Abstract
Bone cancer pain (BCP) profoundly impacts patient’s quality of life, demanding more effective pain management strategies. The aim of this systematic review was to investigate the role of inflammatory cytokines as potential molecular targets in BCP. A systematic search for animal rodent models of bone cancer pain studies was conducted in PubMed, Scopus, and Web of Science. Methodological quality and risk of bias were assessed using the SYRCLE RoB tool. Twenty-five articles met the inclusion criteria, comprising animal studies investigating molecular targets related to inflammatory cytokines in BCP. A low to moderate risk of bias was reported. Key findings in 23 manuscripts revealed upregulated classic pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-17, IL-18, IL-33) and chemokines in the spinal cord, periaqueductal gray, and dorsal root ganglia. Interventions targeting these cytokines consistently mitigated pain behaviors. Additionally, it was demonstrated that glial cells, due to their involvement in the release of inflammatory cytokines, emerged as significant contributors to BCP. This systematic review underscores the significance of inflammatory cytokines as potential molecular targets for alleviating BCP. It emphasizes the promise of targeted interventions and advocates for further research to translate these findings into effective therapeutic strategies. Ultimately, this approach holds the potential to enhance the patient’s quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone cancer pain (BCP) is a prevalent and distressing type of pain associated with advanced malignancies, mainly from metastasis to the bone, but also from primary bone tumors. BCP can be categorized into ongoing pain, spontaneous breakthrough pain, and movement-evoked breakthrough pain. Ongoing pain, often an initial symptom, starts as a dull and constant throbbing sensation that intensifies over time. As bone cancer progresses, individuals may experience intermittent episodes of intense pain, occurring spontaneously, referred to as spontaneous breakthrough pain, or, more frequently, in response to weight-bearing or movement of the affected bone, known as movement-evoked breakthrough pain [1, 2].
While cancer patients are experiencing longer lifespans due to the advances in diagnostics and treatments, the side effects, which include pain, are substantially compromising the overall quality of life for these individuals [3]. More than half of patients with bone metastasis or advanced osteocarcinoma are undertreated for their BCP, experiencing daily moderate to severe pain [4, 5]. Currently, BCP is largely managed based on the World Health Organization’s “analgesic ladder” [6]. In addition, other adjuvant therapies including antiepileptics, steroids, antidepressants, radiotherapy, nerve blocks, nerve lesions, and surgery are often used to control cancer pain [4, 7, 8]. This approach faces several limitations due to tolerance, side effects, and inadequate analgesia [9]. Additionally, BCP is inadequately managed partly due to the current insufficient understanding of the specific mechanisms underlying this pain [10, 11].
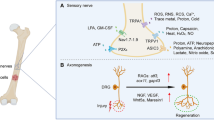
Chronic pain is influenced by physical, social, emotional, cognitive, environmental, and behavioral factors. Its transition from acute to chronic involves complex mechanisms like peripheral and central sensitization, increasing pain perception. In bone cancer pain (BCP), tumor-induced inflammation, nerve reprogramming, and peripheral nerve sensitization play key roles. The daunting nature of BCP arises from the complex interplay of nociceptive, neuropathic, and associated inflammatory mechanisms that evolve and change with disease progression (Fig. 1). The tumor mass comprises several types of cells, such as tumor cells, macrophages, neutrophils, T-lymphocytes, fibroblasts, and endothelial cells that secrete a variety of mediators, including inflammatory cytokines such as TNF-α, IL-1, and IL-6 [12]. Bone nociceptors are sensitized by these mediators, thereby initiating and perpetuating pain. The continued stimulation of the sensory nerve fibers can drive ectopic sprouting of nerve fibers and neuroma formation, leading to peripheral sensitization. Glial cell activation and subsequent release of inflammatory mediators in the central nervous system contribute to neuronal hypersensitive states [10, 11, 13]. If not properly managed, it can evolve to central sensitization and the installation of chronic pain, which is very difficult to manage [14]. Targeting specific cytokines or their receptors presents a promising, tailored approach to mitigating BCP, overcoming some limitations typically associated with traditional management.
Schematic representation of the mechanisms behind BCP. The release of pronociceptive factors by tumor and immune cells, direct tissue damage, and bone degradation through osteoclast (yellow) activation leads to the activation of nociceptors and the perception of pain. If prolonged over time, continuous nociceptive stimuli can lead to peripheral sensitization, with neuronal damage and ectopic appearance of nerve fibers. Because of peripheral events, central excitability changes happen, such as microglial activation and inflammatory mediator release, leading to central sensitization
Currently, most of the research on BCP relies on rodent models of BCP. These models are typically created through inoculation of tumor cells, such as Walker 256 mammary gland carcinoma cells, fibrosarcoma NCTC 2472 cells, or prostate cancer cells, into the bone marrow, mainly of tibia and femur. BCP has been modeled in many domestic animals; however, rodent models predominate in BCP preclinical studies. These models allow investigators to correlate cancer-induced bone remodeling, pain behavioral signs, and neurochemical changes in the spinal cord and primary afferent neurons. Pain is a subjective experience, so animal models often rely on observing pain-related behaviors, like reflex responses to a given stimulus, such as mechanical (e.g., paw withdrawal threshold to von Frey filaments) or thermal (e.g., hot plate test) stimuli. Additionally, spontaneous activities like limb use, flinching, or guarding time can be evaluated. These pain-related behaviors seem to mirror the complex clinical manifestations of ongoing pain, movement-evoked pain, and spontaneous breakthrough pain [15,16,17,18].
The present systematic review aimed to provide a comprehensive assessment of the current state of knowledge regarding potential molecular targets in rodent models within the domain of inflammatory cytokines in the context of BCP. This effort potentially enables the development of targeted cytokine-based therapeutic strategies for the management of BCP, ultimately improving the quality of life for individuals facing with this challenging symptomatology.
Materials and methods
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [19]. The PICO question was: In animal models of bone cancer pain (P), how does the implementation of targeted cytokine-based therapeutic strategies (I) compared to control groups without cytokine modulation (C) influence pain behavior (O)?
For this review, only publications including rodent animal models of BCP were evaluated, focusing primarily on studies investigating the role of inflammatory cytokines as potential molecular targets. The included outcomes of interest were pain behavior and the identification of potential molecular targets.
Studies not published in English, those involving animal models other than rodents, review articles, case reports, and studies not providing relevant data on inflammatory cytokines were excluded. Duplicate publications were also omitted from consideration.
To gather relevant studies, we systematically searched three electronic databases, namely PubMed, Scopus, and Web of Science, starting on November 23, 2022, and retrieving all articles published until October 10, 2023. For the PubMed research, the following Mesh terms were used: “Cytokines” [Mesh] AND “Cancer Pain” [Mesh]. For Web of Science, the keywords were “cytokines” AND “cancer pain”. Finally, the Scopus search for articles used was TITLE-ABS-KEY (“cytokine” AND “cancer pain” AND (“target” OR “treatment”) AND (“animal model” OR “animal study” OR “animal experiment” OR “animal” OR “rat” OR “mice”)).
