Abstract
Parasitic infections potentially drive host’s life-histories since they can have detrimental effects on host’s fitness. Telomere dynamics is a candidate mechanism to underlie life-history trade-offs and as such may correlate with observed fitness reduction in infected animals. We examined the relationship of chronic infection with two genera of haemosporidians causing avian malaria and malaria-like disease with host’s telomere length (TL) in a longitudinal study of free-ranging blue tits. The observed overall infection prevalence was 80% and increased with age, constituting a potentially serious selective pressure in our population. We found longer telomeres in individuals infected with a parasite causing lesser blood pathologies i.e. Haemoproteus compared to Plasmodium genus, but this only held true among males. Female TL was independent of the infection type. Our results indicate that parasitic infections could bring about other types of costs to females than to males with respect to TL. Additionally, we detected linear telomere loss with age, however a random regression analysis did not confirm significant heterogeneity in TL of first breeders and telomere shortening rates in further life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitic infections have been reported to impair survival in a number of taxa (Valkiūnas 2005; Kilpatrick et al. 2010; Murray et al. 2010; Cooper et al. 2012). Detrimental action of parasites is often delayed, resulting in prolonged coexistence of parasite and host. This fact motivates a query for the proximate mechanism mediating long-term consequences (reduced survival) in animals that were able to withstand acute infection stage. In the light of current knowledge, two main mechanisms could be proposed: behavioural (sick animals often become inefficient in foraging, avoiding predation or defending territory [Owen-Ashley and Wingfield 2007], during both acute and chronic phase of infection) and physiological. In the latter case, resources available to the host can be depleted by the parasite directly and indirectly through activation of the immune system (Hasselquist and Nilsson 2012). Consequently, a trade-off in host’s resource allocation is being compromised by the presence of a parasite, potentially resulting in impaired self-maintenance (Sheldon and Verhulst 1996).
One potential mechanism that could elucidate reduced survival of infected animals is telomere dynamics. Telomeres, non-coding DNA repeats, capping ends of chromosomes, are perceived as a biomarker of viability and quality (Bauch et al. 2013; Boonekamp et al. 2013). Telomeres shorten with each cellular division due to incomplete replication on lagging DNA strand, and as such are associated with the ageing process (Monaghan and Haussmann 2006). Indeed, telomere shortening with increasing age has been confirmed not only in vitro in human fibroblasts (Allsopp et al. 1992), but also across multiple free-living and laboratory animal populations (Dantzer and Fletcher 2015; but see Hoelzl et al. 2016; Rollings et al. 2017; Ujvari et al. 2017) and the rate of erosion may indicate cumulative effects of various stressors (Bauch et al. 2014). While the impact of catch-up growth, sibling competition, reproduction or diet composition has been increasingly explored (Geiger et al. 2012; Boonekamp et al. 2014; Sudyka et al. 2014; Noguera et al. 2015), the association between parasitic infection and telomere dynamics is not well understood yet. To our knowledge, apart from observational studies on negative effects of disease on telomere length (TL) in humans (Kong et al. 2013) and mice (Ilmonen et al. 2008), very few studies considered the direct relationship between parasitic infections and telomere dynamics so far. Asghar et al. (2015) showed that great reed warblers infected with avian malaria lived shorter and their telomeres eroded at a faster rate while compared to birds being free of infection. This study suggests that disease-induced negative effects on survival may be mediated through telomere degradation. Other studies also report negative effect of parasitic infections on TL in siskins and tawny owls (Asghar et al. 2016; Karell et al. 2017), however some recent studies failed to show such relationship (Slowinski 2017; Stauffer et al. 2017). This inspires further query on assessing telomere dynamics in various populations in response to parasite-induced diseases.
