Abstract
The selection of drought-tolerant genotypes from the existing gene pool is a preliminary step for breeding for drought tolerance. Research efforts aimed at exploring the ability of crops to withstand drought stress have not yet touched the realm of reproductive drought performance of local genotypes. To examine how local crop accessions demonstrate varying reproductive performance under drought conditions, twenty accessions of maize (Zea mays L.) were evaluated for six quantitative traits in a two-factor factorial completely randomized design with two replications between February to June of 2023. All the traits under study showed significant differences among the genotypes (p < 0.05). Owing to their grain yield, ear weight, and hundred grain weight, accessions NGRC05592, NGRC05582, NGRC05564, NGRC05565, NGRC05571, and NGRC05569 performed better under drought condition than other tested accessions. Accession NGRC05592 showed the highest yield under drought condition, whereas, NGRC05573 and NGRC05576 showed poor performance. GGE Biplot analysis for grain yield revealed that NGRC05571 and NGRC05592 had the highest mean yield, with the accession NGRC05592 standing out as the stable variety under changing soil moisture levels and performing best among all the tested accessions under drought condition. The possibility of accession NGRC05592 being used as a potential genetic resource for drought breeding programs has been observed, but further studies aiming at its stability under field conditions in diverse agro-climatic regions across different years are encouraged to assure its prospect for developing cultivars suited to drought-affected regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Maize (Zea mays L.), renowned as 'the queen of cereals' because of its high yield potential among cereals [1], is the second most important crop after rice in terms of area and production in Nepal [2]. Maize provides a source of livelihood and a means of lifestyle for the majority of hill farmers in Nepal, serving various utilities such as food, feed, and fodder [2]. In the face of global agriculture being increasingly challenged by population growth and climate change, the world cereal equivalent (CE) food demand is projected to increase from 10,094 million tons in 2030 to 14,886 million tons in 2050 [3]. On one hand, the United Nations has projected the world’s population to reach 8.5 billion in 2030, 9.7 billion in 2050, and 10.4 billion in 2100 [4]. On the other hand, the Intergovernmental Panel on Climate Change (IPCC) has warned of a ‘code red’ for humanity as the global warming value reached 1.1 °C in 2021, very close to the threshold levels of 1.5 °C and 2 °C, which depicts the transition of the world into hazardous climate change [5]. With further warming trends, an increase in the frequency and intensity of extreme events, including prolonged drought spells is anticipated [5,6,7], which raises questions regarding the food security of the exponentially increasing global population.
In Nepal, out of the total agricultural land, 52% of the land remains rainfed, and only 48% of the land is irrigated, out of which, only 39% of the land receives year-round irrigation [8]. Maize requires approximately 0.04 mm of moisture to yield 1 kg of grain [9] and uses 350–450 mm of water throughout its lifetime to sustain the life processes [10]. In Northern China, 1 mm of water yields 22.3 kg of grains per hectare in maize crop [9]. In terms of volume, the maize plant consumes a total of 250 L of water until it reaches maturity [10, 11]. The reproductive stage is critical in terms of soil moisture requirement. Restriction of water during the reproductive stage can seriously reduce maize cultivar growth and yield components: such as the number of kernel rows per ear, number of kernels per row, hundred seed weight, seed yield, and biological yield [12]. Yield reduction in maize due to drought can be as much as 70% [13]. Drought stress in maize plants resulted in increased barrenness, delay in days to 50% silking, and reduction in the number of ears per plant and kernels per ear [14], and in many places, it is expected that this will worsen with climate change [15].
Despite the prevailing agrobiodiversity, our local maize varieties are underutilized in national maize breeding programs. Out of 86 maize varieties released or registered in Nepal, only nine included Nepalese landraces in their genetic makeup, either by combining or crossing them with exotic populations [16]. The narrow genetic base of commercial maize varieties emphasizes the importance of conserving, understanding, and utilizing the genetic resources stored in gene banks [17]. NAGRC- Gene Bank of Nepal holds more than 500 maize landraces collected from different parts of the country [16]. Despite the vast genetic potential present in gene bank collections, the valuable variations remain largely untapped by plant breeders [17]. As local genotypes exhibit immense diversity, it is essential to utilize this diversity in mainstream breeding programs to develop new cultivars catering to the challenges of various biotic and abiotic stresses imposed by climate change in crop plants.