Screening of titles and abstracts of identified records was carried out independently by two reviewers to determine eligibility. Full texts of potentially relevant studies were then assessed for a comprehensive analysis. The level of agreement between the authors was assessed using the Kappa test.
Data from eligible studies was extracted. The information collected from each study included information about the study’s authors, publication year, species and characteristics of the animals used, BCP model, molecular mechanisms investigated, experimental interventions, pain behavior assessments, and other outcome measures such as molecular expression and key findings. Data collection involved two independent reviewers, reaching a consensus on discrepancies.
A narrative synthesis was conducted to summarize the findings of the included studies. Given the expected heterogeneity in animal models and outcome measures, it was decided to present a qualitative synthesis instead of a meta-analysis. To assess the methodological quality and risk of bias in the included studies, the SYRCLE RoB tool was employed, specifically designed for animal studies.
Results
A comprehensive literature search in PubMed, Web of Science, and Scopus databases resulted in 365 potential records identified. Following the removal of duplicate records, 303 records remained for the title and abstract review. After the analysis of the title and abstract, 72 articles were selected for full-text examination. The final inclusion criteria were met by 25 articles, comprising animal experiments investigating potential molecular targets related to inflammatory cytokines in BCP. Figure 2 shows the entire literature search process. The Kappa coefficient for interrater agreement was 1, indicating perfect agreement between the reviewers.
Characteristics of included studies
Of the selected 25 articles, two kinds of rodents were included: rats (n = 22) [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] and mice (n = 3) [42,43,44]. Regarding their strains, Sprague–Dawley (SD) rats (n = 17) [20, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], Wistar rats (n = 3) [21, 22, 39], Copenhagen rats (n = 1) [40], Fisher F344/NHsd rats (n = 1) [41], C3H/HeN mice (n = 1) [42], BALB/c mice (n = 1) [43], C57Bl/6J mice (n = 1) [44], and transgenic mice (n = 2) [43, 44] were included. Twenty studies selected female animals [20, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] [41] [43], while five studies selected male animals [21, 22, 40, 42, 44]. Regarding the BCP model, the majority of studies (n = 20) conducted an intramedullary injection of Walker 256 rat breast cancer cells into the tibia [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. The summary of key findings is depicted in Table 1.
The molecular targets investigated encompassed a range of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) [20,21,22, 44], granulocyte–macrophage colony-stimulating factor (GM-CSF), and a variety of interleukins including IL-1β [21, 40], IL-6 [21, 22, 41], IL-17 [42], IL-18 [23], IL-33 [43], and IL-24 [24]. A variety of chemokines and their receptors were also analyzed: CCL2/CCR2 [26, 27], CXCL1/CXCR2 [28, 29], CXCL10/CXCR3 [30], CXCL12/CXCR4 [31,32,33], CXCL13/CXCR5 [34, 35].
Pain behavior assessments involved measuring mechanical allodynia through paw withdrawal threshold (PWT) in response to the stimulation with von Frey filaments in most studies (n = 20) [20,21,22,23,24,25,26,27,28,29,30, 32, 34, 35, 37, 38, 40,41,42,43]. Five studies [31, 33, 36, 39, 44] utilized the 50% PWT (50% paw withdrawal threshold) meaning the mechanical force required to elicit a paw withdrawal response in 50% of animals.
Thermal hyperalgesia was assessed according to paw withdrawal latency (PWL) in ten studies [21,22,23, 25, 29, 31,32,33, 36, 43]. Other pain behavior assessment methods were also used, such as spontaneous pain (through guarding and spontaneous flinching, n = 4) [33, 42,43,44] or limb use score (n = 4) [23, 33, 43, 44] to evaluate movement-evoked pain.
Protein expression and localization were assessed in the dorsal root ganglia (DRG) (n = 3) [22, 32, 36], spinal cord segments (n = 18) [20, 23, 26, 27, 29,30,31,32,33,34,35, 37,38,39,40, 42,43,44], and periaqueductal gray (PAG) (n = 2) [21, 28], mostly through western blotting and immunofluorescence.
Summary of key findings
All studies that evaluated PWT and PWL as pain behavior assessments demonstrated a decrease in these values in bone cancer models compared to control animals. Additionally, all other pain behavior indicators were increased after tumor cell implantation across all included studies.
Studies evaluating the expression of classic pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-17, IL-18, IL-33) in the spinal cord, PAG, and DRG revealed upregulation following inoculation of cancer cells to establish the BCP models [20,21,22,23, 27, 37,38,39,40, 42,43,44]. Interventions that inhibited these cytokines attenuated mechanical [20,21,22,23, 40,41,42,43,44] and thermal [21, 22, 43] allodynia and reduced other pain behaviors [23, 41,42,43,44]. Four studies targeted TNF or its receptor [20,21,22, 44] resulting in reduced pain behaviors. The inhibition of IL-1β signaling was the intervention in two studies [21, 40] leading to attenuated hypersensitive responses. Three studies focused their intervention on blocking IL-6 [21, 22, 41] resulting in pain reduction. Inhibiting IL-17 [42], IL-18 [23], and IL-33 [43] also suppressed pain behaviors. One study evaluated the possible role of IL-24, a member of the IL-10 family, in inhibiting cancer pain. It was demonstrated that IL-24 mediated by adenovirus could significantly attenuate BCP and increase β-endorphin levels while decreasing IL-6 concentration in the plasma of animals [24]. It was demonstrated that targeting GM-CSF significantly alleviated both mechanical and thermal hyperalgesia [25].
Chemokines and their role were also evaluated in several studies of this review. In BCP models, the expression of all chemokines assessed CCL2/CCR2 [26, 27], CXCL1/CXCR2 [28, 29], CXCL10/CXCR3 [30], CXCL12/CXCR4 [31,32,33], and CXCL13/CXCR5 [34, 35] was increased in the spinal cord after BCP induction. Targeting these molecules attenuated pain hypersensitivity consistently throughout every study.
One study’s findings include the significant downregulation of SOCS3 in DRG following tumor cell injection, and subsequent overexpression attenuated pain hypersensitivity probably via reversing TLR4 upregulation [36].
Another study demonstrated that after TCI, the expression of NLRP3 inflammasome was upregulated in the spinal cord. Treatment of the NLRP3 inhibitor MCC950 markedly alleviated BCP-related mechanical allodynia suppressing the activation of NLRP3 inflammasome and spinal inflammatory cytokines in BCP rats [37].
