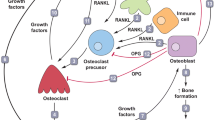
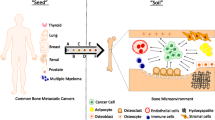
Abstract
Osteolytic bone destruction is found in approximately 60% of advanced breast cancer patients. With the pathogenesis of bone metastasis being unclear, traditional antiresorptive therapeutic strategies might not be ideal for treatment. The Wnt pathway is a highly organized cascade involved in multiple stages of cancer bone metastasis, and Wnt-targeted therapeutic strategies have shown promise in achieving favorable outcomes. In this review, we summarize the current progress of pharmacological Wnt modulators against breast cancer bone metastasis, discuss emerging therapeutic strategies based on Wnt pathway-related targets for bone therapy, and highlight opportunities to better harness the Wnt pathway for bone metastasis therapeutics to further reveal the implications of the Wnt pathway in bone metastasis pathology and provide new ideas for the development of Wnt-based intervention strategies against breast cancer bone metastasis.



Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Kumar D, Myers MR, Al Homsi U, Ilyin V (2018) Role of ESR pathway genes in breast cancer: a review. Adv Breast Cancer Res 7(2):134–186
Woolf DK, Padhani AR, Makris A (2015) Assessing response to treatment of bone metastases from breast cancer: what should be the standard of care? Anna Oncol 26(6):1048–1057
Brook N, Brook E, Dharmarajan A, Dass CR, Chan A (2018) Breast cancer bone metastases: pathogenesis and therapeutic targets. Int J Biochem Cell B 96:63–78
Goldvaser H, Amir E (2019) Role of bisphosphonates in breast cancer therapy. Curr treat options Oncol 20(4):26
Chandran T, Venkatachalam I (2019) Efficacy and safety of denosumab compared to bisphosphonates in improving bone strength in postmenopausal osteoporosis: a systematic review. Singapore Med J 60(7):364–378
Hart IR (1982) ‘Seed and soil’ revisited: mechanisms of site-specific metastasis. Cancer Metastasis Rev 1(1):5–16
Vaupel P, Harrison L (2004) Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 9 S5:4–9
Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A, Kayama T, Saito M, Marumo K (2019) The regulation of bone metabolism and disorders by Wnt signaling. Int J Mol Sci 20(22):5525
Kobayashi Y, Uehara S, Udagawa N, Takahashi N (2016) Regulation of bone metabolism by Wnt signals. J Biochem 159(4):387–392
Li X, Yang J, Bao M, Zeng K, Fu S, Wang C, Ye L (2018) Wnt signaling in bone metastasis: mechanisms and therapeutic opportunities. Life Sci 208:33–45
MacDonald BT, Tamai K, He X (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev cell 17:9–26
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Giles RH, Van Es JH, Clevers H (2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochimi Biophys Acta 1653(1):1–24
Prestwich TC, MacDougald OA (2007) Wnt/β-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19(6):612–617
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA (2000) Inhibition of adipogenesis by Wnt signaling. Science 289(5481):950–953
Breuer EK, Fukushiro Lopes D, Dalheim A, Burnette M, Zartman J, Kaja S, Wells C, Campo L, Curtis KJ, Romero Moreno R et al (2019) Potassium channel activity controls breast cancer metastasis by affecting β-catenin signaling. Cell Death Dis 10(3):1–15
Satriyo PB, Bamodu OA, Chen JH, Aryandono T, Haryana SM, Yeh CT, Chao TY (2019) Cadherin 11 inhibition downregulates β-catenin, deactivates the canonical WNT signalling pathway and suppresses the cancer stem cell-like phenotype of triple negative breast cancer. J Clin Med 8(2):148
Xi Y, Chen Y (2014) Wnt signaling pathway: implications for therapy in lung cancer and bone metastasis. Cancer lett 353(1):8–16
Li H, Yue L, Xu H, Li N, Li J, Zhang Z, Zhao RC (2019) Curcumin suppresses osteogenesis by inducing miR-126a-3p and subsequently suppressing the WNT/LRP6 pathway. Aging (Albany NY) 11(17):6983
Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T (2004) DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene 23(52):8520–8526
Scimeca M, Trivigno D, Bonfiglio R, Ciuffa S, Urbano N, Schillaci O, Bonanno E (2020) Breast cancer metastasis to bone: from epithelial to mesenchymal transition to breast osteoblast-like cells. Semin Cancer Biol 72:155–164
Lambert AW, Pattabiraman DR, Weinberg RA (2017) Emerging biological principles of metastasis. Cell 168(4):670–691
Eyre R, Alférez DG, Santiago Gómez A, Spence K, McConnell JC, Hart C, Simões BM, Lefley D, Tulotta C, Storer J et al (2019) Microenvironmental IL1β promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat commun 10(1):1–15
Aielli F, Ponzetti M, Rucci N (2019) Bone metastasis pain, from the bench to the bedside. Int J Mol Sci 20(2):280
Evenepoel P, D’haese P, Brandenburg V (2015) Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney Int 88(2):235–240
Le Pape F, Vargas G, Clézardin P (2016) The role of osteoclasts in breast cancer bone metastasis. J Bone Oncel 5(3):93–95
Owen R, Reilly GC (2018) In vitro models of bone remodelling and associated disorders. Front Bioeng Biotech 6:134
Bai SB, Liu DZ, Cheng Y, Cui H, Liu M, Cui MX, Mei QB, Zhou SY (2019) Osteoclasts and tumor cells dual targeting nanoparticle to treat bone metastases of lung cancer. Nanomedicine 21:102054
Dilshara MG, Molagoda IM, Jayasooriya RG, Choi YH, Park C, Kim GY (2021) Indirubin-3′-monoxime induces paraptosis in MDA-MB-231 breast cancer cells by transmitting Ca2+ from endoplasmic reticulum to mitochondria. Arch Biochemi Biophys 698:108723
Braig S, Bischoff F, Abhari BA, Meijer L, Fulda S, Skaltsounis L, Vollmar AM (2014) The pleiotropic profile of the indirubin derivative 6BIO overcomes TRAIL resistance in cancer. Biochem Pharmacol 91(2):157–167
Damiens E, Baratte B, Marie D, Eisenbrand G, Meijer L (2001) Anti-mitotic properties of indirubin-3′-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest. Oncogene 20(29):3786–3797
Braig S, Kressirer CA, Liebl J, Bischoff F, Zahler S, Meijer L, Vollmar AM (2013) Indirubin derivative 6BIO suppresses metastasis. Cancer Res 73(19):6004–6012
Czapka A, König S, Pergola C, Grune C, Vougogiannopoulou K, Skaltsounis AL, Fischer D, Werz O (2020) The indirubin derivative 6-bromoindirubin-3′-glycerol-oxime ether (6BIGOE) potently modulates inflammatory cytokine and prostaglandin release from human monocytes through GSK-3 interference. Biochem Pharmacol 180:114170
Chen W, Mook R, Premont R, Wang J (2017) Niclosamide: beyond an antihelminthic drug. Cell Signal 41:89–96
Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y (2011) Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin pathway. PLoS One 6(12):e29290
Jiao Y, Chen C, Hu X, Feng X, Shi Z, Cao J, Li Q, Zhu Y (2020) Niclosamide and its derivative DK-520 inhibit RANKL-induced osteoclastogenesis. FEBS Open Bio 10(8):1685–1697
Cheon YH, Kim JY, Baek JM, Ahn SJ, So HS, Oh J (2016) Niclosamide suppresses RANKL-induced osteoclastogenesis and prevents LPS-induced bone loss. Biochem Biophys Res Commun 470(2):343–349
Morin F, Kavian N, Nicco C, Cerles O, Chereau C, Batteux F (2016) Improvement of sclerodermatous graft-versus-host disease in mice by niclosamide. J Invest Dermatol 136(11):2158–2167
Bajaj J, Zimdahl B, Reya T (2015) Fearful symmetry: subversion of asymmetric division in cancer development and progression. Cancer Res 75(5):792–797
Pal A, Nandave M, Kaithwas G (2020) Chemoprophylactic activity of nitazoxanide in experimental model of mammary gland carcinoma in rats. 3 Biotech 10(8):338
Lee HH, Lim CA, Cheong YT, Singh M, Gam LH (2012) Comparison of protein expression profiles of different stages of lymph nodes metastasis in breast cancer. Int J Biol Sci 8(3):353–362
