Abstract
Extracellular vesicles (EVs) are produced by various cells and exist in most biological fluids. They play an important role in cell–cell signaling, immune response, and tumor metastasis, and also have theranostic potential. They deliver many functional biomolecules, including DNA, microRNAs (miRNA), messenger RNA (mRNA), long non-coding RNA (lncRNA), lipids, and proteins, thus affecting different physiological processes in target cells. Decreased immunogenicity compared to liposomes or viral vectors and the ability to cross through physiological barriers such as the blood–brain barrier make them an attractive and innovative option as diagnostic biomarkers and therapeutic carriers. Here, we highlighted two types of cells that can produce functional EVs, namely, mesenchymal stem/stromal cells (MSCs) and regulatory T cells (Tregs), discussing MSC/Treg-derived EV-based therapies for some specific diseases including acute respiratory distress syndrome (ARDS), autoimmune diseases, and cancer.
Similar content being viewed by others
Introduction
Extracellular vesicles (EVs) play an important role in cell–cell communication and extracellular matrix remodeling [1, 2]. They are secreted by most cell types including mesenchymal stromal cells (MSCs), immune cells, endothelial cells, epithelial cells, neuronal cells, embryonic stem cells, and cancer cells [3,4,5]. Cell-free therapies, being the safe, available, and cost-effective alternative to cell-based therapies [6], have boosted the EV research over the past years.
Although several classes of EVs have been discovered, the International Society for Extracellular Vesicles emphasizes that potential heterogeneity and undefined biogenesis of the obtained samples should imply the use of the term EVs rather than referring to the specific EV subgroup [7]. To avoid any bias or misunderstanding and to support this important point raised in the MISEV2018 guidelines, in this article, we will stick to this term.
MSC-derived EVs (MSC-EVs) seem to be the most researched ones, which is hardly surprising given the wide use of MSCs in cell therapy. Not only MSCs’ potential for self-renewal and multi-lineage differentiation, but also their paracrine activity is currently believed to contribute to their unique therapeutic properties. Moreover, there is evidence that conditioned media of MSCs demonstrate therapeutic effects similar to those of transplanted cells [8,9,10], which means that cytokines, chemokines, and most importantly EVs come to the fore as the probable key factors determining MSCs’ therapeutic potential [11, 12]. Hence, MSC-EVs are being extensively investigated and employed to treat a wide range of diseases, including cardiovascular, neurological, immunological, and kidney pathologies [13].
Immune regulation could become an unequaled tool in treating plenty of pathologic conditions from inflammatory and autoimmune ones to cancer. While MSCs and their EVs exhibit some immunomodulatory properties, immune cells, and most importantly regulatory T cells (Tregs), might be a possible source of EVs with even more potent and specific effects.
Tregs are a subpopulation of CD4 + T lymphocytes that play a crucial role in the creation of immunological self-tolerance unlike CD8 + cytotoxic T lymphocytes [23, 24]. Although the mechanism of their regulatory effects is not fully elucidated, Tregs are reported to produce anti-inflammatory factors including interleukin-10 (IL-10), transforming growth factor beta 1 (TGF-β), and IL-13, thus suppressing the inflammatory response and reducing the inflammatory damage [25, 26]. Accordingly, numerous studies have indicated that type 1 diabetes, multiple sclerosis, myasthenia gravis, rheumatoid arthritis, and other autoimmune diseases are caused by Treg deficiencies [27]. At the same time, Tregs were reported to promote tumor formation by reducing the anticancer activity of immune cells [28]. Hence, a reduction in Treg’s activity may facilitate anticancer immune responses in vivo [29], and targeting Tregs may improve tumor treatment efficacy [30].
In contrast to MSC-EVs, which have been extensively studied, Treg-EV-based studies are still in their infancy. However, Treg-EVs are starting to gain more interest due to their immunosuppressive effects, such as extending survival in animal allograft models [31].
Up to now, there have been 257 clinical trials (www.clinicaltial.gov) evaluating the Treg’s effects in treating different conditions and no clinical trials evaluating Treg-EV effects. However, both Tregs and their EVs hold great promise in increasing the efficacy of autoimmune disease therapies or improving the transplant patients’ condition.
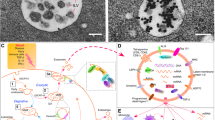
This review aims to discuss the research of understudied Treg-EVs by contrast with the widely studied MSC-EVs (Table 1; Fig. 1) and encourage further studies in this field. We aimed to focus on the immunomodulatory properties of the EVs and therefore chose to discuss the pathological conditions that would illustrate the duality of their effect. Thus, we chose those characterized by immune system over-reactivity (ARDS accompanied by cytokine storm, autoimmune diseases, allograft rejection) and cancer, characterized by immune system suppression.
MSC-EVs and Treg-EVs: contents and immunomodulatory functions
Multiple studies reported MSC-EVs’ specific cargo and associated biological effects, including immunomodulatory ones. In addition to numerous miRNAs, which will be further discussed in this review, there is evidence that MSC-EVs contain growth factors and cytokines, including hepatocyte growth factor (HGF), TGFβ, interleukin-6 (IL-6), and IL-10, which contribute to immunoregulation mechanisms [54].
In contrast, the contents of Treg-EVs are not so thoroughly explored. Like their parent cells, Treg-EVs were reported to express CD73, CD39, and CD25 [22] (Table 2). Both CD73 and CD 39 are involved in the adenosinergic pathway, which plays a key role in modulating immune responses [55]. At the same time, CD25 is speculated to promote T-effector apoptosis; however, this assumption requires further validation [22].
ARDS/ALI
ARDS, as well as its milder form, acute lung injury (ALI), is a complex inflammatory lung disease which leads to substantial reductions in lung diffusing capacity and is characterized by high morbidity and mortality in intensive care unit–admitted patients. It results from a variety of pathological conditions, including pulmonary (e.g., aspiration, pneumonia) and non-pulmonary ones (e.g., trauma, sepsis) [56]. The pathogenesis of ARDS involves alveolar-capillary membrane damage, pulmonary edema due to increased inflammation, inactivation of surfactant, reduced alveolar fluid clearance, impairment of oxygenation, and pulmonary fibrosis [57, 58]. It is thought that uncontrolled inflammation underlies all these pathophysiological mechanisms [59], often leading to life-threatening complications [60]. Despite the therapeutic advances [61], new effective ARDS treatments are urgently needed because of the high mortality rate (34.9–46.1%) [62]. The increase in deaths due to COVID-19-related ARDS during the pandemic in 2019–2022 has once again emphasized the importance of developing new therapeutic options [63, 64].