Two studies focused on HMGB1, finding that spinal HMGB1 upregulation contributes to bone cancer pain and that treatment with anti-HMGB1 significantly reversed bone cancer-induced mechanical allodynia [38, 39].
Several studies implicated the involvement of glial cells, such as microglia and astrocytes, in the modulation of BCP [20, 23, 28, 30, 32, 38, 42, 43].
Risk of bias assessment
Regarding the assessment of the risk of bias (Table 2; Fig. 3), using the SYRCLE tool, no study clearly detailed the methods used to generate a random allocation of animals. In terms of baseline characteristics, all studies exhibited similar starting conditions. Four studies were clear about allocation concealment. Twenty-one studies indicated randomized housing. None of the studies reported blinded allocation or randomization of outcome evaluation. Sixteen studies indicated blinding of the outcome assessor. All these studies have comprehensive outcome-based data and published intended results. Concerning other sources of bias, all studies showed no conflict of interest between authors.
Since many items were not explained in detail in the included studies, this leads to great difficulties and deviations in the interpretation of research bias. Nonetheless, the overall risk of bias was considered low to moderate.
Discussion
The main results indicate that targeting various inflammatory pathways shows promising results in alleviating BCP, mainly through the inhibition of pro-inflammatory cytokines and chemokines. Furthermore, IL-24 and SOCS3 demonstrated pain relief effects by modulating inflammatory mediators, and glial cells were identified as significant contributors to the development of BCP.
Among pro-inflammatory cytokines, TNF-α is recognized as a potent cytokine, rapidly produced, mainly by macrophages, leading to an increase in its levels in response to inflammatory stimuli or injury [45]. TNF-α interacts with target cells through high-affinity membrane receptors, namely TNF receptor type 1 (TNFR1) and type 2 (TNFR2) [46]. TNF-α triggers cytokine cascades, particularly IL-1β, IL-6, and IL-8 [47], driving both inflammatory and neuropathic hyperalgesia [48,49,50,51,52,53,54,55,56,57]. Its correlation with pain is noted across different conditions [58,59,60,61], and it also influences cancer-related chronic inflammation and progression [62, 63]. XPro1595, a TNF-α inhibitor, dose-dependently reduced mechanical allodynia in bone cancer rats, accompanied by decreased astrocyte and microglia activation and diminished pro-inflammatory cytokine production [20]. Injection of PTX (TNF-α synthesis inhibitor, pentoxifylline) and TRPA1 modulation attenuated mechanical and thermal hypersensitivity induced by bone cancer [22]. TNF-α (and IL-6) has a role in modifying the TRPA1 signal pathway [22]. TNF-α inhibition with etanercept attenuates pain response, achieved through downregulation of PI3K-mTOR expression in the dlPAG [21]. The combined absence of TNFR1 and TNFR2 attenuates cancer-related pain and concomitant spinal astrogliosis. However, TNF-α has the capability to promote tumor growth [44]. Nevertheless, exploring TNF-α’s mechanisms could unveil new targets, and combination therapies may provide comprehensive pain control with fewer side effects. Overall, TNF-α-targeted strategies hold potential for improving cancer pain management in the future personalized medicine approach.
In cancer pain, key ILs implicated include IL-1, IL-6, IL-10, IL-17, IL-18, and IL-33. These ILs contribute to the inflammatory responses and pain modulation associated with malignancy. IL-1β is acknowledged as an inflammatory mediator, mainly released by monocytes and macrophages, along with nonimmune cells like endothelial cells and fibroblasts [64]. The upregulation of IL-1β has been reported in the spinal cord during both inflammatory [65, 66] and neuropathic pain [67, 68], playing a role in facilitating the transmission and processing of noxious inputs at the spinal level [66, 69,70,71]. In BCP, IL-1β is consistently upregulated after tumor cell inoculation [20, 21, 34, 38,39,40, 43]. Spinal IL-1β increases NR1 phosphorylation to facilitate pain, and the administration of anakinra, an IL-1RI receptor antagonist, significantly reduces mechanical hyperalgesia and NR1 phosphorylation [40]. Inhibition of the IL-1β receptor relieved mechanical and thermal hyperalgesia in BCP, accompanied by downregulation of PI3K-mTOR [21].
IL-6 is recognized for its involvement in promoting pain by sensitizing nociceptors and amplifying signaling at the site of injury or disease. Its causal role extends to chronic inflammatory and immune diseases [72, 73], playing a crucial role in the pathogenesis of neuropathic pain, inflammatory pain, and BCP [74]. IL-6 also exhibits significant pro-tumorigenic activity, impacting bone metabolism, tumor cell proliferation and survival, angiogenesis, and inflammation [75]. During tumor growth, IL-6 was elevated in the plasma. Additionally, acute IL-6 signaling blockade with TB-2–081, an IL-6 signaling antagonist, demonstrated partial relief only in mechanical allodynia. On the other hand, continuous IL-6 blockade from tumor onset also effectively decreased ongoing pain and tumor-induced bone remodeling [41]. Administration of SC144, a complexed IL-6R-gp130 inhibitor, alleviated mechanical and thermal hypersensitivity in BCP rats [21, 22], reversing the upregulation of TRPA1, p-p38-MAPK, p-JNK [22], and PI3K-mTOR [21] induced by cancer. Crafting tailored treatments centered on IL suppression, such as utilizing tocilizumab and anakinra, holds the potential for more precise and efficient pain management. Tailoring treatments based on individual IL levels and responses could optimize pain relief for patients.
Numerous cytokines are recognized for their ability to induce chemotaxis; a specific subgroup of structurally related cytokines is known as chemokines. While their primary role involves recruiting white blood cells to the inflammation site, chemokines also play additional roles in angiogenesis and immune response, also contributing to the regulation of fever [76]. Chemokine receptors are extensively distributed in white blood cells, neurons, and glial cells [77]. It appears that chemokines play a role in enhancing pain sensitivity and spontaneous pain, either through direct action or by modulating the activity of nociceptors [78,79,80]. An important chemokine, MCP-1 (also known as CCL2), induced mechanical allodynia in naïve rats, and CCR2 antagonist RS-504393 reduced pain sensitivity [26]. PI3K/Akt [26] and NF-kB signaling pathways are involved in the regulation of the MCP-1/CCR2 axis in the spinal cord of BCP rats, contributing to the maintenance of pain by influencing the inflammatory process [27]. PAG administration of exogenous CXCL1 induced mechanical allodynia, while neutralizing antibodies against CXCR2 and CXCL1 attenuated BCP. NF-κB is implicated in the production of CXCL1 in astrocytes, and its inhibition results in decreased mechanical allodynia [28]. JNK activation is an upstream step in the production of CXCL1 in BCP [29]. CXCL10/CXCR3 signaling is also a contributor to the development and maintenance of BCP, most likely through microglial activation [30]. CXCL12/CXCR4 pathway leads to BCP through the activation of astrocytes and microglia and sensitizing neurons [31,32,33]. Spinal RhoA/ROCK2 pathway [31] and CaMKII/CREB pathway [33] have been identified as key downstream targets in CXCR4-mediated hyperalgesia and neuronal sensitization. The role of chemokine CXCL13 is particularly interesting; besides contributing to the development of BCP in rats, CXCL13 acts as a negative regulator in morphine analgesia [34, 35], possibly via p38, ERK, and AKT pathway [35]. Blocking CXCL13 signaling may therefore be a target to improve morphine analgesia in patients with cancer pain.
