Abstract
Traditionally, transfer RNAs (tRNAs) specifically decoded messenger RNA (mRNA) and participated in protein translation. tRNA-derived fragments (tRFs), also known as tRNA-derived small RNAs (tsRNAs), are generated by the specific cleavage of pre- and mature tRNAs and are a class of newly defined functional small non-coding RNAs (sncRNAs). Following the different cleavage positions of precursor or mature tRNA, tRFs are classified into seven types, 5′-tRNA half, 3′-tRNA half, tRF-1, 5′U-tRF, 3′-tRF, 5′-tRF, and i-tRF. It has been demonstrated that tRFs have a diverse range of biological functions in cellular processes, which include inhibiting protein translation, modulating stress response, regulating gene expression, and involvement in cell cycles and epigenetic inheritance. Emerging evidences have indicated that tRFs in extracellular vesicles (EVs) seem to act as regulatory molecules in various cellular processes and play essential roles in cell-to-cell communication. Furthermore, the dysregulation of EV-associated tRFs has been associated with the occurrence and progression of a variety of cancers and they can serve as novel potential biomarkers for cancer diagnosis. In this review, the biogenesis and classification of tRFs are summarized, and the biological functions of EV-associated tRFs and their roles as potential biomarkers in human diseases are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, advances in new technologies such as next-generation deep sequencing have led to the discovery of tens of thousands of non-coding RNAs (ncRNAs), which exist extensively in organisms and have multiple known functions. ncRNAs that are transcribed from DNA and do not translate into proteins include long ncRNAs (lncRNAs, > 200 nucleotides (nts)) and small ncRNAs (sncRNAs, < 200 nts) [1]. Small ncRNAs consist of various RNA species, including small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), microRNAs (miRNAs), endogenous small interfering RNAs (endo-siRNAs), Piwi-interacting RNAs (piRNAs), snoRNA-derived small RNAs (sdRNAs), ribosomal RNA (rRNA)-derived fragments (rRFs), and tRNA-derived fragments (tRFs) [2,3,4]. tRNAs are one of the most abundant cellular ncRNAs discovered so far and account for 4–10% of all cellular RNA [5]. There are about 500 tRNA genes or tRNA gene-like sequences in the human genome. The recent discovery of tRNA-lookalikes has doubled this number [6]. Traditionally, tRNAs specifically recognize messenger RNA (mRNA) codons, transport amino acids to ribosomes, convert genetic information into corresponding polypeptide chains, and participate in protein translation.
Recently, fragments originating from tRNA, known as tRNA-derived fragments (tRFs), have gradually gained wider attention [4]. Similar to miRNAs, expression of some tRFs is commonly observed in cells and tissues from a diverse breadth of organisms, ranging from E. coli to humans [1]. tRFs are first identified in prostate carcinoma cell lines by Lee et al. [4], and later are also called tRNA-derived RNAs (tDRs) or tRNA-derived small RNA (tsRNA) [7, 8]. They are derived from tRNA precursors (pre-tRNAs) or mature tRNAs and can generally be classified into seven categories: 5′-tRNA half, 3′-tRNA half, tRF-1, 5′U-tRF, 3′-tRF, 5′-tRF, and i-tRF, following their differing lengths and cleavage positions [7, 9]. Although the cleavage of tRNA was found as early as 1958 [10], understanding the functions of these tRNA processing intermediates has been neglected until recently [11]. A growing body of studies have demonstrated that tRFs are not always by-products of random tRNA cleavage, but play crucial roles in numerous cellular biological processes, such as regulation of gene and protein expression, stress granule (SG) assembly, RNA processing, modulation of the DNA damage response, inheritance of acquired characteristics, cancer progression, and neurodegeneration [12].
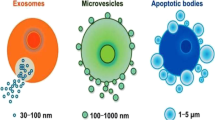
Extracellular vesicles (EVs) are small, lipid membrane particles secreted by almost all kinds of normal and diseased cells into the extracellular environment in different ways. Based on the size and biogenesis, EVs are divided into three main categories: exosomes, microvesicles, and apoptotic bodies [13, 14]. The most extensively researched exosomes originate from cytoplasmic multivesicular bodies (MVBs) and are released following fusion with plasma membranes. They typically range in diameter from 30 to 150 nm and are actively secreted by a variety of living cell types, including immune, neural, muscle, epithelial, and stem cells. Microvesicles range in size from 100 nm to 1 μm and arise via outward budding from the plasma membrane. Apoptotic bodies measuring 1 to 5 μm are derived from apoptotic cells during programmed cell death [15, 16].
So far, EVs have been isolated from all human body fluids, such as blood, urine, cerebrospinal fluid, saliva, and milk [17, 18]. Since their initial definition in 1983, it is currently known that EVs are central players in intercellular communication and signaling [19, 20]. Many evidences have also demonstrated their promising functions within tumorigenic processes, immune responses, cardiovascular diseases, nervous system-related diseases, and interplay between pathogens and hosts [21]. The cargoes of EVs contain membrane proteins, cytoplasmic proteins, nuclear proteins, extracellular matrix proteins, specific lipids, and nucleic acids including DNA, mRNA, tRNAs, miRNAs, lncRNAs, circRNAs, tRFs, and ribosomal RNAs [22,23,24,25]. To promote the research on extracellular RNA (exRNA), the Extracellular RNA Communication Consortium (ERCC) has established an extracellular RNA atlas across 5 human biofluids, which integrates a diverse set of exRNA-seq and qPCR sample profiles from 19 different studies [26, 27]. Further investigation reveals 6 major exRNA cargo types in vesicle, ribonucleoprotein, and lipoprotein carriers [26].
EVs are good natural carriers of small RNAs with regulatory function between cells. MiRNAs are the most deeply studied RNA types within EVs. Work on miRNA has dominated this field; nevertheless, emerging evidence has shown that EV-associated tRFs can contribute towards signaling between cells and can also serve as potential biomarkers for diseases. However, the related mechanisms remain unclear. We suggest that the reader refers to two recent reviews by Tosar and Cayota [28] and Torres and Martí [29], which have reviewed the current descriptions of extracellular tRFs. In more recent years, tRFs have been identified in EVs from the epididymis. Some specific tRFs are conveyed from somatic cells to maturing sperm and finally to embryos [30]. This review first explores the biogenesis and classification of tRFs and then describes the biological functions of EV-associated tRFs and their potential applications as biomarkers in human diseases.
Biogenesis of tRNAs
In the nucleus, the tRNAs are transcribed from the tRNA gene using RNA polymerase III (Pol III). The initial tRNA transcripts, also known as pre-tRNAs, have 5′-leader and 3′-tailer sequences. During tRNA maturation, the 5′-leader sequence and 3′-tailer sequence are cleaved by endonuclease P (RNase P) and endonuclease Z (RNase Z), respectively [31, 32]. Subsequently, the trinucleotide CCA is added to the 3′ end via tRNA nucleotidyltransferase to promote aminoacylation of the tRNA. Before being transported to the cytoplasm, tRNAs undergo extensive post-transcriptional modification, which further affects the structure, stability, and function of the tRNA. Mature tRNAs are70–90 nt long and fold into an L-shaped tertiary structure comprising a D-loop, an anticodon loop, a T-loop, a variable loop, an acceptor arm and a D arm, an anticodon arm, and a T arm [33].
Types of tRFs
On the basis of their mapping positions on pre-tRNA or mature tRNA transcripts, tRFs can be classified into seven categories, 5′-tRNA half, 3′-tRNA half, tRF-1, 5′U-tRF, 3′-tRF, 5′-tRF, and i-tRF (Fig. 1). These tRF subclassifications can be found in organisms ranging from yeasts to humans [34].