Pendas-Franco N, García JM, Pena C, Valle N, Palmer HG, Heinaniemi M, Carlberg C, Jimenez B, Bonilla F, Munoz A et al (2008) DICKKOPF-4is induced by TCF/β-catenin and upregulated in human colon cancer, promotestumour cell invasion and angiogenesis and is repressed by 1α,25-dihydroxyvitamin D3. Oncogene 27(32):4467–4477
Johnson AL, Zinser GM, Waltz SE (2015) Vitamin D3-dependent VDR signaling delays ron-mediated breast tumorigenesis through suppression of β-catenin activity. Oncotarget 6(18):16304–16320
Shi YC, Worton L, Esteban L, Baldock P, Fong C, Eisman JA, Gardiner EM (2007) Effects of continuous activation of vitamin D and Wnt response pathways on osteoblastic proliferation and differentiation. Bone 41(1):87–96
Lope V, Castelló A, Mena-Bravo A, Amiano P, Aragonés N, Fernández-Villa T, Guevara M, Dierssen-Sotos T, Fernandez-Tardón G, Castaño-Vinyals G et al (2018) Serum 25-hydroxyvitamin D and breast cancer risk by pathological subtype (MCC-Spain). J Steriod Biochem Mol Biol 182:4–13
Estébanez N, Gómez-Acebo I, Palazuelos C, Llorca J, Dierssen-Sotos T (2018) Vitamin D exposure and risk of breast cancer: a meta-analysis. Sci Rep (1)8:9039
Horas K, van Herck U, Maier GS, Maus U, Harrasser N, Jakob F, Weissenberger M, Arnholdt J, Holzapfel BM, Rudert M (2020) Does vitamin D deficiency predict tumour malignancy in patients with bone tumours? Data from a multi-center cohort analysis. J Bone Oncol 25:100329
Maier GS, Horas K, Kurth AA, Lazovic D, Seeger JB, Maus U (2015) Prevalence of vitamin D deficiency in patients with bone metastases and multiple myeloma. Anticancer Res 35(11):6281–6285
Ellegård L, Kurlberg G, Bosaeus I (2013) High prevalence of vitamin D deficiency and osteoporosis in out-patients with intestinal failure. Clin Nutr 32(6):983–987
Lind T, Sundqvist A, Hu L, Pejler G, Andersson G, Rasmusson A, Melhus H (2013) Vitamin A is a negative regulator of osteoblast mineralization. PloS one 8(12):e82388
He J, Gu Y, Zhang S (2018) Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer 18(6):e1389–e1400
Peraita Costa I, Garcia P, Morales Suárez-Varela M (2020) Is there an association between β-carotene and breast cancer? A systematic review on breast cancer risk. Nutr Cancer 1–16
Ahmad I, Ahmed M, Ashraf MF, Naeem A, Tasleem A, Ahmed M, Farooqi M (2018) Pain management in metastatic bone disease: a literature review. Cureus 10(9):e3286
Trautman MS, Edwin SS, Collmer D, Dudley DJ, Simmons D, Mitchell MD (1996) Prostaglandin H synthase-2 in human gestational tissues: regulation in amnion. Placenta 17(4):239–245
Pountos I, Georgouli T, Calori GM, Giannoudis PV (2012) Do nonsteroidal anti-inflammatory drugs affect bone healing? A critical analysis. Scientific World J 2012:606404
Sostres C, Gargallo CJ, Arroyo MT, Lanas A (2010) Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Prac Res Clin Gastroenterol 24(2):121–132
Krishnamurthy K, Wang G, Rokhfeld D, Bieberich E (2008) Deoxycholate promotes survival of breast cancer cells by reducing the level of pro-apoptotic ceramide. Breast Cancer Res 10(6):R106
Pai R, Tarnawski AS, Tran T (2004) Deoxycholic acid activates beta-catenin signaling pathway and increases colon cell cancer growth and invasiveness. Mol Biol Cell 15(5):2156–2163
Jeon OC, Seo DH, Kim HS, Byun Y, Park JW (2016) Oral delivery of zoledronic acid by non-covalent conjugation with lysine-deoxycholic acid: in vitro characterization and in vivo anti-osteoporotic efficacy in ovariectomized rats. Eur J Pharm Sci 82:1–10
Lipton A, Fizazi K, Stopeck AT, Henry DH, Smith MR, Shore N, Martin M, Vadhan-Raj S, Brown JE, Richardson GE et al (2016) Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur J Cancer 53:75–83
Hwang SR, Seo DH, Byun Y, Park JW (2016) Preparation and in vivo evaluation of an orally available enteric-microencapsulated parathyroid hormone (1–34)-deoxycholic acid nanocomplex. Int J Nanomed 11:4231–4246
Swami S, Johnson J, Bettinson LA, Kimura T, Zhu H, Albertelli MA, Johnson RW, Wu JY (2017) Prevention of breast cancer skeletal metastases with parathyroid hormone. JCI Insight 2(17):e90874