In the central nervous system, neurons and glial cells interact intricately to process and modulate pain, playing a crucial role in both acute and chronic pain states, particularly in BCP [20, 32, 42, 66, 68, 69, 81, 82]. Following tumor cell implantation, microglia and astrocytes become activated, manifesting as microgliosis and astrogliosis. Activated glial cells release numerous pro-inflammatory mediators, upregulate cell surface receptors, and activate intracellular signaling pathways. These processes enhance pain sensitivity and persistence. Activation of spinal cord microglia via the neuronal complement pathway (involving C1, C2, and C3) through the complement 3 receptor (C3R) enhances pain sensitivity [83]. Astrocytes contribute via the GABAergic pathway, leading to disinhibition and heightened pain sensitivity [84]. Additionally, the CXCR4-RhoA/ROCK2 signaling pathway in spinal neurons, activated by increased CXCR4 expression, promotes glial activation and pain hypersensitivity [31]. Microglial activation involves the release of pro-inflammatory cytokines like IL-18, which activate NMDA receptors on neurons, enhancing pain signals [23]. The P2X7 receptor on microglia triggers the NLRP3 inflammasome, releasing IL-1β and exacerbating pain through inflammation [85]. These complex neuron-glial interactions induce long-term neuronal changes, enhancing excitability and promoting sensitization, underscoring the potential for targeting glial pathways in developing effective BCP treatments, necessitating further research to optimize therapeutic strategies.
When it comes to studies in humans, there is still a significant journey ahead to better understand the link between inflammation and BCP. It was demonstrated that women with advanced breast cancer frequently experience pain and have high systemic levels of TNF-α and IL-1β [86]. A positive correlation between increased IL-6 levels and pain intensity in cancer patients undergoing chemotherapy has been found in clinical practice [87]. Recently, an exploratory analysis of serum cytokines in 57 metastatic breast cancer patients identified nine cytokines (GM-CSF, IFNγ, IL-1β, IL-2, IL-4, IL-5, IL-12p70, IL-17A, and IL-23) that could best predict pain severity [88].
Therapeutic translation is always deeply challenging, particularly in the case of the pathophysiologically diverse condition that is BCP. Despite encouraging outcomes in animal models, targeting pro-inflammatory cytokines and chemokines has not yet been properly studied in clinical trials of BCP. Nevertheless, clinical studies in other chronic pain conditions have supported the analgesic potential of suppressing pro-inflammatory cytokines, such as IL-6 and TNF-α [89,90,91,92,93]. Two cases have been reported where targeted administration of etanercept in an anatomical site proximal to bone metastasis in patients experiencing refractory pain resulted in prompt, significant, and prolonged relief of their complaints. This relief is most likely linked to the role of TNF-α in inhibiting osteoclast-mediated bone reabsorption [94].
The evidence under review suggests that pharmacological interventions targeting inflammatory cytokines hold promise for managing pain in advanced cancer patients struggling with BCP. However, preserving normal cytokine function is crucial, through a delicate balance between mitigating excessive inflammation and maintaining vital immune response and homeostasis [95]. The challenge in administering cytokine or chemokine inhibitors lies in their pleiotropic nature; any cytokine-targeted therapy may act as a double-edged sword, with both beneficial and detrimental effects on human health [96,97,98].
Something worth considering in clinical BCP research is stratifying human patient populations based on excessive/dysregulated inflammatory responses. Identifying inflammatory biomarkers holds the potential to discern patients who might benefit from targeted cytokine therapies, a pivotal step toward precision medicine [99, 100]. Apart from directly measuring cytokine levels, it is important to note that C-reactive protein (CRP) also acts as a biomarker for systemic inflammation and reflects IL-6 levels since its production depends on IL-6 [99, 101].
To bridge the gap between animal and clinical pain research in BCP, preclinical studies must be more rigorous, and animal models should closely replicate human pathology. Using markers of bone remodeling and imaging techniques from human studies can enhance model validity. Due to the varied manifestations of BCP across different cancers and metastasis sites, multiple animal models are necessary to explore variations in pain etiology and drug responsiveness. Effective BCP management involves polypharmacy or agents with polypharmacology. Align animal models with human BCP is essential to identify clinically efficacious agents and compatibility with adjunct therapies. Chemotherapy’s modulation and its potential impact on immune-targeting BCP interventions highlight the need for evaluating therapeutic compatibility in preclinical studies. Given the interrelation between pain, bone wasting, and tumor burden, pharmacological interventions must address the multiple aspects of BCP [15, 16, 18].
Previous reviews have explored the association between cytokines and chemokines with pain [102,103,104,105], including specific molecules such as CXCL3 [106] or IL-17 [107]. Furthermore, cytokines and chemokines can be potential targets for glial cell modulation [82] and morphine tolerance management [108] in chronic pain states. However, the present review specifically targets BCP, providing a comprehensive examination of the unique pathophysiological mechanisms and potential therapeutic targets associated with this condition. Additionally, it also underscores the significant role of glial cells in releasing inflammatory cytokines. Moreover, the present review emphasizes the promise and challenges of translating these findings into effective therapeutic strategies, bridging the gap between preclinical findings and clinical advancements, ultimately aiming to enhance patient quality of life.
The limitations of this research included only animal studies, as the extent to which findings in animal models mirror the human experience of BCP remains a critical question. Differences in disease pathophysiology, host responses, and genetic diversity between rodents and humans limit the applicability of animal models to human BCP. Animal studies often focus on reflexive behaviors, which may not capture the chronic and complex nature of human BCP. Furthermore, variations in treatment responses between species necessitate careful validation of preclinical findings in human clinical trials to ensure translational relevance [18]. Additionally, the research methods of the included manuscripts were conducted in rodent models with samples collected from the CNS, which makes it difficult to extrapolate findings to humans. It should also be considered that the studies were conducted in rats of only one sex, either male or female, and none of them investigated both sexes simultaneously. A meta-analysis was not possible to perform due to the high variability of the experimental protocols included in this study. These protocols often lack details about the methodology used, a problem previously identified in animal studies by the authors of the SYRCLE tool themselves. Nevertheless, there was no high risk of bias in the included studies.