Avian malaria and malaria-like disease, caused by vector-borne haemosporidian parasites from genera Plasmodium and Haemoproteus, are among the most common diseases in bird populations (Atkinson and van Riper 1991; Scheuerlein and Ricklefs 2004). The infection becomes chronic in individuals surviving the initial acute phase of the disease (Valkiūnas 2005). While the life cycle of both parasite genera involves development in blood cells, Haemoproteus is expected to cause less damage to blood cells. Merogony (asexual reproduction) of Haemoproteus occurs in other tissues, whereas Plasmodium multiplies also in red blood cells leading to their disintegration (Valkiūnas 2005; Valkiūnas and Iezhova 2017). Blood pathologies induced by parasitaemia are expected to increase demand for new blood cells (Schoenle et al. 2017), which can be attained through higher number of cellular divisions, possibly leading to pronounced telomere erosion. Due to the differences in life cycles between the haemosporidian parasites, lesser impact of Haemoproteus on blood TL is expected.
Here, we aim to determine the relationship between infection with blood parasites (from genus Plasmodium and Haemoproteus) and telomere length (TL) in a wild population of a small, short-lived passerine, the blue tit (Cyanistes caeruleus). To this end, we examined if the presence of blood parasites correlates with TL of host’s red blood cells. We expected to (i) detect shorter telomeres among infected individuals while compared to uninfected ones and (ii) find differences in TL among hosts infected with parasite genera with varying life cycles, as the action of each parasite is expected to have diverse direct impact on red blood cells. A longitudinal setup of our study allowed to test if (iii) red blood cell telomeres become shorter with chronological age of an individual and (iv) components of telomere dynamics: first breeders’ TL and TL erosion rate vary among individuals.
Materials and methods
Study object
The study was performed in a wild population of nest-box breeding blue tits inhabiting the southern part of the Baltic island of Gotland, Sweden (see Przybylo et al. (2000) for detailed study site description). This population provides a convenient framework for longitudinal studies of age-related parameters due to relatively high survival rate and recapture rate (40% and 41% respectively, (Podmokła et al. 2017)). We have continuously monitored this population since 2002, and for the present analysis we considered data from breeding seasons 2008–2015.
The blue tit is a small passerine (~ 12 g) with a maximum longevity of 12 years recorded in the wild (EURING database). The median lifespan of birds that were observed in our population as adults is 2 years, and maximum lifespan recorded in the study period is at least 6 years. Birds seem to contract infection locally (adult blue tits in this part of Europe rarely migrate (Smith and Nilsson 1987), and even if they do, their wintering grounds are devoid of active malaria vectors), during the activity period of their vectors (May–October): mosquitoes (Culicidae) in the case of Plasmodium and biting midges (Ceratopogonidae) and louse flies (Hippoboscidae) in the case of Haemoproteus (Valkiūnas 2005). Since the prepatent period of infection usually lasts several weeks (Zehtindjiev et al. 2008; Asghar et al. 2016) and seasonal prevalence of parasites is bimodal with the most pronounced peak in autumn (Cosgrove et al. 2008), the infections that we observe (all samplings took place in May–June) were generally chronic, i.e., had to be contracted at least a few months before the sampling.
Adults were caught while feeding nestlings, 10–14 days after hatching of their young either with mist nets set in the vicinity of nest-boxes or self-releasing traps installed inside nest-boxes. All individuals were ringed, blood sampled, and then immediately released. Birds were aged on the basis of a distinctive moult limit visible between the great and the primary wing coverts (Svensson 1992), and their age was assured on the basis of ringing data. Sex was determined based on the presence (female) or absence (male) of a brood patch. Considering the primary importance of longitudinal sampling in TL analyses (Nussey et al. 2008), we chose a subset of birds that were caught at least twice and the first capture took place in their first breeding season (i.e. 1-year-old birds) to standardise age-related effects across all individuals. Such individuals, surviving beyond their first reproductive season, are presumably of better quality than the birds dying earlier. However, in our population, we did not find any effect of infection status (Podmokła et al. 2017) or TL dynamics (Sudyka et al. 2014) on survival, therefore we do not expect our results to be prone to the potential quality-dependent bias in the studied parameters.
According to our database, 160 individuals were captured at least twice. From the analyses we had to exclude birds that underwent an experimental brood size enlargement in their first breeding season, because we have demonstrated that this manipulation alters TL in the study population (Sudyka et al. 2014). For some individuals a blood sample was not available, therefore our final dataset comprised 246 samples belonging to 112 individuals (up to four samples per individual collected in yearly intervals in 54 males and 58 females).