Tai N, Inoue D (2014) Anti-Dickkopf1 (Dkk1) antibody as a bone anabolic agent for the treatment of osteoporosis. Clin Calcium 24(1):75–83
Wall JA, Klempner SJ, Arend RC (2020) The anti-DKK1 antibody DKN-01 as an immunomodulatory combination partner for the treatment of cancer. Expert Opin Investig Drugs 29(7):639–644
Jaschke N, Hofbauer LC, Göbel A, Rachner TD (2020) Evolving functions of Dickkopf-1 in cancer and immunity. Cancer Lett 482:1–7
Kasoha M, Juhasz Böss I, Solomayer EF (2016) The Wnt inhibitor Dickkopf-1; a link between breast cancer and bone metastases. Clin Exp Metastasis 32(8):857–866
Zhou SJ, Zhuo SR, Yang XQ, Qin CX, Wang ZL (2014) Serum Dickkopf-1 expression level positively correlates with a poor prognosis in breast cancer. Diagn Pathol 9:161
Klempner SJ, Bendell J, Meucci VV, Tenner L, Stein S, Sirard CA, Newman W, Kagey M, Schlienger K, Strickler J (2018) Safety and efficacy of a DKK1 inhibitor (DKN-01) in combination with pembrolizumab (P) in patients (Pts) with advanced gastroesophageal (GE) malignancies. Ann Oncol 29(8):viii222
Iyer SP, Beck JT, Stewart AK, Shah J, Kelly KR, Isaacs R, Bilic S, Sen S, Munshi NC (2014) A phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br J Haematol 167(3):366–375
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33(5):747–783
Ominsky MS, Boyce RW, Li X, Ke HZ (2017) Effects of sclerostin antibodies in animal models of osteoporosis. Bone 96:63–75
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM et al (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370(5):412–420
Sølling ASK, Harsløf T, Langdahl B (2018) The clinical potential of romosozumab for the prevention of fractures in postmenopausal women with osteoporosis. Ther Adv Musculoskelet Dis 10(5–6):105–115
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375(16):1532–1543
Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H et al (2015) A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res 30(2):216–224
Ferrari SL (2018) Osteoporosis: romosozumab to rebuild the foundations of bone strength. Nat Rev Rheumatol 14(3):128
Glorieux FH, Devogelaer JP, Durigova M, Goemaere S, Hemsley S, Jakob F, Junker U, Ruckle J, Seefried L, Winkle PJ (2017) BPS804 anti-sclerostin antibody in adults with moderate osteogenesis imperfecta: results of a randomized phase 2a trial. J Bone Miner Res 32(7):1496–1504
McDonald MM, Reagan MR, Youlten SE, Mohanty ST, Seckinger A, Terry RL, Pettitt JA, Simic MK, Cheng TL, Morse A, Le LMT et al (2017) Inhibiting the osteocyte specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood 129(26):3452–3464
Florio M, Gunasekaran K, Stolina M, Li XD, Liu L, Tipton B, Salimi-Moosavi H, Asuncion FJ, Li CY, Sun BH et al (2016) A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun 27(7):11505
Spranger S, Dai D, Horton B, Gajewski TF (2017) Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31(5):711–723
Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, Gajewski AP, Andrade J, Gajewski TF (2016) Density of immunogenic antigens does not explain the presence or absence of the T-cell–inflamed tumor microenvironment in melanoma. Proc Nat Acad Sci USA 113(48):E7759–E7768
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Pénault-Llorca F et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271
Haseeb M, Pirzada RH, Ain QU, Choi S (2019) Wnt signaling in the regulation of immune cell and cancer therapeutics. Cells 8(11):1380
El-Sahli S, Xie Y, Wang L, Liu S (2019) Wnt signaling in cancer metabolism and immunity. Cancers (Basel) 11(7):904
Shang S, Hua F, Hu ZW (2017) The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget 8(20):33972
Katoh M (2017) Canonical and non-canonical WNT signaling in cancer stem cells and their niches: cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity. Int J Oncol 51(5):1357–1369
