Abstract
Urbanisation is one of the biggest environmental challenges of our time, yet we still lack an integrative understanding of how cities affect behaviour, physiology and parasite susceptibility of free-living organisms. In this study, we focus on carotenoids, strictly dietary micronutrients that can either be used as yellow-red pigments, for integument colouration (signalling function), or as antioxidants, to strengthen the immune system (physiological function) in an urban predator, the Eurasian kestrel (Falco tinnunculus). Kestrels are specialised vole hunters but shift to avian prey in cities where diurnal rodents are not sufficiently available. This different foraging strategy might determine the quantity of carotenoids available. We measured integument colouration, circulating carotenoids in the blood and ectoparasite burden in kestrels along an urban gradient. Our results showed that nestlings that were raised in more urbanised areas displayed, unrelated to their ectoparasite burden, a paler integument colouration. Paler colours were furthermore associated with a lower concentration of circulating carotenoids. These findings support the hypothesis that the entire urban food web is carotenoid deprived and only prey of low quality with low carotenoid content is available (e.g. fewer carotenoids in urban trees, insects, small birds and finally kestrels). The alternative hypothesis that nestlings allocate carotenoids to reduce physiological stress and/or to cope with parasites rather than invest into colouration could not be supported. Our study adds to existing evidence that urban stressors negatively affect carotenoid production in urban areas, a deficiency that dissipate into higher trophic levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanisation has greatly altered the environment to serve the need of one species: humans Seto, Güneralp et al. (2012). The process of urbanisation transforms natural areas to artificial environments characterised by high levels of night light, noise, and air pollution, changes in resource availability and quality (Sumasgutner, Cunningham et al. 2023), competition with alien species and alteration of species composition which includes pets, livestock and high densities of humans (Faeth et al. 2005; Shanahan et al. 2014). These new challenges presented by cities cause a huge negative impact in reducing the local biodiversity, where only a few species persist (so-called biotic homogenisation; McKinney 2006). Typical behavioural adjustments seen in urban environments (Lowry, Lill et al. 2013) include the usage of anthropogenic food (Meyrier, Jenni et al. 2017; Stofberg, Cunningham et al. 2019), nesting structures or nesting material (Reynolds, Ibáñez-Álamo et al. 2019). The success of an animal in urban areas could therefore depend on its behavioural flexibility (or phenotypic plasticity) that might include personality traits like boldness or aggressiveness (Lowry, Lill et al. 2013).
Urban exploiter species reach higher densities in cities than in a surrounding non-urban habitat (Møller, Diaz et al. 2012). These higher densities are usually explained by the high availability of attractive artificial nesting sites, especially for cavity nesters (Sumasgutner, Schulze et al. 2014a; Meyrier, Jenni et al. 2017). However, limited accessibility of high-quality food resources in cities could have negative health or fitness consequences (Sumasgutner, Nemeth et al. 2014b; Meyrier, Jenni et al. 2017; Catto, Sumasgutner et al. 2021). Thus, urban areas do not necessarily constitute optimal habitat for urban exploiters, as they can mislead animals by, for example, a high number of nesting opportunities into areas with low food availability and quality.
Raptors have successfully colonised cities across the globe (Boal and Dykstra 2018; Kettel, Gentle et al. 2018; McPherson, Sumasgutner et al. 2021; Headland, Colombelli-Négrel et al. 2023). Whether a raptor species is more or less urban tolerant largely depends on the body size, with smaller raptors being more abundant in cities (Cooper, Shultz et al. 2022; Headland, Colombelli-Négrel et al. 2023), and on the prey type, with avian specialists showing higher breeding success in cities (Kettel, Gentle et al. 2018). For example, peregrine falcons (Falco peregrinus), which are specialised in hunting birds, show larger clutch and brood sizes (Kettel, Gentle et al. 2019; Sumasgutner, Jenkins et al. 2020), while Eurasian kestrels as typical rodent hunters suffer from lower breeding success (Sumasgutner, Nemeth et al. 2014a) in urban centres. These findings highlight that diet has an important role to play in the success of urban-living raptors.
Every prey type has characteristic nutritional components and differentiates in caloric content and micronutrient composition (Fargallo, Navarro-Lopez et al. 2020). While voles are rich in calories, they are restricted in their carotenoid content (Goodwin 1980). In contrast, insectivores like some birds, shrews and lizards, and insects themselves, have an overall higher carotenoid content from the plant they feed on (Goodwin 1980) but might be more energy-demanding to catch and/or provide less calories. Carotenoids are micronutrients that are strictly dietary in vertebrates and can thus constitute a limited resource (Hill and McGraw 2006). Health-related functions of carotenoids include antioxidant capacities to limit oxidative damage triggered by unfavourable environmental conditions such as pollution or to deal with parasite infections as immune-stimulant (von Schantz, Bensch et al. 1999; Martinez-Padilla, Mougeot et al. 2007; Pérez-Rodríguez, Martínez-Padilla et al. 2013). Correlations between carotenoids and parasite infections have been demonstrated in several avian systems, including passerines (Pap, Vágási et al. 2011; Figuerola, López et al. 2014), grouse (Martinez-Padilla, Mougeot et al. 2007) and raptors (De Neve, Fargallo et al. 2008). The different signalling and physiological functions of carotenoids can thus result in a trade-off whereby intense yellow-red colouration is known to be important for mate selection, signalling a strong, healthy individual (Weaver, Santos et al. 2018).
