Abstract
Phytophthora infestans is the causal agent of late blight in potato. The Mexican species Solanum demissum is well known as a good resistance source. Among the 11 R gene differentials, which were introgressed from S. demissum, especially R8 and R9 differentials showed broad spectrum resistance both under laboratory and under field conditions. In order to gather more information about the resistance of the R8 and R9 differentials, F1 and BC1 populations were made by crossing Mastenbroek (Ma) R8 and R9 clones to susceptible plants. Parents and offspring plants were examined for their pathogen recognition specificities using agroinfiltration with known Avr genes, detached leaf assays (DLA) with selected isolates, and gene-specific markers. An important observation was the discrepancy between DLA and field trial results for Pi isolate IPO-C in all F1 and BC1 populations, so therefore also field trial results were included in our characterization. It was shown that in MaR8 and MaR9, respectively, at least four (R3a, R3b, R4, and R8) and seven (R1, Rpi-abpt1, R3a, R3b, R4, R8, R9) R genes were present. Analysis of MaR8 and MaR9 offspring plants, that contained different combinations of multiple resistance genes, showed that R gene stacking contributed to the Pi recognition spectrum. Also, using a Pi virulence monitoring system in the field, it was shown that stacking of multiple R genes strongly delayed the onset of late blight symptoms. The contribution of R8 to this delay was remarkable since a plant that contained only the R8 resistance gene still conferred a delay similar to plants with multiple resistance genes, like, e.g., cv Sarpo Mira. Using this “de-stacking” approach, many R gene combinations can be made and tested in order to select broad spectrum R gene stacks that potentially provide enhanced durability for future application in new late blight resistant varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato is the world’s most important non-cereal food crop (Park et al. 2009; Rauscher et al. 2006). In many regions where potatoes are grown, one of the most notorious and economically damaging pathogens is Phytophthora infestans (Pi), causing late blight (Halterman et al. 2008; Haverkort et al. 2008). The negative economic impact is $3.5 billion annually in developing countries alone (White and Shaw 2010). Fungicides and healthy seed tubers have been used to control potato late blight in developed countries. However, the use of expensive chemical sprays that have unfavorable environmental consequences, and the emergence of new fungicide resistant isolates, have increased the interest and use of host resistance in potato breeding programs (Grunwald et al. 2001; Fry and Goodwin 1997).
So far, over 20 functional late blight R genes, including the Solanum demissum genes R1, R2, R3a, and R3b, have been cloned (Ballvora et al. 2002; Huang et al. 2005; Lokossou et al. 2009; Li et al. 2011). The cloned genes all belong to the CC-NB-LRR class and, besides S. demissum, they were derived from wild Solanum species like S. bulbocastanum (Song et al. 2003; van der Vossen et al. 2003, 2005), S. stoloniferum and S. papita, (Vleeshouwers et al. 2008), S. venturii and S. mochiquense (Pel et al. 2009; Foster et al. 2009).
Solanum demissum, a hexaploid Mexican wild Solanum species, is an important source of resistance to late blight. The major resistance (R) genes from S. demissum show late blight race-specificity, which is associated with a hypersensitive response that is triggered by the cognate avirulence (Avr) genes of incompatible isolates of Pi (Vleeshouwers et al. 2011). Eleven R gene differentials (Black et al. 1953; Malcolmson and Black 1966; Eide et al. 1959) containing R genes introgressed into potato from S. demissum, have been collected by Mastenbroek (1952) and are referred to as the Mastenbroek differential set: MaR1 until MaR11. Seven genes responsible for resistance within this set have been mapped: R1 on chromosome 5 (Leonards-Schippers et al. 1992), R2 on chromosome 4 (Li et al. 1998), R3a, R3b, R6, and R7 on chromosome 11 (Huang et al. 2005; El-Kharbotly et al. 1996), and R8 on chromosome IX (Jo et al. 2011). The differential set was originally thought to represent single late blight resistance factors (classified as R1–R11). However, R1 was also found in the MaR5, MaR6, and MaR9 differentials (Trognitz and Trognitz 2007). Also the MaR3 differential plant contained two R genes, R3a and R3b (Huang et al. 2005).
In the past, R genes of MaR1, -2, -3, -4, and -10 from S. demissum have been introgressed into potato cultivars but were rapidly overcome by the Pi populations. In order to enhance durability of domesticated late blight resistance, stacking of two R genes, was shown to result in a delay in the onset of late blight disease (Tan et al. 2010). R gene stacking using conventional- or marker-assisted breeding is a complicated and time-consuming process due to the multitude of genes to be introgressed and due to the simultaneous introgression of undesired traits. Sarpo Mira is one of the few potato cultivars obtained by conventional breeding, which expresses signicant levels of durable late blight resistance. Recently, it was shown that at least five R genes are stacked in cv Sarpo Mira (Rietman 2011). Besides traditional and marker-assisted breeding for late blight resistance, transformation of cloned R genes into existing potato varieties has gained renewed interest (Haverkort et al. 2008). Recently, it was shown that simultaneous transformation of multiple R genes into susceptible cultivars can be used to produce late blight resistant varieties (Zhu et al. 2011; Forch et al. 2010; BASF on line publication 2011).
Remarkably, among the set of 11 potato R gene differentials, MaR8 and MaR9 have shown a broad spectrum resistance in field trials as well as in detached leaf assays (DLA) against several complex isolates of Pi (Lehtinen et al. 2008). Lehtinen et al. proposed that this is probably due to a lack of selection pressure because R9 has never been introduced into commercial cultivars. Zhang and Kim (2007) collected 261 Pi isolates during 3 years from Korea and found all races carried multiple virulence genes and showed virulence to the potato differential plants R1, R3, R5, R6, R7, R10, and R11, but not on R8 and R9. Also Bisognin et al. (2002) found Pi isolates that were virulent on all R gene differentials except R9 in DLA and on both R8 and R9 in field trials. More recently, it was shown that virulence towards R8 and R9 differential plants is relatively rare in European Pi populations (Chmielarz et al. 2010). In this and other studies (Corbiere et al. 2010; White and Shaw 2010) also cv Sarpo Mira, was included and was shown to express similar levels of resistance to late blight as the MaR8 and MaR9 in multiple years of field trials in Europe and Northern Africa.
