Abstract
Key message
The diploid wheat recessive stem rust resistance gene SrTm4 was fine-mapped to a 754-kb region on chromosome arm 2AmL and potential candidate genes were identified.
Abstract
Race Ug99 of Puccinia graminis f. sp. tritici (Pgt), the causal agent of wheat stem (or black) rust is one of the most serious threats to global wheat production. The identification, mapping, and deployment of effective stem rust resistance (Sr) genes are critical to reduce this threat. In this study, we generated SrTm4 monogenic lines and found that this gene confers resistance to North American and Chinese Pgt races. Using a large mapping population (9522 gametes), we mapped SrTm4 within a 0.06 cM interval flanked by marker loci CS4211 and 130K1519, which corresponds to a 1.0-Mb region in the Chinese Spring reference genome v2.1. A physical map of the SrTm4 region was constructed with 11 overlapping BACs from the resistant Triticum monococcum PI 306540. Comparison of the 754-kb physical map with the genomic sequence of Chinese Spring and a discontinuous BAC sequence of DV92 revealed a 593-kb chromosomal inversion in PI 306540. Within the candidate region, we identified an L-type lectin-domain containing receptor kinase (LLK1), which was disrupted by the proximal inversion breakpoint, as a potential candidate gene. Two diagnostic dominant markers were developed to detect the inversion breakpoints. In a survey of T. monococcum accessions, we identified 10 domesticated T. monococcum subsp. monococcum genotypes, mainly from the Balkans, carrying the inversion and showing similar mesothetic resistant infection types against Pgt races. The high-density map and tightly linked molecular markers developed in this study are useful tools to accelerate the deployment of SrTm4-mediated resistance in wheat breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common wheat (Triticum aestivum L., 2n = 6x = 42, AABBDD) is a major crop used for consumption by humans and domestic animals in many areas of the world and further increases in production are required to accommodate a growing human population. However, these increases are limited by losses generated by pathogens, with fungal pathogens among the major constraints for increasing global wheat production (Figueroa et al. 2018). Among these pathogens, Puccinia graminis f. sp. tritici (Pgt) causes stem (or black) rust of wheat and is potentially a devastating disease worldwide. Severe wheat stem rust epidemics occurred in the United States and Europe in the nineteenth and early twentieth centuries causing large yield losses (Roelfs 1978; Zadoks 1967). Due to efforts to eradicate the alternate host barberry (Berberis vulgaris L.) and breed for resistant wheat varieties, this disease was successfully controlled over the past several decades (Peterson 2001; Roelfs 1982; Singh et al. 2015).
However, stem rust re-emerged as a major concern with the appearance of Pgt race TTKSK (also known as Ug99) in Uganda in 1998 (Pretorius et al. 2000). Ug99 was the first Pgt race to defeat the widely deployed stem rust resistance gene Sr31 (Jin et al. 2007; Pretorius et al. 2000). Subsequently, Ug99 and its derivatives spread throughout the major wheat-growing countries of eastern and southern Africa (Pretorius et al. 2020; Shahin et al. 2020; Singh et al. 2015) and into the Middle East (Nazari et al. 2009, 2021), and gained virulence to additional Sr genes, including Sr24 (Jin et al. 2008), Sr36 (Jin et al. 2009), and SrTmp (Newcomb et al. 2016).
In recent years, other highly virulent Pgt races outside the Ug99 race group, such as TKTTF, TRTTF, RRTTF and TTRTF, were detected in wheat stem rust outbreaks. Race TKTTF was responsible for severe stem rust epidemics in Ethiopia in 2013/14 on the Ug99 resistant wheat cultivar “Digalu” (Olivera et al. 2015), and was subsequently found in more than ten countries, including Ethiopia, Azerbaijan, Egypt, Iraq, Iran, Sudan, Lebanon, Turkey, Germany, Sweden, Denmark and the UK (Lewis et al. 2018; Patpour et al. 2017). Race TRTTF and the closely related race RRTTF were detected in Pakistan, Yemen, Ethiopia, and Ecuador (Barnes et al. 2018; Fetch et al. 2016), and were virulent to at least three Sr genes (Sr36, SrTmp, and Sr1RSAmigo) that are effective against race TTKSK (Olivera et al. 2012). Another Pgt race of concern is TTRTF, which caused a severe epidemic of stem rust on thousands of hectares of durum and bread wheat in Southern Italy in 2016 (Bhattacharya 2017). This race overcame the resistance provided by Sr13b, Sr21, Sr35, Sr45, Sr50 and several other Sr genes (Barnes et al. 2018; Bhattacharya 2017; Patpour et al. 2020), and was recently reported in more countries, including Hungary, Egypt, Ethiopia, Eritrea and Iran (Olivera et al. 2019; Patpour et al. 2020; Tesfaye et al. 2020). Additionally, several Pgt races caused large scale wheat stem rust outbreaks in Northern Kazakhstan and Western Siberia generating yield losses in more than one million hectares of spring wheat (Rsaliyev et al. 2020; Skolotneva et al. 2020). The recent evolution and spread of new virulent Pgt races have prompted widespread efforts to identify new Sr genes and to develop new wheat cultivars with durable resistance.
So far, over 60 Sr genes (Sr1-Sr63) have been assigned official designations (Mago et al. 2022; Yu et al. 2022), among which a significant proportion were introgressed into wheat from wild relatives (Singh et al. 2015). Triticum monococcum (2n = 2x = 14, AmAm), is commonly known as einkorn wheat. This diploid species is closely related to Triticum urartu (AuAu), the A-genome donor of polyploid wheat (Dvorak et al. 1988). Triticum monococcum has contributed five Sr genes, including Sr21 (Chen et al. 2015; The 1973), Sr22a/Sr22b (Gerechter-Amitai et al. 1971; Luo et al. 2022), Sr35 (Saintenac et al. 2013), Sr60 (Chen et al. 2018a), and SrTm4 (Briggs et al. 2015). Except for the recessive gene SrTm4, all previously mapped Sr genes in T. monococcum have been cloned (Chen et al. 2018b, 2020; Luo et al. 2022; Saintenac et al. 2013; Steuernagel et al. 2016).
SrTm4, discovered in cultivated T. monococcum accession PI 306540, is a recessive resistance gene effective against all Pgt races tested, including race TTKSK (Briggs et al. 2015). Using two mapping populations, this gene was previously mapped within a 2.1 cM interval on the distal region of chromosome arm 2AmL (Briggs et al. 2015). The objectives of this study were to: (1) obtain an SrTm4 monogenic line; (2) generate a precise map of SrTm4; and (3) identify candidate genes within the physical maps of the SrTm4 region.
Material and methods
Plant materials and mapping populations
T. monococcum accession PI 306540 was identified as having a unique resistance response to multiple Pgt races (Rouse and Jin 2011a, b), which was subsequently associated with the presence of four Sr genes: Sr21, Sr60, Sr22b, and SrTm4 (Briggs et al. 2015; Chen et al. 2018b, 2020; Luo et al. 2022). PI 306540 was crossed with both wild T. monococcum ssp. aegilopoides accession G3116 (Dubcovsky et al. 1996) and cultivated T. monococcum ssp. monococcum accession PI 272557 (Rouse and Jin 2011b) to generate two segregating populations. Wild accession G3116 was selected as a susceptible parent because it is highly polymorphic compared to cultivated PI 306540 (Chen et al. 2018a; Dubcovsky et al. 1996). The other susceptible parent PI 272557 does not possess any known Sr genes (Rouse and Jin 2011b).
A high-density genetic map of SrTm4 was constructed using 9522 recombinant gametes from the cross of G3116 × PI 306540. From cross PI 272557 × PI 306540, we selected two F3 families homozygous for the presence (TmR4-260; lacking Sr21, Sr60, and Sr22b) or absence (TmS4-110; carrying no Sr gene) of SrTm4 using molecular markers. Markers BQ461276 and DR732348 flanking the SrTm4 resistance gene (Briggs et al. 2015) were used to confirm the presence of the PI 306540 segment in the selected family, whereas diagnostic markers from cloned genes Sr21 (Chen et al. 2018b), Sr60 (Chen et al. 2020), and Sr22b (Luo et al. 2022) were used to determine absence of the other Sr genes. Finally, we explored the distribution of a chromosomal inversion within the SrTm4 candidate region in a collection of 79 wild and cultivated T. monococcum accessions.