The carotenoid content of prey determines the carotenoid availability for the raptor, which can then be allocated either as yellow-red pigments for integument colouration or as antioxidants to health-related functions (Casagrande, Costantini et al. 2009). Within this physiological trade-off, both components of the carotenoid pathway can be measured, as carotenoid-based colouration of skin or feathers or as circulating carotenoids in the bloodstream.
The Eurasian kestrel population in Vienna, Austria, is one of the densest breeding populations of raptors in any city worldwide (Sumasgutner, Nemeth et al. 2014a; Sumasgutner, Schulze et al. 2014b. However, high population density does not necessarily indicate a viable population: high nestling mortality with low overall productivity (Sumasgutner, Nemeth et al. 2014a; Sumasgutner, Schulze et al. 2014b) and impaired physiological health (Sumasgutner, Adrion et al. 2018; Wemer, Hegemann et al. 2021) are characteristics of this study system that cannot be explained by density dependence alone (Sumasgutner, Nemeth et al. 2014a). Urban kestrels display a more generalists’ diet (Sumasgutner, Krenn et al. 2013) and include more nocturnal rodents (house mice and Apodemus species), birds, reptiles and insects compared to their vole specialist conspecifics in suburban and rural areas. The high proportion of alternative prey in the city centre (Sumasgutner, Krenn et al. 2013) and the higher breeding success in proximity to green spaces, even within urban areas (Sumasgutner, Schulze et al. 2014a), both suggest that kestrels are likely to hunt near their nests. Indeed, first tracking studies in Rome, Italy, confirm them to be city-bound with rather small home range sizes (Costantini and Dell’Omo 2020). These dietary shifts and/or decreased foraging efficiencies in catching alternative prey result in an overall lower amount of food for nestlings. Furthermore, the constant exposure to stressors in urban areas (i.e. light, noise and air pollution or urban heat; see review in Sumasgutner, Cunningham et al. (2023)) might impact health and fitness.
Previously, we have shown that urban nestlings show paler integument colouration (Sumasgutner et al. 2018), but thus far, we could not disentangle if this is due to a lower carotenoid content in urban prey or due to a higher demand for carotenoids as dietary antioxidants in the immune system. We identified circulating carotenoids in the bloodstream as an important missing link to draw a more complete picture on the carotenoid trade-off in the city. Our findings on paler colouration in urban kestrels were already surprising, as the known change of main prey types from voles to birds, lizards and insects in urban kestrels would predict a larger intake of carotenoids in the city, which could then be visible in a more intense colouration (i.e. the trophic chain would start with a high carotenoid availability through birds, lizards and insects (García-Heras, Arroyo et al. 2017)). However, carotenoid content of primary producers can in turn be reduced due to pollution in urban areas in a way that plants synthesise less carotenoids when exposed to environmental stress. This environmental paucity of carotenoids could be an alternative explanation for the found paler colouration in kestrels (Sumasgutner, Adrion et al. 2018) and other urban-dwelling species (Sillanpaa, Salminen et al. 2008; Jensen, Ziegler et al. 2022).
Given this background, we explore two possible explanations for paler carotenoid-based colouration found in urban kestrels. First, if the urban environment is overall lower on carotenoids, we would expect both integument colouration and the level of circulating carotenoids in urban kestrels to be lower than in suburban and rural kestrels. Second, if carotenoids are preferentially allocated towards maintaining a high circulating level to defeat reactive oxygen species as a consequence of exposure to urban stressors and/or ectoparasite infection, we would expect paler colouration but elevated levels of circulating carotenoids in the blood together with higher ectoparasite burdens.
Material and methods
Study system
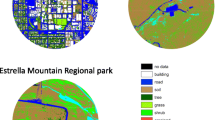
The study area is the city of Vienna, Austria (48° 12′N, 16° 22′E; 415 km2; 150 ± 500 m asl; 1.9 million inhabitants), where the density of kestrels is estimated to be 89–122 breeding pairs per 100 km2 in urbanised areas (Sumasgutner, Nemeth et al. 2014a). We quantify the degree of urbanisation GIS-based as percentage of sealed surfaces within a buffer circle of r = 500 m (78.5 ha) around each nest (Fig. 1; see details in the method in Sumasgutner, Nemeth et al. (2014b)), corresponding to kestrel home range sizes in urban areas (Beichle 1980; Costantini and Dell’Omo 2020). The resulting continuous measurement of urbanisation (Seress, Lipovits et al. 2014) quantifies variation in several urban factors simultaneously, i.e. infrastructure (physical measure of sealed surface area), human population (disturbance) and the exposure to anthropogenic stressors including artificial light at night (see Stathakis et al. 2015; Zhang & Seto 2013 for validation studies). The nesting sites were sampled for carotenoid-based integument colouration and for plasma volumes of circulating carotenoids in 2020 (n = 19) and 2021 (n = 17) and were located in areas between 25 and 95% sealed surface area. Because some nest sites were sampled in both years, the effective sample size is 28 nests for the analysis on integument colouration and 22 nests for the analysis on circulating carotenoids (Fig. 2). Year was included as covariate in both models to account for a potential annual variation.