Host’s telomere length analysis
Immediately after sampling, whole blood was placed in 96% ethanol until extracted with the Blood Mini kit (A&A Biotechnology, Gdynia, Poland; modified for overnight incubation in 37 °C). The DNA concentration and purity was measured with a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and the integrity of each sample was confirmed by an electrophoresis on a 1% agarose gel.
TL was assessed by the real-time quantitative PCR assay adapted for birds (Criscuolo et al. 2009). We used relative TL, expressed as the ratio (T/S) of a telomere copy number (T) and a single control gene copy number (S, which was GAPDH, (Cawthon 2002)). We used the primers designed for zebra finch but these have been previously validated for the blue tit by us (see Sudyka et al. 2014, 2016). To generate a standard curve for amplification efficiency, each plate included serial twofold dilutions of a reference DNA (mixed DNA of five birds not included in the study) run in duplicate from 10 to 0.31 ng for telomere and from 10 to 0.62 ng for GAPDH. The same DNA was used as the golden sample to account for inter-plate variation and run in triplicate on every plate. Mean amplification efficiency and the determination coefficient (r2) of the standard curve were 96% (range 82–109%) and 0.985 (range 0.963–0.990) for GAPDH and 86% (range 77–103%) and 0.974 (range 0.949–0.993) for telomeres respectively. Mean intra-plate SD for the Ct values (repeatability as defined by the MIQE guidelines (Bustin et al. 2009)) was 0.23 (coefficient of variation (100 × SD/mean value), CV = 2.00%) for telomeres and 0.12 (CV = 0.48%) for GAPDH, whereas inter-plate CV (reproducibility as defined by the MIQE guidelines) and SD calculated on the golden sample’s T/S ratios were 17% and 0.09 respectively. All samples from one individual were assayed on the same plate to avoid between plate variations within individual, whereas sexes were evenly distributed on a plate. If the variation between technical replicates (Ct SD) exceeded 0.5, all samples for each individual were measured again. In total, we ran 21 plates. For further details on primers, reagent concentration, reaction setup and T/S ratio calculation please refer to Sudyka et al. (2014). From the analyses of TL, we had to exclude one female due to DNA degradation in the second sample. As a result for TL, we analysed 244 samples from 111 individuals (57 females, 54 males). Repeatability for TL values within individual (calculated with rptR package (Stoffel et al. 2017)) was R = 0.223 ± 0.081, CI = 0.056–0.376, P = 0.004.
Malaria status, lineages and infection intensity analyses
To obtain malaria status, the DNA extracted for TL analysis was screened for the presence of blood parasites (genera Haemoproteus and Plasmodium) by amplifying a 478-bp fragment of the mitochondrial cyt b gene, using a nested polymerase chain reaction (Waldenström et al. 2004). After confirming the presence of a parasite, we assessed its lineage by sequencing purified PCR products and aligning DNA sequences with the MalAvi database (Bensch et al. 2009). Intensity of infection (parasite DNA copy number) was then quantified via qPCR, comparing a focal sample with standard curves created using full-length cyt b PCR products from P. circumflexum and H. majoris (as described in (Knowles et al. 2011). (For more details on primers, reaction setups, reagent concentrations and instruments, please see Podmokła et al. (2014a, b)).
Statistical analyses
To study inter-individual longitudinal variation in TL changes according to malaria infection (parasite-genus wise), we employed random regression analysis, a type of a linear mixed model allowing to study inter-individual variation in intercepts (first breeder TL) and slopes (TL loss with advancing age). To this end, we modelled TL (log-transformed for normality) as a response variable and fitted age (in years, defined as a fixed continuous covariate), sex and infection type as fixed explanatory variables. Infection type was categorised into three levels: none, Plasmodium and Haemoproteus; mixed infections with both genera (7 cases) were included in Plasmodium category because more detrimental effects are expected to arise from the action of this parasite (Valkiūnas 2005); however, we also repeated the analysis excluding mixed infections and the results remained qualitatively the same. We also introduced individual identity to account for the same individual being entered more than once in the analyses, nest identity (some birds attended the same nest), year of data collection and plate id from TL analyses (to account for among-plate variance) as random variables. To test for non-linearity of age effect in initial models, we introduced polynominal (quadratic) age term, but it turned out to be insignificant, so was culled from the final model. We began with full models, and then we culled non-significant (P > 0.05) interaction terms. Additionally, to evaluate infection status dynamics (response variable: infected vs uninfected) with individual age (explanatory continuous variable), we performed an analysis accounting for individual id in random effects structure. Analyses were performed in R (v.3.3.1) (R Core Team 2016) using the ASReml-R package (Butler 2009).