Classification of tRNA-derived fragments (tRFs). tRFs can be divided into 7 subtypes, 5′-tRNA half, 3′-tRNA half, tRF-1, 5′U-tRF, 3′-tRF, 5′-tRF, and i-tRF. tRNA half can be categorized into 2 types, 5′-tRNA half and 3′-tRNA half. They are cleaved by angiogenin (ANG) at the anticodon loop. tRF-1 is derived from precursor tRNAs digested by RNase Z or ELAC2. 5′U-tRF comes from 5′ leader of pre-tRNAs. 3′-tRF and 5′-tRF originate from mature tRNAs using ANG, Dicer, or other RNases. i‐tRF is from the internal region of mature tRNAs by ANG and Rny1
tRNA halves are created by angiogenin (ANG, Rny1 in yeast) cleavage within anticodon loops in mature tRNAs [35,36,37]. Depending on whether the 5′- or 3′-sequence includes the anticodon cleavage sites, tRNA halves can be classified into two subclasses, 5′‐tRNA halves and 3′‐tRNA halves [35, 38, 39]. 5′‐tRNA halves are 30–35 nt long and initiate from the 5′ end of mature tRNAs to the anticodon loop. 3′‐tRNA halves are 40–50 nt and begin at the anti-codon loop to the 3′ end of mature tRNAs [40]. In addition, it has been reported that other RNases than ANG might also create tRNA half [41]. tRNA halves are usually produced under certain stress conditions, such as oxidative stress, hypoxia, starvation, heat shock, ultraviolet irradiation, and virus infection [35, 38].
tRF-1, 3′-tRF, and 5′-tRF are the three principal classical categories. Each unique tRF may have an identification depending on the databases developed for tRFs [42, 43]. Generally, tRF-1 series are present at a lower abundance than 3′-tRF or 5′-tRF series [34].
tRF-1s, also named 3′U tRF, is derived from the cleavage of the 3′ ends of pre-tRNAs using RNase Z in the nucleus or tRNA 3′-endonuclease ELAC2 in the cytoplasm [4, 44]. tRF-1s are generally 16–48 nt. Previous studies have indicated that tRF-1s are processed and accumulated in the nucleus and are subsequently exported to the cytoplasm [45]. This suggests that tRF-1s may play regulatory roles in some unknown steps.
5′U-tRFs originate from the 5′ leader of pre-tRNAs and most of them are 17 nt long. Certain 5′U-tRFs are obtained from the sequences just next to, or only 1 nt away from the sequence of mature tRNA, indicating that they are products of pre-tRNAs processing. These fragments have been identified in prostate cancer patient samples [10, 46].
3′-tRFs, also called tRF-3s, are generated from the 3′ ends of mature tRNAs and are produced via cleavage of Dicer, ANG, and other ribonuclease superfamily members at the TψC loop. They usually end with a universal “CCA” trinucleotide. Depending on the length of 3′-tRFs, they can be further divided into two subtypes: (1) tRF-3a, cleaved site right before the TψC loop; (2) tRF-3b, cleaved site within the TψC loop [1].
5′-tRFs, also refer to tRF-5s, originate from the 5′ ends of mature tRNAs and are cleaved at D-loop or the arm region between the D-loop and the anticodon loop in a Dicer-dependent manner. However, they can also be produced by the actions of other nucleases, such as ANG [35]. The 5′-tRFs are grossly abundant in the nucleus, while 3′-tRFs and tRF-1 s are primarily present in the cytoplasm [34, 47]. Plenty of 5′-tRFs have been identified using deep sequencing of cervical cancer HeLa cells, cleaved by Dicer with a length of 19 nt. These small tRNA fragments combine poorly with Argonaute (Ago) 1 and Ago 2 [48]. In addition, there are 5′-phosphate and 3′-hydroxyl ends in 3′-tRFs and 5′-tRFs, which are similar to miRNAs.
i‐tRFs, also referred to as internal tRFs, mainly originate from the internal regions of mature tRNAs [49]. i‐tRFs have only just been discovered and their classification is based upon their starting location at the 5′-end of tRNA. D-tRFs and A-tRFs are produced by cutting at the D stem and anticodon loop, respectively, and V-tRFs are derived from cleavage at the variable loop [50]. Moreover, i‐tRFs are highly abundant and may vary depending on gender, population, race, amino acid characteristics, anticodons, tissues, diseases, and disease subtypes [49].
In addition to above mentioned tRFs, other types of tRNA fragments have also been observed [51, 52]. Sex hormone-dependent tRNA-derived RNAs (SHOT-RNAs) are a category of tRNA half and have been discovered in sex hormone-dependent cancers. In particular, they are enriched explicitly in cell lines from estrogen receptor (ER) positive breast cancer and androgen receptor (AR) positive prostate cancers. The SHOT-RNAs are derived from ANG-mediated cleavage at the anticodon loop of aminoacylated tRNAs, promoted by sex hormone signaling pathways [53]. Thus, 5′-SHOT-RNAs have a phosphate at the 5′-terminal and a 2′,3′-cyclicphosphate at the 3′-terminal, whereas 3′-SHOT-RNAs contain a 5′-hydroxyl at the 5′-terminal and an amino acid at the 3′-terminal. tRF-2s consist of an anticodon loop and a stem structure, and the 5′ and 3′ parts of the primary or mature tRNA are excluded. They have been detected in breast cancer cells and originate from tRNAGlu, tRNAAsp, tRNAGly, and tRNATyr [54]. Schaffer et al. identified a novel 5′ leader exon generated from impaired pre-tRNA cleavage, with loss of cleavage and polyadenylation factor I subunit 1 (CLP1) kinase activity and destabilized tRNA endonuclease complex (TSEN), which correlated with a progressive loss of neurons [51]. Furthermore, Haussecker et al. identified two categories of tRFs (type I and type II tRFs) based on their cleavage enzymes. Type I tRFs are Dicer-dependent. However, type II tRFs require RNase Z to cleave pre-tRNAs in the nucleus [47].
Nomenclature and databases of tRFs
It is vital to establish a standardized nomenclature for tRFs to facilitate academic communication and research. However, there is lack of consistency of naming system for tRFs so far. To promote research and academic exchange, scientists have created various databases of tRFs, including tRFdb (http://genome.bioch.virginia.edu/trfdb/), MINTbase (http://cm.jefferson.edu/MINTbase/), tRFexplorer (https://trfexplorer.cloud/), and others (Table 1) [7, 42, 43, 55,56,57,58,59,60,61,62]. Currently, the nomenclature of tRFs varies among different databases and researches. In tRFdb, tRFs were specified a unique ID starting with “3” (e.g., tRF-3019a, tRF-3017a), mapping at the 3′ end of mature tRNA [42, 63]. The first identified tRF-1 was named tRF-1001 according to the order of discovery [4]. A tRF is given a unique MINTbase ID (e.g., tRF-19-3L7L73JD) by the License Plate nomenclature, which are based only on tRF sequence and transcend species [43]. Additionally, differentially expressed tRFs were identified and named in small RNA libraries constructed in different studies (e.g., tRF-03357, tDR-5334) [64, 65]. In some studies, tRFs were also named by the length of the tRFs (e.g., tRF-25, tRF-18) or labeled by the authors (e.g., tRF-315, tRF-544) [46, 66].
Several problems lead to the difficulties in setting up a standardized naming scheme for tRFs. Challenges of obtaining the precise origin of tRFs were due to tRNA incomplete annotation, isotypes, and extensive chemical modifications. Moreover, only a few tRFs have been experimentally validated [67].
Different abundance of EV-associated tRFs in human tissues and fluids
Some studies have revealed that the abundance of tRFs varies greatly among a variety of different tissues (Table 2) [27, 68,69,70,71,72,73]. The same parental tRNA can generate different tRFs depending on different tissues [49]. It has been found that tRNA-Gly-GCC-5–1 can produce a tRNA half in colon tissues, but generates a 5′-tRF in liver tissues and seminal fluid and a 3′-tRF in neural progenitor samples, respectively [7].