Therefore, MaR8 and MaR9 differentials are expected to harbor important sources of late blight resistance and it is essential to know more about the genetic background of the resistance in these plants. A combination of DLAs with specific isolates, responsiveness to Avr genes and R gene-specific molecular markers was used to characterize resistance in MaR8 and MaR9 plants. We found that both MaR8 and MaR9 contained multiple resistance genes. In order to test whether broad spectrum resistance is a result of R gene stacking or a result of any of the individual R genes, plants with single R genes and plants with combinations of multiple R genes have been selected. Using an “on site” Pi virulence monitoring system, these plants were tested for their ability to resist high Pi infection pressure.
Materials and methods
Plant material
The Mastenbroek differential set MaR1, 2, 3, 4, 8, and 9 (Mastenbroek 1952), cultivars Sarpo Mira, Concurrent, Bintje, and diploid clone RH89-039-16 (RH) were used for crosses and inoculation experiments. Concurrent is a descendent from the R10 containing cultivar Estima (Vleeshouwers et al. 2000). F1 individuals (two populations; n = 100) derived from the crosses MaR8 × Concurrent and MaR9 × Concurrent were produced and maintained in vitro at Wageningen UR plant breeding. 2-week-old in vitro propagated plants of both populations were transferred into the greenhouse. 2 to 4 weeks after transfer, DLA or agroinfiltration was performed.
R gene-specific markers
R1- and R3a-specific genetic markers were used for validation of the presence or absence of these genes in MaR8 and MaR9 (Ballvora et al. 2002; Huang et al. 2005). The R8-specific marker was described by Jo et al. (2011) and is a cluster directed profiling marker (CDP3). Rpi-abpt1 (an R2 ortholog) specific molecular markers were developed based on alignments of R2, Rpi-abpt, R2-like (Lokossou et al. 2009) and nonfunctional homologs (Lokossou 2010). Stretches of inserted/deleted nucleotides were found, and three conserved primer sets were designed to detect R2. All three R2 primer sets worked well with genomic DNA of MaR1, MaR2, MaR3, MaR8, MaR9, cv. Concurrent, and RH89-039-16 (RH). The R2 primer set that produced the longest polymerase chain reaction (PCR) product was selected (Table 1) and used in this study. Similarly, the R3b marker was designed after alignment of the recently cloned R gene R3b (Li et al. 2011) with non-functional homologs. DNA was extracted from plantlets using a high-throughput method (Huang et al. 2005). The PCR primers listed in Table 1 were used in standard PCR reactions with the indicated annealing temperatures. PCR products were separated in a 1% agarose gel and stained with ethidium bromide (Fig. 1).
R gene diagnostic markers. PCR products representing a R1-, b R2-, c R3a-, and d R3b-specific markers in MaR1, MaR2, MaR3, MaR8, and MaR9, RH89-039-16 (RH), Kathadin (KT), Sarpo Mira (SM), and Concurrent (Co). On the left, the size of the DNA marker (M) in bp is provided. The 1,500 bp band produced by the R3a marker in RH was an a-specific product
Phytophthora infestans assays
The DLA was performed according to Vleeshouwers et al. (2000) using the Pi isolates IPO-C, USA618, 99177, 90128, and 99189. These selected isolates were used because of their race specific virulence spectra. The maintenance and preparation of inoculum was according to previously published protocols (Vleeshouwers et al. 2000). Six days after inoculation, resistance versus susceptibility patterns were scored.
“On site” virulence monitoring was performed at three geographically isolated field locations in The Netherlands (Valthermond in the NorthEast, Lelystad in the center and Vredepeel in the SouthEast) in the years 2006–2010. All three locations represent important potato growing areas with high Pi infection pressure. MaR9, MaR8 and the F1 plants MaR8-18, MaR9-43, MaR9-89, and MaR9-53 were analyzed. Bintje (R0), Sarpo Mira and MaR1 till MaR4, were used as controls. All plants were propagated from in vitro culture and six plants per genotype were planted at each of the three different locations. This way, the plant material was exposed to the local Pi population and weather conditions from beginning of June to mid September without protection by fungicides. Every week, the plants were examined for potato late blight like symptoms. Infected plant material was sampled and used to obtain Pi pure cultures. To avoid a local epidemic, dominated by one or a few Pi genotypes, all six plants of a genotype were destroyed at the affected location as soon as two pure Pi cultures were recovered from this genotype.
The first blight observation date within an experiment was set as day 0. For each genotype, within each experiment and year the first late blight infection was observed and the number of days since the first observation in the field recorded. To estimate the delay in the onset of Pi symptoms for the different genotypes, the Censor method was used (Taylor 1973). For statistical analysis, the number of days delay since the start of the epidemic was LOG10(x + 1) transformed. An observation is said to be censored if it is known only that it is less than (or greater than) a particular value. In this case, it is known that some genotypes were not infected during the sampling period. Therefore, the genotype remained free from infection by Pi for a fixed number of days (bound value) at a given location in a specific year. Therefore, it is known that the delay at this location and year is longer than the bound value, so this observation is censored. The censored observations were replaced by estimated values for delay, using the method outlined by Taylor (1973).
This method estimates the expected value of each censored observation iteratively conditional on the fact that the value must be greater than the fixed bound, and using the relevant information from the other observations in the experiment. Successively, a T test was performed to estimate significant differences between estimated delays of the different genotypes.
R2 and R3a allele mining
R2 allele mining primers were described by Lokossou et al. (2009; Table 1). The high fidelity proofreading DNA polymerase Phusion was used to amplify R2-like genes in the following PCR program: 98°C for 30 s. followed by 35 cycles of 98°C for 10 s, 62°C for 10 s and 72°C for 150 s, followed by 72°C for 10 min.