Future research should prioritize establishing standardized methodologies, outcome measures, and reporting practices. Bridging the translational gap between preclinical findings and clinical applications remains crucial, necessitating robust investigations into the relevance of identified molecular targets in human BCP. Longitudinal studies exploring neuroinflammatory changes at different stages of cancer progression are vital for a comprehensive understanding of BCP. Moreover, considering the complex interplay of multiple molecular targets, research on combinatorial therapeutic approaches tailored to individual molecular profiles holds promise for more effective BCP management in the era of personalized medicine.
Conclusions
Based on the comprehensive assessment provided in this systematic review, targeting inflammatory cytokines holds significant promise in managing BCP, as demonstrated in animal models. Glial cells, due to their involvement in the release of inflammatory cytokines, emerged as significant contributors to BCP. Targeting specific cytokine pathways, such as TNF-α, IL-6, and IL-1β, highlights the potential for new therapeutic options, ultimately leading to an enhanced quality of life for individuals grappling with the debilitating symptoms of BCP. This work underscores the importance of continued research in this area to translate these findings into effective clinical treatments. As we move forward, these findings imply a paradigm shift toward personalized, precision medicine approaches, fostering optimism in the pursuit of more effective therapeutic strategies.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
References
Mercadante S, Arcuri E (1998) Breakthrough pain in cancer patients: pathophysiology and treatment. Cancer Treat Rev 24:425–432. https://doi.org/10.1016/s0305-7372(98)90005-6
Portenoy RK, Payne D, Jacobsen P (1999) Breakthrough pain: characteristics and impact in patients with cancer pain. Pain 81:129–134. https://doi.org/10.1016/s0304-3959(99)00006-8
Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW (2013) Cancer-induced bone pain: mechanisms and models. Neurosci Lett 557(Pt A):52–59. https://doi.org/10.1016/j.neulet.2013.08.003
Colosia A, Njue A, Bajwa Z, Dragon E, Robinson RL, Sheffield KM, Thakkar S, Richiemer SH (2022) The burden of metastatic cancer-induced bone pain: a narrative review. J Pain Res 15:3399–3412. https://doi.org/10.2147/JPR.S371337
Smith HS, Barkin RL (2014) Painful boney metastases. Am J Ther 21:106–130. https://doi.org/10.1097/MJT.0b013e3182456dff
Anekar AA, Hendrix JM, Cascella M (2024) WHO Analgesic LadderStatPearls. Treasure Island (FL)
Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M, Ripamonti CI, Committee EG (2018) Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol 29:iv166–iv191. https://doi.org/10.1093/annonc/mdy152
Desandre PL, Quest TE (2010) Management of cancer-related pain. Hematol Oncol Clin North Am 24:643–658. https://doi.org/10.1016/j.hoc.2010.03.002
Jing D, Zhao Q, Zhao Y, Lu X, Feng Y, Zhao B, Zhao X (2023) Management of pain in patients with bone metastases. Front Oncol. https://doi.org/10.3389/fonc.2023.1156618
Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW (2013) Cancer-induced bone pain: mechanisms and models. Neurosci Lett 557:52–59. https://doi.org/10.1016/j.neulet.2013.08.003
Falk S, Dickenson AH (2014) Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol 32:1647–1654. https://doi.org/10.1200/JCO.2013.51.7219
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9:239–252. https://doi.org/10.1038/nrc2618
Zheng XQ, Wu YH, Huang JF, Wu AM (2022) Neurophysiological mechanisms of cancer-induced bone pain. J Adv Res 35:117–127. https://doi.org/10.1016/j.jare.2021.06.006
Mantyh P (2013) Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 154(Suppl 1):S54–S62. https://doi.org/10.1016/j.pain.2013.07.044
Currie GL, Delaney A, Bennett MI, Dickenson AH, Egan KJ, Vesterinen HM, Sena ES, Macleod MR, Colvin LA, Fallon MT (2013) Animal models of bone cancer pain: systematic review and meta-analyses. Pain 154:917–926. https://doi.org/10.1016/j.pain.2013.02.033
Slosky LM, Largent-Milnes TM, Vanderah TW (2015) Use of animal models in understanding cancer-induced bone pain. Cancer Growth Metastasis 8:47–62. https://doi.org/10.4137/CGM.S21215
Mao-Ying QL, Zhao J, Dong ZQ, Wang J, Yu J, Yan MF, Zhang YQ, Wu GC, Wang YQ (2006) A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun 345:1292–1298. https://doi.org/10.1016/j.bbrc.2006.04.186
Currie GL, Sena ES, Fallon MT, Macleod MR, Colvin LA (2014) Using animal models to understand cancer pain in humans. Curr Pain Headache Rep 18:423. https://doi.org/10.1007/s11916-014-0423-6
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Zhou KX, He XT, Hu XF, Zhao WJ, Li CX, Zhang C, Zhang T, Gu ZX, Deng JP, Dong YL (2019) XPro1595 ameliorates bone cancer pain in rats via inhibiting p38-mediated glial cell activation and neuroinflammation in the spinal dorsal horn. Brain Res Bull 149:137–147. https://doi.org/10.1016/j.brainresbull.2019.04.009
Zhang J, Wang LP, Wang HS, Su ZB, Pang XC (2019) Neuroinflammation and central PI3K/Akt/mTOR signal pathway contribute to bone cancer pain. Mol Pain. https://doi.org/10.1177/1744806919830240
Zhao D, Han DF, Wang SS, Lv B, Wang X, Ma C (2019) Roles of tumor necrosis factor-alpha and interleukin-6 in regulating bone cancer pain via TRPA1 signal pathway and beneficial effects of inhibition of neuro-inflammation and TRPA1. Mol Pain 15:1744806919857981. https://doi.org/10.1177/1744806919857981
Liu S, Liu YP, Lv Y, Yao JL, Yue DM, Zhang MY, Qi DY, Liu GJ (2018) IL-18 contributes to bone cancer pain by regulating glia cells and neuron interaction. J Pain 19:186–195. https://doi.org/10.1016/j.jpain.2017.10.003
Zhao Y, Tian L, Sheng W, Miao J, Yang J (2013) Hypalgesia effect of IL-24, a quite new mechanism for IL-24 application in cancer treatment. J Interferon Cytokine Res 33:606–611. https://doi.org/10.1089/jir.2012.0146