EV-associated tRFs
To avoid degradation catalyzed by RNase in the extracellular environment, exRNAs are packaged or associated with a variety of exRNA carriers, including EVs, ribonucleoproteins (RNPs), and lipoprotein (LPP). A diverse group of small RNAs have been identified in EVs, including mRNAs, miRNAs, rRNAs, lncRNAs, tRNA fragments, circRNAs, piRNAs, and Y RNA. Recently, research into tRFs in EVs has grown, due to their regulatory functions in molecular processes and their prospects as biomarkers of disease.
EV-associated tRFs in blood
So far, EVs have been found in the bloodstream, including in plasma and serum. Circulating EVs can be taken up by the recipient cells and deliver signaling molecules, thereby mediating intercellular communication. In a previous study, RNA sequencing analysis of human plasma-derived exosomes indicated that tRNA accounted for only 1.24% of all mappable reads, contrasting with the most abundant of miRNAs (76.20%) [74]. Similarly, Yuan et al. detected that tRNAs occupied a small proportion (~ 2.1%) of mappable reads in RNA sequencing on plasma extracellular vesicles from a large sample [75].
Syncytiotrophoblast-derived extracellular vesicles (STB-EVs), which are released by the placenta into the maternal blood during the pregnancy, are considered to play an essential role in the adaptive changes during gestation. Wei et al. reported that the small RNAs in STB-EVs from placentae included miRNAs (60–65%), rRNAs (20.2–21.7%), and tRFs (12.8–17%) [68]. In this study, 5′-tRFs from three different tRNAs were also present at different levels between preeclamptic and normotensive trophoblast tissues from the placenta [68]. Lately, another study demonstrated that tRNA species were the most predominant (> 95%) type of short RNAs from STB-EV, in contrast to < 50% in whole placenta tissue [69]. Among these tRNA species within STB-EV, most are 5′‐tRNA halves [69]. However, Amorim et al. indicated that rRNA was reported to be the most abundant RNAs (73%) in plasma-derived EVs [76].
Generally, EV-associated tRFs seem unlikely to be the main form of tRFs in the human circulating bloodstream. Conversely, RNP, another carrier of exRNAs in the blood, has been confirmed to be highly abundant in 5′‐tRNA halves [77].
EV-associated tRFs in other body fluids
To date, EVs have been detected in almost all body fluids. It was identified that seminal exosomes (SE) could transmit small RNAs that serve as regulatory molecules to the recipient mucosa [70]. In a more recent study, five biofluids were compared using 10 exRNA isolation methods, including ultracentrifugation to pre-enrich the EVs. The results showed that 5′‐tRNA halves and 5′-tRFs were highly abundant in bile and urine [27]. This was consistent with the findings in the blood samples. However, 3′-tRFs and i-tRFs were more highly enriched in cells [27]. Small RNAs in mesenteric lymph (ML) may play a crucial part in critical illness. It was reported that the tRNA proportion (> 90%) in ML was more significant than those in plasma (about 45%) from sham rats and was predominantly tRNA halves 32 nt in length [71]. Among these tRNA halves, 5′‐tRNA halves from tRNA-Gly-GCC were the most abundant. Further investigation revealed that tRNA halves only occupied 1% of the ML-EV reads, whereas 76% of the total reads were miRNAs [71].
EV-associated tRFs in cell lines
A study on dendritic cells (DCs) demonstrated that tRFs were selectively present in EVs. Nolte-’t Hoen et al. performed small RNA analysis in both EVs and cells simultaneously [78]. Strikingly, tRFs mapped to either the 5′ or 3′ end of tRNA were both observed in the cells, whereas only 5′ fragments were highly enriched in EVs. Furthermore, most of the abundant tRFs in EVs covered regions of 40–50 nt, compared to the 30–35-nt fragments in cellular RNA. This indicated the existence of two different fragments of the same tRNA. It also confirmed that one specific tRF was observed only in EVs [78].
Another study was performed on exosomes isolated from human bone marrow- and adipose-mesenchymal stem cells (BMSCs and ASCs) [72]. It was observed that tRFs constituted the majority composition of the total tRNA in cells and exosomes. Importantly, 33-nt 5′‐tRNA halves from tRNA-Glu-CTC and tRNA-Gly-GCC were predominantly present in exosomes from ASC and BMSC. However, exosomes released by BMSC preferentially packaged the full-length tRNA-Glu-CTC and 33-nt tRNA halves of another abundant tRNA. Taken together, the different distributions of the tRFs between exosomes of bone marrow and adipose may be linked to the origins and stem of the mesenchymal stem cells (MSCs) [72]. Bioinformatics analysis revealed that several potential targets were related to the self-renewal of stem cells and MSC differentiation [72].
Research on glioblastoma (GBM) by Wei et al. exhibited that some specific 5′‐tRNA halves originated from tRNA-Gly-GCC and tRNA-Glu-CTC and showed prominence in exRNA [73]. Notably, a remarkable abundance of ANG and 5′‐tRNA half was noted in the exosomes released by GBM-derived stem-like cells that could represent therapeutic resistance, which might imply tRNA cleavage in the exosomes [73].
Another study by Sork and coworkers analyzed the diversity of RNA species within cells and EVs from five different cell types. The results revealed a remarkable richness of tRNA-Gly-GCC, tRNA-Glu-CTC, and tRNA-His-GTG, in agreement with previous research [72, 79].
Overall, many studies have indicated that tRFs are more highly enriched in EVs than those in parental cells and that they may regulate essential biological functions, particularly concerning mediating intercellular communication. In contrast, miRNAs in EVs represent a relatively small proportion, which is a different situation in cells. Interestingly, higher enrichment in non-EV or RNP fraction was observed, which is consistent with the finding in blood [73, 80].
Biological functions of EV-associated tRFs
Much evidence has recently indicated that EV-associated tRFs play essential roles in many biological processes, particularly in regulating epigenetic inheritance, gene expression, protein synthesis, and immune activations [30, 81, 82].
Modulating epigenetic inheritance
Some reports have shown that EV-associated tRFs are related to epigenetic inheritance in mammals. Paternal diet can influence the metabolism of subsequent generations. Research in mice exhibited that consuming a low protein diet altered the levels of multiple small RNAs in sperm [81]. 5′ fragments of tRNA-Gly-CCC, TCC, and GCC were upregulated [81]. Of note was that EVs derived from the epididymis, also known as epididymosomes, delivered 5′-tRFs to sperms and oocytes in succession. Furthermore, the authors found that tRF-Gly-GCC which was upregulated in sperm from mice fed with a low protein diet could suppress the endogenous retroelement MERVL-regulated genes in preimplantation embryos [81]. Consequently, these findings might be responsible for the metabolic alterations in offspring.
tRFs in sperm can act as an epigenetic factor that may affect the metabolic phenotypes of future generations (Fig. 2). Chen and colleagues identified 5′‐tRNA halves in a paternal mouse model treated with a high-fat diet (HFD), which exhibited altered expression levels and RNA modifications [83]. Furthermore, they injected tRFs (30–40 nt) from the sperm of HFD mice into normal zygotes. The F1 offspring displayed changes in embryonic gene expression and RNA modifications and subsequently presented with metabolic disorders [83]. These findings demonstrated that diet-induced metabolic abnormalities could be transmitted from father to offspring, unlinked to the DNA methylation of CpG-enriched regions.
The regulation of epigenetic inheritance by 5′-tRNA halves. Epididymosomes (a type of EVs) delivered tRFs to mature sperm. 5′-tRNA halves were discovered in a paternal mouse model with high-fat diet (HFD). Injection of these tRFs generated diet-induced metabolic disorders in F1 offspring. ND, normal diet
Interestingly, tRF types differ in abundance between paternal diet (low protein and high fat) in Sharma et al. [81] and in Chen et al. [83], which deserve further investigation.
Modulating gene expression
So far, it has been reported that EV-associated tRFs might also be relevant to regulating gene expression. Sharma and coauthors noted that epididymosomes (small EVs) could deliver 5′-tRFs to caput sperm in mice [30]. tRF-Gly-GCC can repress the expression of genes related to an endogenous retroelement (MERVL) during the development of preimplantation embryos [30]. Furthermore, this specific tRF regulates the stability and activity of ncRNAs and the histone levels, consequently affecting global chromatin production [84].