Stem rust assays
Infection types of PI 306540, PI 272557, and G3116 to Pgt races TTTTF (isolate 01MN84A-1-2), TTKSK (04KEN156/04), TRTTF (06YEM34-1), MCCFC (59KS19), TPMKC (74MN1409), RKQQC (99KS76A-1), RCRSC (77ND82A-1), QTHJC (75ND717C), QFCSC (06ND717C), and SCCSC (09ID73-2) were reported in previous studies (Briggs et al. 2015; Rouse and Jin 2011a). Race TTTTF is virulent to resistance genes Sr21, Sr60, and Sr22b, but avirulent to SrTm4 (Briggs et al. 2015; Chen et al. 2018a). Moreover, SrTm4 showed a mesothetic resistant infection type (intermediate reaction with both resistant and susceptible infection types present) (Rouse and Jin 2011a).
In the current study, the parental lines, selected families TmR4-260 and TmS4-110, and segregating populations were evaluated with race TTTTF at the United States Department of Agriculture, Agricultural Research Service (USDA-ARS) Cereal Disease Laboratory. For plants carrying recombination events in the candidate gene region, we carried out progeny tests using at least 25 individuals from each F2:3 family with race TTTTF. We further confirmed the phenotypes of these critical lines in the next generation by challenging 25 F3:4 plants homozygous for the recombination with the same race.
We also evaluated the selected families TmR4-260 and TmS4-110 with the Chinese Pgt race 34C3RTGQM (isolate 20IAL32) at Peking University Institute of Advanced Agricultural Sciences. The virulence/avirulence formulae of the Pgt races are provided in Table S1. Plants were grown in growth chambers at 18 °C day/15 °C night with a 16 h light/8 h darkness photoperiod. Procedures for inoculation and scoring disease reactions were as described previously (Briggs et al. 2015; Stakman et al. 1962).
RNA-seq and qRT-PCR analysis
From the population G3116 × PI 306540, we selected two F4 lines homozygous for the resistant SrTm4 haplotype (R-F14 and R-K18) and two sister control lines (S-A13 and S-E14) carrying the susceptible haplotype. The selected lines and parental lines G3116 and PI 306540 (total replications = 3) were inoculated with Pgt race 34C3RTGQM and mock-inoculated with water in two independent chambers under the environmental conditions described above: 18 °C day/15 °C night and 16 h light/8 h darkness. Leaf samples from different plants were collected immediately before inoculation (0 h) and 3-, 6- and 14-days post inoculation (dpi).
Total RNAs were isolated using the Spectrum Plant Total RNA Kit (MilliporeSigma, MO, USA). We performed RNA-seq for Pgt-inoculated RNA samples of G3116 and PI 306540 and the selected F4 lines (R-F14, R-K18, S-A13 and S-E14) at 14 dpi (accession number PRJNA932462). RNA-seq library preparation and sequencing was carried out at Beijing Novogene Bioinformatics Technology Co., Ltd.. Sequencing data quality control, sequence alignment, and variant calling were performed as described previously (Jiang et al. 2023). Differentially expressed genes (DEGs) were identified using edgeR software (Robinson et al. 2010) with a false discovery rate (FDR) of 0.05. The significance of the differences in transcript levels between the two groups was estimated using Student’s t-tests. DEGs within the SrTm4 candidate region of chromosome 2A were analyzed and a heatmap was created using the pheatmap package (Kolde and Kolde 2018). Principal component analysis (PCA) of RNA-seq data from the homozygous resistant lines (PI 306540, R-F14 and R-K18) and the susceptible lines (G3116, S-A13 and S-E14) was presented in supplemental Figure S1.qRT-PCR was carried out on an ABI QuantStudio 5 Real-Time PCR System (Applied Biosystems, CA, USA) using PowerUp SYBR Green Master Mix. Transcript levels were expressed as fold-ACTIN levels using the 2−ΔCT method as described previously (Pearce et al. 2013).
Development of molecular markers
Based on the RNA-seq data, single nucleotide polymorphisms (SNPs) between the two parental lines G3116 and PI 306540 spaced throughout the candidate gene region were selected to develop markers. The sequences flanking the target polymorphisms were obtained from the T. aestivum reference genome of ‘Chinese Spring’ (CS) RefSeq v2.1 (Zhu et al. 2021). Primers were designed using the software Primer3 web version 4.1.0 (https://primer3.ut.ee/) to amplify intronic regions carrying the target polymorphisms. PCR amplification products were sequenced using the Sanger method to confirm sequence polymorphisms between parents. The detected polymorphisms were used to develop Insertion-Deletion (InDel), or Cleaved Amplified Polymorphic Sequence (CAPS), or derived Cleaved Amplified Polymorphic Sequence (dCAPS) markers (Bhattramakki et al. 2002; Konieczny and Ausubel 1993; Neff et al. 1998).
BAC library screening and sequencing
Bacterial artificial chromosome (BAC) libraries from T. monococcum accessions DV92 (Lijavetzky et al. 1999) and PI 306540 (Chen et al. 2020; Luo et al. 2022) were available at the Wheat Molecular Genetics Laboratory, University of California, Davis. High quality DNAs from the selected BAC clones were extracted using QIAGEN Large-Construct Kit (Qiagen, Hilden, Germany). BAC DNAs were fingerprinted using restriction enzyme HindIII. Selected BACs were sequenced using WideSeq at Purdue University (https://purdue.ilabsolutions.com/landing/808) or/and Illumina HiSeq 4000 platform at Beijing Novogene Bioinformatics Technology Co., Ltd.. We blocked the repetitive sequences in the SrTm4 region using the Triticeae Repeat Sequence Database (http://wheat.pw.usda.gov/ITMI/Repeats/blastrepeats3.html), and searched the unblocked region using TBLASTX available at National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/).
Statistical analyses
Genetic linkage maps were generated using MapChart 2.2 software (Voorrips 2002). The released reference genomes of diploid, tetraploid and hexaploid wheat varieties (Avni et al. 2017; Ling et al. 2018; Luo et al. 2017; Maccaferri et al. 2019; Walkowiak et al. 2020) were used in our analyses. The transcriptome databases of DV92 and G3116 (Fox et al. 2014) were also used to detect the expressions of candidate genes. The “Sorting Intolerant from Tolerant” (SIFT) algorithm was used to predict the effect of coding variants on protein function (Ng and Henikoff 2003). The assay for transposase-accessible chromatin (ATAC)-seq data from tetraploid wheat seedling roots was reported previously (Debernardi et al. 2022).
Results
Characterization of stem rust responses
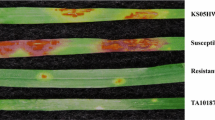
A total of 388 F2 plants from cross PI 272557 × PI 306540 were used to separate SrTm4 from the other three Sr genes (Sr21, Sr60 and Sr22b) present in PI 306540. From this population, we selected F3 family TmR4-260 carrying only SrTm4 and family TmS4-110 carrying no Sr gene. Seedlings from the TmR4-260 family exhibited mesothetic resistant infection types (ITs = ‘3’ to ‘31’) to Pgt races TTTTF and 34C3RTGQM, whereas seedlings from family TmS4-110 displayed susceptible infection types of ‘3+’ to ‘4’ (Fig. S2). Pgt race TTTTF, which is virulent on parental plants carrying resistance genes Sr21, Sr60, and Sr22b, but avirulent on plants carrying SrTm4 (Briggs et al. 2015; Chen et al. 2018a), was used to determinate disease reactions in the G3116 × PI 306540 population. F3:4 seedlings homozygous for the presence of SrTm4 showed ITs ranging from ‘;3’ to ‘31’ (similar to PI 306540), whereas plants homozygous for the absence of the gene displayed susceptible infection types (ITs = ‘3 + ’ to ‘4’, similar to G3116) (Fig. 1).