There is a large body of knowledge evaluating the performance of improved maize varieties. However, the evaluation of local maize genotypes in the face of drought stress is not explored in Nepal, highlighting the need for dedicated research in support of local genotypes. Although some studies have explored vegetative drought tolerance of local maize genotypes [18], the exploration of their reproductive drought performance has been an untapped topic in Nepal so far. This research aims to fulfill the existing knowledge gap by delving into their differential performance under drought stress and yield stability across changing soil moisture conditions. Considering the projected climate change, maintaining stable crop productivity will be increasingly vital for securing food security. Employing diverse multivariate statistical analyses across various genotypes cultivated in different conditions enables the identification of genotypes possessing desirable traits for incorporation into plant breeding initiatives [19]. Gaining knowledge about drought-tolerant genotypes is helpful in their utilization in modern breeding procedures, and such knowledge is thus important to plant breeders, conservationists, and policymakers.
2 Materials and methods
2.1 Experimental site
The research was conducted in a greenhouse present on the farm of the Institute of Agriculture and Animal Science, Lamjung Campus. Located in Sundarbazzar municipality of Lamjung district, the research site prevails in the mid-hill region of Nepal with an altitude of 610 m, latitude of 28.14°N, and longitude of 84.41°E.
2.2 Climatic conditions and growing season
The research was started in the spring of 2023 and extended up to the summer between mid-February and June 2023. The average temperature and relative humidity during the research period were 27.8 °C and 62.8% respectively. The monthly progression of temperature and RH of the research site throughout the experimental period are presented in Fig. 1.
2.3 Genotypes tested
A total of 20 accessions comprising local genotypes acquired from the National Agriculture Genetic Resources Centre (NAGRC), Khumaltar were tested in the experiment (Table 1). These accessions were collected from local farmers across different parts of Nepal by NAGRC for germplasm conservation. The vegetative drought tolerance performance of accessions NGRCO5571 and NGRC05589 has been highlighted by Tripathi et al. [18].
3 Research design and layout of the experiment
The experiment was conducted under Completely Randomized Design with two factors:
-
1)
Watering condition: (a) Well-watered throughout the crop period.
(b) Imposed drought condition in the reproductive stage
-
2)
Genotypes: 20 different maize accessions.
Each factor was replicated two times. The experiment was conducted in the planting bags of dimensions 24 × 24 × 40 cm.
Total genotypes used: 20
Watering conditions: 2
Total replications under each condition: 2
So, a total of 20 × 2 × 2, i.e. 80 planting bags were used in the study containing one plant in each bag.
3.1 Cultivation practices
3.1.1 Planting media
The planting media was prepared by thoroughly mixing soil (sandy-loam texture) and FYM in a ratio of 60:40. Each planting bag was filled to its capacity with media weighing 19 kg.
3.1.2 Nutrient management
By considering the national recommended fertilizer dose for maize crop i.e., 120:60:40 kg NPK/ha, the fertilizers required per plant were calculated to be 1.48 g Urea, 2.43 g DAP, and 1.24 g MoP for basal dose. For a split dose, 1.21 g of Urea was applied during knee-high and tasseling stages of growth at 35 and 60 DAS respectively.
3.1.3 Seeding
Two seeds were planted per bag on 14th February 2023. After seedling establishment, only one seedling was maintained per bag. The seeds were sown at a depth of 6 cm below the soil surface.
3.1.4 Water management
Two watering conditions were imposed upon the experimented plants i.e., well-watered condition by maintaining soil moisture at 65% of Field Capacity (FC) and drought condition by abstaining irrigation just after pollination for 12 days (3 irrigation cycles). The computation of water required for irrigation was done in the following manner:
Weight of the planting bag = 0.3 kg.
Weight of air-dried media = 19 kg.
Weight of oven-dried media = 15.70 kg.
Thus, in 19 kg of our planting media, the composition of soil solid is 15.70 kg, and that of soil water = 19–15.70 = 3.3 kg.
Weight of soil at FC = 24.12 kg.
Where, Soil solid = 15.70 kg.
And, Soil Water = 24.12–15.70 = 8.42 kg.
We have, soil water at FC = 8.42 kg.
So, soil water at 65% FC would be = 0.65 × 8.42 = 5.47 kg.
Therefore, the amount of water that must be added to bring our media to 65% FC would be:
Weight of soil water at 65% FC−Weight of water already prevailing in the media = 5.47–3.3 = 2.17 kg.
With the density of water being 1 g/cc, 2.17 L of water was initially added to the soil. So, the final weight of our grow bag media at 65% FC turned out to be 21.47 (2.17 + 19 + 0.3) kg. For subsequent irrigations, grow bags were weighed and the amount of water required to be added to make the final weight of the grow bag 21.47 kg was supplied.
3.2 Data collection and determination of grain yield
All the studied characters with the method of data recording are presented in Table 2. For the computation of grain yield, the moisture percentage of the grains of individual plants was taken by using a moisture meter. Fresh ear weight was taken by weighing machine. The following formula was employed for the computation of the grain yield:
where, MC = Moisture Content of the grains at harvest (in %), 0.8 = Shelling coefficient of maize, 12.5 = Desirable moisture percent of grains for storage.