Based on a multiple sequence alignment containing R3a homologs from publically accessible databases, a phylogenetic tree was drawn and four clades could be distinguished (Figure S1). Clade-specific degenerate primers were designed and the sequence CACC was added to the 5′ end of the Forward primers to allow directional cloning into D-TOPO vector (Invitrogen, San Diego, CA, USA; Table 1). PCR reactions were done with Phusion™ DNA Polymerase using MaR9 and MaR8 genomic DNA as template (Figure S2). PCR conditions for R3 clade one, two, and four specific primers: 30 s at 98°C, followed by 35 cycles of 98°C for 10 s, 65°C for 45 s, 72°C for 180 s, then followed by 72°C for 10 min. Using R3 clade three specific primer: 30 s at 98°C, followed by 35 cycles of 98°C for 10 s, 62°C for 45 s, 72°C for 180 s; plus a final extension step at 72°C for 10 min.
Both the R2 and R3a PCR products were purified by precipitation using 30% PEG8000/30 mM MgCl2 solution and was inserted into D-TOPO vector (Invitrogen). The recombinant plasmids were transformed into competent cells of Escherichia coli strain TOP10 and cultured in Luria-Bertani (LB) broth supplemented with Kanamycin (50 mg/ml). DNA sequencing was conducted on an ABI Prism 3700 DNA sequencer with primer M13F and R2- and R3a-derived primers inside of inserts.
Sequence similarity was analyzed with Seqman v.7 software (Lasergene, DNAStar, Madison, WI, USA). Sequence comparison was performed with MEGALIGN v.7.
Agroinfiltration assay
Fifteen days after transfer into the greenhouse, F1 population plants derived from MaR8 and MaR9 and their controls were used for agroinfiltration experiments with Avr1, Avr2, Avr3a, and Avr4. Safety regulations for containment determined the use of a separate greenhouse compartment for agro-treatments. 3, 5, and 7 days after agroinfiltration, symptoms which are caused by the interaction between R genes and the corresponding Avr genes were observed and scored. Agroinfiltration assay followed previously described methodology (Vleeshouwers et al. 2008).
Results
Low virulence levels in Pi populations in the Netherlands towards MaR8 and MaR9 plants
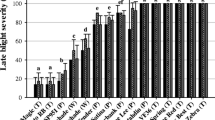
In order to study whether MaR8 and MaR9 plants expressed useful resistance to the Pi populations in The Netherlands, an “on site virulence monitoring” system was set up. Cv Bintje (R0) was included as reference plant that lacks Pi resistance and cv Sarpo Mira was included in the last 2 years of these studies as reference plant with a high level of Pi resistance. In addition, four plants from the Mastenbroek differential set were included (MaR1, MaR2, MaR3, MaR4) because their R gene content is well described. In all 5 years both MaR8 and MaR9 performed significantly better than cv Bintje, MaR1, MaR2, MaR3, and MaR4 plants, showing that useful resistance is present in MaR8 and MaR9 plants (Table 2). In 4 of the 5 years, MaR9 showed a significantly longer delay in the onset of Pi symptoms when compared with MaR8. In 2009 and 2010 cv Sarpo Mira was also included and MaR9 and MaR8 showed a similar or even longer delay in the onset of Pi symptoms. Despite the observed variations per year, it can be concluded that MaR8 and MaR9 express high levels of resistance to severe Pi challenges.
Multiple known R genes are stacked in MaR8 and MaR9 plants
In order to determine the recognition specificity of the resistance in MaR8 and MaR9, the plants were screened for their ability to recognize any of the known Avr genes. Using an agroinfiltration assay Avr1, Avr2, Avr3a, and Avr4 were infiltrated into MaR8 and MaR9 plants (Fig. 2a; Table 3). Interestingly, MaR8 showed an HR reaction with Avr3a and Avr4. MaR9 showed a HR upon infiltration with all tested Avr genes; Avr1, Avr2, Avr3a, and Avr4 (Table 3). These data show that a broad spectrum of Avr’s is recognized, which could be explained by the presence of multiple R genes or by broad spectrum R genes that recognize multiple Avr’s.
Avr1, -2, -3a, and -4 induced hypersensitive response. Avr1, -2, -3a, and -4 were agroinfiltrated in MaR1, MaR2, MaR3, MaR4, MaR8, MaR9, and Bintje (a) or MaR8 and MaR9 F1 offspring plants (a). pGraB and 1:1 mixture of R3a and Avr3a were infiltrated as negative and positive controls, respectively. Representative leaves from three biological replicates are shown
In order to study whether multiple or single broad spectrum R genes were present in the studied plants, MaR8 and MaR9 were crossed with cv Concurrent. F1 populations were sown and maintained in vitro. Agroinfiltrations of Avr1, Avr2, Avr3a, and Avr4 were performed in these F1 populations. Segregation patterns were different for each Avr (Fig. 2b, Table S1) and it is concluded that different R genes must be responsible for their recognition. Because Avr1, 2, 3a, and 4 are known to be recognized by R1, R2, R3a, and R4, our results suggest that R3a and R4 genes are present in MaR8 and R1, R2, R3a, and R4 are present in MaR9. It is, however, an unexpected result since the MaR8 and MaR9 plants have been selected as R gene differentials and were assumed to contain only one R gene. In order to confirm the presence of multiple R genes in these plants, the molecular basis of the observed effector recognition was investigated.
Solanum demissum late blight R genes R1, R2, and R3a have been cloned and their sequences have been used to develop gene-specific molecular markers. The previously published R1- and R3a-specific markers (Trognitz and Trognitz 2007; Huang et al. 2005) and the R2-specific marker (this study) were used to monitor the presence of these genes in MaR8, MaR9, cv Concurrent and RH (Fig. 1), respectively. Also markers derived from the recently cloned R3b gene (Li et al. 2011) and the recently mapped R8 gene (Jo et al. 2011) were included in this study. MaR8 was positive for the R3a, R3b, and R8 markers. MaR9 showed to be positive for the R1, R2, R3a, R3b, and R8 markers. Remarkably, also cv Concurrent did show the R3b marker band. Cv Concurrent and RH were negative for the R1, R2, R3a, and R8 markers. Therefore, MaR8 is expected to contain R3a, R3b, and R8. MaR9 is expected to contain R1, R2, R3a, R3b, and R8 or closely related homologs thereof. This notion was confirmed upon analysis of the F1 populations. When segregation patterns of Avr responsiveness were compared to R gene marker segregation patterns a co-segregation was observed (Table S1). Plants that produced a high background cell death response to agroinfiltration and plants that responded poorly to agroinfiltration were excluded from the comparative analyses. R gene marker segregation ratios are shown in Table 4. For R4 no marker was available and here segregation ratios were inferred from effector responsiveness only. It can be concluded that MaR8 contains R3a, R3b, R4, and R8 in simplex. Overrepresentation of R3b in the R8 population is caused by the presence of R3b in the male parent cv Concurrent. In MaR9; R1, R2, R4, and R8 genes seemed to be present in simplex. R3a and R3b seemed to be present in duplex.