Wang X, Jung YS, Jun S, Lee S, Wang W, Schneider A, Oh YS, Lin SH, Park BJ, Chen J et al (2016) PAF-Wnt signaling-induced cell plasticity is required for maintenance of breast cancer cell stemness. Nat Commun 7:10633
Hou T, Lou Y, Li S, Zhao C, Ji Y, Wang D, Tang L, Zhou M, Xu W, Qian M et al (2018) Kadsurenone is a useful and promising treatment strategy for breast cancer bone metastases by blocking the PAF/PTAFR signaling pathway. Oncology lett 16(2):2255–2262
Hikiji H, Ishii S, Shindou H, Takato T, Shimizu T (2004) Absence of platelet-activating factor receptor protects mice from osteoporosis following ovariectomy. J Clin Invest 114(1):85–93
Zhang N, Li R, Yu H, Shi D, Dong N, Zhang S, Wang H (2013) Development of an LC-MS/MS method for quantification of kadsurenone in rat plasma and its application to a pharmacokinetic study. Biomed Chromatogr 27(12):1754–1758
Zhong L, Simoneau B, Tremblay PL, Gout S, Simard MJ, Huot J (2014) E-selectin-mediated adhesion and extravasation in cancer. Encycl Cancer 1618–1624
Esposito M, Mondal N, Greco TM, Wei Y, Spadazzi C, Lin SC, Zheng H, Cheung C, Magnani JL, Lin SH (2019) Bone vascular niche E-selectin induces mesenchymal–epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat Cell Biol 21(5):627–639
DeAngelo DJ, Jonas BA, Liesveld J, O'Dwyer M, Bixby D, Advani AS, Marlton P, Magnani J, Thackray HM, Becker PS (2017) GMI-1271, a novel E-selectin antagonist, in combination with chemotherapy in relapsed/refractory AML. J Clin Oncol 35(15-suppl):2520–2520
Natoni A, Smith TAG, Keane N, McEllistrim C, Connolly C, Jha A, Andrulis M, Ellert E, Raab M, Glavey S et al (2017) E-selectin ligands recognised by HECA452 induce drug resistance in myeloma, which is overcome by the E-selectin antagonist, GMI-1271. Leukemia 31(12):2642–2651
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13(4):225–238
Kugel S, Mostoslavsky R (2014) Chromatin and beyond: the multitasking roles for SIRT6. Trends Biochem Sci 39(2):72–81
Wang H, Diao D, Shi Z, Zhu X, Gao Y, Gao S, Liu X, Wu Y, Rudolph KL, Liu G et al (2016) SIRT6 controls hematopoietic stem cell homeostasis through epigenetic regulation of Wnt signaling. Cell Stem Cell 18(4):495–507
Tian X, Firsanov D, Zhang Z, Cheng Y, Luo L, Tombline G, Tan R, Simon M, Henderson S, Steffan J et al (2019) SIRT6 is responsible for more efficient DNA double-strand break repair in long-lived species. Cell 177(3):622–638
Domanskyi S, Nicholatos JW, Schilling JE, Privman V, Libert S (2017) SIRT6 knockout cells resist apoptosis initiation but not progression: a computational method to evaluate the progression of apoptosis. Apoptosis 22(11):1336–1343
Miteva YV, Cristea IM (2014) A proteomic perspective of Sirtuin 6 (SIRT6) phosphorylation and interactions and their dependence on its catalytic activity. Mol Cell Proteomics 13(1):168–183
Tasselli L, Zheng W, Chua KF (2017) SIRT6: novel mechanisms and links to aging and disease. Trends Endocrinol Metab 28(3):168–185
Tanrikulu Y, Krüger B, Proschak E (2013) The holistic integration of virtual screening in drug discovery. Drug Discov Today 18(7–8):358–364
Yu B, Huang Z, Zhang M, Dillard DR, Ji H (2013) Rational design of small-molecule inhibitors for β-catenin/T-cell factor protein–protein interactions by bioisostere replacement. ACS Chem Biol 8(3):524–529
Zheng F, Jewell H, Fitzpatrick J, Zhang J, Mierke DF, Grigoryan G (2015) Computational design of selective peptides to discriminate between similar PDZ domains in an oncogenic pathway. J Mol Biol 427(2):491–510
Chang LC, Chen TC, Chen SJ, Chen CL, Lee CC, Wu SH, Yen Y, Huang HS, Lin JJ (2016) Identification of a new class of WNT1 inhibitor: cancer cells migration. G-quadruplex stabilization and target validation Oncotarget 7(42):67986
Hwang SY, Deng X, Byun S, Lee C, Lee SJ, Suh H, Zhang J, Kang Q, Zhang T, Westover KD et al (2016) Direct targeting of β-catenin by a small molecule stimulates proteasomal degradation and suppresses oncogenic Wnt/β-catenin signaling. Cell Rep 16(1):28–36