Thus obtained value, measured in kg/plant was later converted to g/plant for statistical analysis.
3.3 Statistical analysis
The entry of data and visualization of interaction effect graphs were accomplished through MS Excel (2019). Analysis of variance, mean separation, and F-test were performed at 5% level of significance, and GGE biplot analysis was carried out to figure out G × E effects existing among the tested accessions under two test environments by employing R studio (4.3.0) with packages involving readxl, agricolae, ggplot2, and GGEBiplots.
4 Results
4.1 Comparison of maize accessions for traits
The analysis of variance revealed the existence of significant differences among the genotypes, environment and the interaction between the genotype and the environment for the characters studied (Table 3).
4.2 Mean performance of the genotypes, watering condition, and their interactions
4.2.1 Plant height
The interaction between genotypes and watering conditions was found to be significant. As demonstrated in Fig. 2, under irrigated condition, genotype NGRC05590 showed the greatest plant height, with a mean value of 270.50 cm, which was statistically at par with NGRC05592, and NGRC05583. Under drought condition, genotype NGRC05572 showed the greatest plant height with a mean value of 281 cm, statistically at par with NGRC05568, NGRC05582, NGRC05577, and NGRC05589. As for the main effects, the genotypes showed significant differences in terms of plant height. As illustrated in Table 4, plant height was found to be highest for the genotype NGRC05572 with a mean value of 265.25 cm, and lowest for the genotype NGRC05573 with a mean value of 184.25 cm. Similarly, in terms of watering condition, irrigated and drought conditions exhibited significant differences with mean values of 234.93 cm and 219.56 cm respectively.
4.2.2 Ear height
The interaction between genotypes and watering conditions was found to be significant. As demonstrated in Fig. 3, under irrigated condition, genotype NGRC05590 was found to incur the greatest ear height, with a mean value of 182.5 cm, followed by NGRC05592 and NGRC05583. Under drought condition, NGRC05568 was found to incur the greatest ear height with a mean value of 156.75 cm, which was statistically at par with NGRC05587 and NGRC05581. As for the main effects, the genotypes showed highly significant differences in terms of ear height. As illustrated in Table 4, ear height was found to be the highest for the genotype NGRC05590 with a mean value of 155.62 cm, which was statistically at par with NGRC05583, NGRC05568, and NGRC05587. Ear height was found to be the lowest for the genotype NGRC05573 with a mean value of 81.75 cm, which was statistically at par with NGRC05566. Likewise, for the watering condition, irrigated and drought conditions exhibited highly significant differences with mean values of 123.78 cm and 109.57 cm respectively. The lower plant height under drought condition was thus found to be corresponding with lower ear height in the crop plant.
4.2.3 Anthesis silking interval
The interaction between genotypes and watering conditions was found to be significant. As demonstrated in Fig. 4, under both the irrigated and drought conditions, genotype NGRC05577 was found to incur the lowest ASI, with a mean value of 0.5 days in both conditions. As for the main effects, the genotypes showed highly significant differences in terms of anthesis silking interval. As illustrated in Table 4, the number of days between anthesis and silking ranged from 7.75 days in NGRC05566, which was statistically at par with NGRC05590 to the lowest mean value of 0.50 days in the genotype NGRC05577. Contrastingly, no significant difference was found between the watering conditions in terms of anthesis silking interval, with irrigated and drought conditions exhibiting a mean value of 4.30 and 3.95 respectively.
4.2.4 Ear weight
The interaction between genotypes and watering conditions was found to be significant. As demonstrated in Fig. 5, under irrigated condition, genotype NGRC05571 exhibited the greatest ear weight with a mean value of 95.85 g, which was statistically at par with NGRC05592. Not so differently, under drought condition genotype NGRC05564 exhibited the greatest ear weight with a mean value of 55.21 g, which was statistically at par with NGRC05571, NGRC05569, NGRC05582, and NGRC05592. As for the main effects, the genotypes showed highly significant differences in terms of ear weight. As illustrated in Table 4, ear weight was found to be the highest for the genotype NGRC05571 with a mean value of 74.40 g which was statistically at par with NGRC05592. Ear weight was found to be the lowest for the genotype NGRC05576 with a mean value of 26.09 g. Likewise, for the watering condition, irrigated and drought conditions exhibited highly significant differences with mean values of 58.18 g and 37.49 g respectively.