MSC-EVs and ARDS/ALI
There is evidence that MSC-EVs can target epithelial cell damage in ARDS patients, preventing the endothelial and epithelial lung barrier deficiency, increased alveolus permeability, and decrease pulmonary edema. Thus, Bari et al. reported adipose-derived MSC-EVs to express alpha-1-anti trypsin, the main elastase inhibitor in the lung, which could repress proteolytic enzymes derived from neutrophils and demonstrated anti-inflammatory and immune-regulating properties which could protect lung epithelial cells [65]. Other studies revealed that bone marrow–derived MSC-EVs could reduce pulmonary edema [66] and restore alveolar fluid clearance [67].
Furthermore, there is evidence that bone marrow–derived MSC-EVs can shift the balance between pro-inflammatory M1 and anti-inflammatory M2 macrophages [68]. It could be the turning point in inflammation modulation and enhancing tissue repair for ARDS patients experiencing cytokine storm, which practically uncontrollably leads to cytotoxic tissue damage, exacerbating the disease prognosis. Moreover, the presence of miR-145 in bone marrow–derived MSC-EVs was reported to improve the lung tissue maintenance and regeneration [34].
Treg-EVs and ARDS/ALI
Given that Tregs play a crucial role in inhibiting excessive inflammation, Treg-EVs seem a valid option to manage the uncontrolled inflammation occurring in ARDS.
For instance, Treg-EVs can transport specific miRNAs such as miR-150, miR-142-3p, miR-146a-5p, and let-7d from Tregs to effector T cells and dendritic cells, performing targeted mRNA suppression in these cells [69,70,71]. There is evidence that two of these miRNAs, namely, miR-142-3p and miR-150, alleviate lipopolysaccharide-induced ALI [72, 73].
Although there is no study yet on the use of Treg-EVs in the treatment of ALI/ARDS, their application in other immune system–related conditions may be instructive. As we emphasized earlier, promoted M2 macrophage polarization controls excessive inflammation in ALI/ARDS [74], and Treg-EVs were reported to increase M2 polarization in macrophages in a murine model [50].
It is well known that the maintenance of T helper 17 (Th17)/Treg balance is essential to prevent immunological diseases, but this balance is also crucial for the control of lung inflammation in ALI/ARDS [75,76,77]. In a recent study, Chen et al. showed that Treg-EVs from TGF-β-induced Tregs could maintain the balance of Th17/Tregs and regulate Notch1 signaling via miR-449a-5p in a murine model [51]. Finally, Sullivan et al. reported Treg-EVs to suppress CD8 + cytotoxic T lymphocyte proliferation [78], which could possibly alleviate the cytokine storm-mediated tissue damage.
Autoimmune conditions
Autoimmune conditions are characterized by abnormal immune response resulting in healthy cells’ destruction. They include, but are not limited to, psoriasis, multiple sclerosis (MS), type 1 diabetes (T1D), rheumatoid arthritis (RA), and inflammatory bowel disease (IBD). Allograft rejection also involves an autoimmune component.
Whether they affect the skin, central nervous system (CNS), pancreas, joints, or other tissues and organs, autoimmune diseases are commonly treated with immune system suppressants, which often have limited efficacy [79] while causing a number of adverse effects including nephrotoxicity, hepatotoxicity, hypertension, hyperkalemia, lymphadenopathy, lymphopenia, lipoatrophy, and dyspnea [80,81,82,83,84]. Therefore, the search for alternative treatment strategies in this field is of great need.
MSC-EVs and autoimmune conditions
Psoriasis
Human umbilical cord–derived MSC-EVs were reported to significantly suppress the proliferation of epidermis and to reduce Psoriasis Area and Severity Index (PASI) scores in the imiquimod (IMQ)–induced murine model. These EVs also reduced the expression of inflammatory IL-17, IL-23, and chemokine C–C-motif ligand 20 as well as suppressed phosphorylation of signal transducer and activator of transcription 3 both in the skin of IMQ-induced murine model and in human keratinocytes [49]. A similar study reported embryonic stem cell–derived MSC-EVs to reduce psoriasis-associated inflammation in a murine model of IMQ psoriasis via inhibition of complement stimulation in the stratum corneum and reduction in C5b-9 complex and IL-17 [47].
Multiple sclerosis
MSC-EVs were confirmed to be involved in microglial polarization and improvement of motor function in the experimental autoimmune encephalomyelitis (EAE) in a rat model. Comparing the treated and untreated EAE groups, Li et al. showed that bone marrow–derived MSC-EVs significantly reduced neural behavioral scores, decreased the infiltration of inflammatory cells in CNS, and reduced demyelination in the treated group compared to the untreated one. Moreover, they showed that treatment with EVs significantly increased the M2-related cytokines such as TGF-β and IL-10. However, at the same time, significant increases in the production of M1-related tumor necrosis factor alpha (TNF-α) and IL-12 were observed [35]. Hosseini Shamili et al. bio-conjugated the bone marrow–derived MSC-EVs to an aptamer targeting the oligodendrocyte markers. The armed EVs increased the proliferation rate of oligodendroglia cell line (OLN93) in vitro and alleviated demyelinated CNS lesions in vivo in a murine EAE model [36].
Type 1 diabetes mellitus
Menstrual blood–derived MSC-EVs were reported to induce the islet regeneration via pancreatic and duodenal homeobox 1 pathway [48]. Moreover, there is evidence that bone marrow–derived MSC-EV injection combined with islet transplantation could suppress peripheral blood mononuclear cell proliferation, induce regulatory T cells, and increase survival in the T1D murine model [37]. Similarly, Nojehdehi et al. demonstrated adipose-derived MSC-EVs to alleviate clinical symptoms of the streptozotocin-induced T1D model including blood glucose stabilization [32]. The authors also reported the increase of Tregs population, upregulation of IL-10, IL-4, and TGF-β, and downregulation of IL-17 and IFNγ. It is noteworthy that MSC-EVs could improve not only metabolic-related disorders, but also T1D-associated complications, such as cognitive impairment, promoting neurons and astrocytes repair [38]. Due to these promising results, umbilical cord–derived MSC-EVs have entered the II/III clinical trial phase for T1D treatment (NCT02138331) [85].
Rheumatoid arthritis
Bone marrow–derived MSC-EVs were observed to have an anti-inflammatory effect on T and B lymphocytes in a collagen-induced arthritis (CIA) murine model [39], and this effect was enhanced when MSC-EVs were additionally loaded with specific miRNAs. For example, miR-150-5p-enriched MSC-EVs blocked migration and invasion of rheumatoid arthritis–related fibroblast-like synoviocytes (RA-FLS), decreased hind foot thickness, and inhibited angiogenesis in a murine CIA model [40]. Likewise, bone marrow–derived MSC-EVs miR-124a could block proliferation and promote apoptosis of FLS cell line in vitro [86]. There is also evidence of correlation between the expression of miR-320a and chemokine C-X-C motif ligand 9 in the RA synovial tissue. Being a target of miR-320a, CXCL9 was reported to reestablish the function of RA-FLSs when upregulated and suppress their activation, migration, and invasion when knocked down [87]. Accordingly, bone marrow–derived MSC-EVs containing miR-320a demonstrated the same effect in vitro and decreased arthritis and bone injury in CIA mice. [41].