EV-associated tRFs could have a significant impact on host–pathogen interactions [85]. Garcia-Silva et al. found that EVs secreted by Trypanosoma cruzi could induce gene expression changes in host HeLa cells [85]. Furthermore, specific transcripts were significantly changed upon transfection with two EV-associated tRFs (tRFThr and tRFLeu) in HeLa cells. Likewise, some of these transcripts were also affected by incubation with EVs. However, the specific mechanism of action remains to be further elucidated [85].
Regulating protein synthesis
5′‐tRNA halves might inhibit both transcription and translation. Cooke and coauthors identified different enrichment of tRNA species between STB-EV in normal pregnancy and medium-large vesicles (MLEV). 5′-tRNA halves, not 3′-tRNA halves were the most abundant within STB-EV and confirmed by qPCR. Also, they used fibroblasts in cell cultures to investigate the effects of 5′tiRNA-GlyGCC (5′-tRNA half), which was enriched in placental STB-EV [69]. A fluorescence-labeled methionine analog quantified the global protein synthesis. Human fibroblasts showed a reduction in fluorescence by adding exogenous 5′tiRNA-GlyGCC in an in vitro model, implying that 5′-tRNA halves play an essential part in fetus-maternal signaling in normal pregnancies [69].
Regulating immune activation
Recently, a study on T cell activation was performed by Chiou’s research group [82]. The authors used a two-stage ultracentrifugation procedure to isolate EVs secreted by T cells. It was revealed that 5′-tRFs 18–21 nt in length were predominantly enriched in EVs, whereas 3′CCA-tRF measuring 17–18 and 22 nt were depleted. Further investigations found that enrichment and depletion attenuated the activation of resting T cells [82]. Compared to cellular RNA, 5′-tRFs Leu-TAA and Leu-TAG exhibited a higher abundance in EVs in the stimulated conditions, whereas 3′i-tRF Leu-TAA showed higher enrichment levels under resting conditions. These differences might contribute to the T cell response regarding activating stimulating signals. In addition, the findings demonstrated that T cell activation could downregulate the activation-induced EV-enriched tRFs in cells via MVB formation and secretion [82]. Interestingly, transfecting antisense oligonucleotides that inhibit these tRFs could promote T cell activation, suggesting that removing the activation-induced tRFs by EV-biogenesis pathways might be a key mechanism in suppressing the inhibitory effect of tRFs in T cell activation (Fig. 3).
The regulation of immune activation by 5′-tRFs. T cells release 5′-tRFs into extracellular vesicles (EVs) via the multivesicular body (MVB). Immune activation signal promotion of MVB formation and the secretion of specific tRF-enriched EVs. These 5′-tRFs repress both the activation of T cells and cytokine production within T cells
Clinical potential of tRFs in human diseases
Studies have revealed that tRFs were closely related and could be ideal potential biomarkers for cancer, viral infection, and metabolic and neurological diseases (Table 3) [54, 64, 65, 86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109]. Moreover, the research on EV-associated tRFs is developing rapidly, promising biomarkers for various diseases [28].
EV-associated tRFs as potential biomarkers
Most of the research on tRFs as biomarkers has mainly utilized unfractionated bloodstream with a mixed exRNA carrier. However, a growing number of studies have now focused on tRFs within EVs derived from various body fluids [22, 66, 79, 110,111,112,113,114] (Table 4).
EV-associated tRFs as biomarkers in cancer
Exosomal tRFs in liver cancer
Recently, Zhu et al. demonstrated the presence of tRFs in exosomes from a cultured medium of liver cancer cells [22]. Among these tRFs in exosomes, 5′-tRF was the most abundant (90%). Subsequently, 3′-tRF and i-tRF accounted for 9 and 1%, respectively [22]. In addition, the level of tRFs in plasma exosomes from patients with liver cancer was significantly higher than that from healthy controls. Following the findings in cell culture, 5′-tRF was also the predominant category of tRFs in the plasma exosomes [22]. In particular, four tRFs, tRNA-Val-TAC-3(tRF-40-EFOK8YR951K36D26, 3′-tRF), tRNA-Gly-TCC-5(tRF-34-QNR8VP94FQFY1Q, 5′-tRNA half), tRNA-Val-AAC-5(tRF-32-79MP9P9NH57SJ, 5′-tRNA half), and tRNA-Glu-CTC-5(tRF-31-87R8WP9N1EWJ0, 5′-tRF), from plasma exosomes were remarkably expressed in liver cancer patients, suggesting their potential value in cancer diagnosis [22].
Exosomal tRFs in gastric carcinoma
Gastric carcinoma (GC) is one of the most prevalent cancers caused by gene-environment interaction. Lin et al. identified higher plasma exosomal expression levels of tRF-25(tRF-25-DWY2MJ8F81, i-tRF), tRF-38(tRF-38-QB1MK8YUBS68BFD2, 3′-tRF), and tRF-18(tRF-18-8S68BFD2, 3′-tRF) in GC patients than healthy controls [66]. Besides this, the plasma exosomal of these three tRFs exhibited better diagnostic accuracy for GC detection by receiver operating characteristic (ROC) analyses [66].
EV-associated tRFs in breast cancer
Several studies have shown that tRFs may function as potential biomarkers in breast cancer (BC). Koi and associates confirmed that the expression levels of miR-23a-3p, isomiR of miR-21-5p, and tRF-Lys-TTT (tRF-32-PS5P4PW3FJHP1, 5′-tRNA half) were significantly elevated in BC compared to controls [110]. The model based on these three small RNAs demonstrated high diagnostic accuracy, the area under ROC (AUC) value reached 0.92, and could successfully distinguished stage 0 BC from cancer-free individuals [110]. Furthermore, small RNAs in EVs (mainly exosomes) were isolated from serum and cell culture media and were evaluated. Two miRNAs of the above three small RNAs were present in EVs from serum, with a significantly high expression level in BC. However, there was no significant difference in expression of tRF-Lys-TTT [110].
Similarly, several studies indicated that expression difference of EV-associated tRFs could be detected in BC cell lines. IsomiR of miR-21-5p and miR-23a-3p were more abundant in the EVs from BC cell media, while the expression levels of tRF-Lys-TTT were lower in the EVs than those of normal human breast epithelial telomerase immortalized cells [110]. Tosar and coauthors found that 5′-tRNA halves were significantly enriched in the extracellular spaces derived from the BC cell line MCF-7 compared with intracellular fractions [79]. In contrast, miRNAs presented at very low abundances in extracellular fractions [79]. Similarly, “miRNA-like” tRNA fragments (miR-720 and miR-1274b) were greatly expressed in the MCF-7 EVs uniquely and could not been found in cellular profiles [112]. These showed overexpression of tRNAs in MCF7 cells and an effective export process of tRFs. Consequently, these selected tRFs were amplified in EVs. Moreover, the study suggested a potential way to use high levels of tRFs combined with known tumor miRNAs to identify circulating tumor-derived EVs from EVs derived from other cellular fractions [112]. Furthermore, another study demonstrated that 5ʹ tiRNA-Gly (5′-tRNA half) could be secreted into MCF-7 EVs in a concentration-dependent fashion and closely replicated levels of EVs and the recipient cells [115].
EV-associated tRFs as biomarkers in other diseases
Exosomal tRFs in osteoporosis
Osteoporosis is a disorder characterized by decreased bone mineral density and microarchitectural deterioration, which results in an increased risk of fracture. In a study on plasma exosomes, Zhang et al. identified 11 upregulated tRFs and 18 downregulated tRFs in osteoporosis compared with healthy controls, using small RNA sequence of plasma exosomes [111]. In addition, six categories of tRFs were included in the osteoporosis and healthy control groups, namely, 5′-tRNA half, 3′-tRNA half, tRF-1, 3′-tRF, 5′-tRF, and i-tRF [111]. Furthermore, the expression levels of tRF‐25‐R9ODMJ6B26 (3′-tRF), tRF‐38‐QB1MK8YUBS68BFD2 (3′-tRF), and tRF‐18‐BS68BFD2 (3′-tRF) were significantly higher in osteoporosis samples compared to controls [111]. In this study, a tRF panel including the above three tRFs was developed, with higher sensitivity and specificity for diagnosing osteoporosis than a single tRF [111].