High-resolution genetic map of SrTm4
The SrTm4 locus was previously mapped within a 2.1 cM interval on chromosome arm 2AmL (Briggs et al. 2015). To accelerate the development of markers in the candidate region, we first performed an RNA-seq experiment to identify single nucleotide polymorphisms (SNPs) between parental lines G3116 and PI 306540. Approximately 40.9 million and 28.1 million PE150 reads were generated for PI 306540 and G3116, respectively. After removing low-quality reads and adaptors, ~ 94% of the reads were mapped to ‘Chinese Spring’ wheat reference genome RefSeq v2.1, and a total of 84,495 polymorphisms were identified.
Based on these polymorphisms, we developed 12 new markers in the candidate region (Fig. 2b, Table S2). Screening of 811 F2 plants from the G3116 × PI 306540 cross yielded 48 plants with recombination events between SrTm4-flanking markers BQ461276 (IWGSC RefSeq v2.1: 760,094,323 bp) and gwm526 (763,867,267 bp), a genetic distance of 2.96 cM (Fig. 2b). The new markers were used to genotype the 48 lines with recombination events and to construct a genetic map of the SrTm4 region (Fig. 2b). SrTm4 was mapped within a 0.37 cM interval flanked by markers CD903048 and DK658885 and completely linked to markers CK167245.1, CK167245.2, BJ314745.1 and BJ314745.2 (Fig. 2b).
High-density genetic maps for stem rust resistance locus SrTm4. a Genomic region containing SrTm4 (marked in gray) on the long arm of wheat chromosome 2A. b Genetic map based on 811 F2 plants and 14 molecular markers; c High-density genetic map based on 4761 F2 plants and 10 molecular markers; d Physical map of Chinese Spring. Coordinates are based on CS RefSeq v2.1
To define better the position of SrTm4, we screened another 3950 F2 plants with the new flanking markers CD903048 and DK658885. The genetic distance between these two markers was estimated to be 0.27 cM based on 20 plants with informative recombination events identified in this screen plus another 6 plants found in the previous 811 plants. Using these informative recombination events and six new markers developed in this candidate region (Table S2), SrTm4 was finally mapped within a 0.06 cM interval flanked by marker loci CS4211 (IWGSC RefSeq v2.1: 762.67 Mb) and 130K1519 (IWGSC RefSeq v2.1: 763.67 Mb, Fig. 2c).
The candidate gene region partially overlapped with a 1.8 Mb chromosomal inversion between IWGSC RefSeq v2.1 (763.4 Mb to 765.2 Mb) and IWGSC RefSeq v1.1 (Fig. S3 and Table S3). Comparisons among published reference genomes of diploid, tetraploid and hexaploid wheat showed that this 1.8-Mb inversion is present only in IWGSC RefSeq v1.1 but absent in all other sequenced wheat varieties (Fig. S4), indicating that the IWGSC RefSeq v2.1 is the correct assembly for this region.
Candidate genes for SrTm4 within the colinear region of hexaploid wheat genome
The 0.06 cM candidate region between SrTm4-flanking markers CS4211 and 130K1519 defines a 1.0-Mb region (762.67–763.67 Mb, Fig. 2d) in the reference genome of hexaploid wheat ‘Chinese Spring’ (IWGSC RefSeq v2.1) that contains 19 high-confidence annotated genes (TraesCS2A03G1276000-TraesCS2A03G1280800, Table S4).
Among these 19 candidate genes, four were differentially expressed (FDR < 0.05; p-value < 0.01; and |log2 foldchange|> 1) between the inoculated susceptible and resistant sister lines in the RNA-seq experiment (TraesCS2A03G1276800, TraesCS2A03G1278700, TraesCS2A03G1280400, and TraesCS2A03G1280700). Two of the differentially expressed genes (DEGs) showed that their transcripts were significantly higher in the plants carrying the susceptible SrTm4 allele compared to the resistant allele, whereas the other two DEGs were downregulated in the same lines (Table S5).
Among DEGs identified in the RNAseq data (accession number PRJNA932462), 149 genes were significantly upregulated and 136 genes were significantly downregulated in the homozygous resistant lines (PI 306540, R-F14 and R-K18) relative to the susceptible lines (G3116, S-A13 and S-E14). Principal component analysis (PCA) of the RNAseq samples confirmed that the transcriptomes of the sister monogenic lines with and without SrTm4 are very similar to each other and intermediate between the parental lines (Fig. S1). It also shows that the small number of DEGs detected between the sister lines are not sufficient to generate a clear difference between the two groups in the PCA.
Physical maps of the SrTm4 region
To determine whether additional genes were present in the candidate region in T. monococcum, we screened the BAC libraries of susceptible line DV92 and resistant parent PI 306540 using flanking markers CS4211 and 130K1519 and three markers completely linked to SrTm4 (PRRF4R4, CK167245.2 and BJ314745.1, Fig. 2d). In addition, we developed new markers (Table S2) from genes located in the orthologous region in the ‘Chinese Spring’ reference genome to accelerate the screening process.
By chromosome walking, we identified 12 and 11 overlapping BACs from the BAC libraries of DV92 and PI 306540, respectively, covering the candidate gene region (Fig. S5 and S6). Based on sequencing and assembly of BAC sequences, we determined that the 0.06 cM candidate region defines a contiguous sequence of 754-kb region in T. monococcum accession PI 306540 (GenBank accession QQ503488). The 12 overlapping BACs of DV92 were sequenced at lower depth using the WideSeq approach, which yielded 30 non-overlapping contigs covering 652-kb excluding gaps.
High confidence genes in the SrTm4 candidate region
Our annotation of the 754-kb sequence showed no additional genes in the SrTm4 candidate region in PI 306540 relative to Chinese Spring and DV92. TraesCS2A03G1276100 is a pseudogene in both T. monococcum genotypes (DV92 and PI 306540), suggesting it is not a good candidate gene for SrTm4. For the other genes, we focused on those that were either differentially expressed or polymorphic in their coding regions between the resistant parent PI 306540 and the susceptible line DV92 (Table S5 and S6). We detected amino acid changes between PI 306540 and DV92 for nine expressed candidate genes (Table S6) and calculated SIFT scores to predict their effects on protein structure and function. Six genes had SIFT scores lower than 0.05, indicating high probabilities of deleterious effects (Table S6). Based on the functional annotation of these genes, we prioritized TraesCS2A03G1276200, TraesCS2A03G1276800, and TraesCS2A03G1278900 given their known roles in plant defense against pathogens (Hopkins et al. 2008; Laluk et al. 2011; Qiu et al. 2021; Ruffel et al. 2002; Zhang et al. 2019a, 2021; Zhao et al. 2022).
The four DEGs identified in the candidate gene region are also potential candidates for SrTm4 (Table S5). For each of these genes, we sequenced the promoter and open chromatin regions identified by ATAC-seq (Debernardi et al. 2022) (Fig. S7). We found multiple polymorphisms in these regions that may explain their differential expression.
Among the four DEGs, we eliminated TraesCS2A03G1280400, which is a short putative gene with a single predicted exon encoding a 120-amino acid peptide with no similarity to any known-function protein. Attempts to annotate the orthologous regions on chromosomes 2B and 2D revealed multiple frame-shift mutations, and no possible functional orthologs. Based on these results, we hypothesize that this is not a real gene, and we did not consider it further as a candidate for SrTm4.
