Abstract
Developing stress-tolerant plants continues to be the goal of breeders due to their realized yields and stability. Plant responses to drought have been studied in many different plant species, but the occurrence of stress memory as well as the potential mechanisms for memory regulation is not yet well described. It has been observed that plants hold on to past events in a way that adjusts their response to new challenges without altering their genetic constitution. This ability could enable training of plants to face future challenges that increase in frequency and intensity. A better understanding of stress memory-associated mechanisms leading to alteration in gene expression and how they link to physiological, biochemical, metabolomic and morphological changes would initiate diverse opportunities to breed stress-tolerant genotypes through molecular breeding or biotechnological approaches. In this perspective, this review discusses different stress memory types and gives an overall view using general examples. Further, focusing on drought stress, we demonstrate coordinated changes in epigenetic and molecular gene expression control mechanisms, the associated transcription memory responses at the genome level and integrated biochemical and physiological responses at cellular level following recurrent drought stress exposures. Indeed, coordinated epigenetic and molecular alterations of expression of specific gene networks link to biochemical and physiological responses that facilitate acclimation and survival of an individual plant during repeated stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global warming is one of the most important effects of climate change because it poses the heaviest environmental challenge confronted by mankind at the moment (Rajak 2021). It is not only influencing the air temperature but is also affecting the amount and distribution of precipitation, thereby resulting to future more frequent drought spells (Wang et al. 2014). Drought stress has been reported as one of the most destructive abiotic stress factors globally and generates a huge negative impact on crop production (Vurukonda et al. 2016; Koua et al. 2021). In describing agricultural drought, Trenberth et al. (2014) relate it to deficit in moisture in the topmost of about one meter of soil usually the root zone, thereby impacting crops. A meta-analysis of data collected between 1980 and 2015 showed that drought stress led to 40% yield reductions in maize and 21% yield reductions in wheat (Daryanto et al. 2016). Between the years 2005 and 2015, economic loses induced by drought were estimated to be around 29 billion USD (Trenberth et al. 2014; F.A.O. 2021). Recent droughts have had strong impact on world cereal production and will continue to cause year to year yield fluctuations (F.A.O. 2021), with predictions of having 50% of arable land under drought stress by the year 2050 (Kasim et al. 2013).
Drought stress can occur in every growth stage of a plant and influence the water relations of the plant at all levels including whole plant, organs, cellular and molecular levels (Li et al. 2014; Muscolo et al. 2015). In general, the growth and development of a plant are affected, thereby resulting to production of smaller organs as well as altered production of flowers and grain filling (Farooq, et al. 2009). In addition, stomatal closure is followed by a progressive decline in net photosynthetic activity and water-use efficiency, which greatly impair the productivity of plants (Wu et al. 2022).
Different from other organisms, plants are rooted permanently to one location and only respond to environmental cues through adjustment of growth and development patterns. Thus, flexibility is an essential requirement for plants to survive stress, which they maintain through operation of a signal response network (Amtmann & Armengaud 2009; Cutler et al. 2010) that enables them to reprogram their molecular machinery including transcription factors, stress-responsive proteins and secondary messengers (Tani et al. 2019). Plants also respond to drought by adjusting their metabolism/biochemical machinery like ethylene, proline and auxins alterations (Nair et al. 2008; Sharma et al. 2012). In addition, physiological changes involving cell membrane stability and osmotic adjustment (Abid et al. 2018), and morphological changes (phenotypic plasticity) (Basu et al. 2016) occur in plant during exposure to drought.
Recently, researchers have discovered that the ability of plants to adjust response mechanisms in a continuously changing environment shapes their fitness in future and eventually enables them to live in highly diversified habitats (Fleta-Soriano and Munné-Bosch 2016). Upon exposure to stress, plants alter their epigenetic, physiological and metabolomics machineries that modify responses to future similar stress in the same generation (somatic) and/or in the next generation(s) (intergenerational or transgenerational) to adapt and survive in many ways. This popular phenomenon in which an environmental signal prepares a plant for possible future stress exposure is referred to as priming. Xin and Browse (2000) described it as a resource saving strategy of improving plant tolerance to stress. The preservation of a primed state over time forms the basis of stress memory (Haider et al. 2021). Regardless of what plant’s future holds, the first stress exposure will leave an imprint in the plant that affects how it responds to later stresses (Liu et al. 2021a). Therefore, stress memory in plants is the capability of a plant upon exposure to stressors to store stress information so that it can respond in a different fashion when challenged by the same stress later (Bruce et al. 2007; Avramova 2015; Bilichak et al. 2015; Crisp et al. 2016; Fleta-Soriano and Munné-Bosch 2016). This capability is an integral part of plant resilience under changing climate.
Available studies exploring the topic of stress memory in plants have so far advanced the understanding of priming by detailing epigenetic, transcriptional, proteomic and physiological alterations resulting to imprints that establish stress memory in plants (Liu et al. 2022a; Sharma et al. 2022; Singh & Prasad 2022). While these studies have described variation between epigenetic marks and their effect on stress response, the integration of altered gene expression due to these modifications with physiological, biochemical and morphological responses of plants during recurrent stress is not well explored. We elucidate the interconnection of these mechanisms during recurrent drought episodes by describing the coordinated stress memory changes (imprints) at different OMICS, cellular and organismal levels that prepare plants to be more responsive to future stress within or across generation(s), which could provide new opportunities for crop improvement to ensure food security (Fleta-Soriano and Munné-Bosch 2016; Godwin & Farrona 2020).
In this review, we (1) classify stress memory in plants and give an overall view using general examples; (2) focus on drought stress and summarize the epigenetic modifications associated with gene expression control during recurrent drought episodes; and (3) correlate transcriptional and posttranscriptional memory with various drought memory imprints.
Classifications of stress memory based on time point of stress and mode of inheritance
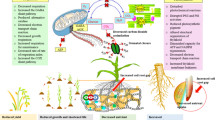
Various terms have been devised to describe the different stress memory types, usually based on the stage of the plant when priming is done and the mode of inheritance (Fig. 1).
Somatic stress memory
Stress memory that is limited to one generation in duration is referred to as somatic stress memory (Lämke and Bäurle 2017). While the abiotic stresses occurring at different stages result in a higher risk of injury, plants can experience stress at an early stage during their growth and development, which can induce short-term stress memory to allow the plants to be tolerant if a similar stress strikes in later developmental stages (Li and Liu 2016). Therefore, somatic stress memory lasts for a short period of time, and its memory imprints are inherited mitotically.
Intergenerational versus transgenerational stress memory
Exposing a plant (parental generation) to drought stress during the reproductive phase also exposes its reproductive cells and the resulting seeds to the same drought stress. Therefore, stress memory in the progeny generation could be mediated by cues introduced into the seed or embryo by the parental plant. This type of stress memory is referred to as intergenerational and implies the direct exposure to the stressor of the parental generation and the following generation (progeny) by means of the developing germ cells (Heard & Martienssen 2014; Lämke & Bäurle 2017).
On the other hand, transgenerational transmission is present when effects of the ancestral exposure to an environment during reproductive stage are present in the generation that is not directly exposed (Klengel et al. 2016). Hence, if grandparental generation was exposed to stress at reproductive stage, true transgenerational inheritance can only be observed in the progeny generation, when the parental generation had been unexposed (recovery period) (Fig. 1).
Stress memory to various stressors in plants
Whether plants can remember is a provocative question that has lately preoccupied scientists. Recent studies addressing priming and stress memory have provided new valuable evidence on responses that are key factors of priming induced stress tolerance (Table 1).
Intensive research has been conducted to study pre-exposure of plants to biotic and abiotic stressors, which trigger stress memory response. These memory imprints enable the plants to be ready to respond to subsequent stressful events (Xin & Browse 2000; Luna et al. 2012; Balmer et al. 2015; Hossain et al. 2015; Wang et al. 2017, 2018; Fan et al. 2018; Leuendorf et al. 2020). For instance, Agrawal (2002) found out that destruction of Raphanus raphanistrum L. following attacks from Pieris rapae L. during the vegetative phase of growth had influenced the induction of resistance on progeny in a later attack when compared to the controls and additionally reported that herbivory in the maternal generation influenced the growth of the progeny especially on seed mass. Furthermore, other studies in the past had indicated the possibility of memory from attacks by aphids, pathogens and other predators, thereby portraying induced resistance on later attacks (Rogers 1966; Roberts 1983; Lammerink et al. 1984; Shattuck 1993).
In a study on three different plant species that had been grown under two CO2 concentrations, Lau et al. (2008) discovered that the maternal CO2 environment during grain filling stage influenced biomass of progeny of all the species. The elevated carbon dioxide (eCO2) memory increased growth response to a future eCO2, a finding that had been contradicted by Huxman et al. (2001), who by using Bromus Rubens L. found out that the effects of maternal exposure to eCO2 reduced the performance of the progeny grown under eCO2 treatment especially by reducing photosynthesis and growth rates.
Whittle et al. (2009) assessed stress memory to find out if Arabidopsis thaliana L. plants adaptively responded to environmental conditions experienced by their ancestors. They examined plants that were exposed to mild heat or cold environments in parental and F1 generation and discovered that previous elevated temperature treatment led to a more than fivefold improvement in fitness in F3 generation. After checking the persistence of previous stress memory, they reported that improvement due to heat memory in F3 generation plants remained even when the heat-exposed parental and F1 plants were grown in a normal temperature regime in F2 generation. Using Arabidopsis thaliana L., the ability of plants to remember salt stress exposure as far as four generations ago was found, and transgenerational as well as somatic effects in almost all analyzed traits were observed (Groot et al. 2016). Similarly, Boyko et al. (2010) had reported that transgenic Arabidopsis thaliana L. offspring from salt stress-exposed parents showed increased tolerance to salt and had higher rates of recombination.