Data accessibility
Data are available in the online Electronic Supporting Information and in the online data repository figshare (Sudyka et al. 2018).
Results
Dynamics of infection prevalence and intensity
Overall, haemosporidian prevalence reached 80% (please see the Electronic Supporting Information (ESI), Table S1A) and in 246 samples, we found 216 cases of infection with various parasite lineages from Plasmodium (7 lineages, prevalence 56.5%) and Haemoproteus genera (3 lineages, 20.3% prevalence), whereas 2.8% of birds yielded mixed infection with the two genera (Table S1B, ESI). Prevalence (proportion of infected individuals) increased with age (estimate for age ± SE = 0.624 ± 0.239, F1,242 = 6.847, P = 0.009). Among 102 individuals, that were recaptured in the subsequent year, probability of contracting infection was high (0.71), whereas losing infection was less likely (0.12) (Table S2, S3, ESI). As a result, prevalence of infection among 1-year-old birds was 73% and it reached 83% in two-year-olds (Table S2, ESI). Infection with Haemoproteus was generally more intensive than with Plasmodium (intensity for all present lineages, excluding mixed infection with both genera, Mann-Whitney U = 594, N = 186, P < 0.001).
Longitudinal telomere dynamics vs avian malaria
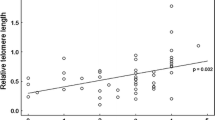
Including a covariance between individual slopes and intercepts did not improve the fit of our model (log(likelihood) of a model including the correlation = 284.13, and of a model without the correlation = 283.99, likelihood-ratio test P = 0.597), thus we applied the model without the covariance. Random regression analysis (Table 1) revealed that the variations in intercepts (first breeder TL) and among individual regression slopes (TL attrition rate) were not significant. We found a significant negative effect of chronological age on TL (Table 1, Fig. 1). TL was found to be significantly correlated with malaria infection type; we found longer telomeres in individuals infected with Haemoproteus compared to Plasmodium, but this only held true among males (Table 1, Fig. 2). Uninfected males showed intermediate levels of TL between the ones infected with the two parasite genera. Infection type was not related to TL among females (Table 1, Fig. 2). Telomere attrition rate (telomere shortening with age) was sex independent (age × sex, F1,100.9 = 1.869; P = 0.175), and more importantly, it was also not related to the type of parasitic infection (age × infection type, F2,166.2 = 0.579; P = 0.562), so these interactions were not included in the final model. We found no association between infection intensity and TL (model description and results in Table S4, ESI).
Relative telomere length [the ratio (T/S) of telomere copy number (T) and single control gene copy (S) in females (dark grey bars) and males (light grey bars) according to infection type. Raw data ± SE are shown. Differences among groups (non-adjusted P values) according to the post hoc pairwise mean difference test with Holm false-positive rate discovery correction
Discussion
In line with our expectations, infection type affected blood TL. We found that males infected with Haemoproteus had longer telomeres than males infected with Plasmodium. Such a finding can be readily explained, considering differential life cycles of the two genera. Haemoproteus causes less blood pathologies during its developmental cycle (Atkinson and van Riper 1991; Valkiūnas 2005), therefore the demand for new blood cells via telomere shortening cellular divisions is lower.
