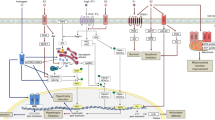
Abstract
Sex differences are evident in the pathophysiology of heart failure (HF). Progression of HF is promoted by cardiac fibrosis and no fibrosis-specific therapies are currently available. The fibrotic response is mediated by cardiac fibroblasts (CFs), and a central event is their phenotypic transition to pro-fibrotic myofibroblasts. These myofibroblasts may arise from various cellular origins including resident CFs and epicardial and endothelial cells. Both female subjects in clinical studies and female animals in experimental studies generally present less cardiac fibrosis compared with males. This difference is at least partially considered attributable to the ovarian hormone 17β-estradiol (E2). E2 signals via estrogen receptors to regulate genes are involved in the fibrotic response and myofibroblast transition. Besides protein-coding genes, E2 also regulates transcription of microRNA that modulate cardiac fibrosis. Sex dimorphism, E2, and miRNAs form multi-level regulatory networks in the pathophysiology of cardiac fibrosis, and the mechanism of these networks is not yet fully deciphered. Therefore, this review is aimed at summarizing current knowledge on sex differences, E2, and estrogen receptors in cardiac fibrosis, emphasizing on microRNAs and myofibroblast origins.
Key messages
• E2 and ERs regulate cardiac fibroblast function.
• E2 and ERs may distinctly affect male and female cardiac fibrosis pathophysiology.
• Sex, E2, and miRNAs form multi-level regulatory networks in cardiac fibrosis.
• Sex-dimorphic and E2-regulated miRNAs affect mesenchymal transition.


Similar content being viewed by others
References
A. Aimo et al., “Sex-related differences in chronic heart failure,” Int J Cardiol., vol. 255, pp. 145–151, maart 2018
Parashar S, Katz R, Smith NL, Arnold AM, Vaccarino V, Wenger NK, Gottdiener JS (2009) Race, gender, and mortality in adults > or =65 years of age with incident heart failure (from the Cardiovascular Health Study). Am J Cardiol 103(8):1120–1127
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB (2016) Executive summary: heart disease and stroke statistics—2016 update. Circulation 133(4):447–454
Appiah D, Schreiner PJ, Demerath EW, Loehr LR, Chang PP, Folsom AR (2016) Association of age at menopause with incident heart failure: a prospective cohort study and meta-analysis. J Am Heart Assoc 5(8):e003769
Zhao D, Guallar E, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Lima JA, Allison MA, Shah SJ, Bertoni AG, Budoff MJ, Post WS, Michos ED (2018) Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol 71(22):2555–2566
Frangogiannis NG (2019) The extracellular matrix in ischemic and nonischemic heart failure. Circ Res 125(1):117–146
Aoki T, Fukumoto Y, Sugimura K, Oikawa M, Satoh K, Nakano M, Nakayama M, Shimokawa H (2011) Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure. Circ J 75(11):2605–2613
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC (2016) Cardiac fibrosis: the fibroblast awakens. Circ Res 118(6):1021–1040
Deb A, Ubil E (2014) Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol 70:47–55
Davis J, Molkentin JD (2014) Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol 70:9–18
Dworatzek E, Baczko I, Kararigas G (2016) Effects of aging on cardiac extracellular matrix in men and women. Proteomics Clin Appl 10(1):84–91
Ambale Venkatesh B et al (2014) Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: the Multi-Ethnic Study of Atherosclerosis study. Hypertens Dallas Tex 1979 64(3):508–515
Treibel TA et al (2018) Sex dimorphism in the myocardial response to aortic stenosis. JACC Cardiovasc Imaging 11(7):962–973
Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, Knosalla C, Golz S, Hetzer R, Regitz-Zagrosek V (2014) Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail 16(11):1160–1167
Villari B, Campbell SE, Schneider J, Vassalli G, Chiariello M, Hess OM (1995) Sex-dependent differences in left ventricular function and structure in chronic pressure overload. Eur Heart J 16(10):1410–1419
Petrov G et al (2014) Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc Imaging 7(11):1073–1080
Petrov G, Regitz-Zagrosek V, Lehmkuhl E, Krabatsch T, Dunkel A, Dandel M, Dworatzek E, Mahmoodzadeh S, Schubert C, Becher E, Hampl H, Hetzer R (2010) Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation 122(11 Suppl):S23–S28
Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ (2000) Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart 84(5):476–482
Xu Y, Arenas IA, Armstrong SJ, Davidge ST (2003) Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res 57(2):388–394
Kang S et al (2012) Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PLoS One 7(10):e48185
Pedram A, Razandi M, Korach KS, Narayanan R, Dalton JT, Levin ER (2013) ERβ selective agonist inhibits angiotensin-induced cardiovascular pathology in female mice. Endocrinology 154(11):4352–4364
Pedram A, Razandi M, O’Mahony F, Lubahn D, Levin ER (2010) Estrogen receptor-β prevents cardiac fibrosis. Mol Endocrinol 24(11):2152–2165
Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER (2008) Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-β to inhibit calcineurin. Endocrinology 149(7):3361–3369
Shenoy V, Grobe JL, Qi Y, Ferreira AJ, Fraga-Silva RA, Collamat G, Bruce E, Katovich MJ (2009) 17β-Estradiol modulates local cardiac renin-angiotensin system to prevent cardiac remodeling in the DOCA-salt model of hypertension in rats. Peptides 30(12):2309–2315
Dennis G et al (2011) Estrogen receptor-β signals left ventricular hypertrophy sex differences in normotensive deoxycorticosterone acetate-salt mice. Hypertension 57(3):648–654
Jessup JA et al (2013) Estrogen therapy, independent of timing, improves cardiac structure and function in oophorectomized mRen2.Lewis rats. Menopause N Y N 20(8):860–868
Jessup JA et al (2011) Neuronal nitric oxide synthase inhibition improves diastolic function and reduces oxidative stress in ovariectomized mRen2.Lewis rats. Menopause N Y N 18(6):698–708
Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L (2012) Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc Res 94(1):96–104
da Silva JS et al (2017) Blunting of cardioprotective actions of estrogen in female rodent heart linked to altered expression of cardiac tissue chymase and ACE2. J Renin-Angiotensin-Aldosterone Syst 18(3):1470320317722270
van Eickels M, Grohe C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA (2001) 17β-Estradiol attenuates the development of pressure-overload hypertrophy. Circulation 104(12):1419–1423
E. Dworatzek et al., 2018 “Sex-specific regulation of collagen I and III expression by 17β-estradiol in cardiac fibroblasts: role of estrogen receptors,” Cardiovasc. Res.
Westphal C, Schubert C, Prelle K, Penkalla A, Fliegner D, Petrov G, Regitz-Zagrosek V (2012) Effects of estrogen, an ERα agonist and raloxifene on pressure overload induced cardiac hypertrophy. PLoS One 7(12):e50802–e50802
Iorga A et al (2016) Rescue of pressure overload-induced heart failure by estrogen therapy. J Am Heart Assoc 5(1)
Iorga A et al (2018) Estrogen rescues heart failure through estrogen receptor beta activation. Biol Sex Differ 9(1)
Wang X, Tan Y, Xu B, Lu L, Zhao M, Ma J, Liang H, Liu J, Yu S (2018) GPR30 attenuates myocardial fibrosis in diabetic ovariectomized female rats: role of iNOS signaling. DNA Cell Biol 37(10):821–830
Menazza S, Murphy E (2016) The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res 118(6):994–1007
Johannes N et al (2004) Upregulation of myocardial estrogen receptors in human aortic stenosis. Circulation 110(20):3270–3275
Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, Weiske J, Pregla R, Hetzer R, Regitz-Zagrosek V (2006) Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB J 20(7):926–934
Klinge CM (2009) Estrogen regulation of microRNA expression. Curr Genomics 10(3):169–183
Creemers EE, van Rooij E (2016) Function and therapeutic potential of noncoding RNAs in cardiac fibrosis. Circ Res 118(1):108–118
Biernacka A, Frangogiannis NG (2011) Aging and cardiac fibrosis. Aging Dis 2(2):158–173
Anastasios L, Giuseppe R, Koch WJ (2013) Adrenergic nervous system in heart failure. Circ Res 113(6):739–753
Zhu B, Liu K, Yang C, Qiao Y, Li Z (2016) Gender-related differences in β-adrenergic receptor-mediated cardiac remodeling. Can J Physiol Pharmacol 94(12):1349–1355
Zhu Y-C, Zhu Y-Z, Lu N, Wang M-J, Wang Y-X, Yao T (2003) Role of angiotensin AT1 and AT2 receptors in cardiac hypertrophy and cardiac remodelling. Clin Exp Pharmacol Physiol 30(12):911–918
Chaney E, Shaw A (2010) Pathophysiology of fluid retention in heart failure. Contrib Nephrol 164:46–53
Basting T, Lazartigues E (2017) DOCA-salt hypertension: an update. Curr Hypertens Rep 19(4):32
Díez J (2007) Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens 9(7):546–550
Aysun K et al (2008) Deoxycorticosterone acetate-salt mice exhibit blood pressure–independent sexual dimorphism. Hypertension 51(4):1177–1183
Jessup JA, Zhang L, Presley TD, Kim-Shapiro DB, Wang H, Chen AF, Groban L (2011) Tetrahydrobiopterin restores diastolic function and attenuates superoxide production in ovariectomized mRen2.Lewis rats. Endocrinology 152(6):2428–2436
Groban L, Yamaleyeva LM, Westwood BM, Houle TT, Lin M, Kitzman DW, Chappell MC (2008) Progressive diastolic dysfunction in the female mRen(2).Lewis rat: influence of salt and ovarian hormones. J Gerontol Ser A 63(1):3–11
Carabello BA (2013) Introduction to aortic stenosis. Circ Res 113(2):179–185
Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Noppinger PR, Kintscher U, Gustafsson JÅ, Regitz-Zagrosek V (2010) Female sex and estrogen receptor-β attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol-Regul Integr Comp Physiol 298(6):R1597–R1606
Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E (2005) Estrogen receptor-β mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol-Heart Circ Physiol 288(2):H469–H476
Russo I, Frangogiannis NG (2016) Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 90:84–93
Chao H-H, Chen JJ, Chen CH, Lin H, Cheng CF, Lian WS, Chen YL, Juan SH, Liu JC, Liou JY, Chan P, Cheng TH (2005) Inhibition of angiotensin II induced endothelin-1 gene expression by 17- -oestradiol in rat cardiac fibroblasts. Heart 91(5):664–669
Wu M, Han M, Li J, Xu X, Li T, Que L, Ha T, Li C, Chen Q, Li Y (2009) 17β-Estradiol inhibits angiotensin II-induced cardiac myofibroblast differentiation. Eur J Pharmacol 616(1):155–159
Stewart JA Jr, Cashatt DO, Borck AC, Brown JE, Carver WE (2006) 17β-Estradiol modulation of angiotensin II-stimulated response in cardiac fibroblasts. J Mol Cell Cardiol 41(1):97–107
Zhou L, Shao Y, Huang Y, Yao T, Lu L-M (2007) 17β-Estradiol inhibits angiotensin II-induced collagen synthesis of cultured rat cardiac fibroblasts via modulating angiotensin II receptors. Eur J Pharmacol 567(3):186–192
Pedram A, Razandi M, Narayanan R, Levin ER (2016) Estrogen receptor beta signals to inhibition of cardiac fibrosis. Mol Cell Endocrinol 434:57–68
Wang H, Zhao Z, Lin M, Groban L (2015) Activation of GPR30 inhibits cardiac fibroblast proliferation. Mol Cell Biochem 405(1–2):135–148
Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V (2010) 17β-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res 85(4):719–728
Lee HW, Eghbali-Webb M (1998) Estrogen enhances proliferative capacity of cardiac fibroblasts by estrogen receptor- and mitogen-activated protein kinase-dependent pathways. J Mol Cell Cardiol 30(7):1359–1368
Mercier I, Colombo F, Mader S, Calderone A (2002) Ovarian hormones induce TGF-beta(3) and fibronectin mRNAs but exhibit a disparate action on cardiac fibroblast proliferation. Cardiovasc Res 53(3):728–739
Dworatzek E, Mahmoodzadeh S (2017) Targeted basic research to highlight the role of estrogen and estrogen receptors in the cardiovascular system. Pharmacol Res 119:27–35
Florijn BW, Bijkerk R, van der Veer EP, van Zonneveld AJ (2018) Gender and cardiovascular disease: are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovasc Res 114(2):210–225
Tsuji M, Kawasaki T, Matsuda T, Arai T, Gojo S, Takeuchi JK (2017) Sexual dimorphisms of mRNA and miRNA in human/murine heart disease. PLoS One 12(7):e0177988–e0177988
Harrington J et al (2017) A systems biology approach to investigating sex differences in cardiac hypertrophy. J Am Heart Assoc 6(8):e005838
Queirós AM, Eschen C, Fliegner D, Kararigas G, Dworatzek E, Westphal C, Sanchez Ruderisch H, Regitz-Zagrosek V (2013) Sex- and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. Int J Cardiol 169(5):331–338
Brønnum H et al (2013) miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving programmed cell death 4 and sprouty-1. PLoS One 8(2):e56280
Regalla K, Ingo V, Virginija J, Seema D, Da-Hee P, Thomas T (2012) Transforming growth factor-β–induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol 32(2):361–369
Sun L-Y, Wang N, Ban T, Sun YH, Han Y, Sun LL, Yan Y, Kang XH, Chen S, Sun LH, Zhang R, Zhao YJ, Zhang H, Ai J, Yang BF (2014) MicroRNA-23a mediates mitochondrial compromise in estrogen deficiency-induced concentric remodeling via targeting PGC-1α. J Mol Cell Cardiol 75:1–11
Wang N, Sun LY, Zhang SC, Wei R, Xie F, Liu J, Yan Y, Duan MJ, Sun LL, Sun YH, Niu HF, Zhang R, Ai J (2015) MicroRNA-23a participates in estrogen deficiency induced gap junction remodeling of rats by targeting GJA1. Int J Biol Sci 11(4):390–403
Papadimitriou E et al (2011) Differential regulation of the two RhoA-specific GEF isoforms Net1/Net1A by TGF-β and miR-24: role in epithelial-to-mesenchymal transition. Oncogene 31:2862
Zhang G, Tian X, Li Y, Wang Z, Li X, Zhu C (2018) miR-27b and miR-34a enhance docetaxel sensitivity of prostate cancer cells through inhibiting epithelial-to-mesenchymal transition by targeting ZEB1. Biomed Pharmacother Biomedecine Pharmacother 97:736–744
Lum-Naihe K, