Abstract
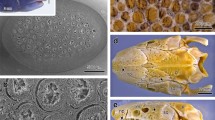
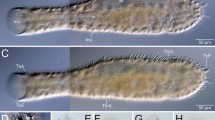
The general integument of reptiles is traditionally defined as being dry, but we report here the discovery of unicellular mucoid glands (UCMG) in the dorsal skin of lizards of the genus Phelsuma (Gekkonidae). To this end, the skin of these lizards and of some others for comparison was studied by scanning electron microscopy and transmission electron microscopy. These photographs showed that the development and function of the UCMGs are related to the skin’s sloughing cycle. The UCMGs differentiate at scattered locations from Oberhäutchen cells of the inner (new) epidermal generation, above the differentiating β-keratin layer. While the inner generation matures, the UCMG increases in size; unlike the surrounding Oberhäutchen cells, it does not develop the spinules that characterize gecko skin. When, upon sloughing, the inner generation becomes the new outer generation, and the Oberhäutchen forms the skin surface, the UCMGs, several per scale, dot the surface as mucus-inflated “blebs” projecting from the surrounding spinulate Oberhäutchen, each nesting in a shallow pit of the underlying β-keratin. On the surface, the UCMGs rupture and the mucus appears to dissipate in cords, flowing over the tips of the spinules, and incorporating minute foreign bodies. It is concluded that, due to the low wettability of the spinulate surface (derived from the spacing of the spinules), the cords brush off easily, with the mucus functioning as a cleaning agent.




Similar content being viewed by others
References
Adam NK (1963) Principles of water repellency. In: Moilliet JL (ed) Waterproofing and water repellency. Elsevier, Amsterdam, pp 1–23
Alibardi L, Maderson PFA (2003a) Observations on the histochemistry and ultrastructure of the epidermis of the tuatara, Sphenodon punctatus (Sphenodontida, Lepidosauria, Reptilia): a contribution to an understanding of the lepidosaurian epidermal generation and the evolutionary origin of the squamate shedding complex. J Morphol 256:111–133
Alibardi L, Maderson PFA (2003b) Observations on the histochemistry and ultrastructure of regenerating caudal epidermis of the tuatara Sphenodon punctatus (Sphenodontida, Lepidosauria, Reptilia). J Morphol 256:134–145
Andres KH, von Duering M, Horn H-G (1999) Fine structure of scale glands and preanal glands of monitors Varanidae. In: Horn H-G, Boehme W (eds) Advances in monitor research II, vol 11. Mertensiella, pp 277-290
Banerjee TK, Mittal AK (1978) Epidermal mucous cells in the chequered water snake Natrix piscator (Colubridae: Squamata). A cytochemical investigation. Zool J Linn Soc 63:289–293
Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202:18
Böhme W, Sering M (1996–1997) Tail squirting in Eurydactylodes: independent evolution of caudal defensive glands in a diplodactyline gecko (Reptilia, Gekkonidae). Zool Anz 235:225–229
Boulenger GA (1885–1887) Catalogue of the lizards in the British Museum (Natural History), vol 13, 2nd edn. British Museum, London
Cannon MS, Davis RW, Weldon PJ (1996) Dorsal glands of Alligator mississippiensis: a histological and histochemical study. J Zool (Lond) 239:625–631
Cassie ABD, Baxter S (1944) Wettability of porous surfaces. Trans Faraday Soc 40:546–551
Cutter WL (1959) An instance of bloodsquirting by Phrynosoma solare. Copeia 1959:176
Goin CJ, Goin OB (1971) Introduction to herpetology. Freeman, San Francisco