In an experiment conducted by Gagliano et al. (2014) using a sensitive Mimosa pudica L. plant, whose leaves close rapidly by folding to respond to mechanical disturbance, it was interesting to realize that when the plant was initially dropped to experience mechanical stress, the leaves reacted by closing tight. However, when they dropped the plant repeatedly, its response changed and did not react as expected but the leaves stayed open. This was a clear indication of training and adaptation that suggested learning and memory mechanisms. The authors also noted that the sensitive plants displayed the learned response also when they were placed for a month in a favorable environment without disturbance. In animal studies, memory is considered as long term if one can store information for 24 h and remember (Sánchez-Andrade and Kendrick 2011). Therefore, based on rules routinely used, the mimosa plants had shown that they were capable of learning and remembering what they had learnt.
Drought stress memory as a mechanism of plant adaptation
Plants’ responses to drought stress have been widely investigated because drought can occur at any stage of growth, from vegetative to grain filling, thereby negatively influencing yield production. Among other mechanisms of adaptation and tolerance to water scarcity, various studies have demonstrated drought stress memory in several species (Table 2) like in Brassica napus L. (Hatzig et al. 2018), Trifolium repens L. (Rendina González et al. 2018), wheat (Liu et al. 2020), rice (Zheng et al. 2013), Polygonum persicaria L. (Herman et al. 2012), Arabidopsis thaliana L. (van Dooren et al. 2020), Leontodon hispidus L., Plantago lanceolata L. and Trifolium pratense L. (Cerda 2020), suggesting that previous drought stress exposure left some stress imprints that were stored to induce improvement in a subsequent stress encounter.
While on the one hand drought stress memory is viewed from an evolutionary perspective as an effective strategy that could prepare a plant for later stress by improving the plant’s potential for local acclimation to changing environments, some studies have nevertheless associated it with negative effects like delayed growth and development and reduced yield (Skirycz & Inzé, 2010; Crisp et al. 2016; Wijewardana et al. 2019a, b). Therefore, although mechanisms of drought stress memory could have evolved as adaptive approaches to enhance resistance against drought, the overall performance may be compromised, thereby leading to tradeoffs between yield and stress survival (Godwin & Farrona 2020).
Molecular mechanisms controlling stress memory in plants
Efforts are made to understand the mechanistic basis of stress memory. Liu et al. (2022a) emphasize that investigations on drought stress memory suggests that regulatory mechanisms on the transcriptional level vary in response to a single stress stimulus and repetitive stress stimulations. Several exposures to drought stress enable plants to respond to a new stress by more rapid adaptive changes to gene expression patterns compared with plants not previously exposed to a drought stress (Li and Liu 2016). Growing evidence points to a stress memory that might involve the maintenance of the response to stress by transcriptional, translational or epigenetic (DNA methylation and Histone modifications) means as summarized in Fig. 2 (Sousa et al. 2022). Epigenetic modifications are either mitotically or/ and meiotically heritable alterations in gene expression, which are independent of primary DNA sequence changes and potentially affect the outcome of a chromosome or locus without changing the underlying DNA (Bird 2007). According to Godwin and Farrona (2020), DNA methylation and histone modification constitute epigenetic marks within chromosomes that stably change gene expression and other chromosomal properties. Over recent years, it has become increasingly evident that transcriptional regulation cannot be fully understood unless the structural context in which it occurs is considered. Moreover, by frequently influencing the distribution of epigenetic marks, noncoding RNAs can act in a sequence-specific manner to regulate gene expression both at transcriptional and posttranscriptional levels, therefore playing an important role in epigenetic control (Thiebaut et al. 2019). On the other hand, regulation of transcription is a result of the combined effects of chromatin structural properties and the interaction of transcription factors. The transcriptional regulation by transcription factors (TFs) is the major step for the establishment of the gene expression network and has been implicated in the control of stress memory (Crisp et al. 2016). Therefore, we summarize the current findings on gene expression regulation mechanisms associated with drought stress memory by showing their integration with drought memory-responsive genes.
A graphic presentation of interactions between gene expression control during repeated exposure and stress responses. Inheritance of epigenetic regulators like histone modifications and DNA methylation, and the alteration of regulatory RNAs and transcription factors affect the expression of genes, thereby causing changes in phenotypes of the plant
Epigenetic regulation of transcription
Histone modifications and drought memory
DNA is in eukaryotes complexed with eight positively charged histone proteins, consisting of two molecules of each histone (H2A, H2B, H3 and H4), wrapped by 147 negatively charged DNA base pairs to make a nucleosome (Cutter & Hayes 2015). Generally, H2A, H2B, H3 and H4 can undergo covalent modification mostly at lysine and arginine residues by methylation, acetylation, ubiquitination, phosphorylation, biotinylation and ADP-ribosylation (Feng and Jacobsen 2011). Histone marks are a type of chromatin modifications that have been associated with drought-responsive memory genes and the subsequent enhancement of transcriptional response to recurrent drought stress. Kim et al. (2012) found a clear enrichment of H3K4me3 in the coding regions of drought-responsive genes RD20, RD29A and AtGOLS2 that increased in response to drought stress and was maintained after gene deactivation by rehydration. In contrast, although H3K9ac increased initially during drought stress, it quickly responded to gene deactivation by rehydration and was drastically reduced on drought-inducible genes. This suggests the possibility of H3K4me3 to function as a stress memory epigenetic mark.
During repeated drought exposures on Arabidopsis thaliana L., even though the RD29B and RAB18 genes returned to their initial non-stressed transcript levels when the plants were rewatered, they remained associated with uncommonly high levels of H3K4me3 and Ser5P polymerase II, demonstrating that RNA polymerase II is delayed or hindered in its activity (Ding et al. 2012). This observation supports the findings by Kim et al. (2012) regarding H3K4me3 as a drought stress memory epigenetic marker. The concept of transcriptional memory was clearly illustrated by the observed return of transcript levels to baseline during recovery and a higher induction of transcript levels on a subsequent stress exposure. In Gossypium hirsutum L., Tian et al. (2022) revealed that H3K4me3 is necessary for the upregulation of memory genes GhNCED9, GhPYL9-11A, GhP5CS1 and GhSnRK2 during repeated drought, and its level on these genes decreased considerably on the 5th day following recovery. Memory genes with enriched H3K4me3 have also been documented, especially in P5CS1 in salt stress and HSP22.0 in heat stress (Feng et al. 2016; Lämke et al. 2016).
DNA methylation and drought memory
DNA methylation is an epigenetic modification where unlike in histone methylation, methylation unvaryingly takes place at the carbon-5 position of cytosine residues (Feng and Jacobsen 2011). Under the action of methylase, the DNA sequence of genes is not altered, but gene function is changed in response to external environmental stimuli. Generally, demethylation events are accompanied by the activation of genes, while methylation in the regulatory or coding regions hampers the expression of target genes (Sousa et al. 2022). This alteration is usually inherited by future generations to form epigenetic memory, which offers the possibility of breeding new crop varieties that are stress-tolerant.
Selfed progenies of drought-stressed plants showed increased DNA demethylation levels in P5CS and δ-OAT genes under subsequent drought than under control treatments. This clearly indicated that proline accumulation during repeated drought is facilitated by DNA demethylation, thereby upregulating the expression of these genes. The stability of DNA demethylation of these genes was observed through the increased proline accumulation in both onetime and two-time stressed plants growing under control environment, and subsequent higher levels of gene expression (Zhang et al. 2013).
Examination of the role of DNA methylation variations on rice adaptation to successive drought stress revealed non-random appearances of drought induced epimutations (Zheng et al. 2017), which was consistent to earlier findings that showed the induction of site-specific DNA methylation (Zheng et al. 2013). The authors noted that drought induced DNA methylation alterations were inherited in advanced generations and the genes associated with the discovered transgenerational DNA methylation changes were directly involved in drought-responsive pathways. Based on the Gene Ontology analysis of the non-TE genes related to both transgenerational and recurring DNA methylation alterations, their products are involved in signal transduction, development of flowers and pollination among others. For example, LOC_Os08g33720 gene encoding a putative lactate/malate dehydrogenase and responding to abiotic stimuli, was found to have 12 hypo-methylated CG-DMPs with recurrence frequencies (Zheng et al. 2017).
The relationship of the expression of memory genes with differentially DNA methylated regions exposed that 5373 drought memory transcripts might be regulated by DNA methylation (Li et al. 2019). Kou et al. (2021) went ahead to examine how DNA methylation is involved in drought stress memory in rice cultivars under recurrent drought stresses and recovery treatments. The study confirmed that the identified differentially methylated regions (DMRs) mediate tolerance by gene expression and transposable elements regulation. Memory (DMRs) were found in promoter region of LOC_Os05g38150 and in gene body of LOC_Os01g62900 to directly regulate rice drought memory genes (Kou et al. 2021). Drought in the vegetative stage altered global DNA methylation levels in rice guard cells, and these modifications remained when drought was recurrent in the reproductive stage due to greater genomic stability at this stage (Auler et al. 2021b). Gene expression analysis in this study revealed that protein abundance had a positive correlation with the expression of their coding genes. Neves et al. (2017) revealed alterations in the global DNA methylation patterns that corresponded to an increase in ABA levels in citrus plants that were subjected to three cycles of drought when compared to plants that had experienced drought stress for the first time. However, a different study that investigated DNA methylome changes in Arabidopsis thaliana L. plants and five successive generations subjected to drought stress failed to link the transgenerational memory to epigenetic methylation (Ganguly et al. 2017). Taken together, much evidence indicates a prominent function of chromatin-based mechanisms in transcriptional memory responses linked to drought stress (Godwin & Farrona 2020).