In order to further confirm the presence of the known R genes in the plants under study, a R2 and R3 (covering both R3a and R3b) homolog mining approach was pursued. Using primer pairs overlapping the start and stop codons of R2 and R3, dozens of open reading frame (ORF) sequences with high levels of homology to R2 and R3, respectively, were identified in MaR8 and MaR9 plants. Using the R2 primers, six clones were identified deriving from MaR9, one clone (9-R-8) was identical at the nucleotide level to Rpi-abpt1 (Fig. 3). Using the R3 primers, among 24 clones two exact copies (9-G-52 and 9-G-81) of R3a were found in MaR9 and no clones identical to R3b were found in MaR9. In MaR8, among 6 clones, no exact copies of either R3a or R3b were found (Fig. 3). The absence of R3a in the screening of MaR8 and the absence of R3b in the screening of both MaR8 and MaR9 is considered to be due to the low saturation of the screen and does not show that R3a or R3b are absent in those plants. These results suggest that the Avr2 and Avr3a recognition by the MaR9 plants is caused by Rpi-abpt1 and R3a, respectively. Also, because of the sequence identity, it can be ruled out that wider Avr spectra are recognized by these individual genes to cause the broad spectrum resistance of the MaR9 plants.
Phylogenetic analysis of the R3a and R2 homologs. Full-length sequences from allele mining studies with 80% or more nucleotide identity to R3a and R2 were aligned with known R3a and R2 homologs using Megalign from the DNAstar software pack. Sequence names starting with 8 or 9 are derived from MaR8 or MaR9, respectively
R8 and at least one additional R gene is present in MaR9
Since it was found that multiple known R genes are present in MaR8 and MaR9, the possibility of additional, unknown, late blight R genes was also investigated. Because of the presence of the multitude of known R genes in the studied plants, it was decided to study the populations using a Pi isolate, IPO-C, that had overcome R1, R2, R3a, R3b, and R4, leaving the possibility to study additional resistance genes in MaR8 and MaR9. Although the parental plants were resistant to IPO-C in our DLAs, analysis of the F1 populations revealed no (in the case of MaR8) or only very few plants (in the case of the MaR9 F1 population) that consistently showed resistance to IPO-C in DLA. However, when the MaR8 and MaR9 populations were tested in field trials in 2009 and 2010, inoculated with the same isolate (IPO-C), 2 to 4 weeks after inoculation a clear distinction could be made between resistant and susceptible plants. Only a few plants could not be classified as resistant or susceptible and were qualified as questionable (Table 4, Table S1).
It had been described that resistance to IPO-C in the MaR8 F1 population, as determined under field conditions, showed a 1:1 segregation and fully co-segregated with the R8 marker (Jo et al. 2011). The segregation of IPO-C resistance in the MaR9 F1 population was severely skewed towards resistance, suggesting that multiple unlinked R genes were present. Interestingly, the R8 marker segregated in a 1:1 fashion (Table 4) in the MaR9 F1 population and all the positive plants were resistant to IPO-C. In addition out of 51 plants that lacked the R8 marker, 46 plants were fully resistant to IPO-C, showing that at least one additional R gene is segregating in the population. In order to reduce the R gene complexity, BC1 populations were generated deriving from MaR9. Eight resistant F1 plants were selected and crossed with susceptible cultivar Kathadin. The BC1 populations were tested in a field trial inoculated with IPO-C. Two BC1 populations showed a 1:1 segregation for resistance and four populations showed a skewing towards resistance. So, even after crossing to a susceptible plant twice, still a multitude of resistance was observed, deriving from MaR9. The R8 marker and IPO-C resistance segregation ratios in two selected BC1 populations (3150 and 3153) are shown in Table 4. A co-segregation from the R8 marker and IPO-C resistance was observed in population 3150. This showed that the R8 marker not only tagged a R gene from MaR8 but also from MaR9 and this gene is referred to as R8 since it is most likely the same gene as the R8 gene derived from MaR8. In population 3153, that was skewed towards resistance, the R8 marker was present exclusively in resistant plants, showing that the R8 gene was also segregating in this population. Also a second R gene, deriving from MaR9, was segregating in population 3153 since four plants without the R8 marker also showed resistance (Table 4). The gene(s) responsible for this resistance is tentatively referred to as R9.
Contribution of R gene stacking to broad spectrum resistance
The R genes within MaR8 and MaR9 were genetically segregating in different F1 and BC1 populations. This allowed the selection of plants with different combinations of R genes that can be used to test for the contribution of each of these R genes to Pi isolate recognition spectrum. In Table 5, the R gene content of selected F1 plants is summarized as derived from R gene-specific markers, effector recognition, and IPO-C resistance segregation in field trials.
Five different Pi isolates were tested on these plants. As described above and in previous studies, F1 plants containing R8 and R9 genes showed no or only weak resistance to IPO-C in DLA. Plant MaR9-71 was an exception. Like the parental plant MaR9, this plant did express resistance to IPO-C in DLAs (Table 5). Due to this lack of sufficient biological activity of the R8 and R9 genes in DLA, the biological activity of the remaining genes R2, R3a, R3b, and R4 could be distinguished by the use of differential isolates. None of the selected plants, except R9-56, contained R1 so no discriminating isolate was used for R1. Isolate 90128 was avirulent on the plants containing Rpi-abpt1. This is in agreement with previous studies and confirms that 90128 expresses Avr2. Isolate USA618 was avirulent on all plants containing R4, suggesting that this isolate expresses Avr4. Isolate 99189 was avirulent on all plants containing R3a and R4. By comparison to the resistance spectrum of USA618, 99189 could be used as a differential isolate for R3a. Isolate 99177 was avirulent on cv Concurrent that was used as the “susceptible” parent for the MaR8 and MaR9 populations. Resistance to isolate 99177 largely co-segregated with the marker for R3b that was derived from parent cv Concurrent. It could, however, not be concluded that isolate 99177 expressed Avr3b since it was compatible on MaR3 plants that also contained the R3b gene. Based on these studies, it can be concluded that stacking of R2, R3a, R3b, and R4 genes contributes to the isolate resistance spectrum of the plants. It remains, however, to be resolved to what extent stacking of these genes contributes to durability of resistance.
