Abstract
Key message
Bulked segregant analysis (BSA) using Affymetrix GeneChips revealed candidate genes underlying the major QTL for Phytophthora capsici resistance in Capsicum . Using the candidate genes, reliable markers for Phytophthora resistance were developed and validated.
Abstract
Phytophthora capsici L. is one of the most destructive pathogens of pepper (Capsicum spp.). Resistance of pepper against P. capsici is controlled by quantitative trait loci (QTL), including a major QTL on chromosome 5 that is the predominant contributor to resistance. Here, to maximize the effect of this QTL and study its underlying genes, an F2 population and recombinant inbred lines were inoculated with P. capsici strain JHAI1-7 zoospores at a low concentration (3 × 103/mL). Resistance phenotype segregation ratios for the populations fit a 3:1 and 1:1 (resistant:susceptible) segregation model, respectively, consistent with a single dominant gene model. Bulked segregant analysis (BSA) using Affymetrix GeneChips revealed a single position polymorphism (SPP) marker mapping to the major QTL. When this SPP marker (Phyto5SAR) together with other SNP markers located on chromosome 5 was used to confirm the position of the major QTL, Phyto5SAR showed the highest LOD value at the QTL. A scaffold sequence (scaffold194) containing Phyto5SAR was identified from the C. annuum genome database. The scaffold contained two putative NBS-LRR genes and one SAR 8.2A gene as candidates for contributing to P. capsici resistance. Markers linked to these genes were developed and validated by testing 100 F1 commercial cultivars. Among the markers, Phyto5NBS1 showed about 90 % accuracy in predicting resistance phenotypes to a low-virulence P. capsici isolate. These results suggest that Phyto5NBS1 is a reliable marker for P. capsici resistance and can be used for identification of a gene(s) underlying the major QTL on chromosome 5.
Similar content being viewed by others
Introduction
Pepper (Capsicum spp.) is an economically important vegetable crop worldwide. Pepper production is challenged by various plant pathogens, and developing cultivars exhibiting multiple disease resistance requires time-consuming gene pyramiding processes. The soil-borne pathogen Phytophthora capsici L. is the causal agent of root rot, stem rot and foliar blight in pepper (Hord and Ristaino 1991; Kim et al. 1989). P. capsici reproduces both sexually and asexually and is a multicyclic disease agent, living on both dead and live plants, as a necrotroph and biotroph, respectively (Bonnet et al. 2007). Under environmental conditions favorable to the pathogen, P. capsici can cause up to 100 % yield loss in tropical and subtropical regions. Although resistance to P. capsici is affected by the virulence of different isolates (Foster and Hausbeck 2010; Jo et al. 2014), the existence of distinct races remains controversial (Oelke et al. 2003).
Numerous sources of resistance against P. capsici have been identified in the cultivated species C. annuum, including Criollo de Morelos 334 (CM334), PI201232, PI201234 and AC2258, among which CM334 shows consistently high levels of resistance (Bartual et al. 1991; Oelke et al. 2003). Phytophthora resistance is often characterized as polygenic or quantitative, although simple inheritance models have been proposed. The inheritance patterns of resistance against P. capsici vary depending on disease screening conditions and isolates of P. capsici. For instance, Reifschneider et al. (1992) proposed a two-gene model for resistance after observing resistance inheritance in an F2 population inoculated with P. capsici at 5 × 104 zoospores/mL. By contrast, single dominant gene inheritance was observed when plants were inoculated with 2,000 zoospores/mL (Sy et al. 2005; Walker and Bosland 1999). Indeed, the introduction of resistance into elite susceptible cultivars has been achieved using simple backcrosses in breeding programs of Korean seed companies and many P. capsici-resistant hot pepper cultivars are available (Lee et al. 2012), suggesting that the inheritance of resistance can be treated as simple Mendelian genetics for practical breeding applications.
In recent years, research efforts have emphasized identification and mapping of quantitative trait loci (QTLs). QTL analyses have been carried out for P. capsici resistance in several intraspecific populations (C. annuum × C. annuum) by different research groups (Nahm 2001; Ogundiwin et al. 2005; Thabuis et al. 2003), and the results suggest that P. capsici resistance is governed by multiple complex quantitative traits. However, the genomic distribution of the QTLs for P. capsici resistance is not random. Despite the fact that data are drawn from different populations subjected to different pathogen isolates, disease screening approaches, and scoring methods, there is a strong tendency for QTLs to aggregate, particularly on chromosome 5. Lefebvre and Palloix (1996) identified one major QTL on chromosome 5 explaining 41–55 % of the phenotypic variance among 13 total QTLs detected. Liu (2006) further summarized QTL studies from different research groups for P. capsici resistance in pepper (Nahm 2001; Ogundiwin et al. 2005; Thabuis et al. 2003, 2004). The most consistently identified QTLs were those on chromosome 5. Out of 67 QTLs belonging to seven QTL groups according to the original studies, 18 were simultaneously aligned on the middle-to-lower arm of P5 on the integrated pepper map, with five to eleven QTLs located on each of four other chromosomes including chromosome 6, 9, 11 and 12. In most cases, reported LOD scores for 18 QTLs on chromosome 5 ranged from 2.28 to 35.69 (Liu 2006). This observation is consistent with recent QTL analysis (Minamiyama et al. 2007) showing that the QTL located on chromosome P5 accounts for the major contribution to Phytophthora resistance (Bonnet et al. 2007; Mallard et al. 2013; Truong et al. 2012).
Despite many QTL studies and identification of the common major QTL for Phytophthora resistance, there is no useful marker for marker-assisted selection (MAS) of Phytophthora resistance in Capsicum. Application of MAS in plant breeding requires markers closely linked to the target locus to be effective for application to a large number of samples and a wide range of crosses in different breeding programs. A sequence-amplified characterized region (SCAR) marker developed from random amplified polymorphic DNA (RAPD) marker OpD04.717 was mapped within 6 cM of the peak of a major QTL located on chromosome P5 in the pepper genetic map AC99 (Quirin et al. 2005). Other Phytophthora resistance-linked markers include bacterial artificial chromosome (BAC)-derived markers converted from restriction fragment length polymorphism (RFLP) markers CDI25 and CT211A, targeting QTLs on chromosome P5 and P9, respectively (Kim et al. 2008). However, these markers are not suitable for MAS in practical breeding programs due to lack of polymorphism between breeding lines or low accuracy in predicting resistance phenotypes.
