Abstract
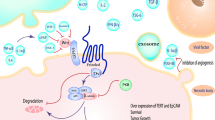
Mesenchymal stem cells (MSCs), with their capacity for self-renewal and differentiation into various cell types, are important seed cells for stem cell therapy. MSCs exhibit potent pathotropic migratory properties that make them attractive for use in tumor prevention and therapy. However, little is known about the underlying molecular mechanisms that link MSCs to the targeted tumor cells. This study investigated the inhibitory effect and mechanism of MSCs on human hepatoma HepG2 cells using co-culture and conditioned medium system and animal transplantation model. The HepG2 cells were co-cultured with MSCs or treated with conditional media derived from MSCs cultures in vitro. Results of methylthiazolyldiphenyl tetrazolium assay and flow cytometric assay showed that the proliferation and apoptosis of HepG2 cells decreased and increased, respectively. Reverse transcription polymerase chain reaction analysis showed that the expression levels of bcl-2, c-Myc, β-catenin, and survivin were downregulated. The results of enzyme-linked immunosorbent assay and Western blot proved that MSCs secreted Dkk-1 to inhibit the expression of Wnt signaling pathway-related factors (bcl-2, c-Myc, β-catenin, and survivin) in tumor cells, consequently inhibiting the proliferation and promoting the apoptosis of HepG2 cells. Animal transplantation experiment showed that tumor growth was significantly inhibited when HepG2 cells were co-injected with MSCs into nude mice. These results suggested that MSCs inhibited the growth and promoted the apoptosis of HepG2 cells in a dose-dependent manner. This study provided a new approach and experimental basis for cancer therapy. This study also proved that the Wnt signaling pathway may have a function in MSC-mediated tumor cell inhibition.










Similar content being viewed by others
References
Molloy AP, Martin FT, Dwyer RM, Griffin TP, Murphy M, Barry FP, et al. Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, and their potential role in mediating interactions with breast cancer cells. Int J Cancer. 2009;124(2):326–32.
Djouad F, Bouffi C, Ghannam S, Noel D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5(7):392–9.
Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203(5):1235–47.
Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, Mohty M, et al. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45(2):180–92.
Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–7.
Maestroni GJ, Hertens E, Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 1999;55(4):663–7.
Deng W, Han Q, Liao L, You S, Deng H, Zhao RC. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol. 2005;24(7):458–63.
Pisati F, Belicchi M, Acerbi F, Marchesi C, Giussani C, Gavina M, et al. Effect of human skin-derived stem cells on vessel architecture, tumor growth, and tumor invasion in brain tumor animal models. Cancer Res. 2007;67(7):3054–63.
Chen X, Lin X, Zhao J, Shi W, Zhang H, Wang Y, et al. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol Ther. 2008;16(4):749–56.
Kang SG, Jeun SS, Lim JY, Yoo DS, Huh PW, Cho KS, et al. Cytotoxicity of rat marrow stromal cells against malignant glioma cells. Childs Nerv Syst. 2005;21(7):528–38.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11.
Menon LG, Picinich S, Koneru R, Gao H, Lin SY, Koneru M, et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25(2):520–8.
Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20(11):1394–404.
Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50.
Raida M, Heymann AC, Gunther C, Niederwieser D. Role of bone morphogenetic protein 2 in the crosstalk between endothelial progenitor cells and mesenchymal stem cells. Int J Mol Med. 2006;18(4):735–9.
Miele L, Miao H, Nickoloff BJ. NOTCH signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 2006;6(4):313–23.
Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281(46):35081–7.
Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–6.
Gregory CA, Singh H, Perry AS, Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278(30):28067–78.
Gaspar C, Fodde R. APC dosage effects in tumorigenesis and stem cell differentiation. Int J Dev Biol. 2004;48(5–6):377–86.
Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101(6):1345–56.
Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M, Ries C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24(8):1892–903.
Yang F, Zeng Q, Yu G, Li S, Wang CY. Wnt/beta-catenin signaling inhibits death receptor-mediated apoptosis and promotes invasive growth of HNSCC. Cell Signal. 2006;18(5):679–87.
Baek SH, Kioussi C, Briata P, Wang D, Nguyen HD, Ohgi KA, et al. Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway. Proc Natl Acad Sci U S A. 2003;100(6):3245–50.
Muncan V, Sansom OJ, Tertoolen L, Phesse TJ, Begthel H, Sancho E, et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol Cell Biol. 2006;26(22):8418–26.
Joubert A, Bianchi P, Maritz C, Joubert F. Influence of prostaglandin A2 on Bax, Bcl-2 and PCNA expression in MCF-7 cells. Biomed Res. 2006;27(4):157–62.
Yang B, Guo H, Zhang Y, Chen L, Ying D, Dong S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS ONE. 2011;6(7):e21679.
Dwyer RM, Khan S, Barry FP, O'Brien T, Kerin MJ. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res Ther. 2010;1(3):25.