Fang L, Zhu Q, Neuenschwander M, Specker E, Wulf-Goldenberg A, Weis WI, Von Kries JP, Birchmeier W (2016) A small-molecule antagonist of the β-catenin/TCF4 interaction blocks the self-renewal of cancer stem cells and suppresses tumorigenesis. Cancer Res 76(4):891–901
Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R (2011) An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Nat Acad Sci USA 108(15):5954–5963
Deshmukh V, Hu H, Barroga C, Bossard C, Kc S, Dellamary L, Stewart J, Chiu K, Ibanez M, Pedraza M et al (2018) A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 26(1):18–27
Hao J, Ao A, Zhou L, Murphy CK, Frist AY, Keel JJ, Thorne CA, Kim K, Lee E, Hong CC (2013) Selective small molecule targeting β-catenin function discovered by in vivo chemical genetic screen. Cell Rep 4(5):898–904
Kim HY, Choi S, Yoon JH, Lim HJ, Lee H, Choi J, Ro EJ, Heo JN, Lee W, No KT et al (2016) Small molecule inhibitors of the Dishevelled-CXXC 5 interaction are new drug candidates for bone anabolic osteoporosis therapy. EMBO Mol Med 8(4):375–387
Placencio VR, Li X, Sherrill TP, Fritz G, Bhowmick NA (2010) Bone marrow derived mesenchymal stem cells incorporate into the prostate during regrowth. PloS one 5(9):e12920
Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J, Hu H, Guan W, Ma Y (2014) Inhibitory effect and mechanism of mesenchymal stem cells on liver cancer cells. Tumor Biol 35(2):1239–1250
Jones EA, Giannoudis PV, Kouroupis D (2016) Bone repair with skeletal stem cells: rationale, progress to date and clinical application. Ther Adv Musculoskelet Dis 8(3):57–71
Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T (2002) Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA 99(13):8932–8937
Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H (2017) Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng Part A 23(21–22):1212–1220
Lin R, Wang S, Zhao RC (2013) Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem 383(1–2):13–20
Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC (2016) In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front Bioeng Biotechnol 4:12
Arrigoni C, Bersini S, Gilardi M, Moretti M (2016) In vitro co-culture models of breast cancer metastatic progression towards bone. Int J Mol Sci 17(9):1405
Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD (2015) Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci 112(1):214–219
Roper J, Tammela T, Cetinbas NM, Akkad A, Roghanian A, Rickelt S, Almeqdadi M, Wu K, Oberli MA, Sánchez-Rivera F et al (2017) In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat Biotechnol 35(6):569–576
Jakubikova J, Cholujova D, Hideshima T, Gronesova P, Soltysova A, Harada T, Joo J, Kong SY, Szalat RE, Richardson PG et al (2016) A novel 3D mesenchymal stem cell model of the multiple myeloma bone marrow niche: biologic and clinical applications. Oncotarget 7(47):77326–77341
Salamanna F, Contartese D, Maglio M, Fini M (2016) A systematic review on in vitro 3D bone metastases models: a new horizon to recapitulate the native clinical scenario? Oncotarget 7(28):44803–44820
Contag CH, Lie WR, Bammer MC, Hardy JW, Schmidt TL, Maloney WJ, King BL (2014) Monitoring dynamic interactions between breast cancer cells and human bone tissue in a co-culture model. Mol Imaging Biol 16(2):158–166
Krzeszinski JY, Schwaid AG, Cheng WY, Jin Z, Gallegos ZR, Saghatelian A, Wan Y (2017) Lipid osteoclastokines regulate breast cancer bone metastasis. Endocrinology 158(3):477–489
Hsu Sh, Huang GS (2013) Substrate-dependent Wnt signaling in MSC differentiation within biomaterial-derived 3D spheroids. Biomaterials 34(20):4725–4738
Fong EL, Wan X, Yang J, Morgado M, Mikos AG, Harrington DA, Navone NM, Farach-Carson MC (2016) A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 77:164–172