Urban study area in Vienna, Austria (243 km2). The urban gradient was quantified by the percentage of sealed soil and displayed from light grey to black (white areas (< 1%) are mainly agriculture areas in the east and large forested areas in the west, which were not systematically monitored). The locations of kestrel nesting sites sampled for this study (n = 28) are represented in orange
Field procedure
Each kestrel was banded with a uniquely coded metal ring (provided by the Austrian Ornithological Centre; “Österreichische Vogelwarte”) and an alpha-alpha–coded colour ring between 10 and 25 days after hatching. Kestrel nestlings hatch asynchronous, why large age discrepancies can exist within one brood, explaining the variation in sampling age. In addition, the birds were measured and weighed (according to the Standard Manual of the Austrian Ornithological Centre), and the number of ectoparasites (species Carnus hemapterus) per individual was counted without removing them from the body. The age of the nestlings was determined based on their wing length according to Kostrzewa and Kostrzewa (1987). Importantly, age was not confounded with the urban gradient (i.e. the sampling age was not earlier or later in more or less urbanised areas; Pearson correlation coefficient r = −0.024), nor was it confounded with the number of ectoparasites (i.e. younger nestlings tend to have more ectoparasites (Roulin, Gasparini et al. 2008), r = −0.23). To account for the hatching asynchrony, each nestling was assigned a hatch rank, as senior (“1”, first hatched/largest), junior (“3”, last-hatched/smallest) or middle (“2”, all the nestlings in between) sibling (see Martínez-Padilla and Viñuela 2011 for more details). To derive a nestlings’ body mass index, we extracted the residuals of a linear model with body mass (in g) as the response and wing length (in mm, linear and quadratic) and sex (males being 20% smaller than females in body mass, Village 1990) as explanatory variables, following Roulin et al. (2007).
Carotenoid-based integument colouration
The colour of the skin (cere, orbital ring and tarsus) was evaluated by comparing the bare parts of the nestlings with a reference colour chart (Michel 2000) and recorded with digital photographs for further extraction of spectral measurements using GIMP 2.10.28. We selected a homogeneous area at the photographs to obtain hue (H), saturation (S) and brightness (B) for each bare part and the corresponding colour chart. The hue represents the perceived colour (e.g. yellow, orange, red) using values ranging from 0° to 360°, the saturation indicates the intensity and purity (in %) of the colouration, and brightness indicates the “quantity of white” (in %) in the colour (see García-Heras et al. 2017 for further details; and illustration in Appendix Figure 8). The colour measurements were taken from 112 nestling across 28 different nest sites over the 2-year period. Due to the high similarity between the colour of the cere and the orbital ring, we averaged the values into one hue, saturation and brightness value, respectively, as “face colouration”. We concluded this “high similarity” by the fact that face colouration could always be matched with the same colour chart reference, while tarsus colouration usually required a different colour field on the chart as reference. Face colouration was furthermore positively correlated with tarsus colouration measurements (hue: r = 0.04, p < 0.0001; saturation: r = 0.56, p < 0.0001; brightness: r = 0.41, p < 0.0001). To reduce the number of variables, a principal component analysis (PCA) was run on the 6 remaining colouration variables (i.e. hue, saturation, brightness of face and tarsus, respectively). The first principal component (PC1-colouration) explained 82.3% of the variance, while the second and third principal components (PC2-colouration and PC3-colouration) explained further 7.2% and 5.8%, respectively (Appendix Table 3). PC1-colouration represents face hue (e.g. bluish or yellowish), where high axis values consist of low face hue values (yellowish face colouration) and low axis values of high face hue values (bluish face colouration) (Appendix Figure 9). The PC2-colouration represents saturation (i.e. intensity) of colouration, which is usually more strongly pronounced in the tarsus than in the face. High axis values of PC2-colouration indicate a lower saturation. The PC3-colouration represents brightness (i.e. whiteness) of colouration, with high axis values indicating lower brightness.
Plasma volumes of circulating carotenoids
Blood samples were collected after disinfecting the skin with an alcohol swab, by puncturing the brachial vein with a sterilised 21-gauge needle and extracting the blood with a 220-μL capillary tube. The blood was stored in a heparinised Eppendorf vial and kept on ice in a thermos until the sample was centrifuged for 15 min at 3000 rpm to separate the plasma from the red blood cells within 4 h after sampling. The plasma was stored at −60 °C until further processing.
After thawing the blood samples for ~30 min on ice, 20-μl plasma was transferred into an Eppendorf tube and mixed with 200-μl high-performance liquid chromatography (HPLC) grade acetone and the internal standard (IS) (600-μM retinyl acetate Sigma + 1-mM tocopheryl acetate Sigma). The tube was vortexed for 10 sec, a 100-μl tert-butyl methyl ether was added, and then vortexed again for another 10 sec. The samples were centrifuged at 10 °C for 5 min at 13,000 rpm. The supernatant was evaporated with a rotary evaporator (Rotavapor) under vacuum for 30 min. The dried sample was dissolved in a 100 μl of the HPLC mobile phase (methanol-acetonitrile (30:70 v/v)) and transferred to a HPLC glass vial and injected into the HPLC system. The HPLC method was carried out with a Thermo Scientific UltiMate 3000 adapted to a Develosil RPAqueous RP-30 HPLC column (250 × 4.6 mm I.D.; Nomura Chemical Co., Ltd., Japan). An isocratic system (HP 1050 Series Isocratic Pump) was applied at a constant flow rate of 1.2 mL/min for 26 min. Carotenoids were detected at 450 nm using an Ultimate 3000 Diode Array Detector UV/VIS detector, whereas the internal standards were detected at 210 and 325 nm. Lutein and zeaxanthin were identified as the two main carotenoids. The data analysis was carried out with the Thermo Scientific™ Chromeleon™ chromatography data system (CDS) software. The concentration of lutein and zeaxanthin was expressed in nmol/mL and summed to a total “circulating carotenoid” concentration for 88 kestrel nestlings across 22 different nest sites over the 2-year period. We did not consider zeaxanthin any further, due to overall low values, following recommended methods from other raptor species (García-Heras, Arroyo et al. 2017).