4.2.5 Hundred grain weight
The interaction between genotypes and watering conditions was also found to be highly significant. As demonstrated in Fig. 6, under irrigated condition, genotype NGRC05575 exhibited the greatest HGW with a mean value of 27.14 g, which was statistically at par with NGRC05581, NGRC05562, NGRC05577, NGRC05583 and NGRC05592. Whereas, under drought condition, NGRC05568 exhibited the greatest HGW with a mean value of 22.91 g, which was statistically at par with NGRC05566, NGRC05592, and NGRC05571. Regarding main effects, the genotypes showed significant differences in terms of hundred grain weight (HGW). As illustrated in the Table 4, HGW was found to be the highest for the genotype NGRC05566 with a mean value of 24.36 g, which was statistically at par with NGRC05575, NGRC05564, NGRC05568, and NGRC05592, and the lowest for the genotype NGRC05565 with a mean value of 11.61 g. Likewise, for the watering condition, irrigated and drought conditions exhibited highly significant differences with mean values of 22 g and 16.44 g respectively.
4.2.6 Grain yield
The interaction between genotypes and watering conditions was found to be highly significant. As demonstrated in Fig. 7, under irrigated condition, genotype NGRC05571 was found to harbor the greatest yield per plant with a mean value of 67.49 g. Whereas under drought condition, genotype NGRC05592 exhibited the greatest yield per plant with a mean value of 41.63 g, statistically at par with NGRC05564, NGRC05582, and NGRC05571. Regarding main effects, the genotypes showed highly significant differences in terms of grain yield. As illustrated in Table 4, grain yield was found to be the highest for the genotype NGRC05571 with a mean value of 52.27 g per plant, which was statistically at par with NGRC05592. Grain yield was found to be the lowest for the genotype NGRC05576 with a mean value of 18.54 g per plant. As for the watering condition, irrigated and drought conditions exhibited highly significant differences with mean values of 41.82 and 28.52 g per plant respectively. The variability among the genotypes for the tested quantitative traits are also presented in the form of box-and-whisker plots in Fig. 8.
Comparative box-and-whisker plots explaining variability among the genotypes for the tested quantitative traits. The blue box designates a boxplot value under irrigated condition. The pink box designates a boxplot value under drought condition. The horizontal line inside the box indicates the median value. Whiskers extend to the largest and smallest observed values within the box lengths. Dots present beyond the whisker inside the boxplot, as prevalent in ASI represent outliers present in the dataset
4.3 GGE Biplot analysis
GGE Biplot Analysis was carried out to figure out G × E effects existing among the tested accessions under irrigated and drought environments.
4.3.1 Mean performance and the stability of the genotypes
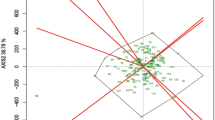
GGE biplot graphically contemplates the performance and the stability of the genotypes by using Average Environment Coordinates (AEC), which is the average of the first two principal components [20], displayed by the arrowhead as shown in Fig. 9. The performance line is denoted by the X-axis of the Average Environment Coordination (AEC) view, which passes through the origin of the biplot with an arrow depicting the positive end of the X-axis (abscissa). The Y-axis (ordinate) of the AEC pertains to the stability axis and is perpendicular to the AEC X-axis.
Average Environment Coordination (AEC) views of the GGE biplots for mean performance and the stability of the genotypes. Treatment numbers assigned in this figure are in accordance to accessions mentioned in Table 1
According to Yan et al. [21], the stability of the genotypes is measured based on their projections onto the AEC ordinate, whereas, the average yield of the genotypes is determined by their projections onto the AEC abscissa. The greater the distance of the genotype along the length of the AEC abscissa in the direction of the arrowhead, the higher the yield performance. On the contrary, the greater the distance of a genotype along the length of the AEC ordinate, the lower the stability. It was thus interpreted from Fig. 9 that genotypes 7 (NGRC05571) and 20 (NGRC05592) had the highest mean yields because of their position covering the largest length of abscissa in the direction of arrowhead (right), and genotype 11 (NGRC05576) had the lowest mean yield. However, between genotypes 20 and 7, the stable genotype was found to be 20. The mean yield of the genotypes followed the hierarchy of 7 > 20 > 3 > 2 > 14 > 6 > 15 > 5 > 12 > 8 > 16. The yield of the genotype 16 was close to the average yield. Genotypes 20, 3, and 6 were the above-average yielders with high stability, whereas, genotypes 7, 2, 15, and 8 were the above-average yielders with low stability. Genotype 11 was the below-average yielder with less stability. Stable genotypes have the least contribution to the variations imposed by genotype by environment interaction [22].