Polymorphic molecular markers based on single nucleotide polymorphisms (SNPs) in DNA sequences are extremely sensitive and can identify individuals within a population. Accordingly, SNPs are believed to represent the most promising marker system for MAS, especially for crop improvement using elite germplasm (Michelmore et al. 1991). The Affymetrix GeneChip microarray provides a high-throughput platform for discovery of single position polymorphisms (SPPs). In this approach, DNA polymorphism is detected from differential hybridization signals of genomic DNA from the tested samples. Bulk segregant analysis (BSA), which has been widely adopted for rapid identification of molecular makers in specific regions of a genome, is based on the underlying principle that bulking of individuals from a segregating population into pools of alternative phenotypes allows the capture of representative genotypes at a particular locus while a random genetic background is generated at all other unlinked loci (Michelmore et al. 1991). Although the combined use of BSA and microarrays could represent a powerful approach for the discovery of genetic makers, there are only few examples of using these tools together to analyze traits in crop plants due to the high cost and the lack of availability of arrays (Kloosterman et al. 2010; Sherman et al. 2013).
The objectives of this study were: (1) to develop SNP markers tightly linked to the major QTL located on pepper chromosome 5 using a combined BSA and SPP (BSA–SPP) approach, (2) to develop and validate SNP markers applicable for marker-assisted selection of P. capsici resistance, and (3) to perform sequence analysis of the major QTL region to reveal candidate genes for contributing to P. capsici resistance.
Materials and methods
Plant materials and DNA extraction
The YT population, consisting of 128 recombinant inbred lines (RILs) at the F8 generation of an intraspecific cross between C. annuum YCM334 and Tean, was used for inheritance analysis and marker development (Truong et al. 2012). YCM334, derived from CM334, served as the resistant parent and the Korean landrace Tean as the susceptible parent. Two hundred F2 segregants were derived from self-pollination of commercial F1 cultivar C. annuum cv. ‘Ildangbaek (IDB)’ (Syngenta Korea Co., Ltd., Korea). This population was used to develop molecular markers linked to the major QTL for P. capsici resistance. The AC99 F2 population originating from an interspecific cross between C. annuum NuMex R Naky (RNaky) and C. chinense ‘PI159234’ (CA4) was used to determine the location of markers (Livingstone et al. 1999). Genomic DNA was extracted with the hexadecyl trimethyl ammonium bromide (CTAB) method from young leaf tissue as described by Yang et al. (2012).
Phytophthora root rot resistance screening and phenotyping
Phytophthora capsici isolate JHAI1-7 was used as inoculum for Phytophthora root rot resistance screening. JHAI1-7 was isolated from infected pepper plants in the pepper field in Chungbuk province in Korea and was provided by Dr. Heung Tae Kim. JHAI1-7 is considered to have medium virulence (Jo et al. 2014). To screen for resistance, plants at the six-to-eight leaf stage were tested by applying 5 mL inoculum adjusted to a concentration of 3 × 103 zoospore/mL. Ten plants from each RIL were used. To avoid disease escape, plant materials were grown in 50-hole trays and kept in a greenhouse where the temperature was controlled at 25 °C, with 12 h light/day. Resistance and susceptibility were scored based on severity of wilting at 7–10 days post-inoculation (dpi) using the following index: 0 = symptomless, 1 = mild wilt in leaves or stems (<25 % of wilt), 2 = severe wilt in leaves or stems (<50 % of wilt), 3 = death. The 0 score was classified as resistance and scores 1–3 as susceptibility (Fig. S1).
Microarray analysis
The pepper SNP genotyping array was purchased from Affymetrix (http://www.affymetrix.com/catalog/prod660100/AFFY/Pepper-(Capsicum)-SNP-Genotyping-Array#1_1). A BSA strategy was used to prepare genomic DNA pools for microarray hybridization. For BSA, equal amounts of genomic DNA from 20 resistant lines and 20 susceptible lines selected from YT RILs were bulked, separately. The bulked DNA (30 μg/array) was randomly fragmented with DNaseI. End labeling of fragmented DNA and hybridization for comparative hybridization were performed according to the modified Affymetrix protocol (Hill et al. 2013). The labeled DNA pools from resistant and susceptible bulks were hybridized on different arrays and hybridization was performed four times for each DNA pool. Probe signals were processed and analyzed based on a non-uniform drop in signal intensity for individual probes within a probe set (Li and Durbin 2009). The R package (http://www.bioconductor.org/) was used to identify SPPs showing a Dstat value of ≥3 or ≤−3 (Borevitz et al. 2003; Gore et al. 2007). EST sequences corresponding to the selected SPPs were identified from the first version C. annuum genome database (http://peppergenome.snu.ac.kr).
SNP marker polymorphism survey
A total of 44 SNP markers located on chromosome 5 were used for polymorphism survey between two parental lines, YCM334 and TEAN. First, 17 intron-based SNP markers were surveyed (Park et al. 2014). Primers were designed based on 30 EST sequences containing SPP probes on chromosome 5 (Hill et al. 2013). SNP marker polymorphisms were surveyed by high-resolution melting (HRM) analysis using a Rotor-Gene™ 6000 thermocycler (Qiagen, Germany). PCR was carried out in 20-μL reaction volumes with 50 ng genomic DNA as template, 1× HiPi buffer (ELPIS-Biotech, Korea), 0.2 mM dNTPs, 500 mM each forward and reverse primers (Bioneer, Korea), 1.5 μM SYTO9 (Invitrogen, USA), 0.6 unit home-made Taq DNA polymerase (Desai and Pfaffle 1995). PCR cycling conditions were 95 °C for 4 min, followed by 95 °C for 20 s, 58 °C for 20 s, and 72 °C for 30 s for 45 cycles. Melting curve analysis was performed after PCR termination, and the temperature was ramped from 70 to 90 °C, raised by 0.1 °C per second.