Contribution of R gene stacking to durability; a new method to study durability of R gene stacks
As described above, the MaR8 and MaR9 plants showed resistance to high Pi infection pressure in “on site” virulence monitoring fields from 2006 to 2010 in three different potato growing areas in the Netherlands. This resistance was similar to the resistance in the durably resistant cultivar Sarpo Mira. In order to set up an R gene stack durability assay, the F1 plants from the MaR8 and MaR9 populations, that contained reduced levels of R gene stacking, can be monitored in the same “on site” virulence monitoring fields as the parental plants MaR8 and MaR9. In 2010, a first trial was performed (Table 2) and MaR8 showed an indistinguishable delay in the onset of late blight symptoms as plant R8-18 which contained, besides R8, no additional known R genes. This suggests that there was no significant contribution from the additional R genes in MaR8. It must be noted that this is only the first year of these trials. Similar experiments in the years to come must be performed in order to assess whether these findings will show consistency. Remarkable is the finding that cv Sarpo Mira, which contains at least five R genes (R3a, R3b, R4, Rpi-smira1, and Rpi-smira2; Rietman 2011), had a similar delay in the onset of Pi symptoms as MaR8 and R8-18 in 2010. However, in 2009 cv Sarpo Mira remained unaffected while MaR8 did show Pi symptoms towards the end of the experiment. This showed that differences in virulence levels towards R genes in the Pi population can vary from year to year. The virtue of R gene stacking was illustrated by plant MaR9-53, that besides R8 also contained R9. MaR9-53 showed a significantly prolonged delay in the onset of Pi symptoms. The delay in MaR9-53 remained, however, below that of MaR9, which remained unaffected in 2010. This suggests that besides R8 and R9 additional factors in MaR9 were enhancing the resistance to Pi.
Discussion
Molecular basis of resistance in MaR8 and MaR9
MaR8 and MaR9 were exposed in sequential years to high Pi infection pressure in trap fields in major potato growing areas in the Netherlands. The onset of late blight symptoms was observed and in every year MaR8 and MaR9 performed significantly better then control cultivar Bintje and in most cases also significantly better then MaR1, MaR2, MaR3, and MaR4 plants (Table 2). In the last 2 years, also comparisons were made to cv Sarpo Mira. In 2009, MaR9 showed a similar delay as cv Sarpo Mira, and in 2010 MaR8 showed a similar delay as cv Sarpo Mira. In 2010, MaR9 even outperformed cv Sarpo Mira. This was in agreement with other comparisons made between MaR8, MaR9 and for cv Sarpo Mira in Western Europe and Northern Africa (Chmielarz et al. 2010; Corbiere et al. 2010; White and Shaw 2010). Using a combination of studies including Avr responsiveness, R gene-specific molecular markers, candidate gene cloning and segregation analysis of these traits in F1 populations, it was possible to show that R3a, R3b, R4, and R8 S. demissum late blight resistance genes were present in MaR8 and that R1, Rpi-abpt1, R3a, R3b, R4, R8, R9 and potentially an additional R gene were present in MaR9. This (these) R9 gene(s) must not be confused with the resistance provided by MaR9 and therefore it is only tentatively referred to as R9. The additional resistance in MaR9 requires further research to identify its molecular and genetic basis. Complicating for this approach will be the difficulty to detect R9 resistance using DLA in the current F1 and BC1 populations.
R8 and R9 resistance is background dependent
In F1 plants, a remarkable discrepancy was found between resistance to IPO-C in DLA and in field trials. Parental plants MaR8 and MaR9 were resistant to IPO-C in DLA and field trials, whereas, only a few F1 plants were consistently resistant to IPO-C in DLA. In field trials, however, at least half of the F1 plants were found to be consistently resistant. Also for cv Sarpo Mira F1 populations, it was reported that DLA resistance to IPO-C was not inherited properly, whereas resistance to IPO-C in field trial conditions was inherited as a dominant trait (Rietman 2011). It has been described before that R genes can be poorly segregating traits (Ordonez et al. 1997; Trognitz 1998). Dominant-, resistance-suppressing factors that can be segregating in the same population have been postulated to explain this phenomenon (El-Kharbotly et al 1995). In our populations, such suppressor genes may have derived from the susceptible parent Concurrent. On the other hand, poor resistance segregation may have been caused by multiple accessory genes, deriving from the resistant parent. These accessory genes, required for resistance, may have been present in a heterozygous state and are, therefore, not all present in all offspring plants (Ordonez et al. 1998). Such accessory genes could encode guardees, host components that are guarded (Jones and Dangl 2006) by the R8 or R9 proteins. Alternatively, they could encode downstream signaling components or upstream transcription factors required for the proper transcriptional regulation of the R8 and R9 genes. This hypothesis implies that the R8 and R9 genes might not be sufficiently active in all genetic backgrounds. Indeed, populations have been found in which the R8 gene is present and segregating but resistance is only expressed to a very limited extent in field trial conditions (JV, unpublished data). The postulated background dependence of R8 and R9 has important implications for GM- and marker-assisted breeding strategies in which multiple broad spectrum R genes are stacked. It can be concluded that additional tools need to be developed to determine and distinguish the biological activity of R genes in stacks.
Extensive R gene stacking in MaR8 and MaR9 caused broad spectrum resistance
It was shown recently that R2 and R3a homologs could have divergent Avr recognition and, therefore, altered Pi recognition spectra (Champouret 2010). This gives rise to the question whether the R2 and R3 homologs in the studied plants are responsible for the observed broad spectrum resistance. Cloning of the R genes responsible for the recognition of Avr2 and Avr3a from MaR9 plants showed sequence identity to Rpi-abpt1 and R3a, respectively. Sequence identity between the genes will result in identical proteins and identical recognition specificities, ruling out the possibility that either of these two genes caused the broad spectrum resistance observed in MaR9.