Treg-EVs and autoimmune conditions
Allograft rejection
While Treg’s role in suppressing alloreactivity has been shown in various studies [52, 88, 89], Treg-EVs in this light have only been studied by Aiello et al. [52]. This study investigated Treg-EVs from dnIKK transgenic mice, and it was demonstrated that modified DnIKK2-Treg-EVs contained a unique molecular cargo of specific miRNAs and iNOS which could convert T cells into regulatory cells. Moreover, inhibiting either miRNAs or NOS alone only partially reduced the suppressive capacity of DnIKK2-Treg-EVs suggesting both are critical. The authors have also found modest but significant improvement with DnIKK2-Treg-EVs in kidney allograft survival in rats arguing that Treg-EVs may provide clinical benefit to transplant patients.
Inflammatory bowel disease models
The effectiveness of Treg-EVs in IBD treatment has been demonstrated by two studies in murine models. Okoye et al.’s studies in mice showed that Tregs utilize miRNAs and deliver them via EVs to T cells to suppress T helper 1 (Th1) response. The authors have demonstrated this by using Dicer-/- as well as Rab27a/Rab27b double knockout mice which are deficient in miRNA or EV biogenesis, respectively [69]. The authors have tested Treg-EVs’ therapeutic potential in an adoptive T cell transfer model of colitis, induced by CD4+CD45RBhi T cells in Rag1-deficient mice. The suppression of inflammatory bowel disease development by Tregs was impaired when Tregs from Dicer-/-, as well as Rab27a/Rab27b double knockout mice-derived Tregs, were used, suggesting that Treg-EVs are indeed contributing to the suppression of inflammation mediated by T cells in vivo. Let-7d has been proposed to be a critical mediator of such suppression [69].
More recently, Liao et al. showed that murine splenic Treg-EVs also ameliorated dextran sodium salt-induced colitis, an acute model of colitis induced by chemical destruction of the intestinal epithelia. This study suggested a role for miR-195a-3p, a miRNA shown to be present in Treg-EVs, in mediating the suppression [53].
Cancer
The crosstalk between the tumor and its microenvironment was proven to have a direct impact on tumor growth and metastasis [90], immune cells including macrophages [91] and Tregs [92] being both the key players and targets in this process. Such orchestrating of the tumor microenvironment is partially provided by the tumor cells’ EVs [93,94,95,96,97], which means that the use of EVs derived from non-tumor cells, either native or loaded with specific miRNAs, could possibly modulate the tumor microenvironment in the way favorable for antitumor therapies.
MSC-EVs and cancer
Native MSC-EVs
There is evidence of both antitumor and tumorigenic action of MSC-EVs on angiogenesis, the tumor cell proliferation, invasion, metastasis, and response to medicines and to radiotherapy [98]. For example, human bone marrow–derived MSC-EVs were reported to inhibit the proliferation of human chronic myeloid leukemia cells in vitro via miR-15a, whereas in vivo, they were shown to increase tumor incidence and speed up tumor growth [42]. This is not an isolated case, and it suggests that the use of native MSCs-EVs, just like the use of MSCs, should require cautiousness in terms of tumorigenicity. However, some pathways determining the possible tumorigenic effect of MSCs-EVs are already determined, which is encouraging, since deepening our knowledge about these pathways will probably give us the chance of managing the unfavorable ones. For example, human bone marrow–derived MSC-EVs were reported to promote gastric cancer cell growth through the activation of the hedgehog signaling pathway [99], while another study reported native umbilical cord–derived MSC-EVs to increase gastric cancer cell proliferative and metastatic potential predominantly via the activation of the protein kinase B signaling pathway [100]. At the same time, adipose-derived MSC-EVs promoted migration and proliferation of breast cancer cell line MCF7 through the activation of the Wnt signaling pathway [101]. Paradoxically, MSC-EV immunomodulatory properties can be another concern regarding their use in oncology. While their ability to switch macrophages’ phenotype from pro-inflammatory M1 to anti-inflammatory M2 is beneficial in treating such inflammatory conditions as ARDS/ALI [68], it can have undesirable effect in tumor treatment.
On the other hand, many studies report the beneficial effect of native MSC-EVs. For example, native bone marrow–derived MSC-EVs were shown to inhibit tumor development and progression in an experimental rat model of diethylnitrosamine-induced hepatocellular carcinoma, which manifested as apoptosis activation, as well as inhibition of angiogenesis and epithelial–mesenchymal transition [102].
Just like reporting the signaling pathways mediating the tumorigenic effect of MSC-EVs, many researchers report specific MSC-EV miRNAs to have antitumor properties. For instance, miR-16 of native bone marrow–derived MSC-EVs was reported to inhibit angiogenesis via downregulation of the expression of vascular endothelial growth factor (VEGF) [43]. Time-dependent downregulation of VEGF in breast cancer cells was also reported to be caused by bone marrow–derived MSC-EVs-miR-100 targeting the mTOR/HIF-1α signaling axis [103]. Another study reported miR-4461 to be a potential target for the diagnosis and treatment of colorectal cancer, since bone marrow–derived MSC-EVs-miR-4461 inhibited the proliferation, migration, and invasion of colorectal cancer cells by downregulating coatomer protein complex subunit beta 2 expression [104]. Furthermore, human bone marrow–derived MSC-EVs carrying miR-205 were reported to suppress rhophilin Rho GTPase binding protein 2, thus enhancing prostate cancer cell apoptosis [105]. miR-143 of human bone marrow–derived MSC-EVs demonstrated the similar effect by downregulating trefoil factor 3 [106].
Functionalized MSC-EVs
Modified or engineered MSC-EVs, however, are reported to have enhanced specificity, reduced immunogenicity, and better targeting capabilities than the native ones [107]. Thus, once the important pathways and essential miRNAs are determined, MSC-EVs can be modified in a way maximizing their beneficial properties and minimizing the unfavorable ones. One way of doing so is loading the EVs with a specific miRNA, since there is evidence that EV-mediated miRNA transport protects them, providing an improved therapeutic effect compared to direct miRNA treatment [108].
Thus, it was determined that miR-1228-loaded human bone marrow–derived MSC-EVs inhibit proliferation, invasion, and migration and accelerate apoptosis of gastric cancer cells, by downregulating the expression of matrix metalloproteinase-14 (MMP-14) [109]. Similar effect was observed in PANC-1 cells treated with human umbilical cord–derived MSC-EVs transfected with hsa-miRNA-128-3p, which targeted Galectin-3 [110]. At the same time, human glioma cell line (U87MG and A172) migration and vasculogenic mimicry formation were suppressed by miR-29a-3p-loaded bone marrow–derived MSC-EVs targeting roundabout guidance receptor 1 [44], while another study reported miRNA-512-5p-loaded bone marrow–derived MSC-EVs to target Jagged Canonical Notch Ligand 1 and inhibit glioblastoma cell proliferation and G1-S phase cell cycle by inhibiting the expression of CDK4, CDK6, and Cyclin D1 [46].