Mapping, linkage and QTL analysis
Single nucleotide polymorphisms (SNP) markers showing polymorphism between the two parental lines were used for linkage and QTL analyses (Table S1). Linkage analysis was performed using CarthaGene software 1.0 (De Givry et al. 2005) and the Kosambi function was used to convert recombination values to genetic distances with a LOD score threshold of 5.0 and a maximum distance of 30 cM. QTL analysis was performed by composite interval mapping (CIM) using QTL Cartographer version 2.5 (Wang et al. 2007) with the forward and backward stepwise regression method. A standard CIM model (8.0 cM walk speed and 5 control markers) was used for control parameters. The threshold value for the QTL significance was determined by permutation test (1,000 replicates) at P < 0.05 significance. For detection of QTLs for Phytophthora root rot resistance, the calculated threshold of LOD scores was set at 11.5.
Genome analysis
DNA sequences obtained from Phyto5SAR located in the QTL region were used for BLAST (blastn) analysis in the C. annuum genome database (http://peppergenome.snu.ac.kr) to identify the corresponding scaffold sequences of the pepper genome. Putative genes were then predicted from scaffold194 sequence using FGENESH (http://linux1.softberry.com) and annotated using BLAST (blastx).
Development and validation of SNP markers using pepper genome sequence
Molecular markers located in scaffold194 were developed for further analysis and validation (Table S1). Four P. capsici-resistant accessions (AC2258, YCM334, CM334 and Perennial) and four susceptible accessions (Tean, Jejujaerae, ShinJoGwang and OngGoalChan) were used for sequence comparison to find SNPs between the resistant and susceptible accessions. DNA extracted from each accession was used as a template for PCR. PCR products were cloned into T-Blunt vector (SolGent, Daejeon, Korea) and sequenced at the National Instrumentation Center for Environmental Management (Seoul National University, Korea). The DNA sequences were assembled and compared using the SeqMan program (DNASTAR, Inc., USA). For validation, the developed SNP markers (Phyto5NBS1, Phyto5NBS2_1 and Phyto5NBS2_2) were tested using 100 commercial F1 hybrid cultivars provided by seven seed companies (NONGWOO BIO Co., Ltd., KOREGON Co., Ltd., Takii Korea Co., Ltd., Sakata Korea Co., Ltd., Syngenta Korea Co., Ltd., Monsanto Korea Co., Ltd. and NONGHYUP R&D Center). For Phytophthora root rot resistance screening, four P. capsici isolates with different levels of virulence were used. The four P. capsici isolates included MY-1 (lowest virulence), KPC-1 and JHAI1-7 (medium virulence), and KPC-7 (highest virulence). Phytophthora root rot resistance screening was performed at the Screening Center for Disease Resistant Vegetable Crops (Korea Research Institute of Chemical Technology, Korea). Resistance and susceptibility were scored based on the percentage of plant wilting after inoculation [0–25 % = resistance (R), 26–100 % = susceptibility (S)].
Results
Screening and phenotyping for Phytophthora root rot resistance
Classical inheritance studies indicated that P. capsici resistance sources in pepper are polygenic; however, single-gene, two-gene and three-gene-models have been also proposed (Reifschneider et al. 1992; Sy et al. 2005; Walker and Bosland 1999). In this study, we hypothesized that Phytophthora resistance in pepper acts as a monogenic trait with resistance being dominant over susceptibility under low disease-pressure conditions, for which we utilized a low intensity inoculation strategy with the Phytophthora concentration adjusted to 3 × 103 zoospore/mL. A qualitative phenotyping method was used where index 0 (without any symptoms) was classified as resistance (R) and disease indexes of 1, 2 and 3 were classified as susceptibility (S). A total of 200 plants from an F2 population derived from a commercial F1 hybrid pepper (Ildangbake) were subjected to Phytophthora root rot screening, resulting in a segregation ratio of 149:45 (3.3:1, R to S), which fits a 3:1 segregation model (X 2 = 0.3367, P > 0.05; Fig. 1a). A segregation ratio of 65:78 (1:1.2, R to S) was obtained for resistance screening of the YT RIL population. This ratio fits a 1:1 segregation model (X 2 = 1.1818, P > 0.05; Fig. 1b). These results indicate that under low disease-pressure conditions, the inheritance of Phytophthora root rot resistance in pepper follows a simple Mendelian inheritance pattern in the tested populations, behaving as a single dominant trait.
Frequency distribution of resistance to P. capsici strain JHAI1-7 displaying medium virulence. The phenotype index was scored with resistance (R) as 0 and susceptibility (S) ranging from 1 to 3. a The ‘Ildangbaek’ F2 population showed a 3:1 (R:S) segregation ratio. b YCM334 × TEAN F8 RILs showed a 1:1 (R:S) segregation ratio. The parental lines YCM334 and TEAN show phenotype indexes of 0 and 3, respectively
Bulk segregation analysis using microarrays and marker development
We combined a BSA approach with SPP analysis using microarray technology to develop molecular markers linked to the Phytophthora resistance exhibiting simple inheritance described above. RILs derived from progeny of a cross between P. capsici-resistant YCM334 and -susceptible TEAN were used for the BSA. Based on a monogenic inheritance hypothesis, the resulting resistant RILs are expected to be homozygous for a resistance allele inherited from YCM334, whereas the susceptible RILs should be homozygous for the susceptibility allele from TEAN. In theory, probes genetically linked to the Phytophthora resistance locus are expected to show signal differences between the resistant and susceptible trait pools in BSA–SPP-based microarray analysis. Using this approach, a total of 65 SPPs with Dstat values ≥3 or ≤−3 representing ten different candidate EST-based unigenes were identified (data not shown). Eight of the ten unigenes originated from P. capsici, possibly due to contamination during pepper RNA preparation after P. capsici infection, and these genes were excluded from further analysis. The two plant-derived EST sequences, CAPS.CONTIG.11101 and CAPS.CONTIG.3667, were annotated as receptor-like protein kinase and SAR82A gene promoter region, respectively. We considered these two genes as candidates to be linked to the Phytophthora resistance locus and used them for SNP marker development. We performed BLAST searches against the first version of the C. annuum genome database (http://peppergenome.snu.ac.kr) to obtain scaffold sequences. CAPS.CONTIG.11101 matched scaffold CAW8S_Contig049087 and CAPS.CONTIG.3667 matched CAW8S_Contig002466. CAW8S_Contig049087 are located on chromosome 11 (Livingstone et al. 1999), whereas CAW8S_Contig002466 is located on chromosome 5. Since previous QTL studies on Phytophthora resistance in pepper indicated that the major QTL is located on chromosome 5 (Quirin et al. 2005; Truong et al. 2012), three SNP markers (7890upSNP_2, SNP002466_2 and Phyto5SAR) were developed using CAW8S_Contig002466 for further analysis. For example, 120-bp DNA fragments containing SPP in CAPS.CONTIG.3667 were obtained from YCM334 and TEAN. In the sequence alignment of the 120-bp DNA fragments, SNPs were found at positions 77, 92 and 93. Based on these SNPs, an HRM marker was developed and named Phyto5SAR (Fig. S2A).
Mapping P. capsici resistance-linked SNP markers on chromosome 5
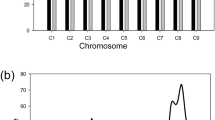
A total of 17 SNP markers located on chromosome 5 were used for QTL analysis. Among these were three markers (7890upSNP_2, SNP002466_2 and Phyto5SAR) derived from CAW8S_Contig002466, nine markers (CONTIG9303, 907783, CONTIG1896, CONTIG1820, CONTIG6473, CONTIG5473, KS14035C05, KS17024G04 and KS17054F01) developed from EST sequences and five intron-based markers (IB1556, IB1558, IB1429, IB1119 and IB834). A total of 128 YT RILs were genotyped using this set of markers, and the results were used to construct a molecular linkage map of chromosome 5. The linkage map length was 131 cM, with 7.70 cM on average between markers (Fig. 2a, b). A QTL peak spanning 18.8 cM flanked by two markers, CONTIG6473 and CONTIG1896, was detected for P. capsci resistance. The QTL region contained nine markers (CONTIG6473, 7890upSNP_2, SNP002466_2, Phyto5SAR, CONTIG1820, 907783, IB1556, IB1558 and CONTIG1896) at map positions between 14.8 and 33.6 cM (Fig. 2a, b; Table 1); primer sequences for the markers are shown in Table S1. The LOD scores within the interval ranged from 14.98 to 30.14 (Fig. 2a, b; Table 1). Phyto5SAR, with a LOD score of 30.14 and accounting for up to 67.68 % of the phenotypic variance, was identified as the most closely linked marker to the major QTL for Phytophthora root rot resistance in this study (Fig. 2a, b; Table 1).
Local genetic linkage map showing the major QTL and the identification of a scaffold located in the major QTL region. a Molecular linkage map and chromosomal locations of the major QTL for P. capsici resistance. The linkage map of chromosome 5 was constructed using the YT RIL mapping population. QTLs detected by composite interval mapping analysis are represented by a solid line. The LOD scale is shown on X axis, while genetic distance (cM) on chromosome 5 is represented on Y axis. (B) Genetic map including the major QTL region between markers 7890upSNP_2 and contig1896 (22.9–33.6 cM). The LOD score of Phyto5SAR is the highest. c Scaffold194 of CM334 from the C. annuum BAC Database (http://peppergenome.snu.ac.kr) in which PhytoSNP5 is located. Newly developed SNP Markers (shown in black text; Phyto5NBS1, Phyto5NBS2_1, and Phyto5NBS2_2) co-segregated with Phyto5SAR. Putative resistance genes against P. capsici, two NBS-LRR genes and SARS.2A, are indicated in red
Genomic sequence analysis of the major QTL on chromosome 5
Further genomic analysis was carried out for the major QTL interval between markers 7890upSNP_2 and CONTIG1896, covering from 22.9 to 33.6 cM on chromosome 5. This interval region was found to explain more than 45 % of the phenotypic variance in Phytophthora resistance (with R 2 ranges from 46.80 to 67.68 %). Sequences of the markers within the region were used as templates to perform BLAST searches in the C. annuum genome database to identify the corresponding scaffold sequences. A total of five scaffolds (scaffold194, scaffold1314, scaffold1306, scaffold419 and scaffold619) were identified. Scaffold194 matched the sequences of three markers (7890up SNP_2, SNP002466-2 and Phyto5SAR) showing the highest LOD in composite mapping analysis. This result suggests that target genes for P. capsici resistance in the major QTL region may be located within scaffold194. More than 500 putative genes were predicted in scaffold194 by the FGENESH program. Among them, 44 putative genes were predicted to be related to disease resistance, including CC-NBS-LRR class genes [R2, recognition of peronospora parasitica (RPP) 8 and RPP13], RLK class genes (cysteine-rich receptor-like protein kinase 8), systemic acquired resistance 8.2A (SAR8.2A) and AVRRPT2-INDUCED GENE 1 (AIG1) (Table S2). However, all candidate genes except two NBS-LRR genes and SAR8.2A were predicted to be pseudogenes. The two NBS-LRR candidate genes, located at 395.9 and 2961.1 kb of scaffold194, were predicted to have the complete NBS-LRR gene structure with a single exon (Fig. 2c; Table S2). SRA8.2A, at 2058.7 kb of scaffold194, and its homologs are highly expressed in P. capsici-infected pepper (Lee and Hwang 2003). These results suggest that the two putative NBS-LRR genes and SAR8.2A are strong candidates for underlying Phytophthora resistance.