A better explanation for the broad spectrum resistance was found in the R gene stacks observed in MaR8 and MaR9 plants. F1 and BC1 studies revealed the presence of plants with many different combinations of the R1, Rpi-abpt1, R3a, R3b, R4, R8, and R9 genes. These different combinations resulted in distinct isolate resistance spectra (Table 5). An interesting observation was the resistance spectrum of cv Concurrent, which was used as crossing parent with MaR8 and MaR9. This cultivar carries the resistance of MaR10 because of its parent cv Estima (Vleeshouwers et al. 2000), which can account for its resistance to isolate 99177. In cv Concurrent, we also found the presence of R3b, which locates, like R10, to the distal end of the short arm of chromosome 11. It was shown recently that in MaR10, R3b and R10 are two different, closely linked genes (Rietman 2011). Therefore, it cannot be ruled out that plants carrying the R3b marker, are in fact containing the R3b and R10 genes, contributing to a different isolate resistance spectrum as plants carrying R3b and not R10.
Durability in relation to stacking
It is shown that the more R genes are present in a plant the broader the isolate resistance spectrum is (Table 5). Theoretically, broad spectrum or multi-R gene-based resistance, would result in enhanced durability because it is increasingly less likely that all cognate Avrs are lost simultaneously in one Pi spore. In the Pi population in the Netherlands, however, virulence towards plants containing R1, Rpi-abpt1, R3a, R3b, and R4 is very common (Table 2). When F1 clone R8-18, that only contained R8 and not R3a, R3b, and R4, was analyzed by “on site” virulence monitoring, no difference was observed with the MaR8. Although this is only a 1 year observation, the fact that this was observed at three different locations suggests that the additional R genes provided no or minor contribution to the resistance spectrum towards the Pi population in the Netherlands. Recent data show that also in cv Sarpo Mira, which has remained durably resistant already for several years, at least five R genes are stacked (R3a, R3b, R4, Rpi-smira1, and Rpi-smira2; Rietman 2011).
The observation that R8 has a major contribution to resistance in MaR8 and MaR9 can be explained in two ways. A potential Avr8 is hard to lose for Pi or, the worldwide Pi population has not been “trained” yet to evade R8 recognition because R8 has not been applied extensively yet in potato varieties. Also a combination of both arguments could hold. In order to avoid Pi populations to mutate and evade R8 recognition, it would be wise to stack additional R genes that still show broad spectrum resistance on top of the R8 gene. The MaR9 F1 population described in this study can be used to select for additional R genes that combine well with R8. Individuals with different known and unknown R gene combinations will be selected and will be tested by “on site” virulence monitoring. This will prove an important way to select for R gene stacks with potentially improved durability. These stacks could, successively, be selected in marker-assisted breeding programs or could be reconstituted in a GM approach (Jacobsen and Schouten 2007).
Besides cv Sarpo Mira, recently two other potato cultivars have been described to contain durable late blight resistance. C88 is a cultivar that is grown in Asian countries already for more than 10 years and it is now the number one variety in the Chinese province Yunnan due to its late blight resistance (Li et al. 2010). Also the US cultivar Missaukee shows durability already for 7 years (Douches et al. 2010). Of course, durability must prove over the years to come, but also for these two cultivars it would be appropriate to identify the molecular basis of resistance aiding the design of strategies for prolonged durability. Such a strategy could consist of monitoring of virulence within the local Pi populations. Virulence towards the R genes involved can be detected and can be linked to a fungicide spray advice.
Implications for the R gene differential set
A system of tester plants with one R gene is essential to determine the virulence spectrum of individual Pi isolates. The differential set MaR1 till MaR11 was long considered to contain one R gene per tester plant. However, research during cloning of the R3 gene clearly indicated that the differential plants from Mastenbroek and Black were different. MaR3 contained two resistance genes, R3a and R3b, and the BlR3 differential contained only R3a (Huang et al. 2005). Trognitz and Trognitz (2007) showed that the differentials MaR5, MaR6, and MaR9 all contain the R1 gene. The availability of cloned R genes and/or matching Avr genes enabled to test also the MaR8 and MaR9 differentials for R gene stacking. In this study five R gene-specific markers (R1, R2, R3a, R3b, and R8) and four Avr genes matching with R1, R2, R3a, and R4 were used. Unexpectedly, three additional R genes were found in MaR8 and at least five additional R genes were found in MaR9. According to our data, race 9 isolates are predicted to also be race 1, 2, 3, 4, 8. Race 8 isolates are predicted to be also race 3, 4. In literature, however, race combinations are found that seem erroneous in retrospect. Consequently, past and future race typing of Pi isolates might require careful (re)evaluation using the current knowledge.
Our unpublished data show that also MaR5, MaR6, and MaR7 contain multiple R genes. Moreover, Rietman (2011) showed that MaR10 contains R3b and R10. These findings call for an update of the differential set. An improved or “purified” differential set will allow a more accurate analysis of Pi isolate virulence spectra. This could be done by crossing the original differential set plants or their offspring to cultivars without R genes. So far, it was possible to select a plant that contained only R8 (R8-18) but a plant with only R9 remains to be identified. Additional BC2 crosses will be required for this. Such “purified” differential plants will be very useful for future “on site” virulence monitoring. In parallel to an improved differential set, virulence towards R genes could be monitored using PCR assays targeting known Avr genes. If an Avr gene is lost in Pi isolates from a geographic location in a potato growing season, this is a good indication that virulence towards the matching R gene in the potato population has emerged. Rapid PCR (a)-virulence assays could be linked to fungicide spray advice in a defined geographic region in order to reduce fungicide application dosage and frequency.