The other three DEGs are annotated in the genome of Chinese Spring (RefSeq v2.1) as eukaryotic initiation factor 4A-III homolog B-like (TraesCS2A03G1276800), S-acyltransferase 11-like (TraesCS2A03G1278700), and acyl-activating enzyme 5 (TraesCS2A03G1280700). Their transcript levels were analyzed in Pgt-inoculated and mock-inoculated T. monococcum plants by qRT-PCR at 0 h, 3- and 6- days post inoculation (dpi). Since we were not able to detect transcripts of TraesCS2A03G1276800 in PI 306540 and TraesCS2A03G1280700 in G3116 based on RNAseq data (Table S5), their transcripts were evaluated only in PI 306540 or in G3116. We found that transcript levels of TraesCS2A03G1276800 and TraesCS2A03G1278700 in G3116 were significantly higher (P < 0.05) in Pgt-inoculated plants than in mock-inoculated controls only at 6 dpi (Fig. S8a, b). There was no significant difference in the transcript levels of TraesCS2A03G1278700 and TraesCS2A03G1280700 in PI 306540 between Pgt-inoculated and mock-inoculated plants (Fig. S8c, d). In addition, we also detected higher transcript levels of TraesCS2A03G1278700 in PI 306540 than in G3116 (Fig. S8b, c), supporting the RNAseq data analysis (Table S5) (Fig. 3).
Transcript levels of high-confidence genes annotated in the candidate gene region. Differentially expressed genes (DEGs) between homozygous susceptible lines (G3116, S-A13 and S-E14) and homozygous resistant lines (PI 306540, R-F14 and R-K18) were identified using RNA-seq data. The heatmap was generated using the pheatmap package (Kolde and Kolde 2018)
Detection of a 593-kb chromosomal inversion in the candidate region that disrupts a potential candidate gene
Based on chromosomal walking experiments, we observed that the order of the markers was reversed within a ~ 600-kb region between PI 306540 and DV92 (Fig. S5 and S6), indicating a potential chromosomal inversion in the candidate region. We then compared the two SrTm4 physical maps with the Chinese Spring reference genome sequence (RefSeqv2.1) and confirmed the presence of an inverted segment in PI 306540 (Fig. 4a and b) relative to CS and DV92. Figure 4a shows that the PI 306540 inverted region was approximately 0.8 Mb, extending from 762.7 Mb to 763.5 Mb based on CS RefSeq v2.1 coordinates.
Chromosomal inversion in the candidate region. (a) Comparison of the SrTm4 physical map in PI 306540 with the genomic sequence of Chinese Spring (RefSeqv2.1). The figure was generated using the Python drawing library matplotlib (Hunter 2007). The inverted region is highlighted by the red square. (b) Syntenic relationships between Chinese Spring and PI 306540. The figure was created using the NGenomeSyn program (https://github.com/hewm2008/NGenomeSyn). Blue arrows represent the inverted regions. (c) Dominant markers used to characterize the inversion breakpoints. Two dominant markers HNPI30F1R1 and HNPI30F4R4 (Table S2) were developed on the breakpoint junctions. These primers amplify PCR products of 1007-bp and 1859-bp when the 593-kb chromosomal inversion is present and no product when its absent. The amplification products are marked with a red arrow. + , PCR product present; -, no PCR product (colour figure online)
Further sequence analysis revealed the inversion breakpoints in PI 306540, as shown in Figure S9. On the proximal side, we delimited the inversion breakpoint to a 3.3-kb region in PI 306540. This 3.3-kb region includes the border of the inversion located at ~ 762.7 Mb (762,705,407–762,705,985 bp) and the inverted part located at ~ 763.5 Mb (763,515,576–763,513,146 bp) based on CS RefSeq v2.1 coordinates. The two parts of the sequences are located ~ 807 kb apart in the Chinese Spring reference genome but they are adjacent in the 3.3 kb region in PI 306540. On the distal side of the inversion, we also identified a 5.2-kb region in PI 306540 that contains two segments located far apart in Chinese Spring (762,705,509–762,704,291 bp and 763,515,638–763,518,339 bp, Fig. S9). Using the physical map of SrTm4, we were able to determine that the inverted region in PI 306540 was ~ 593 kb.
The proximal inversion breakpoint disrupted one gene, TraesCS2A03G1276600LC.1, which was annotated as encoding an L-type lectin-domain containing receptor kinase protein (designated here as LLK1). The inversion breakpoint is located in the coding region of this gene in PI 306540 and, therefore affects the protein structure and function. TraesCS2A03G1276600LC.1 is a truncated gene in Chinese Spring since it carries premature stop codons, but its B- and D-genome homeologs encode complete proteins of 759 and 764 amino acids, respectively. Using the publicly available transcriptome databases of DV92 and G3116 (Fox et al. 2014) and other wheat genome sequences, we found that LLK1 is expressed in susceptible T. monococcum DV92 and G3116, and encodes proteins containing ~ 763 amino acids in different wheat species (Fig. S10). Using qRT-PCR analysis, we found that the transcript levels of LLK1 in G3116 were significantly higher in Pgt-inoculated than in mock-inoculated plants at both 3- and 6-dpi (P < 0.05; Fig. S11). LLK1 was completely linked to SrTm4 in the population of 4761 F2 plants, and is of particular interest because this type of protein was previously associated with disease resistance (Wang et al. 2015a, 2015b, 2018; Woo et al. 2016).
Distribution of the chromosomal inversion
Based on BLASTN searches using the 3.3-kb and 5.2-kb segments carrying the inversion breakpoints in the published reference genomes, we determined that this chromosomal inversion was not present in sequenced accessions of T. urartu (G1812), Aegilops tauschii (AL8/78), T. turgidum subsp. dicoccoides (Zavitan), T. turgidum subsp. durum (Svevo), and T. aestivum (10 + wheat varieties in the Wheat Pan Genome project).
To characterize the distribution of the inversion in T. monococcum, we developed two dominant markers HNPI30F1R1 and HNPI30F4R4 (Table S2) on the breakpoint junctions. These two pairs of primers amplify PCR products of 1007-bp and 1859-bp fragments when the 593-kb chromosomal inversion is present and no product when it is absent (Fig. 4c). We also developed a dominant marker DV92F1R1 (Table S2) for absence of the inversion. PCR amplification with primers DV92F1R1 at an annealing temperature of 56 °C generates a 488-bp fragment when the inversion is absent and no amplification when it is present.
In a previous screen of 1,061 T. monococcum accessions using five selected Pgt races (Rouse and Jin 2011b), SrTm4 was postulated to be present in five T. monococcum accessions in addition to PI 306540, including PI 352480, PI 306544, PI 355541, PI 435000-R, and PI 221414. We identified the same inversion breakpoints in these lines using the three dominant markers (Table S7). In addition, we evaluated a collection of 73 T. monococcum accessions and identified another four accessions (PI 277131-2, PI 306547, PI 428158, and PI 435001) where the same inversion was present based on the three dominant markers. The presence of the same inversion was confirmed by sequencing of the PCR products of HNPI30F1R1 and HNPI30F4R4, which revealed identical sequences flanking the inversion as in PI 306540. Finally, we challenged these four lines with race TTTTF (isolate 01MN84A-1-2), and observed very similar responses to that conferred by PI 306540 (Fig. 5), although we cannot rule out the presence of additional or other Sr genes in these lines.
Infection types of T. monococcum accessions PI 277131-2, PI 306547, PI 428158, PI 435001, PI 306540, and G3116 in response to Puccinia graminis f. sp. tritici race TTTTF (isolate 01MN84A-1-2). Plants were grown in a growth chamber at 18 °C day/15 °C night with 16 h light/8 h darkness. R, resistant; S, susceptible
In summary, the presence of the inversion seems to be linked to the SrTm4 resistance allele. This inversion was found only in a few domesticated T. monococcum ssp. monococcum but was absent in all tested wild T. monococcum ssp. aegilopoides accessions (Table S7). Most of the accessions carrying the inversion were collected in the Balkans (Fig. 6), suggesting that the inversion event likely originated in this region.