Zhang F, Wang Y, Liu Y, Han H, Zhang D, Fan X, Du X, Gamper N, Zhang H (2019) Transcriptional regulation of voltage-gated sodium channels contributes to GM-CSF-induced pain. J Neurosci 39:5222–5233. https://doi.org/10.1523/JNEUROSCI.2204-18.2019
Yan Y, Liang Y, Ding T, Chu H (2019) PI3K/Akt signaling pathway may be involved in MCP-1-induced P2X4R expression in cultured microglia and cancer-induced bone pain rats. Neurosci Lett 701:100–105. https://doi.org/10.1016/j.neulet.2019.02.024
Wang Y, Ni H, Li H, Deng H, Xu LS, Xu S, Zhen Y, Shen H, Pan H, Yao M (2018) Nuclear factor kappa B regulated monocyte chemoattractant protein-1/chemokine CC motif receptor-2 expressing in spinal cord contributes to the maintenance of cancer-induced bone pain in rats. Mol Pain 14:1744806918788681. https://doi.org/10.1177/1744806918788681
Ni H, Wang Y, An K, Liu Q, Xu L, Zhu C, Deng H, He Q, Wang T, Xu M et al (2019) Crosstalk between NFkappaB-dependent astrocytic CXCL1 and neuron CXCR2 plays a role in descending pain facilitation. J Neuroinflammation 16:1. https://doi.org/10.1186/s12974-018-1391-2
Wang ZL, Du TT, Zhang RG (2016) JNK in spinal cord facilitates bone cancer pain in rats through modulation of CXCL1. J Huazhong Univ Sci Technolog Med Sci 36:88–94. https://doi.org/10.1007/s11596-016-1547-1
Bu H, Shu B, Gao F, Liu C, Guan X, Ke C, Cao F, Hinton AO, Xiang H, Yang H et al (2014) Spinal IFN-γ-induced protein-10 (CXCL10) mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models. Breast Cancer Res Treat 143:255–263. https://doi.org/10.1007/s10549-013-2807-4
Xu H, Peng C, Chen XT, Yao YY, Chen LP, Yin Q, Shen W (2020) Chemokine receptor CXCR4 activates the RhoA/ROCK2 pathway in spinal neurons that induces bone cancer pain. Mol Pain 16:1744806920919568. https://doi.org/10.1177/1744806920919568
Shen W, Hu XM, Liu YN, Han Y, Chen LP, Wang CC, Song C (2014) CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation. https://doi.org/10.1186/1742-2094-11-75
Hu XM, Zhang H, Xu H, Zhang HL, Chen LP, Cui WQ, Yang W, Shen W (2017) Chemokine receptor CXCR4 regulates CaMKII/CREB pathway in spinal neurons that underlies cancer-induced bone pain. Sci Rep 7:4005. https://doi.org/10.1038/s41598-017-04198-3
Bu HL, Xia YZ, Liu PM, Guo HM, Yuan C, Fan XC, Huang C, Wen YY, Kong CL, Wang T et al (2019) The roles of chemokine CXCL13 in the development of bone cancer pain and the regulation of morphine analgesia in rats. Neuroscience 406:62–72. https://doi.org/10.1016/j.neuroscience.2019.02.025
Wang SF, Dong CG, Yang X, Yin JJ (2016) Upregulation of (C-X-C motif) ligand 13 (CXCL13) attenuates morphine analgesia in rats with cancer-induced bone pain. Med Sci Monit 22:4612–4622. https://doi.org/10.12659/msm.897702
Wei J, Li M, Wang D, Zhu H, Kong X, Wang S, Zhou YL, Ju Z, Xu GY, Jiang GQ (2017) Overexpression of suppressor of cytokine signaling 3 in dorsal root ganglion attenuates cancer-induced pain in rats. Mol Pain 13:1744806916688901. https://doi.org/10.1177/1744806916688901
Chen SP, Zhou YQ, Wang XM, Sun J, Cao F, HaiSam S, Ye DW, Tian YK (2019) Pharmacological inhibition of the NLRP3 in fl ammasome as a potential target for cancer-induced bone pain. Pharmacol Res 147:104339. https://doi.org/10.1016/j.phrs.2019.104339
An K, Rong H, Ni H, Zhu C, Xu L, Liu Q, Chen Y, Zheng Y, Huang B, Yao M (2018) Spinal PKC activation - Induced neuronal HMGB1 translocation contributes to hyperalgesia in a bone cancer pain model in rats. Exp Neurol 303:80–94. https://doi.org/10.1016/j.expneurol.2018.02.003
Tong W, Wang W, Huang J, Ren N, Wu SX, Li YQ (2010) Spinal high-mobility group box 1 contributes to mechanical allodynia in a rat model of bone cancer pain. Biochem Biophys Res Commun 395:572–576. https://doi.org/10.1016/j.bbrc.2010.04.086
Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT, Berman BM, Lao L (2008) Interleukin 1β facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience 154:1533–1538. https://doi.org/10.1016/j.neuroscience.2008.04.072
Remeniuk B, King T, Sukhtankar D, Nippert A, Li N, Li F, Cheng K, Rice KC, Porreca F (2018) Disease modifying actions of interleukin-6 blockade in a rat model of bone cancer pain. Pain 159:684–698. https://doi.org/10.1097/j.pain.0000000000001139
Huo W, Liu Y, Lei Y, Zhang Y, Huang Y, Mao Y, Wang C, Sun Y, Zhang W, Ma Z et al (2019) Imbalanced spinal infiltration of Th17/Treg cells contributes to bone cancer pain via promoting microglial activation. Brain Behav Immun 79:139–151. https://doi.org/10.1016/j.bbi.2019.01.024
Zhao J, Zhang H, Liu SB, Han P, Hu S, Li Q, Wang ZF, Mao-Ying QL, Chen HM, Jiang JW et al (2013) Spinal interleukin-33 and its receptor ST2 contribute to bone cancer-induced pain in mice. Neuroscience 253:172–182. https://doi.org/10.1016/j.neuroscience.2013.08.026
Geis C, Graulich M, Wissmann A, Hagenacker T, Thomale J, Sommer C, Schäfers M (2010) Evoked pain behavior and spinal glia activation is dependent on tumor necrosis factor receptor 1 and 2 in a mouse model of bone cancer pain. Neuroscience 169:463–474. https://doi.org/10.1016/j.neuroscience.2010.04.022
Friedman R (2000) Pain at the cellular level: the role of the cytokine tumor necrosis factor-alpha. Reg Anesth Pain Med 25:110–112. https://doi.org/10.1053/rapm.2000.0830110
Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH (2006) Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther 112:116–138. https://doi.org/10.1016/j.pharmthera.2006.04.001
Ohishi S (2000) Evaluation of time course and inter-relationship of inflammatory mediators in experimental inflammatory reaction. Yakugaku Zasshi 120:455–462
Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH (1992) The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 107:660–664. https://doi.org/10.1111/j.1476-5381.1992.tb14503.x
Perkins MN, Kelly D, Davis AJ (1995) Bradykinin B1 and B2 receptor mechanisms and cytokine-induced hyperalgesia in the rat. Can J Physiol Pharmacol 73:832–836. https://doi.org/10.1139/y95-113
Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S (1997) Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol 121:417–424. https://doi.org/10.1038/sj.bjp.0701148