Exosomal tRFs in chronic kidney disease
Urine may be used as a promising biomarker due to its noninvasive collection. Khurana et al. found 30 differentially expressed urinary exosomal ncRNAs in chronic kidney disease (CKD) patients using a novel computational algorithm of RNA-seq [113]. Among these exRNAs, tRFVal and tRFLeu, originating from 5′-ends of tRNAs, were significantly less abundant in CKD patients than controls [113]. The increased expression levels of tRFs in exosomes from healthy controls may reflect an effective process to remove cellular waste from cells, which might impair kidney cells in during CKD [113].
EV-associated tRFs in infection
Several studies have indicated that some EV-associated tRFs were also related to infections [85, 116]. Garcia-Silva and coauthors demonstrated that Trypanosoma cruzi epimastigotes excreted vesicles which carry tRFs and Ago, distinctive to trypanosomatids (TcPIWI-tryp), to extracellular medium under nutrient starvation [114]. A portion of these molecules in EVs were transferred between parasites and to infection susceptible mammalian cells [114]. Ghosal et al. analyzed complements of exRNAs from E. coli and found that the major constituents were tRFs, not full-length tRNAs [117]. Likewise, EV-associated tRFs were identified in mouse serum infected with Schistosoma mansoni [118].
EV-associated tRFs in male fertility
Recently, it was demonstrated that EV-associated tRFs in sperm played notable roles in preimplantation embryo development, by modulating cell cycle–associated genes and retrotransposons [119]. Additionally, Chen et al. indicated that human sperm tRF derived from tRNA-Gln-TTG could affect activation of the embryonic genome via regulation of ncRNAs. Their findings showed that this specific tRF group with 30, 31, 32, 33, and 36 nt could act as a promising diagnostic biomarker and therapeutic target for male infertility [120].
Conclusions
It has been demonstrated that tRFs play vital roles in the development and progress of multiple diseases, particularly cancers. Considerable evidence suggests that tRFs can regulate gene expression, gene translation, epigenetic inheritance, and the cell cycle. Meanwhile, an accumulating body of evidence has confirmed the abundant existence of tRFs in EVs. EV-associated tRFs might lead a key part in intercellular communication and serve as novel biomarkers for diagnosing cancer and other diseases. However, several limitations need to be considered. Firstly, the generation mechanism of tRFs and encapsulation in EVs remains to be fully elucidated. Secondly, more studies should be undertaken to confirm the diagnostic roles of EV-associated tRFs in different types of tumors and other diseases. Thirdly, the correlation of EV-associated tRFs with cancer therapy and cancer prognosis requires further investigation. Fourthly, methodological details for EV isolation vary between different studies, explaining the discrepancy in results and poor reproducibility. However, with the progression of technologies, we believe that EV-associated tRFs will play increasingly important roles in the diagnosis and treatment of human diseases.
Abbreviations
- tRNAs:
-
Transfer RNAs
- mRNA:
-
Messenger RNA
- sncRNAs:
-
Small non-coding RNAs
- tRFs:
-
TRNA-derived fragments
- EVs:
-
Extracellular vesicles
- ncRNAs:
-
Non-coding RNAs
- lncRNAs:
-
Long ncRNAs
- sncRNAs:
-
Small ncRNAs
- snoRNAs:
-
Small nucleolar RNAs
- snRNAs:
-
Small nuclear RNAs
- exRNAs:
-
Extracellular RNAs
- miRNAs:
-
MicroRNAs
- endo-siRNAs:
-
Endogenous small interfering RNAs
- piRNAs:
-
Piwi-interacting RNAs
- sdRNAs:
-
SnoRNA-derived small RNAs
- rRFs:
-
Ribosomal RNA (rRNA)-derived fragments
- tsRNA:
-
Transfer RNA (tRNA)-derived small RNA
- tDRs:
-
TRNA-derived RNAs
- MVBs:
-
Multivesicular bodies
- ERCC:
-
The Extracellular RNA Communication Consortium
- Pol III:
-
Polymerase III
- pre-tRNAs:
-
Precursor tRNAs
- RNase P:
-
Endonuclease P
- RNase Z:
-
Endonuclease Z
- ANG:
-
Angiogenin
- Ago:
-
Argonaute
- CLP1:
-
Cleavage and polyadenylation factor I subunit 1
- TSEN:
-
TRNA endonuclease complex
- SHOT-RNAs:
-
Sex hormone-dependent tRNA-derived RNAs
- RNPs:
-
Ribonucleoproteins
- LPP:
-
Lipoprotein
- STB-EV:
-
Syncytiotrophoblast-derived extracellular vesicle
- SE:
-
Seminal exosome
- ML:
-
Mesenteric lymph
- DCs:
-
Dendritic cells
- BMSC:
-
Bone marrow stem cells
- ASC:
-
Adipose mesenchymal stem cells
- MSCs:
-
Mesenchymal stem cells
- GBM:
-
Glioblastoma
- HFD:
-
High-fat diet
- TNBC:
-
Triple-negative breast cancer
- STAT3:
-
Signal transducer and activator of transcription 3
- NSCLC:
-
Non‐small cell lung cancer
- HGSOC:
-
High-grade serous ovarian cancer
- CRC:
-
Colorectal cancer
- PD:
-
Parkinson’s disease
- CSF:
-
Cerebrospinal fluid
- PLS-DA:
-
Partial least squares discriminant analysis
- OGD:
-
Oxygen-glucose deprivation
- RSV:
-
Respiratory syncytial virus
- GC:
-
Gastric carcinoma
- ROC:
-
Receiver operating characteristic
- BC:
-
Breast cancer
- AUC:
-
The area under ROC
- CKD:
-
Chronic kidney disease
References
Zhu P, Yu J, Zhou P (2020) Role of tRNA-derived fragments in cancer: novel diagnostic and therapeutic targets tRFs in cancer. Am J Cancer Res 10(2):393–402
Klastrup LK, Bak ST, Nielsen AL (2019) The influence of paternal diet on sncRNA-mediated epigenetic inheritance. Molecular genetics and genomics : MGG 294(1):1–11
Cherlin T, Magee R, Jing Y, Pliatsika V, Loher P, Rigoutsos I (2020) Ribosomal RNA fragmentation into short RNAs (rRFs) is modulated in a sex- and population of origin-specific manner. BMC Biol 18(1):38
Lee YS, Shibata Y, Malhotra A, Dutta A (2009) A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev 23(22):2639–2649
Kirchner S, Ignatova Z (2015) Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat Rev Genet 16(2):98–112
Telonis AG, Kirino Y, Rigoutsos I (2015) Mitochondrial tRNA-lookalikes in nuclear chromosomes: could they be functional? RNA Biol 12(4):375–380
Selitsky SR, Sethupathy P (2015) tDRmapper: challenges and solutions to mapping, naming, and quantifying tRNA-derived RNAs from human small RNA-sequencing data. BMC Bioinformatics 16:354
Oberbauer V, Schaefer MR (2018) tRNA-derived small RNAs: biogenesis, modification, function and potential impact on human disease development. Genes 9(12):607
Li S, Xu Z, Sheng J (2018) tRNA-derived small RNA: a novel regulatory small non-coding RNA. Genes 9(5):246
Magee R, Rigoutsos I (2020) On the expanding roles of tRNA fragments in modulating cell behavior. Nucleic Acids Res 48(17):9433–9448
Guzzi N, Bellodi C (2020) Novel insights into the emerging roles of tRNA-derived fragments in mammalian development. RNA Biol 17(8):1214–1222
Schimmel P (2018) The emerging complexity of the tRNA world: mammalian tRNAs beyond protein synthesis. Nat Rev Mol Cell Biol 19(1):45–58
Théry C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8):569–579
Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M (2017) RNA in extracellular vesicles. Wiley interdisciplinary reviews RNA 8(4). https://doi.org/10.1002/wrna.1413
Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, Buzás EI, Lötvall J (2013) Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles 2
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S (2007) Exosomes with immune modulatory features are present in human breast milk. J Immunol (Baltimore, Md : 1950) 179(3):1969–1978
Pisitkun T, Shen RF, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101(36):13368–13373
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33(3):967–978
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262(19):9412–9420
Théry C (2011) Exosomes: secreted vesicles and intercellular communications. F1000 Biology Reports 3:15
Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D, Xu X, Zuo Y, Zhao Y, Wei YQ et al (2019) Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol Cancer 18(1):74
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science (New York, NY) 367(6478):eaau6977
Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, Hendrix A, Mathivanan S (2019) Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res 47(D1):D516-d519
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N et al (2016) ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 428(4):688–692
Murillo OD, Thistlethwaite W, Rozowsky J, Subramanian SL, Lucero R, Shah N, Jackson AR, Srinivasan S, Chung A, Laurent CD et al (2019) exRNA atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell 177(2):463-477.e415
Srinivasan S, Yeri A, Cheah PS, Chung A, Danielson K, De Hoff P, Filant J, Laurent CD, Laurent LD, Magee R et al (2019) Small RNA sequencing across diverse biofluids identifies optimal methods for exRNA isolation. Cell 177(2):446-462.e416
Tosar JP, Cayota A (2020) Extracellular tRNAs and tRNA-derived fragments. RNA Biol 17(8):1149–1167
Torres AG, Martí E (2021) Toward an understanding of extracellular tRNA biology. Front Mol Biosci 8:662620
Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL, Rando OJ (2018) Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev Cell 46(4):481-494.e486
Ceballos M, Vioque A (2007) tRNase Z. Protein Pept Lett 14(2):137–145
Walker SC, Engelke DR (2006) Ribonuclease P: the evolution of an ancient RNA enzyme. Crit Rev Biochem Mol Biol 41(2):77–102
Fu Y, Lee I, Lee YS, Bao X (2015) Small non-coding transfer RNA-derived RNA fragments (tRFs): their biogenesis, function and implication in human diseases. Genomics Inform 13(4):94–101
Kumar P, Anaya J, Mudunuri SB, Dutta A (2014) Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol 12:78
Yamasaki S, Ivanov P, Hu GF, Anderson P (2009) Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol 185(1):35–42
Thompson DM, Lu C, Green PJ, Parker R (2008) tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA (New York, NY) 14(10):2095–2103
Zhu L, Ge J, Li T, Shen Y, Guo J (2019) tRNA-derived fragments and tRNA halves: the new players in cancers. Cancer Lett 452:31–37
Tao EW, Cheng WY, Li WL, Yu J, Gao QY (2020) tiRNAs: a novel class of small noncoding RNAs that helps cells respond to stressors and plays roles in cancer progression. J Cell Physiol 235(2):683–690
Saikia M, Hatzoglou M (2015) The many virtues of tRNA-derived stress-induced RNAs (tiRNAs): discovering novel mechanisms of stress response and effect on human health. J Biol Chem 290(50):29761–29768
Anderson P, Ivanov P (2014) tRNA fragments in human health and disease. FEBS Lett 588(23):4297–4304
Su Z, Kuscu C, Malik A, Shibata E, Dutta A (2019) Angiogenin generates specific stress-induced tRNA halves and is not involved in tRF-3-mediated gene silencing. J Biol Chem 294(45):16930–16941
Kumar P, Mudunuri SB, Anaya J, Dutta A (2015) tRFdb: a database for transfer RNA fragments. Nucleic Acids Res 43(Database issue):D141–145
Pliatsika V, Loher P, Magee R, Telonis AG, Londin E, Shigematsu M, Kirino Y, Rigoutsos I (2018) MINTbase v2.0: a comprehensive database for tRNA-derived fragments that includes nuclear and mitochondrial fragments from all The Cancer Genome Atlas projects. Nucleic Acids Res 46(D1):D152-d159
Balatti V, Nigita G, Veneziano D, Drusco A, Stein GS, Messier TL, Farina NH, Lian JB, Tomasello L, Liu CG et al (2017) tsRNA signatures in cancer. Proc Natl Acad Sci USA 114(30):8071–8076
Liao JY, Ma LM, Guo YH, Zhang YC, Zhou H, Shao P, Chen YQ, Qu LH (2010) Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3’ trailers. PLoS One 5(5):e10563
Olvedy M, Scaravilli M, Hoogstrate Y, Visakorpi T, Jenster G, Martens-Uzunova ES (2016) A comprehensive repertoire of tRNA-derived fragments in prostate cancer. Oncotarget 7(17):24766–24777
Haussecker D, Huang Y, Lau A, Parameswaran P, Fire AZ, Kay MA (2010) Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA (New York, NY) 16(4):673–695
Cole C, Sobala A, Lu C, Thatcher SR, Bowman A, Brown JW, Green PJ, Barton GJ, Hutvagner G (2009) Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA (New York, NY) 15(12):2147–2160
Telonis AG, Loher P, Honda S, Jing Y, Palazzo J, Kirino Y, Rigoutsos I (2015) Dissecting tRNA-derived fragment complexities using personalized transcriptomes reveals novel fragment classes and unexpected dependencies. Oncotarget 6(28):24797–24822
Park EJ, Kim TH (2018) Fine-tuning of gene expression by tRNA-derived fragments during abiotic stress signal transduction. Int J Mol Sci 19(2):518
Schaffer AE, Eggens VR, Caglayan AO, Reuter MS, Scott E, Coufal NG, Silhavy JL, Xue Y, Kayserili H, Yasuno K et al (2014) CLP1 founder mutation links tRNA splicing and maturation to cerebellar development and neurodegeneration. Cell 157(3):651–663
Hanada T, Weitzer S, Mair B, Bernreuther C, Wainger BJ, Ichida J, Hanada R, Orthofer M, Cronin SJ, Komnenovic V et al (2013) CLP1 links tRNA metabolism to progressive motor-neuron loss. Nature 495(7442):474–480
Honda S, Loher P, Shigematsu M, Palazzo JP, Suzuki R, Imoto I, Rigoutsos I, Kirino Y (2015) Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc Natl Acad Sci USA 112(29):E3816-3825
Goodarzi H, Liu X, Nguyen HC, Zhang S, Fish L, Tavazoie SF (2015) Endogenous tRNA-derived fragments suppress breast cancer progression via YBX1 displacement. Cell 161(4):790–802
Xie Y, Yao L, Yu X, Ruan Y, Li Z, Guo J (2020) Action mechanisms and research methods of tRNA-derived small RNAs. Signal Transduct Target Ther 5(1):109
Zuo Y, Zhu L, Guo Z, Liu W, Zhang J, Zeng Z, Wu Q, Cheng J, Fu X, Jin Y et al (2021) tsRBase: a comprehensive database for expression and function of tsRNAs in multiple species. Nucleic Acids Res 49(D1):D1038-d1045
Gupta N, Singh A, Zahra S, Kumar S (2018) PtRFdb: a database for plant transfer RNA-derived fragments. Database 2018:bay063
Thompson A, Zielezinski A, Plewka P, Szymanski M, Nuc P, Szweykowska-Kulinska Z, Jarmolowski A, Karlowski WM (2018) tRex: a web portal for exploration of tRNA-derived fragments in Arabidopsis thaliana. Plant Cell Physiol 59(1):e1