Itakura S, Asari S, Rawson J, Ito T, Todorov I, Liu CP, et al. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant. 2007;7(2):336–46.
Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21(2):304–10.
Ohlsson LB, Varas L, Kjellman C, Edvardsen K, Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol. 2003;75(3):248–55.
Wang J, Shou J, Chen X. Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene. 2000;19(14):1843–8.
Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269(1):67–77.
Zhang L, Xiang J, Li G. The uncertain role of unmodified mesenchymal stem cells in tumor progression: what master switch? Stem Cell Res Ther. 2013;4(2):22.
Shah K. Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev. 2012;64(8):739–48.
Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309(1):232–40.
Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30(4):589–97.
Serakinci N, Christensen R, Fahrioglu U, Sorensen FB, Dagnaes-Hansen F, Hajek M, et al. Mesenchymal stem cells as therapeutic delivery vehicles targeting tumor stroma. Cancer Biother Radiopharm. 2011;26(6):767–73.
Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113(18):4197–205.
Acknowledgment
This work was supported by the Fundamental Research Funds for the Central Universities (2011JBM294), the National Natural Science Foundation of China (81201762), Foundation of State Key Laboratory Cultivation Base for the Chemistry and Molecular Engineering of Medicinal Resources, Ministry of Science and Technology of China (CMEMR2012-B07), and the National Basic Research Program of China (973 Program 2009CB521704). The authors thank Dr. Juan Du and Dr. Honggang Hu for critical reading of the manuscript.
Conflicts of Interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lingling Hou and Xiaoyu Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Apoptosis morphology of HepG2 induced by MSCs. (A) Cell morphology of the MSC:HepG2=5:1 group. HepG2 cell volume was reduced, cytoplasm density was increased, nuclear membrane and nucleolus were fragmented, and membrane structure was still intact, as indicated by an arrow. (B) Cell morphology of the MSC:HepG2=1:1 group, a small number of HepG2 cells showed visible cytoplasm agglutination and became round, as indicated by an arrow. (C) Cell morphology of the MSC:HepG2=1:5 group, apoptosis of HepG2 cells was not obvious, most cells were still in normal shape. (200×) (JPEG 32 kb)
Supplementary Fig. 2
mRNA expression levels of Bcl-2, c-Myc, β-catenin, and survivin in HepG2 cells treated with MSC-conditioned media at different treatment times. Lane 1, HepG2 control group at 24 h; Lane 2, HepG2 cells were treated with 80 % MSC-conditioned media for 24 h; Lane 3, HepG2 cells were treated with 80 % HEK 293-conditioned media for 24 h; Lane 4, HepG2 control group at 48 h; Lane 5, HepG2 cells were treated with 80 % MSC-conditioned media for 48 h; Lane 6, HepG2 cells were treated with 80 % HEK 293-conditioned media for 48 h; Lane 7, HepG2 control group at 72 h; Lane 8, HepG2 cells were treated with 80 % MSC-conditioned media for 72 h; and Lane 9, HepG2 cells were treated with 80 % HEK 293-conditioned media for 72 h (JPEG 18 kb)
Supplementary Fig. 3
mRNA expression levels of bcl-2, c-Myc, β-catenin, and survivin in HepG2 cells co-cultured with MSCs. (A) HepG2:MSC=5:1 co-culture groups; (B) HepG2:MSC=1:1 co-culture groups; (C) HepG2:MSC=1:5 co-culture groups; Lane 1, HepG2 control group at 24 h; Lane 2, HepG2 cells were co-cultured with MSCs for 24 h; Lane 3, HepG2 cells were co-cultured with HEK 293 cells for 24 h; Lane 4, HepG2 control group at 48 h; Lane 5, HepG2 cells were co-cultured with MSCs for 48 h; Lane 6, HepG2 cells were co-cultured with HEK 293 cells for 48 h; Lane 7, HepG2 control group at 72 h; Lane 8, HepG2 cells were co-cultured with MSCs for 72 h; and Lane 9, HepG2 cells were co-cultured with HEK 293 cells for 72 h (JPEG 55 kb)
Supplementary Fig. 4
Pathological examination of tumor tissues (200×). Rich and dense tumor cells were detected in the sections of the HepG2 and HEK 293 control groups (Figs. 14A, 14D, 14E, 14F, and 14H); less tumor cells and more connective tissues in the MSC section: HepG2=1:1 and MSC:HepG2=2:1 groups than the HepG2 and HEK 293 control groups (Figs. 14B and 14C) (JPEG 110 kb)
Rights and permissions
About this article
Cite this article
Hou, L., Wang, X., Zhou, Y. et al. Inhibitory effect and mechanism of mesenchymal stem cells on liver cancer cells. Tumor Biol. 35, 1239–1250 (2014). https://doi.org/10.1007/s13277-013-1165-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1165-5