4.3.2 Winning genotypes and environment
The polygon view of a biplot is propounded as the optimal approach for comprehending the interconnection between genotypes and environments in a biplot, thus helping to extract meaningful insights. The polygon is illustrated by joining together the position of genotypes which are located farthest from the point of origin, such that all the other genotypes are encompassed within the polygon [23]. Genotypes at the vertices of the polygon are either the best or poorest in one or more environments. The genotype at the vertex of the polygon performs best in the environment falling within the sectors [23, 24]. As shown in Fig. 10, the 20 genotypes were categorized into 6 different sectors, with test environments falling under different sectors. The vertex genotypes distinguished by possessing the greatest distance from the origin of the biplot along their respective directions were found to be 2, 20, 7, 15, 9, and 11. On interpreting the polygon, genotypes 7 (NGRC05571) and 15 (NGRC05583) were the most responsive under irrigated environment, thus designated as the winning genotypes under irrigated environments. Under drought environment, genotype 20 (NGRC05592) was the most responsive genotype positioned in the vertex of the polygon and winning under drought environment. Genotypes 9 (NGRC05573) and 11 (NGRC05576) were the poor performers in both environments. The genotypes, that were located inside of the polygon and close to the origin of the biplot were not sensitive to changing environments [25].
4.3.3 Ranking genotypes relative to the ideal genotype
An ideal genotype is characterized by its ability to achieve high mean yield and high stability. The center of the innermost concentric circle (where the arrowhead is located) represents the position of the ideal genotype. A genotype becomes more preferable when it approaches the ideal genotype [26]. Thus, as demonstrated in Fig. 11, contrasting with the other genotypes, genotype 20, which was positioned near the center of the innermost concentric circle, was the ideal genotype both in terms of yield and stability. Genotype 20 (NGRC05592), thus could be used for broad selection due to relative superiority in both yield and stability [22]. Furthermore, positioned on the subsequent concentric circles, genotypes 3 (NGRC05565) and 14 (NGRC05582) could be considered the next best favorable choices. Genotype 7 (NGRC05571), despite achieving the highest mean yield in the ‘Mean vs Stability biplot’, has not shown convincing promise under drought condition as explained by the ‘Which-won-where biplot’, and thus has a prospect for irrigated environment only, because changing environment from irrigated to drought condition has adversely affected its grain yield. A similar approach of deciphering the ideal genotype based on the best yield and high stability was also inherited by Bishwas et al. [20], Nzuve et al. [22], Yan et al. [23], and Farshadfar et al. [26].
4.3.4 Ranking environments relative to the ideal environment
Similar to the earlier explanation, an ideal environment is positioned in the center of the innermost concentric circle. Thus, as shown in Fig. 12, the irrigated environment, being near to the position of the ideal environment, is more favorable to the tested genotypes as compared to the drought environment.
5 Discussion
Based on the results obtained from ANOVA, F-test, paired t-test, and box plots, this research suggests the presence of variability among the tested accessions of local genotypes of maize crop. Agro-morphological diversity associated with vegetative and reproductive traits in maize landraces has been detected by research done by various scientists in different parts of the world including Ghimire et al. [16], Tripathi et al. [18], Wasala et al. [27], and Craufurd et al. [28]. The results of ANOVA displayed significant variations among the genotypes in normal and drought stress conditions in almost all studied traits. This clearly indicates the genetic difference between the studied genotypes for drought tolerance [29]. Different crop varieties have different buffering capacities and exhibit contrasting retaliation towards their changing micro-climate like soil type, nutrient availability, and biotic and abiotic stresses [30]. Numerous studies have discovered variations in the resilience of genotypes of plant species against drought, including in Maize [15].
In the lifecycle of a crop, some growth stages are more vulnerable to the effects of moisture stress than the rest, and imposing drought on such critical stages will result in severe damage to the plant growth and the ultimate yield [31]. If cultivated under off-season rainfed conditions prone to recurring drought spells, a reduction in plant height is observed as compared to irrigated environments [32, 33]. When the plants face drought stress, the level of the stress hormone Abscisic Acid (ABA) culminates in them resulting in closing of stomata and premature kernel maturity. This reduces the photosynthetic activity of the plant resulting into plants with lower biomass and slower growth rate [34, 35], as demonstrated by the treatments subjected to drought condition in general. Also, a decrease in plant height under drought condition is attributed to a decline in cell enlargement, elongation, and division as well as higher leaf senescence [36]. This reduction in internode elongation and stomatal closure can also cause decreased ear height observed under drought.