Statistical analyses
All statistical analyses were carried out in R version 4.0.1 (R Development Core Team 2020) using the “lme4” (Bates, Maechler et al. 2015) package, and results were visualised using the “effects” (Fox 2003) and “ggplot2” (Wickham 2009) packages. Residual distributions of the models were inspected visually to assess model fit by evaluating the model criticism plots produced by the “plot” function in the base package and the “mcp_fnc” function in the “LMERConvenienceFunctions” package (Tremblay and Ransijn 2015). The significance of the fixed effects was calculated with the “Anova” function type III in the “car” package (Fox and Weisberg 2019) and was accepted at p≤0.05, and the confidence intervals were set to 95%. Nest site (ID of nest location) was considered as a random factor in each model to account for pseudoreplication arising from the lack of independence of nestlings within one brood and considering the same nest site in consecutive years (2020 and 2021), potentially used by the same breeding pair. Multiple covariates were added to control for their potential influence on carotenoid trade-off: urban gradient (% cover of sealed surface area) and number of ectoparasites as main predictors as per our hypotheses, age (in days), hatch rank (factor in three levels: junior, middle, senior), body mass index, brood size and study year. All quantitative variables (i.e. concentration of circulating carotenoids (log transformed to archive normality), urban gradient, number of ectoparasites, age, brood size) were scaled to ensure that the effect sizes were on a comparable scale.
First, to determine the relationship between the different colour variables (i.e. face hue: PC1-colouration, saturation: PC2-colouration, and brightness: PC3-colouration) and the circulating carotenoids, a generalised linear mixed model (GLMM) per colour variable was created in which the colour variable was the response variable and the concentration of the circulating carotenoids together with study year were the explanatory variables.
Second, to test the influences of different variables (urban gradient, number of ectoparasites, age, hatch rank, body mass index, brood size and study year) on the colour variables, another set of GLMMs (one per colour variable) was created in which integument colouration was the response variable.
Third, to investigate the effects of the different variables (urban gradient, number of ectoparasites, age, hatch rank, body mass index, brood size and study year) on the concentration of circulating carotenoids, a last GLMM was created.
Results
Carotenoid-based colouration and circulating carotenoids
The saturation of the colouration (PC2-colouration) was negatively correlated with the concentration of circulating carotenoids in nestlings (χ2 = 16.442, p < 0.0001) indicating that higher volumes of circulating carotenoids were associated with a more saturated (purer) colouration of integuments (Fig. 3) and vice versa. The higher the concentration of lutein and zeaxanthin in the plasma, the more yellow the skin appears. There was no obvious association between circulating carotenoids and face hue (PC1-colouration; χ2 = 0.577, p = 0.447) or brightness (PC3-colouration; χ2 = 0.283, p = 0.595).
Association between colouration, urbanisation and ectoparasites
PC1-colouration decreased significantly with an increasing urban gradient, which means that face hue became more bluish and less yellowish towards the city centre (χ2 = 7.705, p = 0.006; Table 1; Fig. 4A). No relationship was found between face hue (PC1-colouration) and the number of ectoparasites (χ2 = 0.171, p = 0.680; Table 1; Fig. 4B).
Results of the model testing for the relation between the face hue (PC1-colouration) and A the urban gradient (in %) (p = 0.006) and B number of ectoparasites (p = 0.680). Plots show raw data in background scatter, effect sizes of GLMM and 95% confidence intervals. Model outputs are provided in Table 1
PC2-colouration increased significantly with an increasing urban gradient, which means that saturation of the colouration (relative to white) of the chicks became weaker towards the city centre (χ2 = 5.39, p = 0.020; Table 1; Fig. 5A). No relationship was found between saturation (PC2-colouration) and the number of ectoparasites (χ2 = 0.292, p = 0.292; Table 1; Fig. 5B).
Results of the model testing for the relation between the saturation (PC2-colouration) and A the urban gradient (in %) (p = 0.02), B number of ectoparasites (p = 0.292) and C the age (in days) (p = 0.019). Plots show raw data in background scatter, effect sizes of GLMM and 95% confidence intervals. Model outputs are provided in Table 1
PC3-colouration decreased almost significantly with an increasing urban gradient, which would mean that brightness of colouration increased towards the city centre (χ2 = 3.824, p = 0.051; Table 1; Fig. 6A). No relationship was found between brightness (PC3-colouration) and the number of ectoparasites (χ2 = 0.679, p = 0.410; Table 1; Fig. 6B). Furthermore, both PC2-colouration and PC3-colouration decreased significantly with an increasing age, whereby older chicks had a more saturated (more intense) colouration than younger ones (χ2 = 5.42, p = 0.019; Table 1; Fig. 5C) and older chicks were also brighter (χ2 = 11.964, p < 0.001; Table 1; Fig. 6C). We found a significant year effect on PC3-colouration, showing that nestlings in 2021 were brighter than nestlings in 2020 (χ2 = 9.901, p = 0.002; Table 1; Fig. 6D).
Results of the model testing for the relation between the brightness (PC3-colouration) and A the urban gradient (in %) (p = 0.051), B number of ectoparasites (p = 0.410), C the age (in days) (p < 0.001) and D year (p = 0.002). Plots show raw data in background scatter, effect sizes of GLMM and 95% confidence intervals. Model outputs are provided in Table 1
Association between circulating carotenoids, urbanisation and ectoparasites
The concentration of circulating carotenoids (sum of lutein and zeaxanthin) decreased significantly with an increasing urban gradient, meaning that chicks in more urbanised areas had lower circulating levels of carotenoids compared to those in more suburban areas (χ2 = 10.714, p = 0.001; Table 2; Fig. 7A). There was no obvious association between circulating carotenoids and the number of ectoparasites (χ2 = 0.574, p = 0.449; Table 2; Fig. 7B), but circulating carotenoids were significantly higher in larger broods (χ2 = 6.831, p = 0.009; Table 2; Fig. 7C) and increased significantly with increasing age, in a way that older siblings had higher blood volumes of circulating carotenoids than younger siblings (χ2 = 4.144, p = 0.042; Table 2; Fig. 7D).