Regulatory RNA and drought memory
Small RNA molecules or microRNAs (miRNAs) are created from intergenic regions, repetitive sequences, transposable elements (TEs) and pseudogenes, accounting for more than 90% of all RNA transcripts (Nguyen et al. 2022). They regulate gene expression in signaling and other developmental pathways. According to Melnyk et al. (2011), systemic movement of drought triggered small RNAs through the symplast and vascular tissues to the meristem leads to DNA methylation by the RNA-directed DNA methylation (RdDM) pathway. Drought stress has been reported to induce expression of miRNAs to suggest their potential use in improving tolerance of plants. Guedes et al. (2018) performed miRNAs expression during the different cycles of drought stress on Coffea canephora L. and identified 198 miRNAs (21-nt sequences), from which most targets transcription factors (TFs). Based on differential expression analysis, miRNA miR408 and miR398 were highly up-regulated in the different drought stress cycles. Liu et al. (2020) uncovered differences in microRNA (miRNA) expression following repeated drought episodes, whose targets have critical molecular roles in stress adaptations. Liu et al. (2021b) have also reported the association of small RNA and their targets with transgenerational effects of drought stress.
LncRNAs were demonstrated to participate in rice short-term drought memory (Li et al. 2019). They acted as memory factors to activate phytohormone signaling genes that participate in drought memory response. The association analysis of lncRNAs and related mRNAs revealed three memory-related mRNA transcripts (TCONS_00028567, OS02T0626200-01 and OS04T0412225-00) that participate in different pathways. In Switchgrass, the levels of lncRNAs targeting the biosynthesis of ABA and trehalose increased in both first and second drought cycles, but lncRNAs regulating ethylene signaling were suppressed in the second cycle, thereby preventing leaf senescence and supporting plant development (Zhang et al. 2018).
Transcription factors and transcriptional regulation during recurrent drought episodes
Accumulation of transcription factors (TFs) has been shown to be another possible drought memory mechanism in plants (Ding et al. 2012). For example, the transcript and protein levels found for ABF TFs indicated that ABF3 and ABF4 exhibited transcriptional memory behavior although a marginally increased protein levels in response to repeated drought stress (Virlouvet et al. 2014). In a study of epigenetic signatures of stress adaptation using Zea mays, Forestan et al. (2020) reveal upregulation of well-characterized transcription factors (TFs) including AP2/EREBP, NAC and WRKY families 7 days following drought recovery.
Gene expression regulation link to physiological, biochemical and morphological responses during repeated drought stress
Gene regulatory networks involved in plants response to drought stress have been studied by examining the genes associated with drought responses, which encode regulatory and functional proteins like transcription factors (Shinozaki and Yamaguchi-Shinozaki 2007; Fujita et al. 2011; Osakabe et al. 2014). Transcriptional reprogramming is a regular aspect of the primed state (Godwin and Farrona 2020). Beyond gene expression control, other aspects have been considered in the study of plant response to reiterated stress including changes in other OMICS approaches like proteomics and metabolomics. A system–biology approach revealed that transcriptional memory correlate with physiological parameters, thereby translating into physiological memory (Virlouvet et al. 2018). In this study, 164 genes classified into four categories related to ABA biosynthesis, stomatal regulation, photosynthesis and pigments pathways were found to encode known drought stress-associated proteins. Taken together, transcripts, proteins and metabolites form interconnected, dynamic networks that mediate drought stress memory in plants (Fig. 3).
An overview of stress memory. Molecular and physiological network of drought stress response (Wojtyla et al. 2020). ROS, Reactive oxygen species
On the one hand, some drought stress-responsive genes have been shown to display regulation at transcript level that are significantly different under repeated drought exposures to the responses during their first drought contact (Ding et al. 2012; D’Urso & Brickner 2014). Memory genes according to Ding et al. (2013), are those genes that show altered responses in a subsequent stress different from the non-memory genes that remain unaltered after each round of stress. Comparable to this definition, Forestan et al. (2020) refer transcriptional memory genes, as genes with stable transcriptional changes that persist after drought recovery. Therefore, transcriptional stress memory is said to be evident when there are sustained alterations in activation or repression of genes or from a changed response following a second cue (Lämke and Bäurle 2017). On the other hand, to optimize growth and reproduction in recurrently varying environments, plants have been shown to exhibit a drought stress memory on the physiological level to reduce water loss, reduce cellular oxidative stress by maintaining reactive oxygen species (ROS) homeostasis, reduce membrane damage, reduce inhibition of enzyme activity, increase CO2 assimilation, alter photosynthetic rates and change the general morphology (Fleta-Soriano and Munné-Bosch 2016; Abid et al. 2018). Our focus here is to review transcriptional memory responses in which production of increased levels of transcript and/or enhanced repression has been shown in memory genes upon recurrent drought exposure and their association with physiological, biochemical and morphological responses during repeated drought.
Alterations in photosynthesis and photorespiration
Alterations in photosynthesis and photorespiration mechanisms have been emphasized in different studies tackling drought memory. Generally, damage to the basic organization structure of the plant negatively affects many metabolic processes, carbon assimilation and the photosynthetic apparatus. However, priming has been shown to induce a better maintenance of photosynthetic efficiency during recurrent drought stress. Drought priming of Triticum aestivum L. (Abid et al. 2016; 2017) and coffee (Menezes-Silva et al. 2017) led to photosynthetic efficiency and increased Ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) during later stress. Wang et al. (2014) also indicated that drought primed plants before anthesis accumulated more proteins such as Rubisco small subunit, Rubisco activase and ascorbate peroxidase when subjected to another drought stress after anthesis. The propagules from drought-stressed sugarcane plants displayed increased photosynthetic water-use efficiency as well as quicker photosynthesis recovery following rehydration (Marcos et al. 2018a; b). Concurrently, drought memory genes have been by Virlouvet et al. (2018) related to photosynthesis. For example, the identified Calvin-Benson-Basham Cycle, NANDP-Me-type, NAD-ME type, PEPC, PEPCK enzyme type and PEPC kinase memory genes encode proteins that play a role in light harvesting, non-photochemical quenching, energy transfer and general photosynthesis. Memory gene that encode a chloroplast ATP synthase was down-regulated in the second stress to achieve protection of the photosynthetic apparatus.
Oñate et al. (2011) observed that when they subjected Urtica dioica L. in a combination of water and nutrient scarcity during juvenile stage, mature leaves revealed improved drought tolerance through modulation of chlorophyll levels during a second stress at reproductive stage. Altered chlorophyll content during subsequent encounter was also observed by Abid et al. (2016; 2017) in Triticum aestivum L. Indeed, among the 13 pigment memory genes noted by Virlouvet et al. (2018), two chlorophyll biosynthesis genes were down-regulated while two chlorophyll degradation genes were up-regulated in the second stress encounter. Although situation differ between plants based on the sink–source relationships during stress, a first water stress can improve plant response to a succeeding stress by diminishing the impact of the second stress on plant photosynthesis and energy mechanisms, thus supporting a better carbon status (Jacques et al. 2021).
Alterations in cell integrity, osmotic and plant water status
Hormones, especially phytohormones, play important roles in the regulation of different processes of plant adaptation to drought environments by modifying cellular functions at molecular levels through diverse cell signaling (Yadav et al. 2021; Iqbal et al. 2022). Abscisic acid (ABA) is a phytohormone that during drought conditions, regulates Ca2+ in the guard cells to induce stomatal closure, thereby preventing water loss (Ali et al. 2020). During repeated drought exposures on Arabidopsis thaliana L., transcriptional stress memory was displayed by an increased transcription rate and increased levels of transcripts of ABA-inducible RAB18 (Ding et al. 2012, 2014). The transcripts accumulated progressively in every subsequent drought treatment. In their study, Forestan et al. (2020) noted higher expression levels of genes that speed up ABA biosyntheis steps (ZEP1, four NCEDs and two AOs) indicating stable transcriptional changes that persist after drought recovery and thereby transcriptional memory. The expression levels of ABA and jasmonic acid (JA)-related genes changed significantly in rice during the first drought exposure and the levels were stably maintained following several rounds of treatment (Li et al. 2019).
Higher ABA levels in primed wheat plants under drought stress were associated with improved tolerance to drought that occurred later during grain filling stage and subsequently to higher grain yield compared to the non-primed wheat plants (Wang et al. 2015). Fleta-Soriano et al. (2015) indicated drought memory mediated by modification in ABA by showing that the levels were raised under drought conditions if there was a previous drought exposure on the plant. Moreover, analysis of plant hormone levels in Aptena cordifolia L. exposed to reiterated drought revealed that Gibberelin acid went down during the first exposure and remained so in the second one, while ABA was observed to be higher in double-stressed plants compared to single-stressed plants (Fleta-Soriano et al. 2015).
Using an RNA-seq approach to investigate how Coffea canephora L. responded to subsequent drought, Guedes et al. (2018) were able to identify differentially expressed genes (DEG) in tolerant and sensitive clones. The findings illustrated that in the tolerant plants acclimatized to multiple drought episodes, memory genes involved in ABA pathways were identified. On the other hand, the sensitive clones were associated with memory genes that triggered an oxidative stress response that probably led to programmed death upon exposure to multiple episodes of drought. The observed transcriptional memory in tolerant and sensitive plant genes suggests the ability of the plants to opt to a mechanism to remember genes that should undergo modulation upon drought stress exposure.