Holen I, Speirs V, Morrissey B, Blyth K (2017) In vivo models in breast cancer research: progress, challenges and future directions. Dis Models Mech 10(4):359–371
Holen I, Nutter F, Wilkinson J, Evans C, Avgoustou P, Ottewell PD (2015) Human breast cancer bone metastasis in vitro and in vivo: a novel 3D model system for studies of tumour cell-bone cell interactions. Clin Exp Metastasis 32(7):689–702
Thibaudeau L, Taubenberger AV, Holzapfel BM, Quent VM, Fuehrmann T, Hesami P, Brown TD, Dalton PD, Power CA, Hollier BG et al (2014) A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis Models Mech 7(2):299–309
Lin SC, Lee YC, Yu G, Cheng CJ, Zhou X, Chu K, Murshed M, Le NT, Baseler L, Abe JI et al (2017) Endothelial-to-osteoblast conversion generates osteoblastic metastasis of prostate cancer. Dev Cell 41(5):467–480
Ferrara F, Staquicini DI, Driessen WH, D’Angelo S, Dobroff AS, Barry M, Lomo LC, Staquicini FI, Cardó Vila M, Soghomonyan S et al (2016) Targeted molecular-genetic imaging and ligand-directed therapy in aggressive variant prostate cancer. Proc Natl Acad Sci USA 113(45):12786–12791
Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler McKay P, Bell D, William WN Jr, Glisson BS, Wick MJ et al (2017) Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J Clin Oncol 35(3):352–360
Leconet W, Chentouf M, Du Manoir S, Chevalier C, Sirvent A, Aït Arsa I, Busson M, Jarlier M, Radosevic Robin N, Theillet C et al (2017) Therapeutic activity of anti-AXL antibody against triple-negative breast cancer patient-derived xenografts and metastasis. Clin Cancer Res 23(11):2806–2816
Guan Z, Lan H, Chen X, Jiang X, Wang X, Jin K (2017) Individualized drug screening based on next generation sequencing and patient derived xenograft model for pancreatic cancer with bone metastasis. Mol Med Rep 16(4):4784–4790
Kim TH, Bae CH, Jang EH, Yoon CY, Bae Y, Ko SO, Taketo MM, Cho ES (2012) Col1a1-cre mediated activation of β-catenin leads to aberrant dento-alveolar complex formation. Anat Cell Biol 45(3):193–202
Wan Y, Wei W, Zeve D, Suh JM, Wang X, Du Y, Zerwekh JE, Dechow PC, Graff JM (2011) Biphasic and dosage-dependent regulation of osteoclastogenesis by β-catenin. Mol Cell Biol 31(23):4706–4719
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M (2010) Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 30(12):3071–3085
Kedlaya R, Kang KS, Hong JM, Bettagere V, Lim KE, Horan D, Divieti Pajevic P, Robling AG (2016) Adult-onset deletion of β-catenin in 10kbDmp1-expressing cells prevents intermittent PTH-induced bone gain. Endocrinology 157(8):3047–3057
Yu J, Cao J, Li H, Liu P, Xu S, Zhou R, Yao Z, Guo X (2016) Bone marrow fibrosis with fibrocytic and immunoregulatory responses induced by β-catenin activation in osteoprogenitors. Bone 84:38–46
Acknowledgements
We thank Dr. Rong Sun (Nantong University) for his kind help in the manuscript editing and revision.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81903033) and the project of the Science & Technology Department of Sichuan Province (2018JY0568) to Dr. Xin Li.
Author information
Authors and Affiliations
Contributions
JC and HC contributed to the conception, design, literature search, analysis, and interpretation and drafted and critically revised the manuscript; KZ contributed to the design, data analysis, and interpretation and drafted and critically revised the manuscript; XL contributed to the conception, design, literature analysis, and interpretation and drafted and critically revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approve the manuscript and concur with its publication in the journal.
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, J., Chen, H., Zhang, K. et al. Targeting the Wnt signaling pathway for breast cancer bone metastasis therapy. J Mol Med 100, 373–384 (2022). https://doi.org/10.1007/s00109-021-02159-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-021-02159-y