Results of the models testing for the relation between circulation carotenoids concentration (in nmol/mL) and a urban gradient (in %) (p = 0.001), b numbers of ectoparasites (p = 0.449), c brood size (p = 0.009) and d age (in days) (p = 0.042). Plots show raw data in background scatter, effect sizes of GLMM and 95% confidence intervals. Models outputs are provided in Table 2
Discussion
Eurasian kestrels are specialised vole hunters but shift to avian prey in urban environments where diurnal rodents are not sufficiently available (Sumasgutner, Schulze et al. 2014a). This different foraging strategy determines the amount of carotenoids that can either be used for integument colouration or for the immune system (Pérez-Rodríguez 2009). Consistently, less intense integument colouration and lower volumes of circulating carotenoids towards the city centre support the idea that the entire urban food web is deprived of carotenoids, as no association between carotenoids and ectoparasite infection intensities was found. Kestrel nestlings in more urbanised areas had a more bluish face colouration, an overall less saturated colouration and were duller, together with a lower carotenoid concentration in the bloodstream. In comparison, nestlings in less urbanised areas were more yellowish coloured—this is despite the known dietary shift that would suggest carotenoid richer prey types (like birds, shrews, lizards and insects) to be taken in urban centres and voles that are poorer in carotenoid content to be the main prey in suburban and rural areas.
Carotenoids have multiple functions in both plants (the synthesisers) and animals (consumers). Apart from being antioxidants, carotenoids play a role in UV protection, and cell membrane stability, and are used as colour pigments in skin, scales and feathers and as photosynthetic pigments in plants (Britton 2008). These functions can be affected by urbanisation and heat (Sumasgutner, Cunningham et al. 2023)—which includes the urban heat island effect whereby urban centres are usually several degrees warmer than the surrounding natural areas (Oke 1982; Imhoff, Zhang et al. 2010). In plants, heat stress, UV exposure and air pollution affect carotenoid function and level (e.g. (Camejo, Rodríguez et al. 2005; Joshi and Swami 2009)). Effects on the carotenoid synthesising trees can influence the carotenoid content in the entire food web (i.e. lower carotenoid levels in city trees (Isaksson 2009; Khosropour, Attarod et al. 2018), followed by a lower carotenoid content in urban caterpillars (Isaksson and Andersson 2007) and urban insectivores that are in turn important prey sources of urban kestrels (Sumasgutner, Krenn et al. 2013)). This together with other changes in diet quality can lead to physiological constraints that affect morphology (Olson and Owens 1998). Carotenoid-based plumage coloration is paler in urban than rural great tits Parus major (Isaksson, Örnborg et al. 2005), and carotenoid-based integument colouration is paler in kestrels ((Sumasgutner, Adrion et al. 2018) and this study). Our results on lower volumes of circulating carotenoids in urban centres add to these previous findings on colouration. Thus, we assume bottom-up effects, with primary producers synthesising less carotenoids—which could be associated to coping with environmental stress, including heat, in more urbanised areas—affecting all trophic levels.
Concerning ectoparasite infection intensity, no correlation with coloration or circulating carotenoids could be revealed—contrasting results of a previous investigation in the same system. Sumasgutner et al. (2018) found that while ectoparasite burden in kestrel nestlings had no direct association to the urban gradient, infection intensity was higher in nestlings with less intense face skin yellowness and was also higher in earlier broods. This inconsistency could be due to two aspects: first, in our study years, many nestlings had no ectoparasites—something we consider unusual in the study population. Thus, the result could be a year effect. Second, we adjusted the methodology from scoring parasite infection in ordinal categories (Sumasgutner, Adrion et al. 2018; Wemer, Hegemann et al. 2021) to an actual count of parasites per individual nestling to increase data resolution and variance.
Well known is an association between carotenoids and developmental stage, whereby integument colouration and blood concentrations increased both with nestlings’ age. This could be due to an improved absorption of carotenoids as well as an accumulation over time and was also reported in other raptor populations (Casagrande, Costantini et al. 2007; García-Heras, Arroyo et al. 2017). However, no association between carotenoids and body mass was seen, which could indicate that integument coloration and blood concentration depend on the quality (carotenoid content) of the ingested prey items rather than the quantity of food, as mentioned in other studies (Eeva, Sillanpää et al. 2009; Sternalski, Mougeot et al. 2010; Sternalski, Mougeot et al. 2012; Nebel, Amar et al. 2021). This could also be one reason why no relationship between carotenoids and hatch rank was found either, because hatch rank is directly informed by nestling size and was assigned in the field. Finally, we found a positive correlation between brood size and circulating carotenoids whereby nestlings in larger broods had a higher concentration of circulating carotenoids than nestlings in smaller broods. This could be directly linked to the fact that kestrel broods in suburban and rural areas are consistently larger (Sumasgutner, Nemeth et al. 2014a; Sumasgutner, Schulze et al. 2014b) than those in inner city districts, supporting the idea of larger broods in areas with higher food quality and hence carotenoid availability—which are suburban and rural areas.