Another approach suggests loading EVs with agents blocking specific miRNAs; for example, LNA-antimiR-142-3p-loaded bone marrow–derived MSC-EVs were demonstrated to decrease expression of the miR-142-3p and miR-150, thus reducing clone-formation and tumor-initiating abilities of the MCF7-derived cancer stem-like cells [111]. Or else, EVs can be engineered in a way allowing to target specific oncogenes. For instance, engineered bone marrow–derived MSC-EVs with the ability to target oncogenic KRAS could induce apoptosis of PANC-1 cells, and their use in a murine model of pancreas ductal adenocarcinoma (PDAC) improved the histopathology of pancreas, reduced tumor burden, and exhibited a trending decrease in tumor weight [112]. Moreover, there is an ongoing clinical trial evaluating the efficacy and safety of MSC-EVs with KrasG12D siRNA in treating patients suffering from metastatic pancreatic cancer with KrasG12D mutation (NCT03608631).
Some miRNAs can enhance the chemosensitivity of tumor cells. For example, human adipose MSC-derived miR-199a-loaded EVs targeting and inhibiting mammalian target of rapamycin increased the chemosensitivity of hepatocellular carcinoma cells to doxorubicin both in vivo and in vitro [33]. At the same time, miR-199a-loaded human bone marrow–derived MSC-EVs enhanced the chemosensitivity of glioma cells to temozolomide by downregulating ankyrin repeat and PH domain 2 [45]. Finally, MSC-EVs can act as a vehicle for targeted therapy and can be directly loaded with therapeutic agents. For instance, taxol-loaded umbilical cord–derived MSC-EVs were demonstrated to significantly decrease SK-OV-3 ovarian cancer cell viability [113].
Treg-EVs and cancer
As it was mentioned earlier, Tregs’ immunosuppressive properties beneficial for treating conditions characterized by excessive inflammation may at the same time impede the establishment of effective antitumor immunity in patients with advanced malignancies and even accelerate tumor progression. Despite the fact that cancer immunotherapy is very promising, new anticancer drugs and vaccines have failed to show promising benefits against cancer, which is at least partly due to Treg infiltration into the tumor region and suppression of anticancer drug and vaccine activities [114].
Therefore, studies involving the use of Treg-EVs in cancer models are lacking since targeting them rather than activating seems the right strategy to improve tumor treatment efficacy [30]. On the other hand, there is evidence that Treg-EVs contain proapoptotic or antiproliferative miRNAs (miR-466 family [115], miR-195 [116], and miR-16 [117]), which suggests that they still have some antitumor potential which can be realized when sufficiently studied.
Furthermore, a detailed characterization of Tregs in tumor areas would help us better understand how Treg transcription changes in tumor-specific contexts [118]. For example, Treg cell death pathways [119], Treg-produced IL-35 [120,121,122,123,124,125], and epigenetic pathways [121, 126] could all be potential therapeutic targets and warrant further study.
Challenges and prospects
Based on the above analysis, it is clear that EVs, and particularly MSC-EVs and Treg-EVs, are a relevant research topic. At the same time, their translation to the clinical practice may be hampered by some considerable challenges. First of all, standardized protocols for different stages of the EV-based biomedical product development are lacking. Although the approaches to the EV isolation, classification, purification, characterization, and storage have been developed in the past years, they need to be standardized for large-scale production and clinical applications.
One of the main problems remains the heterogeneity of the obtained samples [7]. In order to address rigor and reproducibility issues in EV research, many aspects should be considered. Those should include not only their size distribution, but also more specific features, such as characteristics of their lipid membranes, the ratio of membrane lipids to proteins or RNA, and the enzyme activity of the surface proteins [127]. Thorough identification of these attributes might help to overcome another challenge, which is predicting therapeutic potency of the obtained samples. Despite some progress in this field, robust potency assays for EV preparations reflecting their mechanism of action are yet to be developed [128].
It is noteworthy that some researchers suggest protein-mediated mechanism of action to be more likely for EVs than miRNA-mediated one [129]. There is evidence that miRNAs may not be the key players determining the EV biological activity [130] since they may not be present in sufficient quantities in EV preparations [131] and therefore may not elicit a biologically relevant response. However, speaking of MSC-EVs, while numerous studies evaluated their molecular composition and associated functions [132,133,134], the contribution of particular miRNAs is still discussed much more often than that of particular proteins. Therefore, more studies assessing the contribution of certain proteins to EV biological activity would be of great scientific value.
Moreover, speaking of Treg-EVs, in contrast to MSC-EVs, their research is still in the investigative stage. In this regard, more systematic studies and experimental models could probably bridge the knowledge gap that still exists in the field of Treg-EV research.
The critical role of Treg-EVs in Treg-mediated suppression of immune cells demonstrated in recent in vitro [135] and in vivo [22, 50, 51, 136] studies offers the prospect of using Treg-EV-based therapies for conditions characterized by immune system over-reactivity. However, Treg-EV successful use was reported not only in immune-related disorders and transplantation tolerance models, but also in models of myocardial infarction [50], and scar formation in wound healing [137]. This is a very important insight suggesting that the range of Treg-EV possible applications is wider than treating excessive immune reactions.
EVs are known to recapitulate a number of the parent cell’s properties; therefore, possible targets of Treg-EV application can be inspired by the Tregs’ reported effects, such as facilitating blood flow recovery after ischemia [138], controlling adipose tissue inflammation, promoting muscle repair [139], and maintaining tissue/organ homeostasis [140].
Another particular field requiring further investigation is the use of Treg-EVs in oncology, since the immune component in cancer development is well documented. Moreover, Tregs are reported to play a crucial role in tumor formation [28, 29, 141, 142] and are known as a target of tumor cell EVs [93,94,95,96,97]. Hopefully, having disclosed these complex interactions between the pool of cells involved in the tumor development and their EVs, we might get answers to the questions still existing in cancer research.
Conclusions
The variety of available sources and the unique role of cell–cell communication mediators make EVs an accessible and promising tool in modulating many vital processes such as immune signaling, inflammation, and angiogenesis. Furthermore, EVs can be used both as diagnostic biomarkers for different pathologies due to their specific cargo of lipids, proteins, and RNAs and as a drug delivery system due to their ability to protect internal biomolecules from degradation and suitable candidates to pass through physiological barriers such as the blood–brain barrier. MSC-EVs, being widely studied, are known for their immunomodulatory properties, but due to the duality of their effect on the tumor growth, their use in oncology would remain a double-edged sword until the sufficient body of knowledge is accumulated and the tools for their undesired effects management are developed. At the same time, Treg-EVs remain largely unstudied but still hold great promise for treating a wide range of diseases.