An increase in expression of key ABA biosynthesis modulators including 9-CIS-EPOXYCAROTENOID DIOXYGENASE 3 (NCED3) and ALDEHYDE OXIDASE 3 (AAO3) has been indicated in previously stressed plants during recovery phase to reduce transpiration in an event of a subsequent stress attack (Virlouvet and Fromm 2015). This locus encodes a vital enzyme in the ABA biosynthesis pathway and performs an important role in signaling in drought stress. As a result of increase in the transcription of many ABA-induced genes in response to repeated drought episodes, plants reduce rates of transpiration by mediating guard cell-specific stomatal memory to keep up the leaf water content (Ding et al. 2012, 2013; Virlouvet and Fromm 2015).
A large proportion of drought memory genes in maize was by Ding et al. (2014) shown to encode for proteins associated in membrane integrity functions including dehydrins, regulators of water and potassium uptake and transport and transmembrane transporters for inorganic phosphate and sucrose. In this regard, plants that had been exposed to repeated periods of both droughts and recovery periods displayed higher retention of leaf water, reduced wilting and increased tolerance to terminal drought stress when compared to plants experiencing the stress for the first time (Jakab et al. 2005; Maseda & Fernández 2006; Ding et al. 2012; Ramírez et al. 2015). The increase in root water was also discovered in multi-generationally stressed sugarcane plants (Marcos et al. 2018b). Seedlings from drought-stressed seeds also displayed reduced membrane damage and increased water retention than the controls (Selote & Khanna-Chopra 2006, 2010; Wang et al. 2018).
Osmotic adjustment for water status maintenance is implicated in water stress plant memory (Jacques et al. 2021). Proline, an amino acid, has been shown to be a critical component of plant drought tolerance due to its role as an osmolyte. Menezes-Silva et al. (2017) reported that plants exposed to multiple drought events adapted to future stress due to the expression of trainable genes related to drought tolerance, which were associated with a deep metabolite reprogramming with concordant adjustments in central metabolic processes. Transcription memory of Δ1-pyrroline-5-carboxylate synthetase 1 (P5CS1) and the gene encoding of the proline biosynthetic enzyme were found to be critical in drought stress memory in rice. There was an induction of expression of LOC_Os01g62900 and LOC_Os05g38150, which are P5CS1 homologous after the initial drought stress and reached a peak during the rewatering, and then stayed constant throughout the succeeding drought stress treatment, corresponding to the level of free proline concentration (Li et al. 2019). Alves et al. (2020) also noted that proline levels in Dipteryx alata L. plants rose significantly following recurring drought stress. Its accumulation in the second drought exposure was also reported in peanut plants by Qin et al. (2021). However, Leufen et al. (2016) and Nguyen et al. (2020) revealed lower proline concentrations in sugar beet plants and soybeans, respectively, in the second and third drought stress episodes compared to the first stress.
Memory DMRs also regulated alpha-linolenic acid metabolism, linoleic acid metabolism, biosynthesis of amino acids, glycerophospholipid metabolism, cysteine and methionine metabolism and lysine biosynthesis pathways. Alves et al. (2020) had observed alterations in primary metabolism in Dipteryx alata L. plants, especially in osmoprotectants including in sugar, organic acids and amino acid levels. There were significant increases in sucrose, fructose and glucose levels in primed plants. Organic acids like citrate, fumarate, threonic acid and palmitic acid increased their levels in response to successive drought cycles. Amino acids, including glycine, histidine, alanine, GABA and tryptophan increased in plants exposed to three cycles of drought compared to those that experienced just one stress event. Oñate et al. (2011) also observed modulation of malondialdehyde (MDA) in prestressed Urtica dioica L. Plants. These findings indicate that past stress exposure determined the response of mature plant as these plants showed acclimation to subsequent stress.
Key proteomic cues and drought stress memory
The general abundance and activity of proteins regulate changes in metabolic pathway activities, thereby influencing metabolite levels. Posttranscriptional regulation through changed protein abundances is an important mechanism of response to stress events, and proteomic analysis under repeated drought revealed an increased abundance of proteins (Alves et al. 2020; Auler et al. 2021a, b; Ding et al. 2013; Schulze et al. 2021). Recently, Schulze et al. (2021) have examined the proteome profiling of recurrent drought events in maize and related it to stress memory responses. The authors found overrepresentation of heat-shock proteins, ribosomal proteins, starch metabolism proteins and proteins involved in photosynthesis photophosphorylation during the first stress encounter. While rewatering recovered these proteins to basal levels, ribosomal proteins remained elevated. The second cycle of drought exposure resulted in abundances in ribosomal, galactolipid synthesis, gluconeogenesis, photophosphorylation and lipid degradation proteins but not heat shock proteins. However, Ding et al. (2013) indicated downregulation of memory genes encoding ribosomal, chloroplast and photosynthetic proteins that are involved in ribosome structure, amino acid biosynthesis and photosynthesis, in addition to memory genes that encode for thylakoid membrane-associated proteins in Arabidopsis thaliana. Repeated drought cycles in D. alata seedlings led to substantial increase in the activity of superoxide dismutases (SOD), pyruvate oxidase (Pox) and glutathione reductase (GR), which were not activated by a single drought event (Alves et al. 2020). In rice, Auler et al. (2021a) report decrease in the expression of genes that encode D1and D2 proteins of reaction center of the PSII due to a single drought stress exposure, but double drought stress increased their expression. Rehydration caused the genes to portray an expression level equivalent to that of the control plants. TRITD1Av1G156270 gene coding for late embryogenesis abundant (LEA) proteins showed variable memory responses in rice and wheat (Sadder et al. 2022). Kim et al. (2020) observed that genes encoding protein phosphatase 2C (PP2C) family proteins and LEA proteins were differentially induced. A number of ABA- and ethylene-responsive genes encoding a putative ABA 8ʹ-hydroxylase, ABA-responsive protein-related and osmotin 34 were highly upregulated under the second drought conditions in soybeans. Comparative proteomics in guard cells between rice plants exposed only once and those with recurrent drought stress exposures at vegetative or/and reproductive stages identified 12 drought-responsive proteins that belonged to the photosynthetic pathway, oxidative stress response and stress signaling such as glucagon-like peptide-1 (GLP-1), glutathione-S-transferase (GST), SOD and those related to protein processing such as small heat-shock proteins in roots (Auler et al. 2021b). Interestingly, the abundance of proteins such as endo-1,3-beta-glucosidase, peroxidase, S-adenosylmethionine (SAMS) and malate dehydrogenase (MDH) significantly increased in roots or leaves depending on the rice genotype. Qin et al. (2021) observed a rapid increase in the expression of Arachis hypogaea abscisic acid transporter like-1 (AhATL1) protein and its levels in the second recovery periods following drought exposure. In turn, the overexpression of AhATL1 raised ABA concentrations and altered the post-response gene type into memory gene type, thereby enhancing the drought tolerance and ability to recover. Generally, these authors concluded that there were changes in protein abundance according to single or repeated drought episodes affecting many pathways in plant.
ROS metabolism cues and drought stress memory
One of the usual consequences of drought stress is the production of ROS in the different cellular compartments, including the peroxisomes, the chloroplasts and the mitochondria. ROS includes singlet oxygen (1O2), superoxide radical (O2•−), hydroxyl radical (•OH) and hydrogen peroxide (H2O2) (Hasanuzzaman et al. 2020). Its overproduction results in the peroxidation of cellular membrane lipids and degradation of enzyme proteins and nucleic acids (Li and Liu 2016). To alleviate the effect of ROS, plants induce higher antioxidant enzyme activities and higher expression of their related genes, thereby conferring drought stress tolerance and adaptation (Hou et al. 2021). According to Lukić et al. (2023), the anti-oxidative system plays a crucial role in forming a plant stress drought memory through changes in the activity pattern of anti-oxidative enzymes like SOD and peroxidase (POD) as well as non-enzymatic anti-oxidative defense. The authors reported that in Alopecurus pratensis L. both enzymes were upregulated in drought treated offspring if the parents were also stressed. Similarly, Lukić et al. (2020, 2023) and Liu et al. (2022b) have pointed out that upregulation of the anti-oxidative system is one of the major mechanisms that mediate transgenerational drought stress memory. In their study, Lukić et al. (2023) found reduced H2O2 concentrations in drought-exposed offspring of drought-exposed parents due to increased activity of Catalase (CAT) and POX that converts H2O2 to oxygen and water. The upregulation of Superoxide SOD activity and removal of superoxide anion radicals in drought-exposed offspring of drought-exposed parents subsequently resulted to a decrease in oxidative stress levels. Moreover, malondialdehyde levels under transgenerational drought priming could be caused by increased chelation of hazardous ferrous ions that initiate lipid synthesis and the formation of MDA. Menconi et al. (1995) uncovered that two drought periods on wheat obtained by withholding water and rewatering at the end of the first period during seedling stage resulted in improved scavenging of H2O2 and control of ROS levels. A second drought stress encounter following recovery period in wheat plant revealed the enhancement of dehydroascorbate reductase, glutathione reductase and ascorbate peroxidase (Menconi et al. 1995). Correspondingly, Li et al. (2015) reported low concentrations of H2O2 in wheat leaves if drought priming was done, which could be explained by the high levels of glutathione peroxidase (GPx) in the same plants. A transformed cell structure and the expression of genes mainly encoding proteins related to redox enzymes like APX have been observed in primed plants compared with non-primed plants under drought during grain filling (Wang et al. 2014). The authors postulate that the higher APX activity in primed plants contribute to improve ROS scavenging capacity, to reduce lipid peroxidation in response to a later stress. Wang et al. (2018) found out that the O2•− release rate and H2O2 concentration of wheat flag leaves were significantly increased under drought stress, while they were less affected by drought in the primed plants than in the non-primed plants. Moreover, the authors reported that the activities of antioxidant enzymes like SOD, CAT and APX were increased significantly by drought stress and were much higher in the primed plants than in the non-primed plants. GPX activity was much higher in the primed plants under a second drought encounter. However, only APX gene expression was consistent with its activity levels. Primed rice seedlings displayed increased POX and SOD activity to dissuade the harmful effects caused by oxidative damage in response to subsequent drought stress (Li et al. 2011). According to Yang et al., (2021), when compared with unprimed control, the primed plants showed lower CAT activity, whereas increasing the activity of SOD fivefold. In Nicotiana tabacum L., POD activity was linked to reduced H2O2 levels in primed plants under drought treatment (Khan et al. 2020). Moreover, transcriptional levels of related genes CAT, APX1 and GR2 were revealed in drought-hardened treatment against drought stress. The expression levels of these genes were considerably increased in drought primed plants in comparison with control, and the expression of these genes was more pronounced in T3 plants than other treatments. In Glycine max L., Zea mays L. and Arabidopsis thaliana L., drought memory genes that encode proteins involved in protective roles including dehydrins and chaperones were discovered (Ding et al. 2012, 2014). Synchronously, KEGG enrichment analysis results showed that the memory DMRs were involved in sesquiterpenoids, triterpenoid and phenylpropanoid biosynthesis and arginine metabolism pathways (Kou et al. 2021). These results suggest that previous drought events modified ROS scavenging systems. Defensive and detoxifying functions are important for plant stress memory since they diminish the impact of drought-induced oxidative stress by sustaining cellular metabolism (Jacques et al. 2021).