Conclusions
Our results suggest that urbanisation has an impact on prey with accumulative effects into higher trophic levels. Diet quality (carotenoid content) likely decreased with a higher degree of urbanisation, resulting in a more bluish face colouration of kestrel nestlings and especially a less saturated (less intense) integument colouration. Moreover, we found no correlation between carotenoids and ectoparasite load. Our finding supports the hypothesis that the entire urban food web is carotenoid deprived and only low-quality prey with low carotenoid content is available (e.g. fewer carotenoids in urban trees, insects, small birds, kestrels). The alternative hypothesis that nestlings allocate carotenoids as antioxidants to reduce physiological stress and/or to cope with ectoparasites rather than allocate carotenoids as pigments to coloration could therefore not be supported in this descriptive study but would require a more experimental approach. Our results might reinforce the assumption of Costantini and Møller (2008) that carotenoids only play a minor role as antioxidants in birds and that it would be important to conduct further studies considering several species at different stages within their life cycle to achieve a more complete understanding of the carotenoid pathway in avian systems.
Data Availability
The data underlying this study will be made available as supplementary electronic material upon acceptance of the manuscript.
References
ASAB (2018) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 135:I-X. https://doi.org/10.1016/j.anbehav.2017.10.001
Bates D, Maechler M et al (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Beichle U (1980) Siedlungsdichte, Jagdreviere und Jagdweise des Turmfalken (Falco tinnunculus) im Stadtgebiet von Kiel. Corax 8:3–12
Boal CW, Dykstra CR (2018) Urban raptors. Island Press, Washington, DC
Britton G (2008) Functions of intact carotenoids. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids. Springer, Basel, pp 189–212
Camejo D, Rodríguez P et al (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol 162(3):281–289. https://doi.org/10.1016/j.jplph.2004.07.014
Casagrande S, Costantini D et al (2007) Patterns of serum carotenoid accumulation and skin colour variation in kestrel nestlings in relation to breeding conditions and different terms of carotenoid supplementation. J Comp Physiol B: Biochem Sys Environ Physiol 177(2):237–245. https://doi.org/10.1007/s00360-006-0125-4
Casagrande S, Costantini D et al (2009) Phenotypic, genetic, and environmental causes of variation in yellow skin pigmentation and serum carotenoids in Eurasian kestrel nestlings. Ecol Res 24(2):273–279. https://doi.org/10.1007/s11284-008-0503-3
Catto S, Sumasgutner P et al (2021) Pulses of anthropogenic food availability appear to benefit parents, but compromise nestling growth in urban red-winged starlings. Oecologia 197:565–576. https://doi.org/10.1007/s00442-021-05033-3
Cooper DS, Shultz AJ et al (2022) Community science data suggest the most common raptors (Accipitridae) in urban centres are smaller, habitat-generalist species. Ibis 164(3):771–784. https://doi.org/10.1111/ibi.13047
Costantini D, Dell’Omo G (2020) The kestrel: ecology, behaviour and conservation of an open-land predator. Cambridge University Press
Costantini D, Møller AP (2008) Carotenoids are minor antioxidants for birds. Funct Ecol 22(2):367–370. https://doi.org/10.1111/j.1365-2435.2007.01366.x
De Neve L, Fargallo JA et al (2008) Effects of maternal carotenoid availability in relation to sex, parasite infection and health status of nestling kestrels (Falco tinnunculus). J Exp Biol 211(9):1414–1425. https://doi.org/10.1242/jeb.014290
Eeva T, Sillanpää S et al (2009) The effects of diet quality and quantity on plumage colour and growth of great tit Parus major nestlings: a food manipulation experiment along a pollution gradient. J Avian Biol 40(5):491–499. https://doi.org/10.1111/j.1600-048X.2008.04535.x
Faeth SH, Shochat E et al (2005) Trophic dynamics in urban communities. Bioscience 55(5):399–407. https://doi.org/10.1641/0006-3568(2005)055[0399:tdiuc]2.0.co;2
Fargallo JA, Navarro-Lopez J et al (2020) Foraging strategy of a carnivorous-insectivorous raptor species based on prey size, capturability and nutritional components. Sci Rep 10(1):7583. https://doi.org/10.1038/s41598-020-64504-4
Figuerola J, López G et al (2014) Plasma carotenoid levels in passerines are related to infection by (some) parasites. Front Ecol Evol 2:47. https://doi.org/10.3389/fevo.2014.00047
Fox J (2003) Effect displays in R for generalised linear models. J Stat Softw 8(15):1–27
Fox J and Weisberg S (2019) An R companion to applied regression (third) sage.