Development and validation of SNP markers for Phytophthora root rot resistance
BLAST results against the C. annuum genome database indicated that two copies of the Phyto5SAR sequence (Fig. S2A) were present in different scaffolds. To develop single-copy molecular markers using genomic sequences of the major QTL, single-copy sequences were obtained and examined for polymorphisms between the four resistant (AC2258, YCM334, CM334 and Perennial) and four susceptible accessions (Tean, Jejujaerae, ShinJoGwang and OngGoalChan). The Phyto5NBS1, Phyto5NBS2_1 and Phyto5NBS2_2 markers indicated polymorphism between the resistant and susceptible accessions. Phyto5NBS1 contained a SNP (A in resistant and G in susceptible) at position 43 (Fig. 3a), and when Phyto5NBS1 was tested for HRM analysis, the curves for each genotype (resistant, heterozygous, susceptible) were clearly distinguishable (Fig. 3b). Phyto5NBS2_1 indicated two SNPs between resistant and susceptible accessions. The first SNP was used for development of a CAPS marker because only susceptible cultivars had a MluCI restriction enzyme site (Fig. S2B). Phyto5NBS2_2 indicated SNPs at positions 61 and 64 between resistant and susceptible accessions (Fig. S2C), and HRM analysis using that marker also generated clear curves for each genotype (resistant, heterozygous, susceptible).
Sequence and HRM curves of the Phyto5NBS1 marker. a Comparison of Phyto5NBS1 sequence from four P. capsici-resistant cultivars and four P. capsici-susceptible cultivars amplified using the Phyto5NBS1 primers indicated by arrows. One SNP found between the resistant and susceptible cultivars is highlighted with a rectangle. b HRM curves for Phyto5NBS1. Green susceptible homozygote, Red resistant homozygote, Blue heterozygote
To validate these markers for use in monitoring Phytophthora root rot resistance, we genotyped 100 commercial F1 cultivars with the three markers and compared their resistance to P. capsici isolates with different virulence (MY-1: lowest virulence, KPC-1 and JHAI1-7: medium virulence, KPC-7: highest virulence) (Jo et al. 2014). Among the 100 F1 cultivars, cultivars 1–59 are labeled as resistant cultivars (Table S3). In general, phenotypes tested using P. capsici isolates exhibiting lower virulence (for example, MY-1) were better matched to the genotypes of the three markers compared with those tested using P. capsici isolates exhibiting higher virulence (for example, KPC-7). Among the markers tested, the Phyto5NBS1 genotypes were better matched with resistance phenotypes than were those of Phyto5NBS2_1 and Phyto5NBS2_2 (Table S3). The relationships between the Phyto5NBS1 marker genotypes and P. capsici isolates with different virulence are shown in Fig. 4. The Phyto5NBS1 genotyping results showed that 58 cultivars were heterozygous or homozygous for the resistance allele, whereas 42 were homozygous for the susceptible allele (Fig. 4; Table S3). When MY-1 (with the lowest virulence) was tested, 59 cultivars were resistant and 41 cultivars were susceptible. In marker genotype and phenotype comparisons, 91 out of 100 genotypes corresponded to the correct disease phenotype. However, when KPC-1 and JHAI1-7 (with medium virulence) were used, 76 and 73 out of 100 genotypes, respectively, matched the disease phenotype. For the KPC-7 isolate (with the highest virulence), only 54 out of 100 genotypes matched the disease phenotype (Fig. 4; Table S3). These results suggest Phyto5NBS1 is a reliable marker for predicting phenotypes of resistance to Phytophthora when less virulent Phytophthora isolates are used.
Validation of Phyto5NBS1 marker by testing 100 commercial F1 hybrids. X axis shows P. capsici isolates with different virulence (MY-1: lowest virulence, KPC-1 and JHAI1-7: medium virulence, KPC-7: highest virulence). Y axis indicates the degree of disease incidence (from 0 to 100). 0–25 and 26–100 are considered to represent resistance (R) and susceptibility (S), respectively. For the genotyping with the Phyto5NBS1 marker, black triangles indicate susceptible homozygous genotypes and red bars indicate resistant homozygous or heterozygous genotypes
Discussion
Our study establishes the main QTL linked to Phyto5NBS1 on chromosome 5 as a key genomic position in Capsicum for resistance to P. capsici. We hypothesized that this position may represent the major QTL found in quantitative resistance studies or the major gene identified in classical inheritance studies (Bradshaw et al. 2006; Huang et al. 2005; Reifschneider et al. 1992; Sy et al. 2005). By adjusting the inoculum concentration of a P. capsici strain with medium virulence, we were able to obtain 1:1 and 3:1 (R:S) segregation ratios in a RIL population and F2 segregating population, respectively. This experimental set-up allowed us to apply a BSA strategy accompanied with SPP analysis using Affymetrix GeneChips, based on the assumption that resistant bulk and susceptible bulk samples contained the resistant and susceptible alleles, respectively, as well as their corresponding linked genomic factors at the target locus. As a result, we were able to develop P. capsici-resistance-linked SNP markers by comparing the extracted DNA from resistant and susceptible bulk samples in the microarray analysis.
Our results demonstrate that SNP markers can be widely used for Phytophthora resistance breeding programs. SNP markers overcome the major limitation of AFLP- and RAPD-derived markers developed from previous studies, where there was often a lack of polymorphism in working breeding populations (personal communications with breeders). Specifically, we demonstrated that Phyto5NBS1 can clearly distinguish susceptible genotypes from resistant genotypes, allowing flexibility for breeders to introgress P. capsici resistance into their materials from different resistance sources. As demonstrated using the commercial breeding materials and F1 hybrids, Phyto5NBS1 was closely associated with Phytophthora root rot resistant/susceptible traits for P. capsici strains with low virulence. When P. capsici strains with higher virulence were applied, the association between Phyto5NBS1 and Phytophthora root rot resistance tended to decline, but the association between Phyto5NBS1 and Phytophthora root rot susceptibility remained unaffected. This is likely caused by the increased virulence, which may overcome the resistance conferred by the major QTL linked to Phyto5NBS1. Other QTLs on chromosome 6, 9, 11 and 12 are also important for resistance against P. capsici with higher virulence.