EVs exert their effects both on cell-mediated and humoral (including the complement system) immunity. Treg-EVs’ immunosuppressive effect is beneficial for conditions characterized by immune system over-reactivity (ARDS accompanied by cytokine storm, autoimmune diseases, allograft rejection), but at the same time fatal in tumor development, being favorable for the tumor growth. On the other hand, MSC-EVs, while alleviating immune system over-reactivity, exhibit a dual effect on the tumor development, being able to both promote and inhibit tumor growth.
Availability of data and material
Not applicable.
Abbreviations
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- CIA:
-
Collagen-induced arthritis
- CNS:
-
Central nervous system
- EAE:
-
Experimental autoimmune encephalomyelitis
- EVs:
-
Extracellular vesicles
- IBD:
-
Inflammatory bowel disease
- IL:
-
Interleukin
- IMQ:
-
Imiquimod
- MMP:
-
Matrix metalloproteinase
- MS:
-
Multiple sclerosis
- MSCs:
-
Mesenchymal stromal cells
- MSC-EVs:
-
MSC-derived extracellular vesicles
- PASI:
-
Psoriasis Area and Severity Index
- RA-FLS:
-
Rheumatoid arthritis-related fibroblast-like synoviocytes
- RA:
-
rheumatoid arthritis
- T1D:
-
type 1 diabetes
- Th:
-
T-helper
- Tregs:
-
regulatory T cells
- Treg-EVs:
-
regulatory T cells-derived extracellular vesicles
- VEGF:
-
vascular endothelial growth factor
References
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P et al (2006) Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20(5):847–856
Zonneveld MI, Brisson AR, van Herwijnen MJ, Tan S, van de Lest CH, Redegeld FA et al (2014) Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. Journal of extracellular vesicles 3(1):24215
Hade MD, Suire CN, Suo Z (2021) Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells 10(8):1959
Srinivasan S, Duval MX, Kaimal V, Cuff C, Clarke SH (2019) Assessment of methods for serum extracellular vesicle small RNA sequencing to support biomarker development. Journal of extracellular vesicles 8(1):1684425
Théry C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8):569–579
Gimona M, Pachler K, Laner-Plamberger S, Schallmoser K, Rohde E (2017) Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Mol Sci 18(6):1190
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin‐Smith GK, Ayre DC (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Ves 7(1):1535750
Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T et al (2014) Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol 306(11):L975–L985
Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA et al (2009) Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 180(11):1122–1130
Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H et al (2005) Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11(4):367–368
Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS et al (2021) Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther 12(1):1–30
Tan S, Wong J, Sim S, Tjio C, Wong K, Chew J et al (2020) Mesenchymal stem cell exosomes in bone regenerative strategies—a systematic review of preclinical studies. Materials Today Bio 7:100067
Aheget H, Tristán-Manzano M, Mazini L, Cortijo-Gutierrez M, Galindo-Moreno P, Herrera C et al (2020) Exosome: a new player in translational nanomedicine. J Clin Med 9(8):2380
Wang H, Zheng R, Chen Q, Shao J, Yu J, Hu S (2017) Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res Ther 8(1):1–10
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood, The Journal of the American Society of Hematology 99(10):3838–3843
Crain SK, Robinson SR, Thane KE, Davis AM, Meola DM, Barton BA et al (2019) Extracellular vesicles from Wharton’s jelly mesenchymal stem cells suppress CD4 expressing T cells through transforming growth factor beta and adenosine signaling in a canine model. Stem Cells Dev 28(3):212–226
Álvarez V, Sánchez-Margallo FM, Macías-García B, Gómez-Serrano M, Jorge I, Vazquez J et al (2018) The immunomodulatory activity of extracellular vesicles derived from endometrial mesenchymal stem cells on CD4+ T cells is partially mediated by TGFbeta. J Tissue Eng Regen Med 12(10):2088–2098
Bruno S, Deregibus MC, Camussi G (2015) The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol Lett 168(2):154–158
Djouad F, Charbonnier L-M, Bouffi C, Louis-Plence P, Bony C, Apparailly F et al (2007) Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem cells 25(8):2025–2032
Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA (2000) Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immun 164(7):3596–3599
Smyth LA, Ratnasothy K, Tsang JY, Boardman D, Warley A, Lechler R et al (2013) CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol 43(9):2430–2440
Tung SL, Fanelli G, Matthews RI, Bazoer J, Letizia M, Vizcay-Barrena G et al (2020) Regulatory T cell extracellular vesicles modify T-effector cell cytokine production and protect against human skin allograft damage. Front Cell Dev Biol 317
Ohkura N, Kitagawa Y, Sakaguchi S (2013) Development and maintenance of regulatory T cells. Immunity 38(3):414–423
Nathan C (2002) Points of control in inflammation. Nature 420(6917):846–852
Ferrer IR, Hester J, Bushell A, Wood KJ (2014) Induction of transplantation tolerance through regulatory cells: from mice to men. Immunol Rev 258(1):102–116
Sakaguchi S, Miyara M, Costantino CM, Hafler DA (2010) FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10(7):490–500
Dominguez-Villar M, Hafler DA (2018) Regulatory T cells in autoimmune disease. Nat Immunol 19(7):665–673
Chen M-L, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, Von Boehmer H et al (2005) Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo. Proc Natl Acad Sci 102(2):419–424
Tanaka A, Sakaguchi S (2017) Regulatory T cells in cancer immunotherapy. Cell Res 27(1):109–118
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133(5):775–87
La Salvia S, Musante L, Lannigan J, Gigliotti JC, Le TH, Erdbrügger U (2020) T cell-derived extracellular vesicles are elevated in essential HTN. Am J Physiol Renal Physiol 319(5):F868–F875
Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM (2018) Immunomodulatory effects of mesenchymal stem cell–derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem 119(11):9433–9443
Lou G, Chen L, Xia C, Wang W, Qi J, Li A et al (2020) MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res 39(1):1–9
Hao Q, Gudapati V, Monsel A, Park JH, Hu S, Kato H et al (2019) Mesenchymal stem cell–derived extracellular vesicles decrease lung injury in mice. J Immunol 203(7):1961–1972
Li Z, Liu F, He X, Yang X, Shan F, Feng J (2019) Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol 67:268–280
Shamili FH, Alibolandi M, Rafatpanah H, Abnous K, Mahmoudi M, Kalantari M et al (2019) Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Control Release 299:149–164