Morphological adjustments
Plant morphological characteristics are the most valuable tools in monitoring responses to stressors as they can reveal underlying factors that produce changes in plant conditions. Nosalewicz et al. (2016) have reported the transgenerational effect of severe drought stress on shoots and roots of barley (Hordeum vulgare L.). The study revealed that the progeny, whose parental generation was also subjected to drought, showed adaptive morphological alterations such as increased root-to-shoot ratio when compared to the progeny of parental plants that had not been subjected to drought conditions. Backhaus et al. (2014) also reported production of higher amounts of above the ground biomass if there was a pre-exposure to drought when compared to the controls without a previous drought encounter. In agreement with these findings, Marcos et al. (2018b) observed that the plants stored information from the previous stressful events, which led the sugarcane plants that were drought-stressed three times to have increased root dry matter.
Conclusions and future directions for research on drought stress memory and its application in breeding
Changes in the epigenome, transcriptome, proteome and metabolome upon stress encounter confers stress memory, which enable enhanced responses to future stress exposure in plants. Uncovering the potential of this phenomena in crops and how best this discovery can be used in plant breeding programs require an integrated approach. Taken together, the reviewed studies here provide results that point to high variation of species and/ or genotypes specificity to drought stress memory responses. Such studies provide new opportunities for plant breeders and researchers in exploiting different memory capabilities in plants to develop new cultivars in the face of changing climates.
The discovery that plants can memorize past stressful events and pass it to their progeny offers an opportunity to adjust plants’ epigenetic architecture and find out how and which genes are expressed to adjust the growth of plant to adapt to the environment. Indeed, exposure to a priming agent could activate a gene or a set of genes. However, instead of reverting to the transcriptionally silent state once the stimulus is removed, an epigenetic modification could perhaps be left, keeping the region in a ‘permissive’ state. As a result, there is a possibility for quicker and more potent responses to subsequent attacks. This discovery can offer a non-traditional approach to breeding because gene networks that are targeted by this manipulation can be identified without altering the genotype. If a memory gene is identified, it can be regulated to make the plant behave as if it is experiencing the stress, and the mechanisms related to stress tolerance are elicited all the time through the expression of other related genes.
The nature of experiments carried out in the study of stress memory should be assessed for success and applicability. Usually, the recovery period following an initial stress is when stress information is integrated and therefore is crucial for the reinforcement of correct stress memory. In addition, experiments should incorporate different priming stages in the life span of a given crop to evaluate which stage induces most pronounced beneficial impacts. Moreover, it would be necessary to prepare, grow and multiply the seed for an experiment or a selection procedure in exactly the same way, so that the memory does not affect the outcome of the experiment or selection. This would also guarantee that memory effects between experiments are duplicated. Validation is also essential when transforming laboratory or controlled experiment information to the field. As depicted in this review, various imprints including hormones (ABA, Gibberelin acid and JA), enzymes (antioxidants such ascorbate peroxidase) and metabolites like proline are strong causes or consequences of plant memory response. Based on these studies, we propose that investigations on their concentrations during recurrent drought episodes, associated memory genes, as well as related epigenetic marks be carried out. Lastly, there are variable results regarding the usefulness of priming and persistence of the discovered drought stress memory. Therefore, further research is needed to explore the influence of priming on plant population and community structures as it involves plant performances and reproductive success. Researchers should in future also find out how the positive stress memory effects can be increased and prolonged.
Change history
07 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00122-023-04338-6
References
Abid M, Tian Z, Ata-Ul-Karim ST, Liu Y, Cui Y, Zahoor R, Jiang D, Dai T (2016) Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and -sensitive wheat cultivars. Plant Physiol Biochem 106:218–227. https://doi.org/10.1016/j.plaphy.2016.05.003
Abid M, Shao Y, Liu S, Wang F, Gao J, Jiang D, Tian Z, Dai T (2017) Pre-drought priming sustains grain development under post-anthesis drought stress by regulating the growth hormones in winter wheat (Triticum aestivum L.). Planta 246(3):509–524. https://doi.org/10.1007/s00425-017-2698-4
Abid M, Ali S, Qi LK, Zahoor R, Tian Z, Jiang D, Snider JL, Dai T (2018) Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci Rep. https://doi.org/10.1038/s41598-018-21441-7
Agrawal AA (2002) Herbivory and maternal effects: mechanisms and consequences of transgenerational induced plant resistance. Ecology 83(12):3408–3415
Ali S, Hayat K, Iqbal A, Xie L (2020) Implications of abscisic acid in the drought stress tolerance of plants. Agronomy. https://doi.org/10.3390/agronomy10091323
Alves RDFB, Menezes-Silva PE, Sousa LF, Loram-Lourenço L, Silva MLF, Almeida SES, Silva FG, Perez de Souza L, Fernie AR, Farnese FS (2020) Evidence of drought memory in Dipteryx alata indicates differential acclimation of plants to savanna conditions. Sci Rep. https://doi.org/10.1038/s41598-020-73423-3
Amtmann A, Armengaud P (2009) Effects of N, P, K and S on metabolism: new knowledge gained from multi-level analysis. Curr Opin Plant Biol 12(3):275–283. https://doi.org/10.1016/j.pbi.2009.04.014
Auler PA, Souza GM, da Silva Engela MRG, do Amaral MN, Rossatto T, da Silva MGZ, Furlan CM, Maserti B, Braga EJB (2021a) Stress memory of physiological, biochemical and metabolomic responses in two different rice genotypes under drought stress: the scale matters. Plant Sci. https://doi.org/10.1016/j.plantsci.2021.110994
Auler PA, do Amaral MN, Braga EJB, Maserti B (2021b) Drought stress memory in rice guard cells: Proteome changes and genomic stability of DNA. Plant Physiol Biochem 169:49–62. https://doi.org/10.1016/J.PLAPHY.2021.10.028
Avramova Z (2015) Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J 83(1):149–159. https://doi.org/10.1111/tpj.12832
Backhaus S, Kreyling J, Grant K, Beierkuhnlein C, Walter J, Jentsch A (2014) Recurrent mild drought events increase resistance toward extreme drought stress. Ecosystems 17(6):1068–1081. https://doi.org/10.1007/s10021-014-9781-5
Balmer A, Pastor V, Gamir J, Flors V, Mauch-Mani B (2015) The ‘prime-ome’: towards a holistic approach to priming. Trends Plant Sci 20(7):443–452. https://doi.org/10.1016/j.tplants.2015.04.002
Basu S, Ramegowda V, Kumar A, Pereira A (2016) Plant adaptation to drought stress. Research 5:1554
BenDkhil B, Issa A, Denden M (2014) Germination and seedling emergence of primed okra (Abelmoschus esculentus L.) seeds under salt stress and low temperature. Am J Plant Physiol 9(2):38–45
Bilichak A, Ilnytskyy Y, Woycicki R, Kepeshchuk N, Fogen D, Kovalchuk I (2015) The elucidation of stress memory inheritance in Brassica rapa plants. Front Plant Sci 6:1–20. https://doi.org/10.3389/fpls.2015.00005
Bird A (2007) Perceptions of epigenetics. Nature 447(7143):396–398. https://doi.org/10.1038/nature05913
Boyko A, Golubov A, Bilichak A, Kovalchuk I (2010) Chlorine ions but not sodium ions alter genome stability of arabidopsis thaliana. Plant Cell Physiol 51(6):1066–1078. https://doi.org/10.1093/pcp/pcq048
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful ‘memories’ of plants: evidence and possible mechanisms. Plant Sci 173(6):603–608. https://doi.org/10.1016/j.plantsci.2007.09.002
Cerda T (2020) Transgenerational effects of single versus recurrent drought on belowground plant traits of three grassland species
Chen Y, Li C, Yi J, Yang Y, Lei C, Gong M (2020) Transcriptome response to drought, rehydration and re-dehydration in potato. Int J Mol Sci 21(1):159. https://doi.org/10.3390/ijms21010159