NBS-LRR and RLK type genes are key resistance genes (R genes) in plants and are often found in clusters at specific regions (Meyers et al. 2003). The major QTL is found in scaffold194, which is considered to be a disease resistance hotspot where 8 and 34 genes are annotated as NBS-LRR type and RLK type R genes, respectively. However, except for two NBS-LRR genes, all of the annotated genes lack functional domains for disease resistance, and are thus considered pseudogenes. The two genes located at 395.9 and 2961.1 kb (Fig. 2c) have intact NBS-LRR structures, suggesting that they are strong candidate genes for P. capsici resistance. The association between a major QTL and R genes for P. capsici resistance in pepper is not surprising given that many studies have reported that NBS-LRR R genes are observed as QTLs or dominant resistance genes. For instance, the R gene RB/Rpiblb1 has been demonstrated to be a major QTL accounting for 62 % of the genetic variation in progeny populations derived from a Phytophthora-resistant source (Naess et al. 2001; Song et al. 2003). C. annuum SAR8.2A is also located within the target locus in scaffold194 (Fig. 2c). SAR8.2 is a type of systemic acquired resistance (SAR)-related gene and is induced both locally and systemically in pepper plants inoculated with virulent and avirulent strains of Xanthomonas campestris and Pseudomonas fluorescens (Choi and Hwang 2011), suggesting that SAR8.2A is another strong candidate gene for Phytophthora root rot resistance in pepper. Recent studies have shown that NBS-LRR-linked and -unlinked loci can function together to confer disease resistance against pathogens. For example, RPP2A and RPP2B function in Arabidopsis thaliana against oomycete isolates, and wheat Lr10 and RGA2 confer resistance against fungal pathogen (Eitas and Dangl 2010). Emerging studies also indicate that NBS-LRR proteins and SAR might be functionally associated in plant defence responses (Bonardi et al. 2011). Recently, Rehrig et al. (2014) carried out QTL analysis for P. capsici resistance using RILs derived from the resistant accession CM334 and the susceptible accession Early Jalapeño. They positioned the major QTL in the similar region of chromosome 5 as we did. However, the predicted candidate gene was different. They predicted DOWNY MILDEW RESISTANT 1 homolog as a strong candidate gene. Therefore, we cannot rule out a possibility that more than one gene may contribute to the major effect observed on chromosome 5 as we described above. For the validation of candidate genes in two studies, further works are required.
Previous comparative genetic analysis of disease resistance in the Solanaceae has suggested that disease resistance QTLs and major (typically single dominant) R genes are not randomly distributed in the genomes of pepper, potato (Solanum tuberosum) and tomato (Solanum lycopersicum) (Grube et al. 2000). In fact, clustering of R genes and QTLs has also been discussed in other plant species including rice (Oryza sativa) and maize (Zea mays) (Wisser et al. 2005, 2006). R gene clusters typically confer resistance to several unrelated pathogen types. In a previous study, shared genomic locations were not observed for genes that showed similar pathogen specificity, except in the case of Phytophthora spp. resistance (Grube et al. 2000). It appears that the genetic structure of chromosome 5 in pepper has undergone complicated chromosomal rearrangement due to translocation through species divergence. The major QTL region on pepper chromosome 5 shows homology to regions on tomato chromosome T4/potato chromosome IV. (Livingstone et al. 1999). This syntenic region in potato chromosome IV contains at least four R genes, including R2, and one QTL for P. infestans resistance (Grube et al. 2000; Leonards-Schippers et al. 1994; Park et al. 2005a, b, c; Van Der Vossen et al. 2003). Interestingly, the two putative NBS-LRR candidate genes are highly similar to R2, RPP8 and RPP13. R2 in potato and RPP8/RPP13 in Arabidopsis are responsible for resistance to the oomycete pathogens Phytophthora infestans and Peronospora parasitica, respectively (Lokossou et al. 2009; Rose et al. 2004; Takahashi et al. 2002). The conserved syntenic position and functional characteristics of Phytophthora spp. resistance in potato and pepper indicate that the two loci likely arose from the same ancestral sources followed by co-evolutionary processes. The conservation of coding regions among the R gene families might allow the R genes in potato and pepper to recognize the corresponding pathogens P. infestans and P. capsici, respectively, while maintaining their syntenic positions (Dangl and Jones 2001; Hulbert et al. 2001; Michelmore and Meyers 1998).
Author contributions
WYL and JHK prepared manuscript, and HSJ, HJC, and HBY performed research (sequence 2 analysis and marker development). KKT developed and provided RILs, and KJC screened 3 phytophthora resistance. DC, MJ and BCK participated in its design and coordination of 4 research and helped to draft the manuscript.
References
Bartual R, Carbonell EA, Marsal JI, Tello JC, Campos T (1991) Gene action in the resistance of peppers (Capsicum annuum) to Phytophthora stem blight (Phytophthora capsici L.). Euphytica 54:195–200
Bonardi V, Tang S, Stallmann A, Roberts M, Cherkis K, Dangl JL (2011) Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proc Natl Acad Sci USA 108:16463–16468
Bonnet J, Danan S, Boudet C, Barchi L, Sage-Palloix A-M, Caromel B, Palloix A, Lefebvre V (2007) Are the polygenic architectures of resistance to Phytophthora capsici and P. parasitica independent in pepper? Theor Appl Genet 115:253–264
Borevitz JO, Liang D, Plouffe D, Chang HS, Zhu T, Weigel D, Berry CC, Winzeler E, Chory J (2003) Large-scale identification of single-feature polymorphisms in complex genomes. Genome Res 13:513–523