A collection of 79 T. monococcum accessions was used to test the presence/absence of the chromosomal inversion. Dominant markers HNPI30F1R1, HNPI30F4R4, and DV92F1R1 (Table S2) were used to genotype these T. monococcum accessions. Green circles, accessions without the inversion; Red triangles, genotypes with the inversion (colour figure online)
Discussion
SrTm4 shows broad-spectrum resistance to Pgt races
In this study, we generated SrTm4 monogenic line TmR4-260 and sister susceptible line TmS4-110 lacking SrTm4. The monogenic line is useful to determine response profiles for SrTm4 without confounding effects of other resistance genes. In a previous study, we showed that SrTm4 conferred a low hypersensitive reaction to Pgt races TTKSK, TTTTF, TRTTF, QFCSC, MCCFC (Rouse and Jin 2011a), and mesothetic infection types for additional races TPMKC, RKQQC, RCRSC, and SCCSC that were virulent to Sr21 (Briggs et al. 2015). Using the SrTm4 monogenic line, we confirmed that SrTm4 was effective against both North American and Chinese Pgt races (Fig. S2). The broad-spectrum resistance conferred by SrTm4 makes it a potentially valuable genetic resource in breeding for resistance, especially to race Ug99 (TTKSK) and other more recently identified, widely virulent races.
High-resolution mapping of SrTm4 reveals an inversion linked to SrTm4 resistance
Most plant disease resistance genes are dominant or partially dominant, but recessive R genes are also well documented. However, the molecular bases of recessive wheat stem rust recessive genes remain largely unknown, since all 18 Sr genes cloned so far in wheat and its relatives are dominant or partially dominant (Zhang et al. 2022). The recessive nature of SrTm4 and the mesothetic resistant infection type provide increased incentive to clone this gene.
Using our high-resolution genetic map, the published reference genome of hexaploid wheat (The International Wheat Genome Sequencing Consortium 2018), and the available T. monococcum BAC libraries (Chen et al. 2020; Lijavetzky et al. 1999), we delimited the SrTm4 candidate region to a 1.0-Mb region in common wheat Chinese Spring, a 652-kb discontinuous region in DV92, and a 754-kb continuous region in the resistant T. monococcum accession PI 306540.
A comparison of the 754-kb PI 306540 BAC sequence with the available genomic sequence of Chinese Spring and BAC sequence of DV92 revealed a 593-kb chromosomal inversion within the candidate region. Chromosomal inversions cause suppression of recombination, which likely explains the lack of recombination in the SrTm4 candidate region. This inversion precluded a more detailed mapping of SrTm4 in spite of the use of a very large mapping population (9522 gametes).
The geographic distribution of this inversion in the T. monococcum germplasm is limited to a few domesticated accessions that were collected mainly in the Balkans and all display similar mesothetic resistant responses to Pgt races (Fig. 5 and Table S7). Thus far, the presence of the inversion is completely linked to SrTm4.
Chromosomal inversions are important drivers of genome structure evolution in natural populations (Said et al. 2018). Inversions have the potential to disrupt genes at breakpoints, generate linkage blocks that cannot be broken by recombination, and cause positional effects on adjacent chromatin (Allshire et al. 1994; Spofford 1976). In this study, we observed significant gene expression differences in genes located close to the inversion breakpoint regions, such as TraesCS2A03G1276800 and TraesCS2A03G1280400 (Table S5), but we currently do not know if these differences are caused by position effects or disruption of the resistance gene.
Candidate genes linked to SrTm4
We found no typical NLR gene within the candidate gene region. NLR genes are the most frequent gene class associated with pathogen resistance in wheat and other plant species (Li et al. 2021; Saintenac et al. 2013; Wang et al. 2022; Yang et al. 2022; Zhang et al. 2017, 2019b). We did not detect additional genes in the SrTm4 candidate region in the susceptible T. monococcum line DV92 relative to the resistant parent PI 306540. However, we do not have a contiguous BAC sequence of DV92 and therefore cannot rule out the possibility that we missed the susceptibility gene located in the gap regions. However, this is unlikely because no additional gene(s) in the candidate region were found in Chinese Spring and other published wheat reference genomes.
Among the candidate genes, we identified six carrying predicted deleterious variants and four DEGs with polymorphisms in their regulatory regions (Table S5, S6), but we currently do not know if these changes affect their functions. Further functional characterization will be needed to demonstrate if one of these genes is SrTm4.
Except for the candidates described above, we also identified an L-type lectin-domain containing receptor kinase LLK1, that was completely linked to SrTm4 and disrupted by an inversion in the resistant parent. Members of this gene family have been implicated in disease resistance in several plant species (Wang and Bouwmeester 2017; Wang et al. 2015b, 2018; Woo et al. 2016). Functional LLK1 alleles are present in susceptible T. monococcum accessions but absent in all resistant T. monococcum accessions since the proximal inversion breakpoint disrupts its coding sequence. These results agree with the recessive nature of the resistance, and suggest that LLK1 is a potential candidate for SrTm4. To determine if LLK1 is the causal gene, we have initiated the development of loss-of-function mutations using both sequenced ethyl methane sulfonate (EMS)-mutagenized population of durum wheat Kronos (Krasileva et al. 2017) and CRISPR-Cas9 editing. If LLK1 is demonstrated to be the causal gene for SrTm4, then the inversion itself will be the basis for the origin of SrTm4.
Conclusions and practical implications
SrTm4 is a broad-spectrum resistance gene and confers resistance to widely virulent Pgt races recently identified in the United States, Kenya, Yemen, and China (Table S1). Since SrTm4 only confers intermediate levels of resistance when present alone, it would be necessary to combine it with other Sr genes to provide commercially useful levels of resistance. Pyramids of recessive and dominant resistance genes are expected to be an effective strategy for incorporating resistance (Pradhan et al. 2015). A combination of recessive and dominant R genes for resistance breeding has been reported in rice against bacterial blight pathogen (Li et al. 2001), in wild Arachis species against Meloidogyne arenaria (Church et al. 2005), in barley against leaf blotch (Garvin et al. 1997), and in pigeon pea against podfly and podborer (Verulkar et al. 1997).
Since the resistance genes Sr21 (resistant haplotype R2 in PI 306540) and SrTm4 are on the same chromosome arm located ~ 35 cM apart (Briggs et al. 2015; Chen et al. 2018b), it should be possible to introgress a T. monococcum chromosome segment carrying both genes into hexaploid wheat through the use of the ph1b mutation (Dubcovsky et al. 1995; Sears 1977). However, additional studies will be needed to test if the large introgressed T. monococcum segment with both Sr genes carries any undesirable genes. Even if SrTm4 was successfully introgressed into hexaploid wheat, it may be required to also knock out the B- and D-genome homeologs to confer resistance given the recessive nature of SrTm4.
In summary, SrTm4 recessive nature and its broad-spectrum resistance can make this gene a valuable component of gene pyramids combining different types of Sr genes. The high-density map of SrTm4 and the tightly linked molecular markers identified in this study are useful tools to facilitate the cloning of this gene.