Crisp PA, Ganguly D, Eichten SR, Borevitz JO, Pogson BJ (2016) Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci Adv. https://doi.org/10.1126/sciadv.1501340
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679. https://doi.org/10.1146/annurev-arplant-042809-112122
Cutter AR, Hayes JJ (2015) A brief review of nucleosome structure. FEBS Lett. https://doi.org/10.1016/j.febslet.2015.05.016
D’Urso A, Brickner JH (2014) Mechanisms of epigenetic memory. Trends Genet 30(6):230–236. https://doi.org/10.1016/j.tig.2014.04.004
Daryanto S, Wang L, Jacinthe PA (2016) Global synthesis of drought effects on maize and wheat production. PLoS ONE. https://doi.org/10.1371/journal.pone.0156362
de Guedes FAF, Nobres P, Ferreira DCR, Menezes-Silva PE, Ribeiro-Alves M, Correa RL, DaMatta FM, Alves-Ferreira M (2018) Transcriptional memory contributes to drought tolerance in coffee (Coffea canephora) plants. Environ Exp Botany 147:220–233
de Oliveira Sousa AR, Ribas RF, Coelho Filho MA, Freschi L, Ferreira CF, dos Santos Soares Filho, W., Pérez-Molina, J.P. and da Silva Gesteira, A., (2022) Drought tolerance memory transmission by citrus buds. Plant Sci 320:111292. https://doi.org/10.1016/j.plantsci.2022.111292
Ding Y, Fromm M, Avramova Z (2012) Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat Commun. https://doi.org/10.1038/ncomms1732
Ding Y, Virlouvet L, Liu N, Riethoven JJ, Fromm M, Avramova Z (2014) Dehydration stress memory genes of Zea mays; comparison with Arabidopsis thaliana. BMC Plant Biol. https://doi.org/10.1186/1471-2229-14-141
Ding Y, Liu N, Virlouvet L, Riethoven JJ, Fromm M, Avramova Z (2013) Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. http://www.biomedcentral.com/1471-2229/13/229
F.A.O. (2021) The state of food and agriculture 2021. In the state of food and agriculture 2021. FAO. https://doi.org/10.4060/cb4476en
Fan Y, Ma C, Huang Z, Abid M, Jiang S, Dai T, Zhang W, Ma S, Jiang D, Han X (2018) Heat priming during early reproductive stages enhances thermo-tolerance to post-anthesis heat stress via improving photosynthesis and plant productivity in winter wheat (Triticum aestivum L.). Front Plant Sci. https://doi.org/10.3389/fpls.2018.00805
Farhoudi R, Sharifzadeh F, Poustini K, Makkizadeh MT, Kochak Por M (2007) The effects of NaCl priming on salt tolerance in canola (Brassica napus) seedlings grown under saline conditions. Seed Sci Technol 35(3):754–759. https://doi.org/10.15258/sst.2007.35.3.23
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Sustainable agriculture. Springer, Netherlands, pp 153–188
Feng S, Jacobsen SE (2011) Epigenetic modifications in plants: An evolutionary perspective. Curr Opin Plant Biol 14(2):179–186. https://doi.org/10.1016/j.pbi.2010.12.002
Feng XJ, Li JR, Qi SL, Lin QF, Jin JB, Hua XJ (2016) Light affects salt stress-induced transcriptional memory of P5CS1 in Arabidopsis. Proc Natl Acad Sci USA 113(51):E8335–E8343. https://doi.org/10.1073/pnas.1610670114
Fleta-Soriano E, Munné-Bosch S (2016) Stress memory and the inevitable effects of drought: a physiological perspective. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00143
Fleta-Soriano E, Pintó-Marijuan M, Munné-Bosch S (2015) Evidence of drought stress memory in the facultative CAM Aptenia cordifolia: possible role of phytohormones. PLoS ONE. https://doi.org/10.1371/journal.pone.0135391
Forestan C, Farinati S, Zambelli F, Pavesi G, Rossi V, Varotto S (2020) Epigenetic signatures of stress adaptation and flowering regulation in response to extended drought and recovery in Zea mays. Plant Cell Environ 43(1):55–75. https://doi.org/10.1111/pce.13660
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124(4):509–525. https://doi.org/10.1007/s10265-011-0412-3
Gagliano M, Renton M, Depczynski M, Mancuso S (2014) Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 175(1):63–72. https://doi.org/10.1007/s00442-013-2873-7
Ganguly DR, Crisp PA, Eichten SR, Pogson BJ (2017) The arabidopsis DNA methylome is stable under transgenerational drought stress. Plant Physiol 175(4):1893–1912. https://doi.org/10.1104/pp.17.00744
Godwin J, Farrona S (2020) Plant epigenetic stress memory induced by drought: a physiological and molecular perspective. In: Farrona S (ed) Plant Epigenetics and Epigenomics (Second Edition). Springer, Newyork
Groot MP, Kooke R, Knoben N, Vergeer P, Keurentjes JJB, Ouborg NJ, Verhoeven KJF (2016) Effects of multi-generational stress exposure and offspring environment on the expression and persistence of transgenerational effects in Arabidopsis thaliana. PLoS ONE. https://doi.org/10.1371/journal.pone.0151566
Haider S, Iqbal J, Shaukat M, Naseer S, Mahmood T (2021) The epigenetic chromatin-based regulation of somatic heat stress memory in plants. Plant Gene 27:100318. https://doi.org/10.1016/j.plgene.2021.100318
Hasanuzzaman M, Bhuyan MHMB, Parvin K, Bhuiyan TF, Anee TI, Nahar K, Hossen MS, Zulfiqar F, Alam MM, Fujita M (2020) Regulation of ros metabolism in plants under environmental stress: a review of recent experimental evidence. Int J Mol Sci 21(22):1–44. https://doi.org/10.3390/ijms21228695
Hatzig SV, Nuppenau JN, Snowdon RJ, Schießl SV (2018) Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica napus L.). BMC Plant Biol 18:1–13. https://doi.org/10.1186/s12870-018-1531-y
Heard E, Martienssen RA (2014) Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157(1):95–109. https://doi.org/10.1016/j.cell.2014.02.045
Herman JJ, Sultan SE, Horgan-Kobelski T, Riggs C (2012) Adaptive transgenerational plasticity in an annual plant: grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr Comp Biol 52(1):77–88. https://doi.org/10.1093/icb/ics041
Hossain MA, Bhattacharjee S, Armin SM, Qian P, Xin W, Li HY, Burritt DJ, Fujita M, Tran LSP (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00420
Hou P, Wang F, Luo B, Li A, Wang C, Shabala L, Ahmed HAI, Deng S, Zhang H, Song P, Zhang Y, Shabala S, Chen L (2021) Antioxidant enzymatic activity and osmotic adjustment as components of the drought tolerance mechanism in Carex duriuscula. Plants 10(3):436
Huxman TE, Charlet TN, Grant C, Smith SD (2001) THE effects of parental Co2 and offspring nutrient environment on initial growth and photosynthesis in an annual grass. Int J Plant Sci 162(3):617–623
Iqbal S, Wang X, Mubeen I, Kamran M, Kanwal I, Díaz GA, Abbas A, Parveen A, Atiq MN, Alshaya H, Zin El-Abedin TK, Fahad S (2022) Phytohormones trigger drought tolerance in crop plants: outlook and future perspectives. Front Plant Sci. https://doi.org/10.3389/fpls.2021.799318
Jacques C, Salon C, Barnard RL, Vernoud V, Prudent M (2021) Drought stress memory at the plant cycle level: a review. Plants. https://doi.org/10.3390/plants10091873
Jakab G, Ton J, Flors V, Zimmerli L, Métraux JP, Mauch-Mani B (2005) Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol 139(1):267–274. https://doi.org/10.1104/pp.105.065698
Kasim WA, Osman ME, Omar MN, Abd El-Daim IA, Bejai S, Meijer J (2013) Control of drought stress in wheat using plant-growth-promoting bacteria. J Plant Growth Regul 32(1):122–130. https://doi.org/10.1007/s00344-012-9283-7
Khan R, Ma X, Shah S, Wu X, Shaheen A, Xiao L, Wu Y, Wang S (2020) Drought-hardening improves drought tolerance in Nicotiana tabacum at physiological, biochemical, and molecular levels. BMC Plant Biol. https://doi.org/10.1186/s12870-020-02688-7
Kim JM, To TK, Ishida J, Matsui A, Kimura H, Seki M (2012) Transition of chromatin status during the process of recovery from drought stress in arabidopsis thaliana. Plant Cell Physiol 53(5):847–856. https://doi.org/10.1093/pcp/pcs053
Kim YK, Chae S, Oh NI, Nguyen NH, Cheong JJ (2020) Recurrent drought conditions enhance the induction of drought stress memory genes in glycine max L. Front Genet. https://doi.org/10.3389/fgene.2020.576086
Klengel T, Dias BG, Ressler KJ (2016) Models of intergenerational and transgenerational transmission of risk for psychopathology in mice. Neuropsychopharmacology 41(1):219–231. https://doi.org/10.1038/npp.2015.249
Kou S, Gu Q, Duan L, Liu G, Yuan P, Li H, Wu Z, Liu W, Huang P, Liu L (2021) Genome-wide bisulphite sequencing uncovered the contribution of dna methylation to rice short-term drought memory formation. J Plant Growth Regul. https://doi.org/10.1007/s00344-021-10483-3
Koua AP, Oyiga BC, Baig MM, Léon J, Ballvora A (2021) Breeding driven enrichment of genetic variation for key yield components and grain starch content under drought stress in winter wheat. Front Plant Sci. https://doi.org/10.3389/fpls.2021.684205
Lämke J, Bäurle I (2017) Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. https://doi.org/10.1186/s13059-017-1263-6
Lämke J, Brzezinka K, Altmann S, Bäurle I (2016) A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J 35(2):162–175