García-Heras M-S, Arroyo B et al (2017) Pollutants and diet influence carotenoid levels and integument coloration in nestlings of an endangered raptor. Sci Total Environ 603–604:299–307. https://doi.org/10.1016/j.scitotenv.2017.06.048
Goodwin TW (1980) The biochemistry of the carotenoids. Chapman and Hall, London, New York
Headland T, Colombelli-Négrel D et al (2023) Smaller Australian raptors have greater urban tolerance. Sci Rep 13(1):11559. https://doi.org/10.1038/s41598-023-38493-z
Hill GE, McGraw KJ (2006) Bird coloration: mechanisms and measurements. Harvard University Press, Cambridge, Mass
Imhoff ML, Zhang P et al (2010) Remote sensing of the urban heat island effect across biomes in the continental USA. Remote Sens Environ 114(3):504–513. https://doi.org/10.1016/j.rse.2009.10.008
Isaksson C (2009) The chemical pathway of carotenoids: from plants to birds. Ardea 97(1):125–128. https://doi.org/10.5253/078.097.0116
Isaksson C, Andersson S (2007) Carotenoid diet and nestling provisioning in urban and rural great tits Parus major. J Avian Biol 38(5):564–572. https://doi.org/10.1111/j.2007.0908-8857.04030.x
Isaksson C, Örnborg J et al (2005) Plasma glutathione and carotenoid coloration as potential biomarkers of environmental stress in Great Tits. EcoHealth 2(2):138–146. https://doi.org/10.1007/s10393-005-3869-5
Jensen JK, Ziegler AK et al (2022) Quantifying the influence of urban biotic and abiotic environmental factors on great tit nestling physiology. Sci Total Environ 859:160225. https://doi.org/10.1016/j.scitotenv.2022.160225
Joshi PC, Swami A (2009) Air pollution induced changes in the photosynthetic pigments of selected plant species. J Environ Biol 30(2):295–298
Kettel EF, Gentle LK et al (2018) The breeding performance of raptors in urban landscapes: a review and meta-analysis. Journal of Ornithology 159(1):1–18. https://doi.org/10.1007/s10336-017-1497-9
Kettel EF, Gentle LK et al (2019) Breeding performance of an apex predator, the peregrine falcon, across urban and rural landscapes. Urban Ecosyst 22(1):117–125. https://doi.org/10.1007/s11252-018-0799-x
Khosropour E, Attarod P et al (2018) Response of Platanus orientalis leaves to urban pollution by heavy metals. J For Res 30(4):1437–1445. https://doi.org/10.1007/s11676-018-0692-8
Kostrzewa R, Kostrzewa A (1987) Zur Jugendentwicklung des Turmfalken (Falco tinnunculus) ein Altersbestimmungsschlüssel. Ökologie der Vögel 9:119–125
Lowry H, Lill A et al (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88(3):537–549. https://doi.org/10.1111/brv.12012
Martinez-Padilla J, Mougeot F et al (2007) Nematode parasites reduce carotenoid-based signalling in male red grouse. Biol Lett 3(2):161–164. https://doi.org/10.1098/rsbl.2006.0593
Martínez-Padilla J, Viñuela J (2011) Hatching asynchrony and brood reduction influence immune response in common kestrel Falco tinnunculus nestlings. Ibis 153(3):601–610
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127(3):247–260. https://doi.org/10.1016/j.biocon.2005.09.005
McPherson SC, Sumasgutner P et al (2021) South African raptors in urban landscapes: a review. Ostrich 92:41–57. https://doi.org/10.2989/00306525.2021.1900942
Meyrier E, Jenni L et al (2017) Happy to breed in the city? Urban food resources limit reproductive output in Western Jackdaws. Ecol Evol 7(5):1363–1374. https://doi.org/10.1002/ece3.2733
Michel Michel-Farbführer (2000) Schwaneberger Verlag GmbH, www.michel.de, München
Møller AP, Diaz M et al (2012) High urban population density of birds reflects their timing of urbanization. Oecologia 170(3):867–875. https://doi.org/10.1007/s00442-012-2355-3
Nebel C, Amar A et al (2021) Parental morph combination does not influence innate immune function in nestlings of a colour-polymorphic African raptor. Sci Rep 11(1):11053. https://doi.org/10.1038/s41598-021-90291-7
Oke TR (1982) The energetic basis of the urban heat island. Q J Roy Meteorol Soc 108(455):1–24. https://doi.org/10.1002/qj.49710845502
Olson VA, Owens IPF (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13(12):510–514. https://doi.org/10.1016/S0169-5347(98)01484-0
Pap PL, Vágási CI et al (2011) The effect of coccidians on the condition and immune profile of molting house sparrows (Passer domesticus). The Auk 128(2):330–339. https://doi.org/10.1525/auk.2011.10142
Pérez-Rodríguez L (2009) Carotenoids in evolutionary ecology: re-evaluating the antioxidant role. BioEssays 31(10):1116–1126. https://doi.org/10.1002/bies.200900070
Pérez-Rodríguez L, Martínez-Padilla J et al (2013) Carotenoid-based ornaments as signals of health status in birds: evidences from two galliform species, the red-legged partridge (Alectoris rufa) and the red grouse (Lagopus lagopus scoticus). Carotenoids: food sources, production and health benefits. Nova Science Publishers, New York, pp 173–198
R (Development Core Team (2020) A language and environment for statistical computing. In: R version 4.0.0 R Foundation for Statistical Computing, Vienna, Austria
Reynolds JS, Ibáñez-Álamo JD et al (2019) Urbanisation and nest building in birds: a review of threats and opportunities. Journal of Ornithology 160:841–860. https://doi.org/10.1007/s10336-019-01657-8
Roulin A, Christe P et al (2007) Origin-related, environmental, sex, and age determinants of immunocompetence, susceptibility to ectoparasites, and disease symptoms in the barn owl. Biol J Linn Soc 90(4):703–718. https://doi.org/10.1111/j.1095-8312.2007.00759.x
Roulin A, Gasparini J et al (2008) Pre-hatching maternal effects and the tasty chick hypothesis. Evol Ecol Res 10:463–473
Seress G, Lipovits Á et al (2014) Quantifying the urban gradient: a practical method for broad measurements. Landsc Urban Plann 131:42–50. https://doi.org/10.1016/j.landurbplan.2014.07.010
Seto KC, Güneralp B et al (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci U S A 109(40):16083–16088. https://doi.org/10.1073/pnas.1211658109