Sommer C, Schmidt C, George A (1998) Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol 151:138–142. https://doi.org/10.1006/exnr.1998.6797
Wagner R, Myers RR, O’Brien JS (1998) Prosaptide prevents hyperalgesia and reduces peripheral TNFR1 expression following TNF-alpha nerve injection. NeuroReport 9:2827–2831. https://doi.org/10.1097/00001756-199808240-00026
Sommer C, Schafers M (1998) Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res 784:154–162. https://doi.org/10.1016/s0006-8993(97)01327-9
Wagner R, Myers RR (1996) Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience 73:625–629. https://doi.org/10.1016/0306-4522(96)00127-3
Schafers M, Svensson CI, Sommer C, Sorkin LS (2003) Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci 23:2517–2521. https://doi.org/10.1523/JNEUROSCI.23-07-02517.2003
George A, Schmidt C, Weishaupt A, Toyka KV, Sommer C (1999) Serial determination of tumor necrosis factor-alpha content in rat sciatic nerve after chronic constriction injury. Exp Neurol 160:124–132. https://doi.org/10.1006/exnr.1999.7193
Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C (2003) Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci 17:791–804. https://doi.org/10.1046/j.1460-9568.2003.02504.x
Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL (1992) Tumor necrosis factor production in patients with leprosy. Infect Immun 60:1441–1446. https://doi.org/10.1128/iai.60.4.1441-1446.1992
Lindenlaub T, Sommer C (2003) Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol 105:593–602. https://doi.org/10.1007/s00401-003-0689-y
Shafer DM, Assael L, White LB, Rossomando EF (1994) Tumor necrosis factor-alpha as a biochemical marker of pain and outcome in temporomandibular joints with internal derangements. J Oral Maxillofac Surg 52:786–791; discussion 791–782. https://doi.org/10.1016/0278-2391(94)90217-8
Tak PP, Smeets TJ, Daha MR, Kluin PM, Meijers KA, Brand R, Meinders AE, Breedveld FC (1997) Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum 40:217–225. https://doi.org/10.1002/art.1780400206
Balkwill F (2009) Tumour necrosis factor and cancer. Nat Rev Cancer 9:361–371. https://doi.org/10.1038/nrc2628
Chu WM (2013) Tumor necrosis factor. Cancer Lett 328:222–225. https://doi.org/10.1016/j.canlet.2012.10.014
Zhang JM, An J (2007) Cytokines, inflammation, and pain. Int Anesthesiol Clin 45:27–37. https://doi.org/10.1097/AIA.0b013e318034194e
Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ (2001) Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 410:471–475. https://doi.org/10.1038/35068566
Raghavendra V, Tanga FY, DeLeo JA (2004) Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci 20:467–473. https://doi.org/10.1111/j.1460-9568.2004.03514.x
Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA (2001) Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol 439:127–139
Raghavendra V, Tanga F, DeLeo JA (2003) Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 306:624–630. https://doi.org/10.1124/jpet.103.052407
Watkins LR, Milligan ED, Maier SF (2003) Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol 521:1–21
Copray JC, Mantingh I, Brouwer N, Biber K, Kust BM, Liem RS, Huitinga I, Tilders FJ, Van Dam AM, Boddeke HW (2001) Expression of interleukin-1 beta in rat dorsal root ganglia. J Neuroimmunol 118:203–211. https://doi.org/10.1016/s0165-5728(01)00324-1
Reeve AJ, Patel S, Fox A, Walker K, Urban L (2000) Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 4:247–257. https://doi.org/10.1053/eujp.2000.0177
Ebbinghaus M, Segond von Banchet G, Massier J, Gajda M, Brauer R, Kress M, Schaible HG (2015) Interleukin-6-dependent influence of nociceptive sensory neurons on antigen-induced arthritis. Arthritis Res Ther 17:334. https://doi.org/10.1186/s13075-015-0858-0
Kiguchi N, Maeda T, Kobayashi Y, Kondo T, Ozaki M, Kishioka S (2008) The critical role of invading peripheral macrophage-derived interleukin-6 in vincristine-induced mechanical allodynia in mice. Eur J Pharmacol 592:87–92. https://doi.org/10.1016/j.ejphar.2008.07.008
Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK (2016) Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation 13:141. https://doi.org/10.1186/s12974-016-0607-6
Ara T, Declerck YA (2010) Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer 46:1223–1231. https://doi.org/10.1016/j.ejca.2010.02.026
Ransohoff RM (2009) Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31:711–721. https://doi.org/10.1016/j.immuni.2009.09.010
Ubogu EE, Cossoy MB, Ransohoff RM (2006) The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci 27:48–55. https://doi.org/10.1016/j.tips.2005.11.002
Abbadie C (2005) Chemokines, chemokine receptors and pain. Trends Immunol 26:529–534. https://doi.org/10.1016/j.it.2005.08.001
Kiguchi N, Kobayashi Y, Kishioka S (2012) Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol 12:55–61. https://doi.org/10.1016/j.coph.2011.10.007
White FA, Bhangoo SK, Miller RJ (2005) Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov 4:834–844. https://doi.org/10.1038/nrd1852
Ji RR, Berta T, Nedergaard M (2013) Glia and pain: is chronic pain a gliopathy? Pain 154(Suppl 1):S10–S28. https://doi.org/10.1016/j.pain.2013.06.022
Donnelly CR, Andriessen AS, Chen G, Wang K, Jiang C, Maixner W, Ji RR (2020) Central nervous system targets: glial cell mechanisms in chronic pain. Neurotherapeutics 17:846–860. https://doi.org/10.1007/s13311-020-00905-7
Huang X, Li J, Xie J, Li Y, Gao Y, Li X, Xu X, Shi R, Yao W, Ke C (2018) Neuronal complement cascade drives bone cancer pain via C3R mediated microglial activation. Brain Res 1698:81–88. https://doi.org/10.1016/j.brainres.2018.06.011
Ge MM, Chen SP, Zhou YQ, Li Z, Tian XB, Gao F, Manyande A, Tian YK, Yang H (2019) The therapeutic potential of GABA in neuron-glia interactions of cancer-induced bone pain. Eur J Pharmacol 858:172475. https://doi.org/10.1016/j.ejphar.2019.172475
Wu P, Wu X, Zhou G, Wang Y, Liu X, Lv R, Liu Y, Wen Q (2022) P2X7 receptor-induced bone cancer pain by regulating microglial activity via NLRP3/IL-1beta signaling. Pain Physician 25:E1199–E1210