References
Ballvora A, Ercolano MR, Weiss J, Meksem K, Bormann CA, Oberhagemann P, Salamini F, Gebhardt C (2002) The R1 gene for potato resistance to late blight (Phytophthora infestans) belongs to the leucine zipper/NBS/LRR class of plant resistance genes. Plant J 30(3):361–371
BASF online publication (2011) http://www.basf.com/group/corporate/en/function/conversions:/publish/content/products-and-industries/biotechnology/images/Fortuna_VC.pdf. Accessed 23 June 2011
Bisognin DA, Douches DS, Jastrzebski K, Kirk WW (2002) Half-sib progeny evaluation and selection of potatoes resistant to the US8 genotype of Phytophthora infestans from crosses between resistant and susceptible parents. Euphytica 125:129–138
Black W, Mastenbroek C, Mills WR, Peterson LC (1953) A proposal for an international nomenclature of races of Phytophthora infestans and of genes controlling immunity in Solanum demissum derivatives. Euphytica 2:173–178
Champouret N (2010) Functional genomics of Phytophthora infestans effectors and Solanum resistance genes. PhD thesis, Wageningen University
Chmielarz M, Sobkowiak S, Lebecka R Śliwka J (2010) Chosen characteristics of Polish Phytophthora infestans isolates. PPO-Special Report 14, pp 39–44
Corbiere R, Rekad FZ, Galfout A, Andrivon D, Bouznad Z (2010) Phenotypic and genotypic characteristics of Algerian isolates of Phytophthora infestans. PPO-Special Report 14, pp 291–296
Douches DS, Coombs J, Felcher K, Kirk WW, Long C, Bird G (2010) Missaukee: a roundwhite potato variety combining chip-processing with resistance to late blight, verticillium wilt and golden cyst nematode. Am J Pot Res 87:10–18
Eide CJ, Bonde R, Gallegly ME, Graham K, Mills WR, Niederhauser J, Wallin JR (1959) Report of the late blight investigations committee. Am Pot J 36:421–423
El-Kharbotly A, Palomino-Sanchez C, Salamini R, Jacobsen E, Gebhardt C (1996) R6 and R7 alleles of potato conferring race-specific resistance to Phytophthora infestans (Mont.) de Bary identified genetic loci clustering with the R3 locus on chromosome XI. Theor Appl Genet 92:880–884
El-Kharbotly A, Pereira A, Stiekema W, Jacobsen E (1995) Race specific resistance against Phytophthora infestans in potato is controlled by more genetic factors than only R genes. PhD Thesis, Wageningen University, Wageningen, pp 68–78
Forch M, van den Bosch T, van Bekkum P, Evenhuis B, Vossen JH, Kessel G (2010) Monitoring the Dutch Phytophthora infestans population for virulence against new R genes. PPO-Special Report no. 14, pp 39–1444
Foster SJ, Park TH, Pel MA, Brigneti G, Sliwka J, Jagger L, Van der Vossen EAG, Jones JDG (2009) Rpi-vnt1.1, a Tm-2 homolog from Solanum venturii confers resistance to potato late blight. Mol Plant Microbe Interact 22:589–600
Fry WE, Goodwin SB (1997) Resurgence of the Irish potato famine fungus. BioScience 47:363–371
Grunwald NJ, Flier WG, Sturbaum AK, Garay-Serrano E, van den Bosch TBM, Smart CD, Matuszak JM, Lozoya-Saldana H, Turkensteen LJ, Fry WE (2001) Population structure of Phytophthora infestans in the Toluca valley region of central Mexico. Phytopathology 91:882–890
Halterman DA, Kramer LC, Wielgus S, Jiang J (2008) Performance of transgenic potato containing the late blight resistance gene RB. Plant Dis 92:339–343
Haverkort AJ, Boonekamp PM, Hutten R, Jacobsen E, Lotz LAP, Kessel GJT, Visser RGF, van der Vossen EAG (2008) Societal costs of late blight in potato and prospects of durable resistance through cisgenic modification. Pot Res 51:47–57
Huang S, van der Vossen EAG, Kuang H, Vleeshouwers VGAA, Zhang N, Borm TJA, van Eck HJ, Baker B, Jacobsen E, Visser RGF (2005) Comparative genomics enabled the cloning of the R3a late blight resistance gene in potato. Plant J 42(2):251–261
Jacobsen E, Schouten HJ (2007) Cisgenesis strongly improves introgression breeding and induced translocation breeding of plants. Trends Biotechnol 25:219–223
Jo KR, Arens M, Kim TY, Jongsma MA, Visser RGF, Jacobsen E, Vossen JH (2011) Mapping of the S. demissum late blight resistance gene R8 to a new locus on chromosome IX. Accepted for publication in TAG
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Lehtinen A, Hannukkala A, Andersson B, Hermansen A, Le VH, Nærstad R, Brurberg MB, Nielsen BJ, Hansen JG, Yuen J (2008) Phenotypic variation in Nordic populations of Phytophthora infestans in 2003. Plant Pathol 57:227–234
Leonards-Schippers C, Gieffers W, Salamini F, Gebhardt C (1992) The R1 gene conferring race-specific resistance to Phytophthora infestans in potato is located on potato chromosome V. Mol Gen Genet 233:278–283
Li C, Wang J, Chien DH, Chujoy E, Song B, VanderZaag P (2010) Cooperation-88: a high yielding, multi-purpose, late blight resistant cultivar growing in Southwest China. Am J Pot Res 88:190–194
Li G, Huang S, Guo X, Li Y, Yang Y, Guo Z, Kuang H, Rietman H, Bergervoet M, Vleeshouwers VGAA, van der Vossen E, Qu D, Visser RGF, Jacobsen E, Vossen JH (2011) Cloning and characterization of R3b; members of the R3 superfamily of late blight resistance genes show sequence and functional divergence. Mol Plant Microbe Interact (in press). http://apsjournals.apsnet.org/doi/pdf/10.1094/MPMI-11-10-0276
Li X, van Eck HJ, Rouppe van der Voort JNAM, Huigen DJ, Stam P, Jacobsen E (1998) Autotetraploids and genetic mapping using common AFLP markers: the R2 allele conferring resistance to Phytophthora infestans mapped on potato chromosome 4. Theor Appl Genet 96:1121–1128
Lokossou AA, Park T, van Arkel G, Arens M, Ruyter-Spira C, Morales J, Whisson SC, Birch PRJ, Visser RGF, Jacobsen E, van der Vossen EAG (2009) Exploiting knowledge of R/Avr genes to rapidly clone a new LZ-NBS-LRR family of late blight resistance genes from potato linkage group IV. Mol Plant Microbe Interact 22:630–641