Bradshaw JE, Bryan GJ, Lees AK, McLean K, Solomon-Blackburn RM (2006) Mapping the R10 and R11 genes for resistance to late blight (Phytophthora infestans) present in the potato (Solanum tuberosum) R-gene differentials of Black. Theor Appl Genet 112:744–751
Choi HW, Hwang BK (2011) Systemic acquired resistance of pepper to microbial pathogens. J Phytopathol 159:393–400
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411:826–833
De Givry S, Bouchez M, Chabrier P, Milan D, Schiex T (2005) Carh ta gene: multipopulation integrated genetic and radiation hybrid mapping. Bioinformatics 21:1703–1704
Desai UJ, Pfaffle PK (1995) Single-step purification of a thermostable DNA polymerase expressed in Escherichia coli. Biotechniques 19(780–782):784
Eitas TK, Dangl JL (2010) NB-LRR proteins: pairs, pieces, perception, partners, and pathways. Curr Opin Plant Biol 13:472–477
Foster JM, Hausbeck MK (2010) Resistance of pepper to Phytophthora crown, root, and fruit rot is affected by isolate virulence. Plant Dis 94:24–30
Gore M, Bradbury P, Hogersc R, Kirstd M, Verstegec E, van Oeverenc J, Pelemanc J, Bucklere E, van Eijk M (2007) Evaluation of target preparation methods for single-feature polymorphism detection in large complex plant genomes. Crop Sci 47:S135–S148
Grube RC, Radwanski ER, Jahn M (2000) Comparative genetics of disease resistance within the Solanaceae. Genetics 155:873–887
Hill TA, Ashrafi H, Reyes-Chin-Wo S, Yao J, Stoffel K, Truco M-J, Kozik A, Michelmore RW, Van Deynze A (2013) Characterization of Capsicum annuum genetic diversity and population structure based on parallel polymorphism discovery with a 30 K unigene pepper GeneChip. PLoS One 8:e56200
Hord MJ, Ristaino JB (1991) Effects of physical and chemical factors on the germination of oospores of Phytophthora capsici in vitro. Phytopathology 81:1541–1546
Huang S, Van Der Vossen EAG, Kuang H, Vleeshouwers VGAA, Zhang N, Borm TJA, Van Eck HJ, Baker B, Jacobsen E, Visser RGF (2005) Comparative genomics enabled the isolation of the R3a late blight resistance gene in potato. Plant J 42:251–261
Hulbert SH, Webb CA, Smith SM, Sun Q (2001) Resistance gene complexes: evolution and utilization. Annu Rev Phytopathol 39:285–312
Jo SJ, Shim SA, Jang KS, Choi YH, Kim JC, Choi GJ (2014) Resistance of chili pepper cultivars to isolates of Phytophthora capsici. Korean J Hortic Sci Technol 32:66–76
Kim YJ, Hwang BK, Park KW (1989) Expression of age-related resistance in pepper plants infected with Phytophthora capsici. Plant Dis 73:745
Kim HJ, Nahm SH, Lee HR, Yoon GB, Kim KT, Kang BC, Choi D, Kweon OY, Cho MC, Kwon JK, Kim BD (2008) BAC-derived markers converted from RFLP linked to Phytophthora capsici resistance in pepper (Capsicum annuum L.). Theor Appl Genet 118:15–27
Kloosterman B, Oortwijn M, America T, de Vos R, Visser RG, Bachem CW (2010) From QTL to candidate gene: genetical genomics of simple and complex traits in potato using a pooling strategy. BMC Genom 11:158
Lee SC, Hwang BK (2003) Identification of the pepper SAR8. 2 gene as a molecular marker for pathogen infection, abiotic elicitors and environmental stresses in Capsicum annuum. Planta 216:387–396
Lee WP, Lee JD, Han JH, Kang BC, Yoon JB (2012) Validity test for molecular markers associated with resistance to Phytophthora root rot in chili pepper (Capsicum annuum L.). Korean J Hortic Sci Technol 30:64–72
Lefebvre V, Palloix A (1996) Both epistatic and additive effects of QTLs are involved in polygenic induced resistance to disease: a case study, the interaction pepper-Phytophthora capsici Leonian. Theor Appl Genet 93:503–511
Leonards-Schippers C, Gieffers W, Schäfer-Pregl R, Ritter E, Knapp SJ, Salamini F, Gebhardt C (1994) Quantitative resistance to Phytophthora infestans in potato: a case study for QTL mapping in an allogamous plant species. Genetics 137:67–77
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760
Liu WY (2006) Comparative genetics of resistance to Phytophthora in pepper and strategies to improve marker resources. MS Thesis, Cornell University, USA
Livingstone KD, Lackney VK, Blauth JR, Van Wijk RIK, Jahn MK (1999) Genome mapping in Capsicum and the evolution of genome structure in the Solanaceae. Genetics 152:1183–1202
Lokossou AA, T-h Park, van Arkel G, Arens M, Ruyter-Spira C, Morales J, Whisson SC, Birch PR, Visser RG, Jacobsen E (2009) Exploiting knowledge of R/Avr genes to rapidly clone a new LZ-NBS-LRR family of late blight resistance genes from potato linkage group IV. MPMI 22:630–641
Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15:809–834
Michelmore RW, Meyers BC (1998) Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res 8:1113–1130
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Minamiyama Y, Tsuro M, Kubo T, Hirai M (2007) QTL analysis for resistance to Phytophthora capsici in pepper using a high density SSR-based map. Breed Sci 57:129–134
Naess S, Bradeen J, Wielgus S, Haberlach G, McGrath J, Helgeson J (2001) Analysis of the introgression of Solanum bulbocastanum DNA into potato breeding lines. Mol Genet Genomics 265:694–704
Nahm SH (2001) QTL mapping of Phytophthora root rot resistance in chili pepper (Capsicum annuum L.). Ph. D. Dissertation, Seoul National University, Republic of Korea
Oelke LM, Bosland PW, Steiner R (2003) Differentiation of race specific resistance to Phytophthora root rot and foliar blight in Capsicum annuum. J Am Soc Hortic Sci 128:213–218
Ogundiwin EA, Berke TF, Massoudi M, Black LL, Huestis G, Choi D, Lee S, Prince JP (2005) Construction of 2 intraspecific linkage maps and identification of resistance QTLs for Phytophthora capsici root-rot and foliar-blight diseases of pepper (Capsicum annuum L.). Genome 48:698–711