Grant C (1931) The sphaerodactyls of Porto Rico, Culebra and Mona Islands. J Dep Agric P R 15:199–213
Greenberg B (1943) Social behavior of the western banded gecko, Coleonyx variegatus Baird. Physiol Zool 16:110–122
Han D, Zhou K, Bauer AM (2004) Phylogenetic relationships among gekkotan lizards inferred from Cmos nuclear DNA sequences and a new classification of the Gekkota. Biol J Linn Soc 83:353–368
Hiller U (1968) Untersuchungen zum Feinbau und zur Funktion der Haftborsten von Reptilien. Z Morphol Tiere 62:307–362
Hiller U (1970) Morphologische Untersuchungen der Haftborstenbildung und Häutung bei Tarentola mauritanica (Rept.). Forma Functio 2:169–177
Hiller U (1972) Licht- und elektronenmikroskopische Untersuchungen zur Haftborstenentwicklung bei Tarentola m. mauritanica L. (Reptilia, Gekkonidae). Z Morphol Tiere 73:263–278
Hiller U (1976a) Elektronenmikroskopische Untersuchungen zur funktionellen Morphologie der borstenführenden Hautsinnesorgane bei Tarentola mauritanica L. (Reptilia, Gekkonidae). Zoomorphologie 84:211–222
Hiller U (1976b) Ultrastructure, degeneration and regeneration of cutaneous sensilla in gekkonid lizards. In: Sixth European Congress on Electron Microscopy, Jerusalem, September 1976, pp 577–578
Hiller U (1978) Morphology and electrophysiological properties of cutaneous sensilla in agamid lizards. Pflügers Arch 377:189–191
Hiller U (1995) Ultrastructural studies on the conjunctiva in agamid lizards. Ann Anat 177:553–562
Hiller U (1998) The conjunctiva and nictitating membrane of the eyelids in some reptilian species: a SEM overview. Eur J Morphol 36:165–171
Irish FJ, Williams EE, Seling E (1988) Scanning electron microscopy of changes in epidermal structure occurring during the shedding cycle in squamate reptiles. J Morphol 197:105–126
Jared C, Antoniazzi MM, Silva JRMC, Freymüller E (1999) Epidermal glands in Squamata: microscopical examination of precloacal glands in Amphisbaena alba (Amphisbaenia, Amphisbaenidae). J Morphol 241:197–206
Kessing SV (1968) Mucous gland system of the conjunctiva. A quantitative normal anatomical study. Acta Ophthalmol (Kbh) 95(Suppl):11–31
Kluge AG (1967) Higher taxonomic categories of gekkonid lizards and their evolution. Bull Am Mus Nat Hist 135:1–59
Maderson PFA (1966) Histological changes in the epidermis of the Tokay (Gekko gecko) during the sloughing cycle. J Morphol 119:39–50
Maderson PFA (1967) The histology of the escutcheon scales of Gonatodes (Gekkonidae) with a comment on the squamate sloughing cycle. Copeia 1967:743–752
Maderson PFA (1968a) The epidermal glands of Lygodactylus (Gekkonidae, Lacertilia). Breviora Mus Comp Zool 288:1–35
Maderson PFA (1968b) On the presence of “escutcheon scales” in the eublepharine gekkonid Coleonyx. Herpetologica 24:99–103
Maderson PFA (1972) The structure and evolution of holocrine epidermal glands in sphaerodactyline and eublepharine gekkonid lizards. Copeia 1972:559–571
Maderson PFA (1985) Some developmental problems of the reptilian integument. In: Gans C, Billett F, Maderson PFA (eds) Biology of the reptilia, vol 14. Wiley, New York, pp 523–598
Maderson PFA, Chiu KW (1970) Epidermal glands in gekkonid lizards: evolution and phylogeny. Herpetologica 26:233–238
Maderson PFA, Rabinowitz T, Tandler B, Alibardi L (1998) Ultrastructural contributions to an understanding of the cellular mechanisms involved in lizard skin shedding with comments on the function and evolution of a unique lepidosaurian phenomenon. J Morphol 236:1–24
