declarations
Conflict of Interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alzoubi, L., Aljabali, A.A.A. & Tambuwala, M.M. Empowering Precision Medicine: The Impact of 3D Printing on Personalized Therapeutic. AAPS PharmSciTech 24, 228 (2023). https://doi.org/10.1208/s12249-023-02682-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02682-w