References
Allshire RC, Javerzat J-P, Redhead NJ, Cranston G (1994) Position effect variegation at fission yeast centromeres. Cell 76:157–169
Avni R, Nave M, Barad O, Baruch K, Twardziok SO, Gundlach H, Hale I, Mascher M, Spannagl M, Wiebe K (2017) Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science 357:93–97
Barnes CW, Ordóñez ME, Hambleton S, Dadej K, Szabo LJ, Fetch T (2018) Detection of Puccinia graminis f. sp. tritici race RRTTF in Ecuador in 2016. Plant Dis 102:448
Bhattacharya S (2017) Deadly new wheat disease threatens Europe’s crops. Nature 542:145–146
Bhattramakki D, Dolan M, Hanafey M, Wineland R, Vaske D, Register JC, Tingey SV, Rafalski A (2002) Insertion-deletion polymorphisms in 3’ regions of maize genes occur frequently and can be used as highly informative genetic markers. Plant Mol Biol 48:539–547
Briggs J, Chen S, Zhang W, Nelson S, Dubcovsky J, Rouse MN (2015) Mapping of SrTm4, a recessive stem rust resistance gene from diploid wheat effective to Ug99. Phytopathology 105:1347–1354
Chen S, Guo Y, Briggs J, Dubach F, Chao S, Zhang W, Rouse MN, Dubcovsky J (2018a) Mapping and characterization of wheat stem rust resistance genes SrTm5 and Sr60 from Triticum monococcum. Theor Appl Genet 131:625–635
Chen S, Rouse MN, Zhang W, Jin Y, Akhunov E, Wei Y, Dubcovsky J (2015) Fine mapping and characterization of Sr21, a temperature-sensitive diploid wheat resistance gene effective against the Puccinia graminis f. sp. tritici Ug99 race group. Theor Appl Genet 128:645–656
Chen S, Rouse MN, Zhang W, Zhang X, Guo Y, Briggs J, Dubcovsky J (2020) Wheat gene Sr60 encodes a protein with two putative kinase domains that confers resistance to stem rust. New Phytol 225:948–959
Chen S, Zhang W, Bolus S, Rouse MN, Dubcovsky J (2018b) Identification and characterization of wheat stem rust resistance gene Sr21 effective against the Ug99 race group at high temperature. PLoS Genet 14:e1007287
Church G, Starr J, Simpson C (2005) A recessive gene for resistance to Meloidogyne arenaria in interspecific Arachis spp. hybrids. J Nematol 37:178
Debernardi JM, Burguener G, Bubb K, Liu Q, Queitsch C, Dubcovsky J (2022) Optimization of ATAC-seq in wheat seedling roots using INTACT-isolated nuclei. Research Square: https://doi.org/10.21203/rs.21203.rs-2058892/v2058891
Dubcovsky J, Luo M-C, Zhong G-Y, Bransteitter R, Desai A, Kilian A, Kleinhofs A, Dvořák J (1996) Genetic map of diploid wheat, Triticum monococcum L., and its comparison with maps of Hordeum vulgare L. Genetics 143:983–999
Dubcovsky J, Luo M, Dvorak J (1995) Differentiation between homoeologous chromosomes 1A of wheat and 1Am of Triticum monococcum and its recognition by the wheat Ph1 locus. Proc Natl Acad Sci USA 92:6645–6649
Dvorak J, McGuire PE, Cassidy B (1988) Apparent sources of the A genomes of wheats inferred from polymorphism in abundance and restriction fragment length of repeated nucleotide sequences. Genome 30:680–689
Fetch T, Zegeye T, Park R, Hodson D, Wanyera R (2016) Detection of wheat stem rust races TTHSK and PTKTK in the Ug99 race group in Kenya in 2014. Plant Dis 100:1495–1495
Figueroa M, Hammond-Kosack KE, Solomon PS (2018) A review of wheat diseases-a field perspective. Mol Plant Pathol 19:1523–1536
Fox SE, Geniza M, Hanumappa M, Naithani S, Sullivan C (2014) De novo transcriptome assembly and analyses of gene expression during photomorphogenesis in diploid wheat Triticum monococcum. PLoS ONE 9:e96855
Garvin D, Brown A, Burdon J (1997) Inheritance and chromosome locations of scald-resistance genes derived from Iranian and Turkish wild barleys. Theor Appl Genet 94:1086–1091
Gerechter-Amitai Z, Wahl I, Vardi A, Zohary D (1971) Transfer of stem rust seedling resistance from wild diploid einkorn to tetraploid durum wheat by means of a triploid hybrid bridge. Euphytica 20:281–285
Hopkins MT, Lampi Y, Wang T-W, Liu Z, Thompson JE (2008) Eukaryotic translation initiation factor 5A is involved in pathogen-induced cell death and development of disease symptoms in Arabidopsis. Plant Physiol 148:479–489
Hunter JD (2007) Matplotlib: A 2D graphics environment. Comput Sci Eng 9:90–95
Jiang D, Hua L, Zhang C, Li H, Wang Z, Li J, Wang G, Song R, Shen T, Li H (2023) Mutations in the miRNA165/166 binding site of the HB2 gene result in pleiotropic effects on morphological traits in wheat. Crop J 11:9–20
Jin Y, Singh RP, Ward RW, Wanyera R, Kinyua M, Njau P, Pretorius ZA (2007) Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f. sp tritici. Plant Dis 91:1096–1099
Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, Fetch T (2008) Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp tritici. Plant Dis 92:923–926
Jin Y, Szabo LJ, Rouse MN, Fetch T, Pretorius ZA, Wanyera R, Njau P (2009) Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f. sp tritici. Plant Dis 93:367–370
Kolde R, Kolde MR (2018) Package ‘pheatmap’. R Package 1, https://cran.r-project.org/web/packages/pheatmap/index.html
Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4:403–410
Krasileva KV, Vasquez-Gross H, Howell T, Bailey P, Paraiso F, Clissold L, Simmonds J, Ramirez-Gonzalez RH, Wang X, Borrill P, Fosker C, Ayling S, Phillips A, Uauy C, Dubcovsky J (2017) Uncovering hidden variation in polyploid wheat. Proc Natl Acad Sci USA 114:E913–E921
Laluk K, AbuQamar S, Mengiste T (2011) The Arabidopsis mitochondria-localized pentatricopeptide repeat protein PGN functions in defense against necrotrophic fungi and abiotic stress tolerance. Plant Physiol 156:2053–2068
Lewis CM, Persoons A, Bebber DP, Kigathi RN, Maintz J, Findlay K, Bueno-Sancho V, Corredor-Moreno P, Harrington SA, Kangara N (2018) Potential for re-emergence of wheat stem rust in the United Kingdom. Commun Biol 1:13
Li H, Hua L, Rouse MN, Li T, Pang S, Bai S, Shen T, Luo J, Li H, Zhang W (2021) Mapping and characterization of a wheat stem rust resistance gene in durum wheat “Kronos.” Front Plant Sci 12:751398
Li Z-K, Sanchez A, Angeles E, Singh S, Domingo J, Huang N, Khush GS (2001) Are the dominant and recessive plant disease resistance genes similar?: a case study of rice R genes and Xanthomonas oryzae pv. oryzae races. Genetics 159:757–765
Lijavetzky D, Muzzi G, Wicker T, Keller B, Wing R, Dubcovsky J (1999) Construction and characterization of a bacterial artificial chromosome (BAC) library for the a genome of wheat. Genome 42:1176–1182
Ling HQ, Ma B, Shi X, Liu H, Dong L, Sun H, Cao Y, Gao Q, Zheng S, Li Y (2018) Genome sequence of the progenitor of wheat a subgenome Triticum urartu. Nature 557:424–428
Luo J, Rouse MN, Hua L, Li H, Li B, Li T, Zhang W, Gao C, Wang Y, Dubcovsky J, Chen S (2022) Identification and characterization of Sr22b, a new allele of the wheat stem rust resistance gene Sr22 effective against the Ug99 race group. Plant Biotechnol J 20:554–563
Luo MC, Gu YQ, Puiu D, Wang H, Twardziok SO, Deal KR, Huo N, Zhu T, Wang L, Wang Y (2017) Genome sequence of the progenitor of the wheat D genome Aegilops tauschii. Nature 551:498–502