Zheng LL, Xu WL, Liu S, Sun WJ, Li JH, Wu J, Yang JH, Qu LH (2016) tRF2Cancer: a web server to detect tRNA-derived small RNA fragments (tRFs) and their expression in multiple cancers. Nucleic Acids Res 44(W1):W185-193
La Ferlita A, Alaimo S, Veneziano D, Nigita G, Balatti V, Croce CM, Ferro A, Pulvirenti A (2019) Identification of tRNA-derived ncRNAs in TCGA and NCI-60 panel cell lines and development of the public database tRFexplorer. Database 2019:baz115
Yao D, Sun X, Zhou L, Amanullah M, Pan X, Liu Y, Liang M, Liu P, Lu Y (2020) OncotRF: an online resource for exploration of tRNA-derived fragments in human cancers. RNA Biol 17(8):1081–1091
Loher P, Telonis AG, Rigoutsos I (2017) MINTmap: fast and exhaustive profiling of nuclear and mitochondrial tRNA fragments from short RNA-seq data. Sci Rep 7:41184
Zhang F, Shi J, Wu Z, Gao P, Zhang W, Qu B, Wang X, Song Y, Wang Z (2020) A 3’-tRNA-derived fragment enhances cell proliferation, migration and invasion in gastric cancer by targeting FBXO47. Arch Biochem Biophys 690:108467
Zhang M, Li F, Wang J, He W, Li Y, Li H, Wei Z, Cao Y (2019) tRNA-derived fragment tRF-03357 promotes cell proliferation, migration and invasion in high-grade serous ovarian cancer. Onco Targets Ther 12:6371–6383
Huang Y, Ge H, Zheng M, Cui Y, Fu Z, Wu X, Xia Y, Chen L, Wang Z, Wang S et al (2020) Serum tRNA-derived fragments (tRFs) as potential candidates for diagnosis of nontriple negative breast cancer. J Cell Physiol 235(3):2809–2824
Lin C, Zheng L, Huang R, Yang G, Chen J, Li H (2020) tRFs as potential exosome tRNA-derived fragment biomarkers for gastric carcinoma. Clin Lab 66(6)
Xu WL, Yang Y, Wang YD, Qu LH, Zheng LL (2017) Computational approaches to tRNA-derived small RNAs. Non-coding RNA 3(1):2
Wei J, Blenkiron C, Tsai P, James JL, Chen Q, Stone PR, Chamley LW (2017) Placental trophoblast debris mediated feto-maternal signalling via small RNA delivery: implications for preeclampsia. Sci Rep 7(1):14681
Cooke WR, Cribbs A, Zhang W, Kandzija N, Motta-Mejia C, Dombi E, Ri R, Cerdeira AS, Redman C, Vatish M (2019) Maternal circulating syncytiotrophoblast-derived extracellular vesicles contain biologically active 5’-tRNA halves. Biochem Biophys Res Commun 518(1):107–113
Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R, Tewari M et al (2014) Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res 42(11):7290–7304
Hong J, Blenkiron C, Tsai P, Premkumar R, Nachkebia S, Tun SM, Petzer A, Windsor JA, Hickey AJ, Phillips AR (2019) Extracellular RNA profile in mesenteric lymph from exemplar rat models of acute and critical illness. Lymphat Res Biol 17(5):512–517
Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N et al (2015) Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 6(1):127
Wei Z, Batagov AO, Schinelli S, Wang J, Wang Y, El Fatimy R, Rabinovsky R, Balaj L, Chen CC, Hochberg F et al (2017) Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun 8(1):1145
Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M et al (2013) Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 14:319
Yuan T, Huang X, Woodcock M, Du M, Dittmar R, Wang Y, Tsai S, Kohli M, Boardman L, Patel T et al (2016) Plasma extracellular RNA profiles in healthy and cancer patients. Sci Rep 6:19413
Amorim MG, Valieris R, Drummond RD, Pizzi MP, Freitas VM, Sinigaglia-Coimbra R, Calin GA, Pasqualini R, Arap W, Silva IT et al (2017) A total transcriptome profiling method for plasma-derived extracellular vesicles: applications for liquid biopsies. Sci Rep 7(1):14395
Dhahbi JM, Spindler SR, Atamna H, Yamakawa A, Boffelli D, Mote P, Martin DI (2013) 5’ tRNA halves are present as abundant complexes in serum, concentrated in blood cells, and modulated by aging and calorie restriction. BMC Genomics 14:298
Nolte-’t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH (2012) t Hoen PA: Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res 40(18):9272–9285
Tosar JP, Gámbaro F, Sanguinetti J, Bonilla B, Witwer KW, Cayota A (2015) Assessment of small RNA sorting into different extracellular fractions revealed by high-throughput sequencing of breast cell lines. Nucleic Acids Res 43(11):5601–5616
Savelyeva AV, Baryakin DN, Chikova ED, Kuligina EV, Richter VA, Semenov DV (2016) Vesicular and extra-vesicular RNAs of human blood plasma. Adv Exp Med Biol 924:117–119
Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, Belleannee C, Kucukural A, Serra RW, Sun F et al (2016) Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science (New York, NY) 351(6271):391–396
Chiou NT, Kageyama R, Ansel KM (2018) Selective export into extracellular vesicles and function of tRNA fragments during T cell activation. Cell Rep 25(12):3356-3370.e3354
Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, Feng GH, Peng H, Zhang X, Zhang Y et al (2016) Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science (New York, NY) 351(6271):397–400
Boskovic A, Bing XY, Kaymak E, Rando OJ (2020) Control of noncoding RNA production and histone levels by a 5’ tRNA fragment. Genes Dev 34(1–2):118–131
Garcia-Silva MR, Cabrera-Cabrera F, das Neves RF, Souto-Padrón T, de Souza W, Cayota A (2014) Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: relevance of tRNA-derived halves. BioMed Res Int 2014:305239
Cui Y, Huang Y, Wu X, Zheng M, Xia Y, Fu Z, Ge H, Wang S, Xie H (2019) Hypoxia-induced tRNA-derived fragments, novel regulatory factor for doxorubicin resistance in triple-negative breast cancer. J Cell Physiol 234(6):8740–8751
Dhahbi JM, Spindler SR, Atamna H, Boffelli D, Martin DI (2014) Deep sequencing of serum small RNAs identifies patterns of 5’ tRNA half and YRNA fragment expression associated with breast cancer. Biomarkers in cancer 6:37–47
Maute RL, Schneider C, Sumazin P, Holmes A, Califano A, Basso K, Dalla-Favera R (2013) tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc Natl Acad Sci USA 110(4):1404–1409
Chen Z, Qi M, Shen B, Luo G, Wu Y, Li J, Lu Z, Zheng Z, Dai Q, Wang H (2019) Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res 47(5):2533–2545
Zhou K, Diebel KW, Holy J, Skildum A, Odean E, Hicks DA, Schotl B, Abrahante JE, Spillman MA, Bemis LT (2017) A tRNA fragment, tRF5-Glu, regulates BCAR3 expression and proliferation in ovarian cancer cells. Oncotarget 8(56):95377–95391
Li S, Shi X, Chen M, Xu N, Sun D, Bai R, Chen H, Ding K, Sheng J, Xu Z (2019) Angiogenin promotes colorectal cancer metastasis via tiRNA production. Int J Cancer 145(5):1395–1407
Zhu L, Li T, Shen Y, Yu X, Xiao B, Guo J (2019) Using tRNA halves as novel biomarkers for the diagnosis of gastric cancer. Cancer Biomark 25(2):169–176
Shen Y, Xie Y, Yu X, Zhang S, Wen Q, Ye G, Guo J (2021) Clinical diagnostic values of transfer RNA-derived fragment tRF-19-3L7L73JD and its effects on the growth of gastric cancer cells. J Cancer 12(11):3230–3238