Lammerink J, Macgibbon DB, Wallace AR (1984) Effect of the cabbage aphid (Brevicoryne brassicae) on total glucosinolate in the seed of oilseed rape (Brassica napus). N Z J Agric Res 27(1):89–92. https://doi.org/10.1080/00288233.1984.10425735
Lau JA, Peiffer J, Reich PB, Tiffin P (2008) Transgenerational effects of global environmental change: Long-term CO2 and nitrogen treatments influence offspring growth response to elevated CO2. Oecologia 158(1):141–150. https://doi.org/10.1007/s00442-008-1127-6
Leuendorf JE, Frank M, Schmülling T (2020) Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci Rep. https://doi.org/10.1038/s41598-019-56797-x
Leufen G, Noga G, Hunsche M (2016) Drought stress memory in sugar beet: mismatch between biochemical and physiological parameters. J Plant Growth Regul 35(3):680–689. https://doi.org/10.1007/s00344-016-9571-8
Li X, Liu F (2016) Drought stress memory and drought stress tolerance in plants: biochemical and molecular basis. Drought Stress Tolerance Plants Physiol Biochem. https://doi.org/10.1007/978-3-319-28899-4
Li X, Zhang L, Li Y (2011) Preconditioning alters antioxidative enzyme responses in rice seedlings to water stress. Proc Environ Sci 11:1346–1351. https://doi.org/10.1016/j.proenv.2011.12.202
Li X, Cai J, Liu F, Dai T, Cao W, Jiang D (2014) Cold priming drives the sub-cellular antioxidant systems to protect photosynthetic electron transport against subsequent low temperature stress in winter wheat. Plant Physiol Biochem 82:34–43. https://doi.org/10.1016/j.plaphy.2014.05.005
Li X, Topbjerg HB, Jiang D, Liu F (2015) Drought priming at vegetative stage improves the antioxidant capacity and photosynthesis performance of wheat exposed to a short-term low temperature stress at jointing stage. Plant Soil 393(1–2):307–318. https://doi.org/10.1007/s11104-015-2499-0
Li P, Yang H, Wang L, Liu H, Huo H, Zhang C, Liu A, Zhu A, Hu J, Lin Y, Liu L (2019) Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Front Genet. https://doi.org/10.3389/fgene.2019.00055
Liu H, Able AJ, Able JA (2020) Transgenerational effects of water-deficit and heat stress on germination and seedling vigour—new insights from durum wheat microRNAs. Plants. https://doi.org/10.3390/plants9020189
Liu H, Able AJ, Able JA (2021a) Priming crops for the future: rewiring stress memory. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2021a.11.015
Liu H, Able AJ, Able JA (2021b) Small RNAs and their targets are associated with the transgenerational effects of water-deficit stress in durum wheat. Sci Rep. https://doi.org/10.1038/s41598-021-83074-7
Liu L, Cao X, Zhai Z, Ma S, Tian Y, Cheng J (2022a) Direct evidence of drought stress memory in mulberry from a physiological perspective: antioxidative, osmotic and phytohormonal regulations. Plant Physiol Biochem 186:76–87. https://doi.org/10.1016/j.plaphy.2022.07.001
Liu X, Quan W, Bartels D (2022b) Stress memory responses and seed priming correlate with drought tolerance in plants: an overview. Planta. https://doi.org/10.1007/s00425-022-03828-z
Lukić N, Kukavica B, Davidović-Plavšić B, Hasanagić D, Walter J (2020) Plant stress memory is linked to high levels of anti-oxidative enzymes over several weeks. Environ Exp Botany. https://doi.org/10.1016/j.envexpbot.2020.104166
Lukić N, Schurr FM, Trifković T, Kukavica B, Walter J (2023) Transgenerational stress memory in plants is mediated by upregulation of the antioxidative system. Environ Exp Bot 205:105129
Luna E, Bruce TJA, Roberts MR, Flors V, Ton J (2012) Next-generation systemic acquired resistance. Plant Physiol 158(2):844–853. https://doi.org/10.1104/pp.111.187468
Marcos FCC, Silveira NM, Marchiori PER, Machado EC, Souza GM, Landell MGA, Ribeiro RV (2018a) Drought tolerance of sugarcane propagules is improved when origin material faces water deficit. PLoS ONE. https://doi.org/10.1371/journal.pone.0206716
Marcos FCC, Silveira NM, Mokochinski JB, Sawaya ACHF, Marchiori PER, Machado EC, Souza GM, Landell MGA, Ribeiro RV (2018b) Drought tolerance of sugarcane is improved by previous exposure to water deficit. J Plant Physiol 223:9–18. https://doi.org/10.1016/j.jplph.2018.02.001
Maseda PH, Fernández RJ (2006) Stay wet or else: three ways in which plants can adjust hydraulically to their environment. J Exp Botany 57(15):3963–3977. https://doi.org/10.1093/jxb/erl127
Melnyk CW, Molnar A, Baulcombe DC (2011) Intercellular and systemic movement of RNA silencing signals. EMBO J 30(17):3553–3563. https://doi.org/10.1038/emboj.2011.274
Menconi M, Sgherri CLM, Pinzino C, Navari-Lzzo F (1995) Activated oxygen production and detoxification in wheat plants subjected to a water deficit programme. J Exp Botany. https://doi.org/10.1093/jxb/46.9.1123
Menezes-Silva PE, Sanglard LMVP, Ávila RT, Morais LE, Martins SCV, Nobres P, Patreze CM, Ferreira MA, Araújo WL, Fernie AR, DaMatta FM (2017) Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J Exp Bot 68(15):4309–4322. https://doi.org/10.1093/jxb/erx211
Muscolo A, Junker A, Klukas C, Weigelt-Fischer K, Riewe D, Altmann T (2015) Phenotypic and metabolic responses to drought and salinity of four contrasting lentil accessions. J Exp Bot 66(18):5467–5480. https://doi.org/10.1093/jxb/erv208
Nair AS, Abraham TK, Jaya DS (2008) Studies on the changes in lipid peroxidation and antioxidants in drought stress induced cowpea (Vigna unguiculata L.) varieties. J Environ Biol 29(5):689–691
Neves DM, Almeida LADH, Santana-Vieira DDS, Freschi L, Ferreira CF, Soares Filho WDS, Costa MGC, Micheli F, Coelho Filho MA, Gesteira ADS (2017) Recurrent water deficit causes epigenetic and hormonal changes in citrus plants. Sci Rep. https://doi.org/10.1038/s41598-017-14161-x
Nguyen NH, Vu NT, Cheong JJ (2022) Transcriptional stress memory and transgenerational inheritance of drought tolerance in plants. Int J Mol Sci 23(21):12918. https://doi.org/10.3390/ijms232112918
Nosalewicz A, Siecińska J, Śmiech M, Nosalewicz M, Wiącek D, Pecio A, Wach D (2016) Transgenerational effects of temporal drought stress on spring barley morphology and functioning. Environ Exp Bot 131:120–127. https://doi.org/10.1016/j.envexpbot.2016.07.006
Oñate M, Blanc J, Munné-Bosch S (2011) Influence of stress history on the response of the dioecious plant Urtica dioica L. to abiotic stress. Plant Ecol Divers 4(1):45–54. https://doi.org/10.1080/17550874.2011.557400
Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol 202(1):35–49. https://doi.org/10.1111/nph.12613
Phung KH, Lee SH, Cheong JJ (2020) Evaluation of proline, soluble sugar and ABA content in soybean Glycine max (L.) under drought stress memory. AIMS Bioeng 7:114–123. https://doi.org/10.3934/bioeng.2020011
Qin M, Li X, Tang S, Huang Y, Li L, Hu B (2021) Expression of AhaTL1, an ABA transport factor gene from peanut, is affected by altered memory gene expression patterns and increased tolerance to drought stress in Arabidopsis. Int J Mol Sci. https://doi.org/10.3390/ijms22073398
Rajak J (2021) A preliminary review on impact of climate change and our environment with reference to global warming. Int J Environ Sci 10:11–14
Ramírez DA, Rolando JL, Yactayo W, Monneveux P, Mares V, Quiroz R (2015) Improving potato drought tolerance through the induction of long-term water stress memory. Plant Sci 238:26–32. https://doi.org/10.1016/j.plantsci.2015.05.016
Rendina González AP, Preite V, Verhoeven KJF, Latzel V (2018) Transgenerational effects and epigenetic memory in the clonal plant trifolium repens. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01677
Roberts DA (1983) Short communications. Virology, 124
Rogers FW (1966) The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet 288(7473):1087–1093
Sadder MT, Musallam A, Allouzi M, Duwayri MA (2022) Dehydration stress memory genes in Triticum turgidum L. ssp. durum (Desf). Biotech. https://doi.org/10.3390/biotech11030043
Sánchez-Andrade G, Kendrick KM (2011) Roles of α- and β-estrogen receptors in mouse social recognition memory: effects of gender and the estrous cycle. Horm Behav 59(1):114–122. https://doi.org/10.1016/j.yhbeh.2010.10.016
Schulze WX, Altenbuchinger M, He M, Kränzlein M, Zörb C (2021) Proteome profiling of repeated drought stress reveals genotype-specific responses and memory effects in maize. Plant Physiol Biochem 159:67–79. https://doi.org/10.1016/j.plaphy.2020.12.009
Selote DS, Khanna-Chopra R (2006) Drought acclimation confers oxidative stress tolerance by inducing co-ordinated antioxidant defense at cellular and subcellular level in leaves of wheat seedlings. Physiol Plant 127(3):494–506. https://doi.org/10.1111/j.1399-3054.2006.00678.x
Selote DS, Khanna-Chopra R (2010) Antioxidant response of wheat roots to drought acclimation. Protoplasma 245(1):153–163. https://doi.org/10.1007/s00709-010-0169-x