Conversely to the previous studies in some bird populations (Asghar et al. 2015, 2016; Karell et al. 2017), we did not show that uninfected birds had longer telomeres. Uninfected males showed intermediate TL between the ones infected with Plasmodium and Haemoproteus. This lack of effect has already been reported in other animals (Slowinski 2017; Stauffer et al. 2017) and adds to the evidence that parasitic infections do not always entail negative consequences on TL. In general, the information on the relationship between telomere dynamics and parasitic infections is still lacking, and comparisons among taxa have to be treated with great caution since telomere dynamics and telomerase activity varies substantially within animal kingdom (Olsson et al. 2018). Alternatively, temporal resolution of our sampling points—we miss the information on autumn prevalence—could be not fine enough to detect the differences (expected especially due to the acute stage of infection (Asghar et al. 2016)). For example, in humans, malaria infection considerably shortened telomeres up to 3 months post infection, but after 1 year, TL was restored (Asghar et al. 2018). It appears that varying results among studies examining the impact of parasitic infections on TL may stem from different phases of infection investigated. Since we demonstrate that Haemoproteus-infected males have longer telomeres than the other groups, we would expect that the rate of their telomere loss is lower. However, since we did not detect significant differences in shortening rates and TL as first breeders among individuals (Table 1), telomere dynamics of these males could have been shaped prior to the first sampling. To our knowledge, this is the first study to show diverse relationship of parasite genera varying in life cycles on TL and stresses the importance of accounting for ecological structure (parasite genera with different developmental patterns in this case) while addressing parasite–host interactions. Indeed, it has already been demonstrated that avian malaria can yield multiple effects on their hosts (Lachish et al. 2011), yet exploring early-life telomere dynamics in response to such interactions could be particularly needed.
We show that effect of host interaction with parasite may also be sex-specific. Unlike males, females infected with Haemoproteus did not show longer telomeres in the studied population. It is possible that infection with Haemoproteus, incurs other types of costs to females than to males. For example, anti-malarial treatment reduced Haemoproteus infection intensity and ultimate fitness cost, i.e. survival only in females, whereas males did not benefit from such a manipulation (Martínez-de la Puente et al. 2010). While males are generally more susceptible to parasitic infections, endocrine-immune interactions may cause increased resistance to some parasites in males (Klein 2004).
We demonstrated that TL decreases linearly with advancing individual age in both sexes and parasitic infection type does not alter the general pattern. The number of longitudinal studies in natural populations indicating telomere erosion with age slowly, but persistently increases (Dantzer and Fletcher 2015; Sudyka et al. 2016). However, the causal involvement of telomeres in ageing process still remains an open question (Simons 2015). This discussion is further inspired by recent longitudinal studies indicating no telomere erosion (Ujvari et al. 2017) or even telomere elongation with age (Hoelzl et al. 2016), suggesting that divergent life-histories among taxa may shape lifetime telomere dynamics. A longitudinal approach is particularly useful for disentangling within-individual reactions from between-individual heterogeneity (Nussey et al. 2008). Although several studies show variability in individual TL dynamics (Hall et al. 2004; Bize et al. 2009; Heidinger et al. 2012), here we did not confirm statistical significance of heterogeneity neither in TL among first breeders, nor in TL erosion rates. We were only able to detect larger contribution of first breeders TL, rather than telomere erosion rate, to overall variation in TL (Table 1). However, it is important to note, that the lack of significant differences in individual starting TL and erosion rates, does not denote that all individuals share the same erosion rate or have identical TL as first breeders (the variances are non-zero), but that we failed to statistically detect these differences. Even though the number of individuals that we sampled appears to be sufficient, the total sample size falls below the one recommended for individual heterogeneity modelling (van de Pol 2012). While the number of replicates within an individual are restricted by the blue tit’s lifespan, our result motivates and justifies a query in search for factors contributing to the phenotypic TL variation among individuals in other study systems.
To conclude, the idea that TL dynamics may be shaped by various life’s insults is already well established (Monaghan and Haussmann 2006) and here, we showed that parasitic infections may be one of such TL modulating factors in a wild population of a short-lived vertebrate. For the first time, we demonstrated the contrasting associations between two parasite genera varying in life cycles with blood TL. Consistently with majority of the studies on endotherms, in the blue tit telomeres eroded with age on a within-individual level, and this erosion rate was not significantly variable among individuals. Since our study provides correlational evidence for sex-specific differences in TL in response to different parasite genera, it has to be treated as a prerequisite for further exploration of the topic. For example, it could be fruitful to experimentally examine the impact of varying parasite genera on TL in multiple body tissues in a sex-specific context.