Maccaferri M, Harris NS, Twardziok SO, Pasam RK, Gundlach H, Spannagl M, Ormanbekova D, Lux T, Prade VM, Milner SG, Himmelbach A, Mascher M, Bagnaresi P, Faccioli P, Cozzi P, Lauria M, Lazzari B, Stella A, Manconi A, Gnocchi M, Moscatelli M, Avni R, Deek J, Biyiklioglu S, Frascaroli E, Corneti S, Salvi S, Sonnante G, Desiderio F, Mare C, Crosatti C, Mica E, Ozkan H, Kilian B, De Vita P, Marone D, Joukhadar R, Mazzucotelli E, Nigro D, Gadaleta A, Chao S, Faris JD, Melo ATO, Pumphrey M, Pecchioni N, Milanesi L, Wiebe K, Ens J, MacLachlan RP, Clarke JM, Sharpe AG, Koh CS, Liang KYH, Taylor GJ, Knox R, Budak H, Mastrangelo AM, Xu SS, Stein N, Hale I, Distelfeld A, Hayden MJ, Tuberosa R, Walkowiak S, Mayer KFX, Ceriotti A, Pozniak CJ, Cattivelli L (2019) Durum wheat genome highlights past domestication signatures and future improvement targets. Nat Genet 51:885–895
Mago R, Chen C, Xia X, Whan A, Forrest K, Basnet BR, Perera G, Chandramohan S, Randhawa M, Hayden M, Bansal U, Huerta-Espino J, Singh RP, Bariana H, Lagudah E (2022) Adult plant stem rust resistance in durum wheat Glossy Huguenot: mapping, marker development and validation. Theor Appl Genet 135:1541–1550
Nazari K, Al-Maaroof EM, Kurtulus E, Kavaz H, Hodson D, Ozseven I (2021) First report of Ug99 race TTKTT of wheat stem rust (Puccinia graminis f. sp. tritici) in Iraq. Plant Dis 105:2719
Nazari K, Mafi M, Yahyaoui A, Singh R, Park R (2009) Detection of wheat stem rust (Puccinia graminis f. sp. tritici) race TTKSK (Ug99) in Iran. Plant Dis 93:317–317
Neff MM, Neff JD, Chory J, Pepper AE (1998) dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J 14:387–392
Newcomb M, Olivera PD, Rouse MN, Szabo LJ, Johnson J, Gale S, Luster DG, Wanyera R, Macharia G, Bhavani S (2016) Kenyan isolates of Puccinia graminis f. sp. tritici from 2008 to 2014: Virulence to SrTmp in the Ug99 race group and implications for breeding programs. Phytopathology 106:729–736
Ng PC, Henikoff S (2003) SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 31:3812–3814
Olivera P, Newcomb M, Szabo LJ, Rouse M, Johnson J, Gale S, Luster DG, Hodson D, Cox JA, Burgin L (2015) Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis f. sp. tritici that caused a wheat stem rust epidemic in southern Ethiopia in 2013–14. Phytopathology 105:917–928
Olivera PD, Jin Y, Rouse M, Badebo A, Fetch T, Singh RP, Yahyaoui A (2012) Races of Puccinia graminis f. sp. tritici with combined virulence to Sr13 and Sr9e in a field stem rust screening nursery in Ethiopia. Plant Dis 96:623–628
Olivera PD, Sikharulidze Z, Dumbadze R, Szabo LJ, Newcomb M, Natsarishvili K, Rouse MN, Luster DG, Jin Y (2019) Presence of a sexual population of Puccinia graminis f. sp. tritici in Georgia provides a hotspot for genotypic and phenotypic diversity. Phytopathology 109:2152–2160
Patpour M, Hovmoller M, Hansen J, Justesen A, Thach T, Rodriguez-Algaba J, Hodson D, Randazo B (2017) Epidemics of yellow rust and stem rust in Southern Italy 2016–2017. BGRI 2018 Technical Workshop, pp https://www.globalrust.org/content/epidemics-yellow-and-stem-rust-southern-italy-2016-2017
Patpour M, Justesen AF, Tecle AW, Yazdani M, Yasaie M, Hovmøller MS (2020) First report of race TTRTF of wheat stem rust (Puccinia graminis f. sp. tritici) in Eritrea. Plant Dis 104:973
Pearce S, Vanzetti LS, Dubcovsky J (2013) Exogenous gibberellins induce wheat spike development under short days only in the presence of VERNALIZATION 1. Plant Physiol 163:1433–1445
Peterson PD (2001) Stem rust of wheat: from ancient enemy to modern foe. American Phytopathological Society (APS Press)
Pradhan SK, Nayak DK, Mohanty S, Behera L, Barik SR, Pandit E, Lenka S, Anandan A (2015) Pyramiding of three bacterial blight resistance genes for broad-spectrum resistance in deepwater rice variety, Jalmagna. Rice 8:1–14
Pretorius ZA, Prins R, Wessels E, Bender CM, Visser B, Boshoff WH (2020) Accomplishments in wheat rust research in South Africa. S Afr J Sci 116:1–8
Pretorius ZA, Singh RP, Wagoire WW, Payne TS (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f sp tritici in Uganda. Plant Dis 84:203
Qiu T, Zhao X, Feng H, Qi L, Yang J, Peng YL, Zhao W (2021) OsNBL3, a mitochondrion-localized pentatricopeptide repeat protein, is involved in splicing nad5 intron 4 and its disruption causes lesion mimic phenotype with enhanced resistance to biotic and abiotic stresses. Plant Biotechnol J 19:2277–2290
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformat (oxford, England) 26:139–140
Roelfs AP (1978) Estimated losses caused by rust in small grain cereals in the United States, 1918–76. United States Department of Agriculture, Washington DC
Roelfs AP (1982) Effects of barberry eradication. Plant Dis 66:177
Rouse M, Jin Y (2011a) Genetics of resistance to race TTKSK of Puccinia graminis f. sp. tritici in Triticum monococcum. Phytopathology 101:1418–1423
Rouse M, Jin Y (2011b) Stem rust resistance in A-genome diploid relatives of wheat. Plant Dis 95:941–944
Rsaliyev A, Yskakova G, Maulenbay A, Zakarya K, Rsaliyev S (2020) Virulence and race structure of Puccinia graminis f. sp. tritici in Kazakhstan. Plant Protect Sci 56:275–284
Ruffel S, Dussault MH, Palloix A, Moury B, Bendahmane A, Robaglia C, Caranta C (2002) A natural recessive resistance gene against potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E). Plant J 32:1067–1075
Said I, Byrne A, Serrano V, Cardeno C, Vollmers C, Corbett-Detig R (2018) Linked genetic variation and not genome structure causes widespread differential expression associated with chromosomal inversions. Proc Natl Acad Sci USA 115:5492–5497
Saintenac C, Zhang W, Salcedo A, Rouse MN, Trick HN, Akhunov E, Dubcovsky J (2013) Identifcation of wheat gene Sr35 that confers resistance to Ug99 stem rust race group. Science 341:783–786
Sears ER (1977) An induced mutant with homoeologous pairing in common wheat. Can J Genet Cytol 19:585–593
Shahin A, Youssif W, EL-Naggar D, (2020) Detection of Ug99 (TTKSK) of wheat stem rust fungus and new virulence races of Puccinia graminis f. sp. tritici in Egypt. Egyptian J Phytopathol 48:14–28
Singh RP, Hodson DP, Jin Y, Lagudah ES, Ayliffe MA, Bhavani S, Rouse MN, Pretorius ZA, Szabo LJ, Huerta-Espino J (2015) Emergence and spread of new races of wheat stem rust fungus: continued threat to food security and prospects of genetic control. Phytopathology 105:872–884
Skolotneva ES, Kosman E, Patpour M, Kelbin VN, Morgounov AI, Shamanin VP, Salina EA (2020) Virulence phenotypes of Siberian wheat stem rust population in 2017–2018. Front Agronomy 2:6
Spofford JB (1976) Position-effect variegation in Drosophila. In The Genetics and Biology of Drosophila, M Ashburner and E Novitski, eds (New York: Academic Press, Incorporated) 1: 955–1018
Stakman EC, Stewart DM, Loegering WQ (1962) Identification of physiologic races of Puccinia graminis var. tritici. United States Department of Agriculture Research Service E-617, Washington DC
Steuernagel B, Periyannan SK, Hernandez-Pinzon I, Witek K, Rouse MN, Yu G, Hatta A, Ayliffe M, Bariana H, Jones JD, Lagudah ES, Wulff BB (2016) Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat Biotechnol 34:652–655