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26. https://doi.org/10.1155/2012/217037
Sharma M, Kumar P, Verma V, Sharma R, Bhargava B, Irfan M (2022) Understanding plant stress memory response for abiotic stress resilience: molecular insights and prospects. Plant Physiol Biochem 179:10–24. https://doi.org/10.1016/j.plaphy.2022.03.004
Shattuck VI (1993) Glucosinolates and glucosinolate degradation in seeds from turnip mosaic virus-infected rapid cycle Brassica campestris L. Plants. J Exp Botany. https://doi.org/10.1093/jxb/44.5.963
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58(2):221–227. https://doi.org/10.1093/jxb/erl164
Singh RK, Prasad M (2022) Delineating the epigenetic regulation of heat and drought response in plants. Critical Rev Biotechnol. https://doi.org/10.1080/07388551.2021.1946004
Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Curr Opin Biotechnol 21(2):197–203. https://doi.org/10.1016/j.copbio.2010.03.002
Tajdoost S, Farboodnia T, Heidari R (2007) Salt pretreatment enhance salt tolerance in Zea mays L. seedlings. Pakistan J Biol Sci 10(12):2086–2090. https://doi.org/10.3923/pjbs.2007.2086.2090
Tani E, Chronopoulou E, Labrou N, Sarri E, Goufa Μ, Vaharidi X, Tornesaki A, Psychogiou M, Bebeli P, Abraham Ε (2019) Growth, physiological, biochemical, and transcriptional responses to drought stress in seedlings of Medicago sativa L., Medicago arborea L. and their hybrid (Alborea). Agronomy 9(1):38. https://doi.org/10.3390/agronomy9010038
Thiebaut F, Hemerly AS, Ferreira PCG (2019) A role for epigenetic regulation in the adaptation and stress responses of non-model plants. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00246
Tian Z, Li K, Sun Y, Zhang S, Chen B, Pan Z, PangB, Miao Y, Du X, He S (2022) Physiological and transcriptional analyses reveal formation of memory under recurring drought stresses in Gossypium hirsutum. https://doi.org/10.22541/au.165389768.89159040/v1
Tombesi S, Frioni T, Poni S, Palliotti A (2018) Effect of water stress “memory” on plant behavior during subsequent drought stress. Environ Exp Bot 150:106–114. https://doi.org/10.1016/j.envexpbot.2018.03.009
Trenberth KE, Dai A, van der Schrier G, Jones PD, Barichivich J, Briffa KR, Sheffield J (2014) Global warming and changes in drought. Nat Climate Change 4(1):17–22. https://doi.org/10.1038/nclimate2067
ur Rehman H, Iqbal H, Basra SM, Afzal I, Farooq M, Wakeel A, Ning WANG (2015) Seed priming improves early seedling vigor, growth and productivity of spring maize. J Integrative Agric 14(9):1745–1754. https://doi.org/10.1016/S2095-3119(14)61000-5
van Dooren TJM, Silveira AB, Gilbault E, Jiménez-Gómez JM, Martin A, Bach L, Tisné S, Quadrana L, Loudet O, Colot V (2020) Mild drought in the vegetative stage induces phenotypic, gene expression, and DNA methylation plasticity in Arabidopsis but no transgenerational effects. J Exp Bot 71(12):3588–3602. https://doi.org/10.1093/jxb/eraa132
Virlouvet L, Fromm M (2015) Physiological and transcriptional memory in guard cells during repetitive dehydration stress. New Phytol 205(2):596–607. https://doi.org/10.1111/nph.13080
Virlouvet L, Ding Y, Fujii H, Avramova Z, Fromm M (2014) ABA signaling is necessary but not sufficient for RD29B transcriptional memory during successive dehydration stresses in Arabidopsis thaliana. Plant J 79(1):150–161. https://doi.org/10.1111/tpj.12548
Virlouvet L, Avenson TJ, Du Q, Zhang C, Liu N, Fromm M, Avramova Z, Russo SE (2018) Dehydration stress memory: gene networks linked to physiological responses during repeated stresses of zea mays. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01058
Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. https://doi.org/10.1016/j.micres.2015.12.003
Walter J, Nagy L, Hein R, Rascher U, Beierkuhnlein C, Willner E, Jentsch A (2011) Do plants remember drought? Hints towards a drought-memory in grasses. Environ Exp Bot 71(1):34–40. https://doi.org/10.1016/j.envexpbot.2010.10.020
Wang X, Vignjevic M, Jiang D, Jacobsen S, Wollenweber B (2014) Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat (Triticum aestivum L.) var Vinjett. J Exp Botany 65(22):6441–6456. https://doi.org/10.1093/jxb/eru362
Wang X, Vignjevic M, Liu F, Jacobsen S, Jiang D, Wollenweber B (2015) Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul 75(3):677–687. https://doi.org/10.1007/s10725-014-9969-x
Wang X, Zhang X, Chen J, Wang X, Cai J, Zhou Q, Dai T, Cao W, Jiang D (2018) Parental drought-priming enhances tolerance to post-anthesis drought in offspring of wheat. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00261
Whittle CA, Otto SP, Johnston MO, Krochko JE (2009) Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany 87(6):650–657. https://doi.org/10.1139/B09-030
Wijewardana C, Raja Reddy K, Jason Krutz L, Gao W, Bellaloui N (2019a) Drought stress has transgenerational effects on soybean seed germination and seedling vigor. PLoS ONE. https://doi.org/10.1371/journal.pone.0214977
Wijewardana C, Reddy KR, Krutz LJ, Gao W, Bellaloui N (2019b) Poor seed quality, reduced germination, and decreased seedling vigor in soybean is linked to exposure of the maternal lines to drought stress. BioRxiv. https://doi.org/10.1101/590059
Wojtyla Ł, Paluch-Lubawa E, Sobieszczuk-Nowicka E, Garnczarska M (2020) Drought stress memory and subsequent drought stress tolerance in plants. In: Priming-mediated stress and cross-stress tolerance in crop plants. Academic Press, pp 115–131
Wu Y, Zhong H, Li J, Xing J, Xu N, Zou H (2022) Water use efficiency and photosynthesis of Calamagrostis angustifolia leaves under drought stress through CO2 concentration increase. J Plant Interact 17(1):60–74. https://doi.org/10.1080/17429145.2021.2011444
Xiao WANG, Fu-lai LIU, Dong JIANG (2017) Priming: a promising strategy for crop production in response to future climate. J Integrative Agric 16(12):2709–2716. https://doi.org/10.1016/S2095-3119(17)61786-6
Xin Z, Browse J (2000) Cold comfort farm: the acclimation of plants to freezing temperatures. Plant Cell Environ 23(9):893–902. https://doi.org/10.1046/j.1365-3040.2000.00611.x
Yadav B, Jogawat A, Gnanasekaran P, Kumari P, Lakra N, Lal SK, Pawar J, Narayan OP (2021) An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene. https://doi.org/10.1016/j.plgene.2020.100264
Yan K, Xu H, Cao W, Chen X (2015) Salt priming improved salt tolerance in sweet sorghum by enhancing osmotic resistance and reducing root Na+ uptake. Acta Physiol Plant. https://doi.org/10.1007/s11738-015-1957-x
Yang H, Li P, Jin G, Gui D, Liu L, Zhang C (2020) Temporal regulation of alternative splicing events in rice memory under drought stress. Plant Divers. https://doi.org/10.1016/j.pld.2020.11.004
Yang H, Hu W, Zhao J, Huang X, Zheng T, Fan G (2021) Genetic improvement combined with seed ethephon priming improved grain yield and drought resistance of wheat exposed to soil water deficit at tillering stage. Plant Growth Regul 95(3):399–419. https://doi.org/10.1007/s10725-021-00749-x
Zhang CY, Wang NN, Zhang YH, Feng QZ, Yang CW, Liu B (2013) DNA methylation involved in proline accumulation in response to osmotic stress in rice (Oryza sativa). Genet Mol Res 12(2):1269–1277. https://doi.org/10.4238/2013.april.17.5
Zhang C, Tang G, Peng X, Sun F, Liu S, Xi Y (2018) Long non-coding RNAs of switchgrass (Panicum virgatum L.) in multiple dehydration stresses. BMC Plant Biol. https://doi.org/10.1186/s12870-018-1288-3
Zheng X, Chen L, Li M, Lou Q, Xia H, Wang P, Li T, Liu H, Luo L (2013) Transgenerational variations in DNA methylation induced by drought stress in two rice varieties with distinguished difference to drought resistance. PLoS ONE. https://doi.org/10.1371/journal.pone.0080253
Zheng X, Chen L, Xia H, Wei H, Lou Q, Li M, Li T, Luo L (2017) Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci Rep. https://doi.org/10.1038/srep39843
Acknowledgements
Special thanks to the Deutscher Akademischer Austauschdienst (DAAD) for financially supporting Carolyn Mukiri Kambona
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was obtained to support in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
CMK drafted the manuscript. PAK, JL and AB were responsible for the correction and critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Additional information
Communicated by Rajeev K. Varshney.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kambona, C.M., Koua, P.A., Léon, J. et al. Stress memory and its regulation in plants experiencing recurrent drought conditions. Theor Appl Genet 136, 26 (2023). https://doi.org/10.1007/s00122-023-04313-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04313-1