Matoltsy AG, Huszar T (1972) Keratinization of the reptilian epidermis: an ultrastructural study of the turtle skin. J Ultrastruct Res 38:87–101
Oycka CA, Gugnani HC (1998) Keratin degradation by Scytalidium species and Fusarium solani. Mycoses 41:73–76
Podhoranyi G (1966) Über die Becherzellen der Bindehaut. Albrecht von Graefes Arch Klin Exp Ophthalmol 169:285–293
Quay WB (1986) Glands. In: Bereiter-Hahn J, Matoltsy AG, Richards KS (eds) Biology of the integument, 2, vertebrates. Springer, Berlin Heidelberg New York, pp 188–193
Regamey J (1935) Les caractéres sexuels du lézard (Lacerta agilis L). Rev Suisse Zool 42:87–168
Romer AS (1949) The vertebrate body. Saunders, Philadelphia
Rosenberg HI, Russell AP (1980) Structural and functional aspects of tail squirting: a unique defense mechanism of Diplodactylus, Reptilia: (Gekkonidae). Can J Zool 58:865–881
Rosenberg HI, Russell AP, Kapoor M (1984) Preliminary characterization of the defensive secretion of Diplodactylus (Reptilia: Gekkonidae). Copeia 1984:1025–1028
Sherbrooke WC, Middendorf GA III (2001) Blood-squirting variability in horned lizards (Phrynosoma). Copeia 2001:1114–1122
Simpson GG (1953) The major features of evolution. Columbia University Press, New York
Smith MA (1935) The fauna of British India, reptilia and amphibia, 2: Sauria. Taylor and Francis, London
Starck D (1982) Vergleichende Anatomie der Wirbeltiere auf evolutionsbiologischer Grundlage, vol 3. Springer, Berlin Heidelberg New York
Steuhl P, Rohen JW (1984) Cellular structure of the conjunctival epithelium of rabbits. Albrecht von Graefes Arch Klin Exp Ophthalmol 221:265–271
Stewart GR, Daniel RS (1972) Scales of the lizard Gekko gecko: surface structure examined with the scanning electron microscope. Copeia 1972:252–257
Stewart GR, Daniel RS (1973) Scanning electron microscopy of scales from different body regions of three lizard species. J Morph 139:377–388
Stewart GR, Daniel RS (1975) Microornamentation of lizard scales: some variation and taxonomic correlates. Herpetologica 31:117–130
Taylor EH, Leonard AB (1956) Concerning the relationship of certain neotropical gekkonid lizard genera, with comments on the microscopical structure of their glandular scales. Univ Kans Sci Bull 38:1019–1029
Wagner T, Neinhuis C, Barthlott W (1996) Wettability and contaminability of insect wings as a function of their surface sculptures. Acta Zool (Stockh) 77:213–225
Yamamura S, Morita Y, Hasan Q, Yokoyama K, Tamiya E (2002) Keratin degradation: a cooperative action of two enzymes from Stenotrophomonas sp. Biochem Biophys Res Commun 295:1034
Acknowledgements
This discovery is an offshoot of YLW’s fieldwork on Hawaii, and he thanks Bill Mautz, University of Hawaii at Hilo, for his hospitality and support and Ken Smith and Nurit Werner for their participation in the field. We are deeply indebted to the following persons living in Germany for providing us with samples used in this study: Ulrike Anders (Hamburg), Michael Franken (Münster), Gerhard Hallmann, (Dortmund), Sandra Jager (Berlin), Frank Jäger (Dietzenbach), Hartmut Lipp (Braunschweig), and Gebhardt Roland (Bayreuth). Furthermore, we thank Mrs. I. Schroeder and Mrs. S. Seidel for skillful technical assistance and, last but not least, Nurit Sharet for Fig. 4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiller, U., Werner, Y.L. First evidence of unicellular glands in the general epidermis of terrestrial reptiles. Naturwissenschaften 95, 193–202 (2008). https://doi.org/10.1007/s00114-007-0311-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-007-0311-6