In the context of the Anthesis Silking Interval (ASI), longer ASI is generally observed in plants under drought stress conditions if the moisture stress was subjected before silking, as that would result delay in silk emergence [37, 38]. However, in this experiment, since moisture stress was imposed during the critical stage of grain-filling which occurs after silk emergence and pollination, no significant difference was observed in relation to the changing moisture condition. However, among genotypes, the tested accessions were found to possess ASI ranging from 0 to 10 days (Table 5), which suggests the immense variability existing among local genotypes, providing scope for breeding intervention. As for the yield attributing traits, reduction in ear weight, hundred grain weight, and grain yield under drought condition can be supported by several explanations. Water shortages following anthesis reduce the volume of the embryo and delay the time of grain filling by producing premature endosperm desiccation. Additionally, drought stress shortens the effective grain-filling period, which lowers grain yield [39]. Furthermore, since the ears under drought stress are poorly filled with lower kernel number per cob than those grown in well-watered conditions [33], and are sometimes barren because of exhaustion of pollen grains before the appearance of silks [40], as well as abortion of young embryos on imposing moisture stress during grain filling stage [38], lower ear weight was exhibited by plants subjected to drought stress than the irrigated ones. In addition to that, reduction in the yield of the moisture-stressed plants is also attributed to the underdeveloped kernels, resulting in a potential reduction in the average kernel weight [33], which explains why the plants under drought stress showcased lower hundred grain weight and grain yield. Also, the increased ABA level causing stomatal closure results in decreased photosynthetic activity and limited accumulation of necessary carbohydrates for kernel development. So, decreased kernel set, reduced kernel size, shrunken ears, and premature kernel maturity can be seen as the prime effects of drought stress on maize production. Whereas, since irrigation during the milking stage has a positive impact on the kernel weight [32], the well-irrigated plants exhibited higher hundred grain weight, and consequently, better grain yield.
Selecting genotypes that are appropriate for breeding broadly adaptable cultivars is a more effective strategy than selecting genotypes for breeding locally adapted cultivars [19]. Owing to their yield and yield attributing performances reflected by grain yield, hundred grain weight and ear weight, accessions NGRC05592, NGRC05582, NGRC05564, NGRC05565, NGRC05571, and NGRC05569 have shown better promise under drought condition than other tested accessions. Accession NGRC05592 performed best under drought condition, whereas, NGRC05573 and NGRC05576 showed poor performance. While NGRC05571 shows a fairly good yield under drought stress and the highest mean yield, its stability, as implied by GGE biplots for grain yield indicated that changing environment from irrigated to drought condition adversely affects its yield performance. In this regard, accession NGRC05592, which shows second best mean yield performance, is the most stable accession under changing soil moisture levels performing best among all the tested accessions under drought condition. Accession NGRC05571 was predicted to be drought-tolerant from the findings based on vegetative drought performance entailing root-associated traits, relative water content, and normalized difference vegetation index (NDVI) values by Tripathi et al. [18]. On the contrary, the findings of this research suggest that accession NGRC05571 suffers a significant yield loss in changing environments from irrigated condition to drought condition. Hence, the yield potential shown by accession NGRC05571 under irrigated condition is unmatchable to the one under drought condition, which raises the question based on reproductive performance if the accession can be considered drought tolerant at all. It must however be noted that the interpretation from GGE biplots of this research is based on the data obtained from a single year of experiment, and thus serves as a frame of reference for future research endeavors associated with the stability analysis of these local accessions. Therefore, these short-listed accessions must be subjected to further studies in terms of their agronomic performance and stability under drought stress in field conditions across multiple years. Along with that, marker-assisted identification of their drought stress tolerance is encouraged before recommending them as the best options for cultivar development in regions prone to drought stress.
6 Conclusion
Drought stress presents one of the principal barriers among various abiotic stresses to enhancing crop productivity and meeting the yield potential of maize crops, adversely affecting various physiological, biochemical, and molecular mechanisms. Thus, for genetic improvement, identifying genotypes that show promising performance under drought conditions is one of the primary steps in developing drought-tolerant cultivars. Among the examined accessions, a significant level of genetic differences in response to different watering conditions was observed, with accessions NGRC05592, NGRC05582, NGRC05564, NGRC05565, NGRC05571, and NGRC05569 demonstrating relatively higher values for grain yield, ear weight, and hundred-grain weight, implying their potential to thrive under drought stress. However, on subjecting the yield performance of these accessions to GGE Biplots, accession NGRC05592 was found to be the winning genotype under drought stress, with minimal change in its yield performance on changing soil moisture levels from irrigated to drought conditions. On the contrary, accessions NGRC05573 and NGRC05576 have been identified as poor performers under drought condition. While accession NGRC05592 shows promise under drought condition in this research, further research efforts aiming at its stability under field conditions in diverse agro-climatic regions across different years must be carried out to reach a decisive point for its utilization in drought breeding programs.