Shanahan DF, Strohbach MW et al (2014) The challenges of urban living. In: Gil D, Brumm H (eds) Avian Urban Ecology Behavioural and Physiological Adaptations. Oxford University Press, pp 3–20
Sillanpaa S, Salminen JP et al (2008) Carotenoids in a food chain along a pollution gradient. Sci Total Environ 406(1-2):247–255. https://doi.org/10.1016/j.scitotenv.2008.07.065
Stathakis D, Tselios V et al (2015) Urbanization in European regions based on night lights. Remote Sens Appl: Soc Environ 2:26–34. https://doi.org/10.1016/j.rsase.2015.10.001
Sternalski A, Mougeot F et al (2010) Carotenoids in nestling Montagu’s harriers: variations according to age, sex, body condition and evidence for diet-related limitations. J Comp Physiol B-Biochem Sys Environ Physiol 180(1):33–43. https://doi.org/10.1007/s00360-009-0384-y
Sternalski A, Mougeot F et al (2012) Carotenoid-based coloration, condition, and immune responsiveness in the nestlings of a sexually dimorphic bird of prey. Physiol Biochem Zool 85(4):364–375. https://doi.org/10.1086/665981
Stofberg M, Cunningham SJ et al (2019) Juggling a “junk-food” diet: responses of an urban bird to fluctuating anthropogenic-food availability. Urban Ecosyst 22:1019–1026. https://doi.org/10.1007/s11252-019-00885-3
Sumasgutner P, Adrion M et al (2018) Carotenoid coloration and health status of urban Eurasian kestrels (Falco tinnunculus). PloS One 13(2):e0191956. https://doi.org/10.1371/journal.pone.0191956
Sumasgutner P, Cunningham SJ et al (2023) Interactive effects of rising temperatures and urbanisation on birds across different climate zones: a mechanistic perspective. Glob Chang Biol 29(9):1399–2420. https://doi.org/10.1111/gcb.16645
Sumasgutner P, Jenkins A et al (2020) Nest boxes buffer the effects of climate on breeding performance in an African urban raptor. PloS One 15(6):e0234503. https://doi.org/10.1371/journal.pone.0234503
Sumasgutner P, Krenn HW et al (2013) Diet specialisation and breeding success along an urban gradient: the kestrel (Falco tinnunculus) in Vienna, Austria. Beiträge zur Jagd- und Wildforschung 38:385–397
Sumasgutner P, Nemeth E et al (2014a) Hard times in the city - attractive nest sites but insufficient food supply lead to low reproduction rates in a bird of prey. Front Zool 11:48. https://doi.org/10.1186/1742-9994-11-48
Sumasgutner P, Schulze CH et al (2014b) Conservation related conflicts in the nest-site selection of the Eurasian kestrel (Falco tinnunculus) and the distribution of its avian prey. Landsc Urban Plann 127:94–103. https://doi.org/10.1016/j.landurbplan.2014.03.009
Tremblay A and Ransijn J (2015). LMERConvenienceFunctions: model selection and post-hoc analysis for (G) LMER models. R package version 2(10)
Village A (1990) The Kestrel. T & AD Poyser, London
von Schantz T, Bensch S et al (1999) Good genes, oxidative stress and condition–dependent sexual signals. Proc R Soc London B: Biol Sci 266(1414):1–12
Weaver RJ, Santos ESA et al (2018) Carotenoid metabolism strengthens the link between feather coloration and individual quality. Nat Commun 9(1):73. https://doi.org/10.1038/s41467-017-02649-z
Wemer L, Hegemann A et al (2021) Reduced ectoparasite load, body mass and blood haemolysis in Eurasian kestrels (Falco tinnunculus) along an urban–rural gradient. Sci Nat 108(5):42. https://doi.org/10.1007/s00114-021-01745-x
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Zhang Q, Seto K (2013) Can night-time light data identify typologies of urbanization? A global assessment of successes and failures. Remote Sens (Basel) 5(7):3476–3494. https://doi.org/10.3390/rs5073476
Acknowledgements
We would like to thank everyone who reported kestrel breeding sites in Vienna as part of this citizen science project and especially the Vienna Fire Fighters, as well as the Vienna Chimney Sweepers, for facilitating access to several nesting sites. Without their cooperation, this study would not have been possible. We are grateful to the Research Institute of Wildlife Ecology, University of Veterinary Medicine Vienna, for providing lab space and chemicals for the carotenoid analyses. Last but not the least, we would like to thank Anita Gamauf (*1962–†2018) for her essential role and support when setting up this study system.
Funding
Open access funding provided by University of Vienna. This project was financially supported by the British Ornithologists’ Union (BOU) Small Ornithological Research Grant and the Faculty of Life Science’s Young Investigator Award of the University of Vienna (both to PS).
Author information
Authors and Affiliations
Contributions
PS conceived and designed the study. TN, MM, LH and PS performed all field work. TN and AH conducted the laboratory work based on a protocol provided by CI. LF provided laboratory space, in-kind support, and aided the interpretation of the results. TN performed the statistical analyses with advice by PS and SR. TN and PS wrote the first draft of the manuscript. CI and AH improved the manuscript. All co-authors read, edited and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
The study was performed under licence of the Federal Ministry of Science and Research (permit number: GZ 2020-0.547.690) and was approved by the ethics committee of the University of Vienna. All data were acquired strictly following current Austrian and EU law as well as the guidelines for treatment of animals in behavioural research and teaching (ASAB 2018).
Consent for publication
The manuscript has been approved by all co-authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Matthias Waltert
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sumasgutner, P., Nilles, T., Hykollari, A. et al. Integument colouration and circulating carotenoids in relation to urbanisation in Eurasian kestrels (Falco tinnunculus). Sci Nat 110, 48 (2023). https://doi.org/10.1007/s00114-023-01874-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-023-01874-5