Shen Y, Yu X, Ruan Y, Li Z, Xie Y, Yan Z, Guo J (2021) Global profile of tRNA-derived small RNAs in gastric cancer patient plasma and identification of tRF-33-P4R8YP9LON4VDP as a new tumor suppressor. Int J Med Sci 18(7):1570–1579
Joilin G, Gray E, Thompson AG, Bobeva Y, Talbot K, Weishaupt J, Ludolph A, Malaspina A, Leigh PN, Newbury SF et al (2020) Identification of a potential non-coding RNA biomarker signature for amyotrophic lateral sclerosis. Brain Communications 2(1):fcaa053
Tong L, Zhang W, Qu B, Zhang F, Wu Z, Shi J, Chen X, Song Y, Wang Z (2020) The tRNA-derived fragment-3017A promotes metastasis by inhibiting NELL2 in Human Gastric Cancer. Front Oncol 10:570916
Shao Y, Sun Q, Liu X, Wang P, Wu R, Ma Z (2017) tRF-Leu-CAG promotes cell proliferation and cell cycle in non-small cell lung cancer. Chem Biol Drug Des 90(5):730–738
Zhao C, Tolkach Y, Schmidt D, Muders M, Kristiansen G, Müller SC, Ellinger J (2018) tRNA-halves are prognostic biomarkers for patients with prostate cancer. Urol Oncol 36(11):503.e501-503.e507
Telonis AG, Loher P, Magee R, Pliatsika V, Londin E, Kirino Y, Rigoutsos I (2019) tRNA fragments show intertwining with mRNAs of specific repeat content and have links to disparities. Can Res 79(12):3034–3049
Karousi P, Katsaraki K, Papageorgiou SG, Pappa V, Scorilas A, Kontos CK (2019) Identification of a novel tRNA-derived RNA fragment exhibiting high prognostic potential in chronic lymphocytic leukemia. Hematol Oncol 37(4):498–504
Katsaraki K, Artemaki PI, Papageorgiou SG, Pappa V, Scorilas A, Kontos CK (2019) Identification of a novel, internal tRNA-derived RNA fragment as a new prognostic and screening biomarker in chronic lymphocytic leukemia, using an innovative quantitative real-time PCR assay. Leuk Res 87:106234
Karousi P, Adamopoulos PG, Papageorgiou SG, Pappa V, Scorilas A, Kontos CK (2020) A novel, mitochondrial, internal tRNA-derived RNA fragment possesses clinical utility as a molecular prognostic biomarker in chronic lymphocytic leukemia. Clin Biochem 85:20–26
Katsaraki K, Adamopoulos PG, Papageorgiou SG, Pappa V, Scorilas A, Kontos CK (2021) A 3’ tRNA-derived fragment produced by tRNA(LeuAAG) and tRNA(LeuTAG) is associated with poor prognosis in B-cell chronic lymphocytic leukemia, independently of classical prognostic factors. Eur J Haematol 106(6):821–830
Londin E, Magee R, Shields CL, Lally SE, Sato T, Rigoutsos I (2020) IsomiRs and tRNA-derived fragments are associated with metastasis and patient survival in uveal melanoma. Pigment Cell Melanoma Res 33(1):52–62
Wang Q, Lee I, Ren J, Ajay SS, Lee YS, Bao X (2013) Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol Ther 21(2):368–379
Ruggero K, Guffanti A, Corradin A, Sharma VK, De Bellis G, Corti G, Grassi A, Zanovello P, Bronte V, Ciminale V et al (2014) Small noncoding RNAs in cells transformed by human T-cell leukemia virus type 1: a role for a tRNA fragment as a primer for reverse transcriptase. J Virol 88(7):3612–3622
Selitsky SR, Baran-Gale J, Honda M, Yamane D, Masaki T, Fannin EE, Guerra B, Shirasaki T, Shimakami T, Kaneko S et al (2015) Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C. Sci Rep 5:7675
Magee R, Londin E, Rigoutsos I (2019) TRNA-derived fragments as sex-dependent circulating candidate biomarkers for Parkinson’s disease. Parkinsonism Relat Disord 65:203–209
Li Q, Hu B, Hu GW, Chen CY, Niu X, Liu J, Zhou SM, Zhang CQ, Wang Y, Deng ZF (2016) tRNA-derived small non-coding RNAs in response to ischemia inhibit angiogenesis. Sci Rep 6:20850
Koi Y, Tsutani Y, Nishiyama Y, Ueda D, Ibuki Y, Sasada S, Akita T, Masumoto N, Kadoya T, Yamamoto Y et al (2020) Predicting the presence of breast cancer using circulating small RNAs, including those in the extracellular vesicles. Cancer Sci 111(6):2104–2115
Zhang Y, Cai F, Liu J, Chang H, Liu L, Yang A, Liu X (2018) Transfer RNA-derived fragments as potential exosome tRNA-derived fragment biomarkers for osteoporosis. Int J Rheum Dis 21(9):1659–1669
Guzman N, Agarwal K, Asthagiri D, Yu L, Saji M, Ringel MD, Paulaitis ME (2015) Breast cancer-specific miR signature unique to extracellular vesicles includes “microRNA-like” tRNA fragments. Molecular cancer research : MCR 13(5):891–901
Khurana R, Ranches G, Schafferer S, Lukasser M, Rudnicki M, Mayer G, Hüttenhofer A (2017) Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA (New York, NY) 23(2):142–152
Garcia-Silva MR, das Neves RF, Cabrera-Cabrera F, Sanguinetti J, Medeiros LC, Robello C, Naya H, Fernandez-Calero T, Souto-Padron T, de Souza W et al (2014) Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol Res 113(1):285–304
Gámbaro F, Li Calzi M, Fagúndez P, Costa B, Greif G, Mallick E, Lyons S, Ivanov P, Witwer K, Cayota A et al (2020) Stable tRNA halves can be sorted into extracellular vesicles and delivered to recipient cells in a concentration-dependent manner. RNA Biol 17(8):1168–1182
Fernandez-Calero T, Garcia-Silva R, Pena A, Robello C, Persson H, Rovira C, Naya H, Cayota A (2015) Profiling of small RNA cargo of extracellular vesicles shed by Trypanosoma cruzi reveals a specific extracellular signature. Mol Biochem Parasitol 199(1–2):19–28
Ghosal A, Upadhyaya BB, Fritz JV, Heintz-Buschart A, Desai MS, Yusuf D, Huang D, Baumuratov A, Wang K, Galas D et al (2015) The extracellular RNA complement of Escherichia coli. MicrobiologyOpen 4(2):252–266
Hoy AM, Lundie RJ, Ivens A, Quintana JF, Nausch N, Forster T, Jones F, Kabatereine NB, Dunne DW, Mutapi F et al (2014) Parasite-derived microRNAs in host serum as novel biomarkers of helminth infection. PLoS Negl Trop Dis 8(2):e2701
Chen X, Zheng Y, Lei A, Zhang H, Niu H, Li X, Zhang P, Liao M, Lv Y, Zhu Z et al (2020) Early cleavage of preimplantation embryos is regulated by tRNA(Gln-TTG)-derived small RNAs present in mature spermatozoa. J Biol Chem 295(32):10885–10900
Chen X, Sun Q, Zheng Y, Liu Z, Meng X, Zeng W, Lu H (2021) Human sperm tsRNA as potential biomarker and therapy target for male fertility. Reproduction (Cambridge, England) 161(2):111–122
Funding
This study was supported by grants from the National Natural Science Foundation of China (no. 81974316), Zhejiang Provincial Natural Science Foundation of China (no. LGF21H200004), Zhejiang Province Public Welfare Technology Application Research Project of China (no. LGF22H160039), Ningbo Nature Fund Project (no. 2021Z133 and no. 2021J253), The Foundation of Zhejiang Key Laboratory of Pathophysiology (Grant no. 202101), and the K.C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Contributions
QW and YX drafted the manuscript. YW, XY, SZ, JG, and ZL collected relevant papers, and GY and JG revised and finalized the review. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weng, Q., Wang, Y., Xie, Y. et al. Extracellular vesicles-associated tRNA-derived fragments (tRFs): biogenesis, biological functions, and their role as potential biomarkers in human diseases. J Mol Med 100, 679–695 (2022). https://doi.org/10.1007/s00109-022-02189-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02189-0