Data availability
The datasets generated and analyzed in the current study are not publicly available for privacy reasons, but can be made available by the corresponding author on plausible request.
References
Singh C. Modern techniques of raising field crops. Delhi: Oxford and IBH publishing; 1983.
Govind KC, Karki TB, Shrestha J, Achhami BB. Status and prospects of maize research in Nepal. J Maize Res Dev. 2015;1:1–9.
Islam SMF, Karim Z. World’s demand for food and water: the consequences of climate change. In: Farahani MHDA, Vatanpour V, Taheri AH, editors. Desalination—challenges and opportunities. London: IntechOpen; 2020. p. 1–27. https://doi.org/10.5772/intechopen.85919.
Togbe VG, Bassarsky L, Gu D, Spoorenberg T, Zeifman L. World population prospects 2022. United Nations Publication. 2022;9:1–52. www.un.org/development/desa/pd/. Accessed 7 Mar 2024.
Francois E, Pedro M. Climate change: the IPCC’s latest assessment report. Quest. 2021;17:34–5. https://doi.org/10.10520/ejc-quest-v17-n3-a12.
Stocker TF, Qin D, Plattner GK. Technical Summary in Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. https://doi.org/10.1007/978-3-030-88654-7_40.
Harrison MT, Tardieu F, Dong Z, Messina CD, Hammer GL. Characterizing drought stress and trait influence on maize yield under current and future conditions. Glob Chang Biol. 2014;20:867–78. https://doi.org/10.1111/gcb.12381.
Irrigation Master Plan 2019. Government of Nepal, Ministry of Energy, Water Resources and Irrigation.
Zheng H, Shao R, Xue Y, Ying H. Water productivity of irrigated maize production systems in Northern China: a meta-analysis. Agric Water Manag. 2020;234:106119. https://doi.org/10.1016/j.agwat.2020.106119.
Du Plessis J. Maize production. South Africa: Department of Agriculture Pretoria. 2003.
Aslam M, Maqbool MA, Cengiz R. Drought stress in maize (Zea mays L.): effects, resistance mechanisms, global achievements. Berlin: Springer; 2015. https://doi.org/10.1007/978-3-319-25442-5.
Sellamuthu R, Dhanarajan A, Marimuthu R. Impact of drought stress on morphological and yield components in maize (Zea mays L.). Res J Biotechnol. 2022;17:77–85. https://doi.org/10.25303/1710rjbt77085.
Kamara AY, Menkir A, Badu-Apraku B, Ibikunle O. The influence of drought stress on growth, yield and yield components of selected maize genotypes. J Agric Sci. 2003;141:43–50. https://doi.org/10.1017/S0021859603003423.
Bolaños J, Edmeades GO. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. F Crop Res. 1996;48:65–80. https://doi.org/10.1016/0378-4290(96)00036-6.
Liu M, Li M, Liu K, Sui N. Effects of drought stress on seed germination and seedling growth of different maize varieties. J Agric Sci. 2015;7:231–40. https://doi.org/10.5539/jas.v7n5p231.
Ghimire KH, Joshi BK, Karkee A, Paudel MN. Assessing genetic diversity of Nepalese maize landraces using multivariate analysis. In: Proc 29th Natl Summer Crop Work 2018;3:105–14.
Popovic A, Polje Z, Prodanovic S. Characterisation and evaluation towards selection of maize. 2019. p. 2–12.
Tripathi A, Poudel R, Gurung R, Ghimire U, Kandel BP, Joshi BK. Drought tolerance screening of maize accessions at early growth stage in the mid-hills of Nepal. Cogent Food Agric. 2024. https://doi.org/10.1080/23311932.2024.2319157.
Mulugeta B. Multivariate analyses of Ethiopian durum wheat revealed stable and high yielding genotypes. PLoS ONE. 2022;17:1–21. https://doi.org/10.1371/journal.pone.0273008.
Bishwas KC, Poudel MR, Regmi D. AMMI and GGE biplot analysis of yield of different elite wheat line under terminal heat stress and irrigated environments. Heliyon. 2021;7: e07206. https://doi.org/10.1016/j.heliyon.2021.e07206.
Yan W, Kang MS, Ma B, Woods S, Cornelius PL. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 2007;47:643–55. https://doi.org/10.2135/cropsci2006.06.0374.
Nzuve F, Githiri S, Mukunya DM, Gethi J. Analysis of genotype × environment interaction for grain yield in maize hybrids. J Agric Sci. 2013;5:75–85. https://doi.org/10.5539/jas.v5n11p75.
Yan W, Kang MS. GGE biplot analysis: a graphical tool for breeders, geneticists, and agronomists. Boca Raton: CRC Press; 2002.