Wen D, Peng Y, Liu D, Weizmann Y, Mahato RI (2016) Mesenchymal stem cell and derived exosome as small RNA carrier and immunomodulator to improve islet transplantation. J Control Release 238:166–175
Nakano M, Nagaishi K, Konari N, Saito Y, Chikenji T, Mizue Y et al (2016) Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep 6(1):1–14
Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C et al (2018) Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8(5):1399
Chen Z, Wang H, Xia Y, Yan F, Lu Y (2018) Therapeutic potential of mesenchymal cell–derived miRNA-150-5p–expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol 201(8):2472–2482
Meng Q, Qiu B (2020) Exosomal microRNA-320a derived from mesenchymal stem cells regulates rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing CXCL9 expression. Front Physiol 11:441
Zhang X, Yang Y, Yang Y, Chen H, Tu H, Li J (2020) Exosomes from bone marrow microenvironment-derived mesenchymal stem cells affect CML cells growth and promote drug resistance to tyrosine kinase inhibitors. Stem Cells Intern
Lee J-K, Park S-R, Jung B-K, Jeon Y-K, Lee Y-S, Kim M-K et al (2013) Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 8(12):e84256
Zhang Z, Guo X, Guo X, Yu R, Qian M, Wang S et al (2021) MicroRNA-29a-3p delivery via exosomes derived from engineered human mesenchymal stem cells exerts tumour suppressive effects by inhibiting migration and vasculogenic mimicry in glioma. Aging (Albany NY) 13(4):5055
Yu L, Gui S, Liu Y, Qiu X, Zhang G, Zhang Xa et al (2019) Exosomes derived from microRNA-199a-overexpressing mesenchymal stem cells inhibit glioma progression by down-regulating AGAP2. Aging (Albany NY) 11(15):5300
Yan T, Wu M, Lv S, Hu Q, Xu W, Zeng A et al (2021) Exosomes derived from microRNA-512-5p-transfected bone mesenchymal stem cells inhibit glioblastoma progression by targeting JAG1. Aging (Albany NY) 13(7):9911
Zhang B, Lai RC, Sim WK, Choo ABH, Lane EB, Lim SK (2021) Topical application of mesenchymal stem cell exosomes alleviates the imiquimod induced psoriasis-like inflammation. Int J Mol Sci 22(2):720
Mahdipour E, Salmasi Z, Sabeti N (2019) Potential of stem cell-derived exosomes to regenerate β islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J Cell Physiol 234(11):20310–20321
Zhang Y, Yan J, Li Z, Zheng J, Sun Q (2022) Exosomes derived from human umbilical cord mesenchymal stem cells alleviate psoriasis-like skin inflammation. J Interferon Cytokine Res 42(1):8–18
Hu H, Wu J, Cao C, Ma L (2020) Exosomes derived from regulatory T cells ameliorate acute myocardial infarction by promoting macrophage M2 polarization. IUBMB Life 72(11):2409–2419
Chen J, Huang F, Hou Y, Lin X, Liang R, Hu X et al (2021) TGF-β-induced CD4+ FoxP3+ regulatory T cell-derived extracellular vesicles modulate Notch1 signaling through miR-449a and prevent collagen-induced arthritis in a murine model. Cell Mol Immunol 18(11):2516–2529
Aiello S, Rocchetta F, Longaretti L, Faravelli S, Todeschini M, Cassis L et al (2017) Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci Rep 7(1):1–19
Liao F, Lu X, Dong W (2020) Exosomes derived from T regulatory cells relieve inflammatory bowel disease by transferring miR-195a-3p. IUBMB Life 72(12):2591–2600
Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G (2016) Stem cell-derived extracellular vesicles and immune-modulation. Frontiers in cell and developmental biology 4:83
Azambuja JH, Ludwig N, Braganhol E, Whiteside TL (2019) Inhibition of the adenosinergic pathway in cancer rejuvenates innate and adaptive immunity. Int J Mol Sci 20(22):5698
Frutos-Vivar F, Nin N, Esteban A (2004) Epidemiology of acute lung injury and acute respiratory distress syndrome. Curr Opin Crit Care 10(1):1–6
Kaku S, Nguyen CD, Htet NN, Tutera D, Barr J, Paintal HS et al (2020) Acute respiratory distress syndrome: etiology, pathogenesis, and summary on management. J Intensive Care Med 35(8):723–737
Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A et al (2019) Acute respiratory distress syndrome. Nat Rev Dis Primers 5(1):1–22
Lin S-H, Fu J, Wang C-J, Gao F, Feng X-Y, Liu Q et al (2016) Inflammation elevated IL-33 originating from the lung mediates inflammation in acute lung injury. Clin Immunol 173:32–43
Khoshdel-Rad N, Zahmatkesh E, Shpichka A, Timashev P, Vosough M (2021) Outbreak of chronic renal failure: will this be a delayed heritage of COVID-19?. Springer p. 3–5
Sreepadmanabh M, Sahu AK, Chande A (2020) COVID-19: advances in diagnostic tools, treatment strategies, and vaccine development. J Biosci 45(1):1–20
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A et al (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315(8):788–800
Hossein-Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M (2020) Novel therapeutic approaches for treatment of COVID-19. J Mol Med 98(6):789–803
Hossein-Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M (2021) An update to “novel therapeutic approaches for treatment of COVID-19.” J Mol Med 99(2):303–310
Bari E, Ferrarotti I, Di Silvestre D, Grisoli P, Barzon V, Balderacchi A et al (2019) Adipose mesenchymal extracellular vesicles as alpha-1-antitrypsin physiological delivery systems for lung regeneration. Cells 8(9):965
Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A et al (2014) Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice. Stem Cells 32(1):116–25
Gennai S, Monsel A, Hao Q, Park J, Matthay M, Lee J (2015) Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Transplant 15(9):2404–2412
He X, Dong Z, Cao Y, Wang H, Liu S, Liao L et al (2019) MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Intern
Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T et al (2014) MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 41(1):89–103
Tung SL, Boardman DA, Sen M, Letizia M, Peng Q, Cianci N et al (2018) Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci Rep 8(1):1–12
Torri A, Carpi D, Bulgheroni E, Crosti M-C, Moro M, Gruarin P et al (2017) Extracellular microRNA signature of human helper T cell subsets in health and autoimmunity. J Biol Chem 292(7):2903–2915
Li P, Yao Y, Ma Y, Chen Y (2019) MiR-150 attenuates LPS-induced acute lung injury via targeting AKT3. Int Immunopharmacol 75:105794
Yang Y, Yang C, Guo Y-F, Liu P, Guo S, Yang J et al (2019) MiR-142a-3p alleviates Escherichia coli derived lipopolysaccharide-induced acute lung injury by targeting TAB2. Microb Pathog 136:103721
Xie K, Chai Y-s, Lin S-h, Xu F, Wang CJ (2021) Luteolin regulates the differentiation of regulatory T cells and activates IL-10-dependent macrophage polarization against acute lung injury. J Immunol Res