References
Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB (1992) Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A 89:10114–10118
Asghar M, Hasselquist D, Hansson B et al (2015) Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science (80- ) 347:436–438. https://doi.org/10.1126/science.1261121
Asghar M, Palinauskas V, Mukhin A et al (2016) Parallel telomere shortening in multiple body tissues owing to malaria infection. Proc R Soc B Biol Sci 283:20161184. https://doi.org/10.1098/rspb.2016.1184
Asghar M, Yman V, Homann MV, Sondén K, Hammar U, Hasselquist D, Färnert A (2018) Cellular aging dynamics after acute malaria infection: a 12-month longitudinal study. Aging Cell 17:e12702. https://doi.org/10.1111/acel.12702
Atkinson CT, van Riper IIIC (1991) Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Haemoproteus, and Leucocytozoon. In: Loye J, Zuk M (eds) Bird – parasite interactions. Oxford University Press, pp 19–48
Bauch C, Becker PH, Verhulst S (2013) Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc R Soc B Biol Sci 280:20122540. https://doi.org/10.1098/rspb.2012.2540
Bauch C, Becker PH, Verhulst S (2014) Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long-lived seabird. Mol Ecol 23:300–310. https://doi.org/10.1111/mec.12602
Bensch S, Hellgren O, Perez-Tris J (2009) MalAvi : a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 1–6 . https://doi.org/10.1111/j.1755-0998.2009.02692.x
Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P (2009) Telomere dynamics rather than age predict life expectancy in the wild. Proc R Soc B Biol Sci 276:1679–1683. https://doi.org/10.1098/rspb.2008.1817
Boonekamp JJ, Simons MJP, Hemerik L, Verhulst S (2013) Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 12:330–332. https://doi.org/10.1111/acel.12050
Boonekamp JJ, Mulder G, Salomons HM et al (2014) Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc R Soc B Biol Sci 281:20133287. https://doi.org/10.1098/rspb.2013.3287
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines : minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
Butler D (2009) Asreml: asreml() fits the linear mixed model. R package version 3.0–1
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47. https://doi.org/10.1093/nar/30.10.e47
Cooper N, Kamilar JM, Nunn CL (2012) Host longevity and parasite species richness in mammals. PLoS One 7:e42190. https://doi.org/10.1371/journal.pone.0042190
Cosgrove CL, Wood MJ, Day KP, Sheldon BC (2008) Seasonal variation in Plasmodium prevalence in a population of blue tits Cyanistes caeruleus. J Anim Ecol 77:540–548. https://doi.org/10.1111/j.1365-2656.2008.01370.x
Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P (2009) Real-time quantitative PCR assay for measurement of avian telomeres. J Avian Biol 40:342–347. https://doi.org/10.1111/j.1600-048X.2008.04623.x
Dantzer B, Fletcher QE (2015) Telomeres shorten more slowly in slow-aging wild animals than in fast-aging ones. Exp Gerontol 71:38–47. https://doi.org/10.1016/j.exger.2015.08.012
Geiger S, Le Vaillant M, Lebard T et al (2012) Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol Ecol 21:1500–1510. https://doi.org/10.1111/j.1365-294X.2011.05331.x
Hall ME, Nasir L, Daunt F, Gault EA, Croxall JP, Wanless S, Monaghan P (2004) Telomere loss in relation to age and early environment in long-lived birds. Proc R Soc B Biol Sci 271:1571–1576. https://doi.org/10.1098/rspb.2004.2768
Hasselquist D, Nilsson JÅ (2012) Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Anim Behav 83:1303–1312. https://doi.org/10.1016/j.anbehav.2012.03.025
Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P (2012) Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A 109:1743–1748. https://doi.org/10.1073/pnas.1113306109
Hoelzl F, Smith S, Cornils JS, Aydinonat D, Bieber C, Ruf T (2016) Telomeres are elongated in older individuals in a hibernating rodent, the edible dormouse (Glis glis). Sci Rep 6:36856. https://doi.org/10.1038/srep36856