Oliveira TRA. Hybrid maize selection through GGE biplot analysis. Bragantia. 2019;78:166–74. https://doi.org/10.1590/1678-4499.20170438.
Rakshit S. GGE biplot analysis to evaluate genotype, environment and their interactions in sorghum multi-location data. Euphytica. 2012;185:465–79. https://doi.org/10.1007/s10681-012-0648-6.
Farshadfar E, Rashidi M, Jowkar MM, Zali H. GGE biplot analysis of genotype× environment interaction in chickpea genotypes. Eur J Exp Biol. 2013;3:417–23.
Wasala SK. Analysis of yield performance and genotype × environment effects on selected maize (Zea mays) landrace accessions of India. Indian J Agric Sci. 2013;83:287–93.
Craufurd PQ, Ellis AMRH, Sallah PYK. Phenotypic Diversity in Maize Landraces in Ghana. Int J Sci Adv Technol. 2012;2:39–70.
Chen X, Min D, Yasir TA, Hu YG. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). F Crop Res. 2012;137:195–201. https://doi.org/10.1016/j.fcr.2012.09.008.
Waluyo B. Stability and adaptability of castor hybrids. Indian J Agric R. 2010;32:293–301.
Poudel R. Effects of drought stress on growth and yield parameters of Zea mays—a comprehensive review. Agribus Manag Dev N. 2023;1:72–5. https://doi.org/10.26480/amdn.02.2023.72.75.
Çakir R. Effect of water stress at different development stages on vegetative and reproductive growth of corn. F Crop Res. 2004;89:1–16. https://doi.org/10.1016/j.fcr.2004.01.005.
Soler CMT, Hoogenboom G, Sentelhas PC, Duarte AP. Impact of water stress on maize grown off-season in a subtropical environment. J Agron Crop Sci. 2007;193:247–61. https://doi.org/10.1111/j.1439-037X.2007.00265.x.
Efeoǧlu B, Ekmekçi Y, Çiçek N. Physiological responses of three maize cultivars to drought stress and recovery. S Afr J Bot. 2009;75:34–42. https://doi.org/10.1016/j.sajb.2008.06.005.
Ghahfarokhi MG, Mansurifar S, Taghizadeh-Mehrjardi R, Saeidi M, Jamshidi AM, Ghasemi E. Effects of drought stress and rewatering on antioxidant systems and relative water content in different growth stages of maize (Zea mays L.) hybrids. Arch Agron Soil Sci. 2015;61:493–506. https://doi.org/10.1080/03650340.2014.943198.
Anjum SA, Xie X, Wang L, Saleem MF, Man C, Lei W. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res. 2011;6:2026–32. https://doi.org/10.5897/AJAR10.027.
Hall AJ, Vilella F, Trapani N, Chimenti C. The effects of water stress and genotype on the dynamics of pollen-shedding and silking in maize. F Crop Res. 1982;5:349–63. https://doi.org/10.1016/0378-4290(82)90036-3.
Herrero MP, Johnson RR. Drought stress and its effects on maize peproductive systems. Crop Sci. 1981;21:105. https://doi.org/10.2135/cropsci1981.0011183x002100010029x.
Ge T, Sui F, Bai L, Tong C, Sun N. Effects of water stress on growth, biomass partitioning, and water-use efficiency in summer maize (Zea mays L.) throughout the growth cycle. Acta Physiol Plant. 2012;34:1043–53. https://doi.org/10.1007/s11738-011-0901-y.
Lu Y. Large-scale screening for maize drought resistance using multiple selection criteria evaluated under water-stressed and well-watered environments. F Crop Res. 2011;124:37–45. https://doi.org/10.1016/j.fcr.2011.06.003.
Funding
The authors declare that no funds, grants, or other financial support were received during the design of the study and preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed in conception and design of the study. Material preparation and data collection were done by Rashmi Poudel, Anubhav Tripathi, Reema Gurung and Unisha Ghimire. Data analysis was performed by Rashmi Poudel, Bishnu Prasad Kandel, and Anubhav Tripathi. The first draft of manuscript was written by Rashmi Poudel. Proof reading and suggestions to address reviewers’ comments were provided by Ankur Poudel and Bishnu Prasad Kandel. All the authors read, commented and approved the final manuscript, and agree to be accountable for all aspects of the submitted work.
Corresponding author
Ethics declarations
Competing interests
The authors do not have any financial or non-financial interests to reveal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Poudel, R., Kandel, B.P., Poudel, A. et al. Performance evaluation and multivariate analysis of maize accessions against drought stress in Lamjung district, Nepal. Discov Agric 2, 30 (2024). https://doi.org/10.1007/s44279-024-00046-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44279-024-00046-x