Kimura A, Kishimoto T (2010) IL-6: regulator of Treg/Th17 balance. Eur J Immunol 40(7):1830–1835
Chen J, Zhang X, Xie J, Xue M, Liu L, Yang Y et al (2020) Overexpression of TGFβ1 in murine mesenchymal stem cells improves lung inflammation by impacting the Th17/Treg balance in LPS-induced ARDS mice. Stem Cell Res Ther 11(1):1–16
Lee GR (2018) The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci 19(3):730
Sullivan JA, Tomita Y, Jankowska-Gan E, Lema DA, Arvedson MP, Nair A et al (2020) Treg-cell-derived IL-35-coated extracellular vesicles promote infectious tolerance. Cell Rep 30(4):1039–51. e5
Gelfand JM (2014) Multiple sclerosis: diagnosis, differential diagnosis, and clinical presentation. Handb Clin Neurol 122:269–290
Kim WB, Jerome D, Yeung J (2017) Diagnosis and management of psoriasis. Can Fam Physician 63(4):278–285
Malatjalian D, Ross J, Williams C, Colwell S, Eastwood B (1996) Methotrexate hepatotoxicity in psoriatics: report of 104 patients from Nova Scotia, with analysis of risks from obesity, diabetes and alcohol consumption during long term follow-up. Can J Gastroenterol 10(6):369–375
Nakhaei-Nejad M, Barilla D, Lee C-H, Blevins G, Giuliani F (2018) Characterization of lymphopenia in patients with MS treated with dimethyl fumarate and fingolimod. Neurol Neuroimmunol Neuroinflam 5(2)
Wang M, Yuan Q, Xie L (2018) Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem cells Intern
Benjamin O, Bansal P, Goyal A, Lappin SL (2018) Disease modifying anti-rheumatic drugs (DMARD)
Kahmini FR, Shahgaldi S (2021) Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles as novel cell-free therapy for treatment of autoimmune disorders. Exp Mol Pathol 118:104566
Meng H-Y, Chen L-Q, Chen L-H (2020) The inhibition by human MSCs-derived miRNA-124a overexpression exosomes in the proliferation and migration of rheumatoid arthritis-related fibroblast-like synoviocyte cell. BMC Musculoskelet Disord 21(1):1–10
Lin K, Su H, Jiang L, Chu T, Li Z, Chen X et al (2019) Influences of miR-320a on proliferation and apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis through targeting MAPK-ERK1/2. Eur Rev Med Pharmacol Sci 23(5):1907–1914
Yu X, Huang C, Song B, Xiao Y, Fang M, Feng J et al (2013) CD4+ CD25+ regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model. Cell Immunol 285(1–2):62–68
Agarwal A, Fanelli G, Letizia M, Tung SL, Boardman D, Lechler R et al (2014) Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation. Front Immunol 5:555
Brücher BL, Jamall IS (2014) Cell-cell communication in the tumor microenvironment, carcinogenesis, and anticancer treatment. Cell Physiol Biochem 34(2):213–243
Lai B, Wang J, Fagenson A, Sun Y, Saredy J, Lu Y et al (2019) Twenty novel disease group-specific and 12 new shared macrophage pathways in eight groups of 34 diseases including 24 inflammatory organ diseases and 10 types of tumors. Front Immunol 10:2612
Xu K, Yang WY, Nanayakkara GK, Shao Y, Yang F, Hu W et al (2018) gaTa3, hDac6, and Bcl6 regulate FOXP3+ Treg plasticity and determine Treg conversion into either novel antigen-presenting cell-like Treg or Th1-Treg. Front Immunol 9:45
Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL (2010) Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS ONE 5(7):e11469
Yin Y, Cai X, Chen X, Liang H, Zhang Y, Li J et al (2014) Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res 24(10):1164–1180
Kalvala A, Wallet P, Yang L, Wang C, Li H, Nam A et al (2019) Phenotypic switching of naïve T cells to immune-suppressive Treg-like cells by mutant KRAS. J Clin Med 8(10):1726
Ning T, Li J, He Y, Zhang H, Wang X, Deng T et al (2021) Exosomal miR-208b related with oxaliplatin resistance promotes Treg expansion in colorectal cancer. Mol Ther 29(9):2723–2736
Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X et al (2018) Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res 6(12):1578–1592
Xunian Z, Kalluri R (2020) Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci 111(9):3100–3110
Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y, Li Y et al (2017) Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol Biochem 42(6):2242–2254
Gu H, Ji R, Zhang X, Wang M, Zhu W, Qian H et al (2016) Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol Med Rep 14(4):3452–3458
Lin R, Wang S, Zhao RC (2013) Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem 383(1):13–20
Alzahrani FA, El-Magd MA, Abdelfattah-Hassan A, Saleh AA, Saadeldin IM, El-Shetry ES et al (2018) Potential effect of exosomes derived from cancer stem cells and MSCs on progression of DEN-induced HCC in rats. Stem Cells Intern
Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F et al (2017) MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol 40(5):457–470
Chen H-L, Li J-J, Jiang F, Shi W-J, Chang G-Y (2020) MicroRNA-4461 derived from bone marrow mesenchymal stem cell exosomes inhibits tumorigenesis by downregulating COPB2 expression in colorectal cancer. Biosci Biotechnol Biochem 84(2):338–346
Jiang S, Mo C, Guo S, Zhuang J, Huang B, Mao X (2019) Human bone marrow mesenchymal stem cells-derived microRNA-205-containing exosomes impede the progression of prostate cancer through suppression of RHPN2. J Exp Clin Cancer Res 38(1):1–16
Che Y, Shi X, Shi Y, Jiang X, Ai Q, Shi Y et al (2019) Exosomes derived from miR-143-overexpressing MSCs inhibit cell migration and invasion in human prostate cancer by downregulating TFF3. Molecular Therapy-Nucleic Acids 18:232–244
Yang J, Zhang L (2022) The roles and therapeutic approaches of MSC-derived exosomes in colorectal cancer. Clin Trans Oncol 1–9
Sheykhhasan M, Kalhor N, Sheikholeslami A, Dolati M, Amini E, Fazaeli H (2021) Exosomes of mesenchymal stem cells as a proper vehicle for transfecting miR-145 into the breast cancer cell line and its effect on metastasis. BioMed Res Intern
Chang L, Gao H, Wang L, Wang N, Zhang S, Zhou X et al (2021) Exosomes derived from miR-1228 overexpressing bone marrow-mesenchymal stem cells promote growth of gastric cancer cells. Aging (Albany NY) 13(8):11808
Xie X, Ji J, Chen X, Xu W, Chen H, Zhu S et al (2022) Human umbilical cord mesenchymal stem cell-derived exosomes carrying hsa-miRNA-128-3p suppress pancreatic ductal cell carcinoma by inhibiting Galectin-3. Clin Transl Oncol 24(3):517–531
Oskuee RK, Jaafari MR (2020) Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem cell reviews and reports 16(3):541–556
Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu C-C, Gagea M et al (2018) Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3(8)
Melzer C, Rehn V, Yang Y, Bähre H, von der Ohe J, Hass R (2019) Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers 11(6):798
Zeng G, Jin L, Ying Q, Chen H, Thembinkosi MC, Yang C et al (2020) Regulatory T cells in cancer immunotherapy: basic research outcomes and clinical directions. Cancer Management and Research 12:10411