Ilmonen P, Kotrschal A, Penn DJ (2008) Telomere attrition due to infection. PLoS One 3:e2143. https://doi.org/10.1371/journal.pone.0002143
Karell P, Bensch S, Ahola K, Asghar M (2017) Pale and dark morphs of tawny owls show different patterns of telomere dynamics in relation to disease status. Proc R Soc B Biol Sci 284:20171127. https://doi.org/10.1098/rspb.2017.1127
Kilpatrick AM, Briggs CJ, Daszak P (2010) The ecology and impact of chytridiomycosis : an emerging disease of amphibians. Trends Ecol Evol 25:109–118. https://doi.org/10.1016/j.tree.2009.07.011
Klein SL (2004) Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol 26:247–264
Knowles SCL, Wood MJ, Alves R et al (2011) Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Mol Ecol 20:1062–1076. https://doi.org/10.1111/j.1365-294X.2010.04909.x
Kong CM, Lee XW, Wang X (2013) Telomere shortening in human diseases. FEBS J 280:3180–3193. https://doi.org/10.1111/febs.12326
Lachish S, Knowles SCL, Alves R, Wood MJ, Sheldon BC (2011) Fitness effects of endemic malaria infections in a wild bird population: the importance of ecological structure. J Anim Ecol 80:1196–1206. https://doi.org/10.1111/j.1365-2656.2011.01836.x
Martínez-de la Puente J, Merino S, Tomás G et al (2010) The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol Lett 6:663–665. https://doi.org/10.1098/rsbl.2010.0046
Monaghan P, Haussmann MF (2006) Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol 21:47–53. https://doi.org/10.1016/j.tree.2005.11.007
Murray CJL, Rosenfeld LC, Lim SS et al (2010) Global malaria mortality between 1980 and 2010 : a systematic analysis. Lancet 379:413–431. https://doi.org/10.1016/S0140-6736(12)60034-8
Noguera JC, Metcalfe NB, Boner W, Monaghan P (2015) Sex-dependent effects of nutrition on telomere dynamics in zebra finches (Taeniopygia guttata). Biol Lett 11:20140938. https://doi.org/10.1098/rsbl.2014.0938
Nussey DH, Coulson T, Festa-Bianchet M, Gaillard JM (2008) Measuring senescence in wild animal populations: towards a longitudinal approach. Funct Ecol 22:393–406. https://doi.org/10.1111/j.1365-2435.2008.01408.x
Olsson M, Wapstra E, Friesen C (2018) Ectothermic telomeres : it’ s time they came in from the cold. Proc R Soc B Biol Sci 373:20160449. https://doi.org/10.1098/rstb.2016.0449
Owen-Ashley NT, Wingfield JC (2007) Acute phase responses of passerine birds: characterization and seasonal variation. J Ornithol 148:583–591
Podmokła E, Dubiec A, Drobniak SM, Arct A, Gustafsson L, Cichoń M (2014a) Avian malaria is associated with increased reproductive investment in the blue tit. J Avian Biol 45:219–224. https://doi.org/10.1111/j.1600-048X.2013.00284.x
Podmokła E, Dubiec A, Drobniak SM, Arct A, Gustafsson L, Cichoń M (2014b) Determinants of prevalence and intensity of infection with malaria parasites in the blue tit. J Ornithol 155:721–727. https://doi.org/10.1007/s10336-014-1058-4
Podmokła E, Dubiec A, Drobniak SM, Sudyka J, Krupski A, Arct A, Gustafsson L, Cichoń M (2017) Effect of haemosporidian infections on host survival and recapture rate in the blue tit. J Avian Biol 48:796–803. https://doi.org/10.1111/jav.01108
Przybylo R, Sheldon BC, Merilä J (2000) Climatic effects on breeding and morphology: evidence for phenotypic plasticity. J Anim Ecol 69:395–403. https://doi.org/10.1046/j.1365-2656.2000.00401.x
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.r-project.org/
Rollings N, Uhrig EJ, Krohmer RW, Waye HL, Mason RT, Olsson M, Whittington CM, Friesen CR (2017) Age-related sex differences in body condition and telomere dynamics of red-sided garter snakes. Proc R Soc B Biol Sci 284:9. https://doi.org/10.1098/rspb.2016.2146
Scheuerlein A, Ricklefs RE (2004) Prevalence of blood parasites in European passeriform birds. Proc R Soc B Biol Sci 271:1363–1370. https://doi.org/10.1098/rspb.2004.2726