Park T-H, Gros J, Sikkema A, Vleeshouwers VGAA, Muskens M, Allefs S, Jacobsen E, Visser RGF, van der Vossen EAG (2005a) The late blight resistance locus Rpi-blb3 from Solanum bulbocastanum belongs to a major late blight R gene cluster on chromosome 4 of potato. MPMI 18:722–729
Park T-H, Vleeshouwers VGAA, Hutten RCB, van Eck HJ, van der Vossen E, Jacobsen E, Visser RGF (2005b) High-resolution mapping and analysis of the resistance locus Rpi-abpt against Phytophthora infestans in potato. Mol Breeding 16:33–43
Park TH, Vleeshouwers VGAA, Huigen DJ, Van Der Vossen EAG, Van Eck HJ, Visser RGF (2005c) Characterization and high-resolution mapping of a late blight resistance locus similar to R2 in potato. Theor Appl Genet 111:591–597
Park SW, Jung JK, Choi EA, Kwon JK, Kang JH, Jahn M, Kang BC (2014) An EST-based linkage map reveals chromosomal translocation in Capsicum. Mol Breed. doi:10.1007/s11032-014-0089-0
Quirin EA, Ogundiwin EA, Prince JP, Mazourek M, Briggs MO, Chlanda TS, Kim KT, Falise M, Kang BC, Jahn MM (2005) Development of sequence characterized amplified region (SCAR) primers for the detection of Phyto. 5.2, a major QTL for resistance to Phytophthora capsici Leon. in pepper. Theor Appl Genet 110:605–612
Rehrig WZ, Ashrafi H, Hill T, Prince J, Van Deynze A (2014) CaDMR1 cosegregates with QTL Pc5.1 for resistance to Phytophthora capsici in pepper (Capsicum annuum). Plant Genome 7:1–12
Reifschneider FJB, Boiteux LS, Della Vecchia PT, Poulos JM, Kuroda N (1992) Inheritance of adult-plant resistance to Phytophthora capsici in pepper. Euphytica 62:45–49
Rose LE, Bittner-Eddy PD, Langley CH, Holub EB, Michelmore RW, Beynon JL (2004) The maintenance of extreme amino acid diversity at the disease resistance gene, RPP13, in Arabidopsis thaliana. Genetics 166:1517–1527
Sherman A, Eshed R, Harel-Beja R, Tzuri G, Portnoy V, Cohen S, Rubinstein M, Schaffer AA, Burger J, Katzir N, Ophir R (2013) Combining bulk segregation analysis and microarrays for mapping of the pH trait in melon. Theor Appl Genet 126:349–358
Song J, Bradeen JM, Naess SK, Raasch JA, Wielgus SM, Haberlach GT, Liu J, Kuang H, Austin-Phillips S, Buell CR (2003) Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc Natl Acad Sci USA 100:9128–9133
Mallard SP, Ml Cantet, Massire A, Bachellez A, Ewert S, Lefebvre V (2013) A key QTL cluster is conserved among accessions and exhibits broad-spectrum resistance to Phytophthora capsici: a valuable locus for pepper breeding. Mol Breed 32:349–364
Sy O, Bosland PW, Steiner R (2005) Inheritance of Phytophthora stem blight resistance as compared to Phytophthora root rot and Phytophthora foliar blight resistance in Capsicum annuum L. J Am Soc Hortic Sci 130:75–78
Takahashi H, Miller J, Nozaki Y, Takeda M, Shah J, Hase S, Ikegami M, Ehara Y, Dinesh-Kumar S (2002) RCY1, an Arabidopsis thaliana RPP8/HRT family resistance gene, conferring resistance to cucumber mosaic virus requires salicylic acid, ethylene and a novel signal transduction mechanism. Plant J 32:655–667
Thabuis A, Palloix A, Pflieger S, Daubeze A-M, Caranta C, Lefebvre V (2003) Comparative mapping of Phytophthora resistance loci in pepper germplasm: evidence for conserved resistance loci across Solanaceae and for a large genetic diversity. Theor Appl Genet 106:1473–1485
Thabuis A, Lefebvre V, Bernard G, Daubeze AM, Phaly T, Pochard E, Palloix A (2004) Phenotypic and molecular evaluation of a recurrent selection program for a polygenic resistance to Phytophthora capsici in pepper. Theor Appl Genet 109:342–351
Truong HTH, Kim KT, Kim DW, Kim S, Chae Y, Park JH, Oh DG, Cho MC (2012) Identification of isolate-specific resistance QTLs to phytophthora root rot using an intraspecific recombinant inbred line population of pepper (Capsicum annuum). Plant Pathol 61:48–56
Van Der Vossen E, Sikkema A, Hekkert BtL, Gros J, Stevens P, Muskens M, Wouters D, Pereira A, Stiekema W, Allefs S (2003) An ancient R gene from the wild potato species Solanum bulbocastanum confers broad-spectrum resistance to Phytophthora infestans in cultivated potato and tomato. Plant J 36:867–882
Walker SJ, Bosland PW (1999) Inheritance of Phytophthora root rot and foliar blight resistance in pepper. J Am Soc Hortic Sci 124:14–18
Wang S, Basten CJ, Zeng ZB (2007) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC
Wisser RJ, Sun Q, Hulbert SH, Kresovich S, Nelson RJ (2005) Identification and characterization of regions of the rice genome associated with broad-spectrum, quantitative disease resistance. Genetics 169:2277–2293
Wisser RJ, Balint-Kurti PJ, Nelson RJ (2006) The genetic architecture of disease resistance in maize: a synthesis of published studies. Phytopathology 96:120–129
Yang HB, Liu WY, Kang WH, Kim JH, Cho HJ, Yoo JH, Kang BC (2012) Development and validation of L allele-specific markers in Capsicum. Mol Breed 30:819–829
Acknowledgments
This study was supported by a grant (Project No. 609002-5) from the Screening Center for Disease Resistance Vegetable Crops of the Technology Development Program, and a Grant (Project No. 710001-07) from the Vegetable Breeding Research Center through the R&D Convergence Center Support Program, Ministry of Agriculture, Food and Rural Affairs (MAFRA) Republic of Korea. This work was also supported a grant from the Next-Generation BioGreen 21 Program (Plant Molecular Breeding Center No. PJ00906501), Rural Development Administration. We would like to thank Dr. Marco van Schriek and Alexander Wittenberg at KeyGene for microarray analysis.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hong-Qing Ling.
W. Y. Liu and J. H. Kang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, WY., Kang, JH., Jeong, HS. et al. Combined use of bulked segregant analysis and microarrays reveals SNP markers pinpointing a major QTL for resistance to Phytophthora capsici in pepper. Theor Appl Genet 127, 2503–2513 (2014). https://doi.org/10.1007/s00122-014-2394-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2394-8