Druz A, Chu C, Majors B, Santuary R, Betenbaugh M, Shiloach J (2011) A novel microRNA mmu-miR-466h affects apoptosis regulation in mammalian cells. Biotechnol Bioeng 108(7):1651–1661
Yang X, Yin J, Yu J, Xiang Q, Liu Y, Tang S et al (2012) miRNA-195 sensitizes human hepatocellular carcinoma cells to 5-FU by targeting BCL-w. Oncol Rep 27(1):250–257
Cai C-K, Zhao G-Y, Tian L-Y, Liu L, Yan K, Ma Y-L et al (2012) miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncol Rep 28(5):1764–1770
Stéphan P, Lautraite R, Voisin A, Grinberg-Bleyer Y (2020) Transcriptional control of regulatory T cells in cancer: toward therapeutic targeting? Cancers 12(11):3194
Yang WY, Shao Y, Lopez-Pastrana J, Mai J, Wang H, Yang XF (2015) Pathological conditions re-shape physiological Tregs into pathological Tregs. Burns & Trauma 3
Yin Y, Li X, Sha X, Xi H, Li Y-F, Shao Y et al (2015) Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol 35(4):804–816
Shao Y, Cheng Z, Li X, Chernaya V, Wang H, Yang X-F (2014) Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction-a novel mechanism for maintaining vascular function. J Hematol Oncol 7(1):1–14
Li X, Fang P, Yang WY, Wang H, Yang X (2019) IL-35, as a newly proposed homeostasis-associated molecular pattern, plays three major functions including anti-inflammatory initiator, effector, and blocker in cardiovascular diseases. Cytokine 122:154076
Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X et al (2012) IL-35 is a novel responsive anti-inflammatory cytokine—a new system of categorizing anti-inflammatory cytokines. PLoS ONE 7(3):e33628
Li X, Fang P, Sun Y, Shao Y, Yang WY, Jiang X et al (2020) Anti-inflammatory cytokines IL-35 and IL-10 block atherogenic lysophosphatidylcholine-induced, mitochondrial ROS-mediated innate immune activation, but spare innate immune memory signature in endothelial cells. Redox Biol 28:101373
Fu H, Sun Y, Shao Y, Saredy J, Cueto R, Liu L et al (2020) Interleukin 35 delays hindlimb ischemia-induced angiogenesis through regulating ROS-extracellular matrix but spares later regenerative angiogenesis. Front Immunol 2662
Lopez-Pastrana J, Shao Y, Chernaya V, Wang H, Yang X-F (2015) Epigenetic enzymes are the therapeutic targets for CD4+ CD25+/highFoxp3+ regulatory T cells. Transl Res 165(1):221–240
Witwer KW, Van Balkom BW, Bruno S, Choo A, Dominici M, Gimona M et al (2019) Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. Journal of extracellular vesicles 8(1):1609206
Gimona M, Brizzi MF, Choo ABH, Dominici M, Davidson SM, Grillari J et al (2021) Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy 23(5):373–380
Toh WS, Lai RC, Zhang B, Lim SK (2018) MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans 46(4):843–853
Albanese M, Chen Y-FA, Hüls C, Gärtner K, Tagawa T, Mejias-Perez E et al (2021) MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Gene 17(12):e1009951
Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM et al (2014) Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci 111(41):14888–14893
Varderidou-Minasian S, Lorenowicz MJ (2020) Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics 10(13):5979
Racchetti G, Meldolesi J (2021) Extracellular vesicles of mesenchymal stem cells: therapeutic properties discovered with extraordinary success. Biomedicines 9(6):667
Kou M, Huang L, Yang J, Chiang Z, Chen S, Liu J et al (2022) Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis 13(7):1–16
Li P, Liu C, Yu Z, Wu M (2016) New insights into regulatory T cells: exosome-and non-coding RNA-mediated regulation of homeostasis and resident Treg cells. Front Immunol 7:574
Chen L, Huang H, Zhang W, Ding F, Fan Z, Zeng Z (2019) Exosomes derived from T regulatory cells suppress CD8+ cytotoxic T lymphocyte proliferation and prolong liver allograft survival. Medical science monitor: international medical journal of experimental and clinical research 25:4877
He L, Marneros AG (2013) Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am J Pathol 182(6):2407–2417
Sharir R, Semo J, Shaish A, Landa-Rouben N, Entin-Meer M, Keren G et al (2014) Regulatory T cells influence blood flow recovery in experimental hindlimb ischaemia in an IL-10-dependent manner. Cardiovasc Res 103(4):585–596
Burzyn D, Benoist C, Mathis D (2013) Regulatory T cells in nonlymphoid tissues. Nat Immunol 14(10):1007–1013
DiSpirito JR, Zemmour D, Ramanan D, Cho J, Zilionis R, Klein AM et al (2018) Molecular diversification of regulatory T cells in nonlymphoid tissues. Sci Immunol 3(27):eaat5861
Lee W-C, Wu T-J, Chou H-S, Yu M-C, Hsu P-Y, Hsu H-Y et al (2012) The impact of CD4+ CD25+ T cells in the tumor microenvironment of hepatocellular carcinoma. Surgery 151(2):213–222
Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T et al (2007) FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 13(3):902–911
Acknowledgements
The authors deeply acknowledge the unique scientific facility of Transgenebank. The authors would also like to thank Anna Shpichka for assisting in drawing Fig. 1.
Funding
Research at Sechenov University (sections “Introduction” including the table and the picture, “MSC-EVs and ARDS/ALI,” “MSC-EVs and autoimmune conditions,” “Challenges and prospects,” and “Conclusions”) was funded by the Ministry of Science and Higher Education of the Russian Federation under the grant agreement no. 075–15-2021–951 (13.2251.21.0022).
Research at Nicolae Testemitanu State University of Medicine and Pharmacy (section “MSC-EVs and cancer”) was funded in the framework of ERA.Net RUS Plus project “Prophylactic And Therapeutic Use Of Regulatory T Cell Derived Exosomes In Murine Models Of Multiple Sclerosis And Psoriasis” (21.80013.8007.2 M).
Author information
Authors and Affiliations
Contributions
AS, NK, and PT outlined the review. MP and DB wrote sections “Introduction,” “Challenges and prospects,” and “Conclusions.” ZH and MV—“MSC-EVs and ARDS/ALI” and “MSC-EVs and autoimmune conditions.” ZBG, AE, AHY, MED, NG, and HA—“Treg-EVs and ARDS/ALI,” “Treg-EVs and autoimmune conditions,” and “Treg-EVs and cancer.” IC, DGA, MU, VV, SG, and MT—“MSC-EVs and cancer.” MP prepared the table, designed the picture, and drafted the manuscript with primary editing and revision support from AS and PT. DB, YO, SG, and MV coordinated the manuscript preparation. PT coordinated the entire work and revised the final draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heydari, Z., Peshkova, M., Gonen, Z.B. et al. EVs vs. EVs: MSCs and Tregs as a source of invisible possibilities. J Mol Med 101, 51–63 (2023). https://doi.org/10.1007/s00109-022-02276-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02276-2