Schoenle LA, Kernbach M, Haussmann MF, Bonier F, Moore IT (2017) An experimental test of the physiological consequences of avian malaria infection. J Anim Ecol 86:1–14. https://doi.org/10.1111/1365-2656.12753
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Simons MJP (2015) Questioning causal involvement of telomeres in aging. Ageing Res Rev 24:191–196
Slowinski SP (2017) How parasites affect, and are affected by, host physiology, behavior, and breeding system. Indiana University
Smith HG, Nilsson J-Å (1987) Intraspecific variation in migratory pattern of a partial migrant, the blue tit (Parus caeruleus): an evaluation of different hypotheses. Auk 104:109–115
Stauffer J, Bruneaux M, Panda B, Visse M, Vasemägi A, Ilmonen P (2017) Telomere length and antioxidant defense associate with parasite-induced retarded growth in wild brown trout. Oecologia 185:365–374. https://doi.org/10.1007/s00442-017-3953-x
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Sudyka J, Arct A, Drobniak SM, Dubiec A, Gustafsson L, Cichoń M (2014) Experimentally increased reproductive effort alters telomere length in the blue tit (Cyanistes caeruleus). J Evol Biol 27:2258–2264. https://doi.org/10.1111/jeb.12479
Sudyka J, Arct A, Drobniak S, Gustafsson L, Cichoń M (2016) Longitudinal studies confirm faster telomere erosion in short-lived bird species. J Ornithol 157:373–375. https://doi.org/10.1007/s10336-015-1304-4
Sudyka J, Podmokła E, Drobniak SM, et al (2018) Data from: Sex-specific effects of parasites on telomere dynamics in a short-lived passerine - the blue tit. figshare. https://doi.org/10.6084/m9.figshare.7635785
Svensson L (1992) Identification guide to European passerines. Naturhistoriska Riksmuseet, Stockholm
Ujvari B, Biro PA, Charters JE, Brown G, Heasman K, Beckmann C, Madsen T (2017) Curvilinear telomere length dynamics in a squamate reptile. Funct Ecol 31:753–759. https://doi.org/10.1111/1365-2435.12764
Valkiūnas G (2005) Avian malaria parasites and other Haemosporidia. CRC Press
Valkiūnas G, Iezhova TA (2017) Exo-erythrocytic development of avian malaria and related haemosporidian parasites. Malar J 16:101. https://doi.org/10.1186/s12936-017-1746-7
van de Pol M (2012) Quantifying individual variation in reaction norms : how study design affects the accuracy , precision and power of random regression models. Methods Ecol Evol 3:268–280. https://doi.org/10.1111/j.2041-210X.2011.00160.x
Waldenström J, Bensch S, Hasselquist D, Östman Ö (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90:191–194
Zehtindjiev P, Ilieva M, Westerdahl H, Hansson B (2008) Dynamics of parasitemia of malaria parasites in a naturally and experimentally infected migratory songbird , the great reed warbler Acrocephalus arundinaceus. Exp Parasitol 119:99–110. https://doi.org/10.1016/j.exppara.2007.12.018
Acknowledgements
We thank Adam Krupski for helping with malaria infection analyses.
Funding
This research was financed by the grant of the Polish National Science Centre no. DEC-2013/09/N/NZ8/03211 awarded to JS.
Author information
Authors and Affiliations
Contributions
JS conceived the idea; SMD, AD, JS, AA, MC and EP collected the samples; JS, EP and AD analysed the samples; JS and SMD analysed the data, LG provided logistical support in the field, JS led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
All applicable institutional and/or national guidelines for the care and use of animals were followed. Permit number: dnr 37-15 issued by Jordbruksverket, Sweden.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by: Agnieszka Szalewska-Palasz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 707 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sudyka, J., Podmokła, E., Drobniak, S.M. et al. Sex-specific effects of parasites on telomere dynamics in a short-lived passerine—the blue tit. Sci Nat 106, 6 (2019). https://doi.org/10.1007/s00114-019-1601-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-019-1601-5