Tesfaye T, Chala A, Shikur E, Hodson D, Szabo LJ (2020) First report of TTRTF race of wheat stem rust, Puccinia graminis f. sp. tritici, in Ethiopia. Plant Dis 104:293–293
The International Wheat Genome Sequencing Consortium (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361:eaar7191
The T (1973) Chromosome location of genes conditioning stem rust resistance transferred from diploid to hexaploid wheat. Nature-New Biol 241:256
Verulkar S, Singh D, Bhattacharya A (1997) Inheritance of resistance to podfly and podborer in the interspecific cross of pigeonpea. Theor Appl Genet 95:506–508
Voorrips R (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Walkowiak S, Gao L, Monat C, Haberer G, Kassa MT, Brinton J, Ramirez-Gonzalez RH, Kolodziej MC, Delorean E, Thambugala D (2020) Multiple wheat genomes reveal global variation in modern breeding. Nature 588:277–283
Wang S, Wang X, Zhang R, Liu Q, Sun X, Wang J, Wang Y, Xing J, Liu Y, Zhao Y (2022) RppM, encoding a typical CC-NBS-LRR protein, confers resistance to southern corn rust in maize. Front Plant Sci 13:951318
Wang Y, Bouwmeester K (2017) L-type lectin receptor kinases: New forces in plant immunity. Plos Pathog 13:e1006433
Wang Y, Cordewener JH, America AH, Shan W, Bouwmeester K, Govers F (2015a) Arabidopsis lectin receptor kinases LecRK-IX. 1 and LecRK-IX. 2 are functional analogs in regulating Phytophthora resistance and plant cell death. Mol Plant Microbe in 28:1032–1048
Wang Y, Weide R, Govers F, Bouwmeester K (2015b) L-type lectin receptor kinases in Nicotiana benthamiana and tomato and their role in Phytophthora resistance. J Exp Bot 66:6731–6743
Wang Z, Cheng J, Fan A, Zhao J, Yu Z, Li Y, Zhang H, Xiao J, Muhammad F, Wang H (2018) LecRK-V, an L-type lectin receptor kinase in Haynaldia villosa, plays positive role in resistance to wheat powdery mildew. Plant Biotechnol J 16:50–62
Woo JY, Jeong KJ, Kim YJ, Paek K-H (2016) CaLecRK-S. 5, a pepper L-type lectin receptor kinase gene, confers broad-spectrum resistance by activating priming. J Exp Bot 67:5725–5741
Yang H, Wang H, Jiang J, Liu M, Liu Z, Tan Y, Zhao T, Zhang H, Chen X, Li J (2022) The Sm gene conferring resistance to gray leaf spot disease encodes an NBS-LRR (nucleotide-binding site-leucine-rich repeat) plant resistance protein in tomato. Theor Appl Genet 135:1467–1476
Yu G, Matny O, Champouret N, Steuernagel B, Moscou MJ, Hernández-Pinzón I, Green P, Hayta S, Smedley M, Harwood W, Kangara N, Yue Y, Gardener C, Banfield MJ, Olivera PD, Welchin C, Simmons J, Millet E, Minz-Dub A, Ronen M, Avni R, Sharon A, Patpour M, Justesen AF, Jayakodi M, Himmelbach A, Stein N, Wu S, Poland J, Ens J, Pozniak C, Karafiátová M, Molnár I, Doležel J, Ward ER, Reuber TL, Jones JDG, Mascher M, Steffenson BJ, Wulff BBH (2022) Aegilops sharonensis genome-assisted identification of stem rust resistance gene Sr62. Nat Commun 13:1607
Zadoks T (1967) Epidemiology of wheat rust in Europe. Int J Pest Manage 13:29–46
Zhang C, Gao H, Li R, Han D, Wang L, Wu J, Xu P, Zhang S (2019a) GmBTB/POZ, a novel BTB/POZ domain-containing nuclear protein, positively regulates the response of soybean to Phytophthora sojae infection. Mol Plant Pathol 20:78–91
Zhang C, Gao H, Sun Y, Jiang L, He S, Song B, Liu S, Zhao M, Wang L, Liu Y, Wu J, Xu P, Zhang S (2021) The BTB/POZ domain protein GmBTB/POZ promotes the ubiquitination and degradation of the soybean AP2/ERF-like transcription factor GmAP2 to regulate the defense response to Phytophthora sojae. J Exp Bot 72:7891–7908
Zhang C, Huang L, Zhang H, Hao Q, Lyu B, Wang M, Epstein L, Liu M, Kou C, Qi J, Chen F, Li M, Gao G, Ni F, Zhang L, Hao M, Wang J, Chen X, Luo MC, Zheng Y, Wu J, Liu D, Fu D (2019b) An ancestral NB-LRR with duplicated 3’UTRs confers stripe rust resistance in wheat and barley. Nat Commun 10:4023
Zhang J, Nirmala J, Chen S, Jost M, Steuernagel B, Karafiátová M, Hewitt T, Li H, Edae E, Sharma K, Hoxha S, Bhatt D, Antoniou-Kourounioti R, Dodds P, Wulff BBH, Doležel J, Ayliffe M, Hiebert C, McIntosh R, Dubcovsky J, Zhang P, Rouse M, Lagudah E (2022) A single amino acid change can alter the specificity of the multi-allelic wheat stem rust resistance locus SR9. Research Square: https://doi.org/10.21203/rs.21203.rs-2208386/v2208381
Zhang W, Chen S, Abate Z, Nirmala J, Rouse MN, Dubcovsky J (2017) Identification and characterization of Sr13, a tetraploid wheat gene that confers resistance to the Ug99 stem rust race group. Proc Natl Acad Sci USA 114:E9483-9492
Zhao M, Ge Y, Xu Z, Ouyang X, Jia Y, Liu J, Zhang M, An Y (2022) A BTB/POZ domain-containing protein negatively regulates plant immunity in Nicotiana benthamiana. Biochem Bioph Res Co 600:54–59
Zhu T, Wang L, Rimbert H, Rodriguez JC, Deal KR, De Oliveira R, Choulet F, Keeble-Gagnère G, Tibbits J, Rogers J (2021) Optical maps refine the bread wheat Triticum aestivum cv. Chinese Spring Genome Assembly Plant J 107:303–314
Acknowledgements
Work at SC laboratory was supported by the Young Taishan Scholars Program of Shandong Province, the Provincial Natural Science Foundation of Shandong (ZR2021ZD30 and ZR2021MC056), and the National Key Research and Development Program of China (2022YFD1201300). Work at JD laboratory was supported by the Howard Hughes Medical Institute and by the Agriculture and Food Research Initiative Competitive Grant 2022-68013-36439 (WheatCAP) from the USDA National Institute of Food and Agriculture (NIFA). Work at MNR laboratory was supported by the USDA-ARS National Plant Disease Recovery System.
Funding
Work at SC laboratory was supported by the Young Taishan Scholars Program of Shandong Province, the Provincial Natural Science Foundation of Shandong (ZR2021ZD30 and ZR2021MC056), and the National Key Research and Development Program of China (2022YFD1201300).
Author information
Authors and Affiliations
Contributions
HL and JL performed most of the experimental work. LH, JW, BX, CY, and GW contributed primers and the SrTm4 monogenic line. WZ created the mapping population, conducted genotyping and sequence analyses. KL obtained some BAC clones from the DV92 BAC library. MNR designed and performed the phenotyping experiments. SC analyzed the data and wrote the first version of the manuscript. SC, MNR, and JD conceived and supervised the project. All authors revised the manuscript and provided suggestions.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Data availability statement
The BAC sequences and raw sequencing data reported in this study are available in the GenBank database as accession numbers QQ503488 and PRJNA932462. Data supporting the findings of this study are within the manuscript or the supplementary file.
Additional information
Communicated by Urmil Bansal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Luo, J., Zhang, W. et al. High-resolution mapping of SrTm4, a recessive resistance gene to wheat stem rust. Theor Appl Genet 136, 120 (2023). https://doi.org/10.1007/s00122-023-04369-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04369-z