Lokossou (2010) Dissection of the major late blight resistance cluster on potato linkage group IV. PhD thesis, Wageningen University
Malcolmson JF, Black W (1966) New R genes in Solnum demissum Lindl. and their complementary races of Phytophthora infestans (Mont.) de Bary. Euphytica 15:199–203
Mastenbroek C (1952) Over de differentiatie van Phytophthora infestans (Mont.) de Bary en de vererving van de resistentie van Solanum demissum Lindl. PhD thesis, Fytopathologie, Landbouwhogeschool Wageningen, Wageningen, The Netherlands
Ordonez ME, Forbes GA, Trognitz BR (1998) Relationship between ineffective R gene and expansion rate of lesions on potato caused by Phytophthora infestans. Plant Pathol 47:130–136
Ordonez ME, Forbes GA, Trognitz BR (1997) Resistance to late blight in potato. A putative gene that suppresses R genes and is elicited by specific isolates. Euphytica 95:167–172
Park T, Foster S, Brigninna G, Jones JDG (2009) Two distinct potato late blight resistance genes from Solanum berthaultii are located on chromosome 10. Euphytica 165:269–278
Pel MA, Foster SJ, Park TH, Rietman H, Van Arkel G, Jones JDG, Van Eck HJ, Jacobsen E, Visser RGF, Van der Vossen EAG (2009) Mapping and cloning of late blight resistance genes from Solanum venturii using an interspecific candidate gene approach. Mol Plant Microbe Interact 22:601–615
Rauscher GM, Smart CD, Simko I, Bonierbale M, Mayton H, Greenland A, Fry WE (2006) Characterization and mapping of Rpi-ber, a novel potato late blight resistance gene from Solanum berthaultii. Theor Appl Genet 112:674–687
Rietman H (2011) Putting the Phytophthora infestans genome sequence at work; identification of many new R and Avr genes in Solanum. PhD thesis, Wageningen University
Song J, Bradeen JM, Naess SK et al (2003) Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc Natl Acad Sci USA 100:9128–9133
Tan MYA, Hutten RCB, Visser RGF, van Eck HJ (2010) The effect of pyramiding Phytophthora infestans resistance genes RPi-mcd1 and RPi-ber in potato. Theor Appl Genet 121:117–125
Taylor J (1973) The analysis of designed experiments with censored observations. Biometrics 29:35–43
Trognitz BR (1998) Inheritance of resistance in potato to lesion expansion and sporulation by Phytophthora infestans. Plant Pathol 47:712–722
Trognitz BR, Trognitz FC (2007) Occurrence of the R1 allele conferring resistance to late blight in potato R gene differentials and commercial cultivars. Plant Pathol 56:150–155
van der Vossen EAG, Sikkema A, Hekkert BTL, Gros J, Stevens P, Muskens M, Wouters D, Pereira A, Stiekema W, Allefs S (2003) An ancient R gene from the wild potato species Solanum bulbocastanum confers broad-spectrum resistance to Phytophthora infestans in cultivated potato and tomato. Plant J 36(6):867–882
van der Vossen EAG, Gros J, Sikkema A, Muskens M, Wouters D, Wolters P, Perira A, Allefs S (2005) The Rpi-blb2 gene from Solanum bulbocastanum is an Mi-1 gene homolog conferring broad-spectrum late blight resistance in potato. Plant J 44:208–222
Vleeshouwers VGAA, Raffaele S, Vossen JH, Champouret N, Oliva R, Segretin ME, Rietman H, Cano LM, Lokossou AA, Kessel GJT, Pel M, Kamoun S (2011) Understanding and exploiting late blight resistance in the age of effectors. Annu Rev Phytopathol 49 (in press). http://www.annualreviews.org/doi/pdf/10.1146/annurev-phyto-072910-095326
Vleeshouwers VGAA, van Dooijeweert W, Govers F, Kamoun S, Colon LT (2000) Does basal PR gene expression in Solanum species contribute to non-specific resistance to Phytophthora infestans. Mol Plant Pathol 57:35–42
Vleeshouwers VGAA, Rietman H, Krenek P, Champouret N, Young C, Oh SK, Wang M, Bouwmeester K, Vosman B, Visser RGF, Jacobsen E, Govers F, Kamoun S, Van der Vossen EAG (2008) Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. Plos One 3(8):e2875
White S, Shaw D (2010) Breeding for host resistance: the key to sustainable potato production. PPO-Special Report 14, pp 125–130
Zhang XZ, Kim B (2007) Physiological Races of Phytophthora infestans in Korea. Plant Pathol J 23:219–222
Zhu S, Li Y, Vossen JH, Visser RGF, Jacobsen E (2011) One step stacking of three potato late blight resistance genes; the use of avirulence genes to select for functionality. Transgenic Res. http://www.springerlink.com/content/ek7183524459724p/fulltext.pdf
Acknowledgments
HJK was supported by the Korea Research Foundation Grant funded by the Korean Government [KRF-2008-357-F00013]. HRL, GK, BE, and JV were supported by the DuRPh program funded by the Ministry of Agriculture in the Netherlands (now Ministry of EL&I). MM was supported by a grant from the University of Tehran. KRJ was financially supported by the international program BO-10-010-112 program of the Ministry of EL&I and the EuropeAid program 128275/C/ACT/KP2 project DCI-FOOD/2009/218-671
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gebhardt.
H.-J. Kim, H.-R. Lee and K.-R. Jo contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2011_1757_MOESM1_ESM.xls
Table S1. Segregation of resistance and susceptibility, R gene markers and Avr responses in MaR8 (3020) and MaR9 (3025) F1 populations. (XLS 120 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kim, HJ., Lee, HR., Jo, KR. et al. Broad spectrum late blight resistance in potato differential set plants MaR8 and MaR9 is conferred by multiple stacked R genes. Theor Appl Genet 124, 923–935 (2012). https://doi.org/10.1007/s00122-011-1757-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-011-1757-7