Panis C, Victorino VJ, Herrera AC, Freitas LF, De Rossi T, Campos FC, Simao AN, Barbosa DS, Pinge-Filho P, Cecchini R et al (2012) Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Res Treat 133:881–888. https://doi.org/10.1007/s10549-011-1851-1
Al-Mazidi S, Farhat K, Nedjadi T, Chaudhary A, Al-Abdin OZ, Rabah D, Al-Zoghaibi M, Djouhri L (2018) Association of interleukin-6 and other cytokines with self-reported pain in prostate cancer patients receiving chemotherapy. Pain Med 19:1058–1066. https://doi.org/10.1093/pm/pnx145
Fazzari J, Sidhu J, Motkur S, Inman M, Buckley N, Clemons M, Vandermeer L, Singh G (2020) Applying serum cytokine levels to predict pain severity in cancer patients. J Pain Res 13:313–321. https://doi.org/10.2147/JPR.S227175
Cohen SP, White RL, Kurihara C, Larkin TM, Chang A, Griffith SR, Gilligan C, Larkin R, Morlando B, Pasquina PF et al (2012) Epidural steroids, etanercept, or saline in subacute sciatica: a multicenter, randomized trial. Ann Intern Med 156:551–559. https://doi.org/10.7326/0003-4819-156-8-201204170-00397
Ohtori S, Miyagi M, Eguchi Y, Inoue G, Orita S, Ochiai N, Kishida S, Kuniyoshi K, Nakamura J, Aoki Y et al (2012) Efficacy of epidural administration of anti-interleukin-6 receptor antibody onto spinal nerve for treatment of sciatica. Eur Spine J 21:2079–2084. https://doi.org/10.1007/s00586-012-2183-5
Cohen SP, Wenzell D, Hurley RW, Kurihara C, Buckenmaier CC 3rd, Griffith S, Larkin TM, Dahl E, Morlando BJ (2007) A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology 107:99–105. https://doi.org/10.1097/01.anes.0000267518.20363.0d
Cohen SP, Galvagno SM, Plunkett A, Harris D, Kurihara C, Turabi A, Rehrig S, Buckenmaier CC 3rd, Chelly JE (2013) A multicenter, randomized, controlled study evaluating preventive etanercept on postoperative pain after inguinal hernia repair. Anesth Analg 116:455–462. https://doi.org/10.1213/ANE.0b013e318273f71c
Sainoh T, Orita S, Miyagi M, Inoue G, Yamauchi K, Suzuki M, Sakuma Y, Kubota G, Oikawa Y, Inage K et al (2016) Single intradiscal injection of the interleukin-6 receptor antibody tocilizumab provides short-term relief of discogenic low back pain; prospective comparative cohort study. J Orthop Sci 21:2–6. https://doi.org/10.1016/j.jos.2015.10.005
Tobinick EL (2003) Targeted etanercept for treatment-refractory pain due to bone metastasis: two case reports. Clin Ther 25:2279–2288. https://doi.org/10.1016/s0149-2918(03)80219-9
Rider P, Carmi Y, Cohen I (2016) Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol 2016:9259646. https://doi.org/10.1155/2016/9259646
Zidek Z, Anzenbacher P, Kmonickova E (2009) Current status and challenges of cytokine pharmacology. Br J Pharmacol 157:342–361. https://doi.org/10.1111/j.1476-5381.2009.00206.x
Bonati L, Tang L (2021) Cytokine engineering for targeted cancer immunotherapy. Curr Opin Chem Biol 62:43–52. https://doi.org/10.1016/j.cbpa.2021.01.007
Rossi JF, Lu ZY, Massart C, Levon K (2021) Dynamic immune/inflammation precision medicine: the good and the bad inflammation in infection and cancer. Front Immunol 12:595722. https://doi.org/10.3389/fimmu.2021.595722
Laird BJA, Scott AC, Colvin LA, McKeon AL, Murray GD, Fearon KCH, Fallon MT (2011) Cancer pain and its relationship to systemic inflammation: an exploratory study. Pain 152:460–463. https://doi.org/10.1016/j.pain.2010.10.035
Chiswick EL, Duffy E, Japp B, Remick D (2012) Detection and quantification of cytokines and other biomarkers. Methods Mol Biol 844:15–30. https://doi.org/10.1007/978-1-61779-527-5_2
Calapai F, Mondello E, Mannucci C, Sorbara EE, Gangemi S, Quattrone D, Calapai G, Cardia L (2021) Pain biomarkers in cancer: an overview. Curr Pharm Des 27:293–304. https://doi.org/10.2174/1381612826666201102103520
Vanderwall AG, Milligan ED (2019) Cytokines in pain: harnessing endogenous anti-inflammatory signaling for improved pain management. Front Immunol 10:3009. https://doi.org/10.3389/fimmu.2019.03009
Goncalves Dos Santos G, Delay L, Yaksh TL, Corr M (2019) Neuraxial cytokines in pain states. Front Immunol 10:3061. https://doi.org/10.3389/fimmu.2019.03061
Kaye AD, Perilloux DM, Hawkins AM, Wester GC, Ragaland AR, Hebert SV, Kim J, Heisler M, Kelkar RA, Chami AA et al (2024) Tumor necrosis factor and interleukin modulators for pathologic pain states: a narrative review. Pain Ther 13:481–493. https://doi.org/10.1007/s40122-024-00603-8
Puerto Valencia LM, He Y, Wippert PM (2024) The changes of blood-based inflammatory biomarkers after non-pharmacologic interventions for chronic low back pain: a systematic review. BMC Musculoskelet Disord 25:209. https://doi.org/10.1186/s12891-024-07289-1
Zhou YQ, Liu DQ, Chen SP, Sun J, Zhou XR, Xing C, Ye DW, Tian YK (2019) The role of CXCR3 in neurological diseases. Curr Neuropharmacol 17:142–150. https://doi.org/10.2174/1570159X15666171109161140
Gao SJ, Liu L, Li DY, Liu DQ, Zhang LQ, Wu JY, Song FH, Zhou YQ, Mei W (2024) Interleukin-17: a putative novel pharmacological target for pathological pain. Curr Neuropharmacol 22:204–216. https://doi.org/10.2174/1570159X21666230811142713
Liu DQ, Zhou YQ, Gao F (2019) Targeting cytokines for morphine tolerance: a narrative review. Curr Neuropharmacol 17:366–376. https://doi.org/10.2174/1570159X15666171128144441
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
Conceptualization: Jacinta Ruivo, Isaura Tavares, and Daniel H. Pozza; methodology: Jacinta Ruivo, Isaura Tavares, and Daniel H. Pozza; writing—original draft preparation: Jacinta Ruivo and Daniel H. Pozza; writing—review and editing: Jacinta Ruivo, Isaura Tavares, and Daniel H. Pozza; supervision: Isaura Tavares and Daniel H. Pozza; project administration: Jacinta Ruivo and Daniel H. Pozza. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruivo, J., Tavares, I. & Pozza, D.H. Molecular targets in bone cancer pain: a systematic review of inflammatory cytokines. J Mol Med (2024). https://doi.org/10.1007/s00109-024-02464-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00109-024-02464-2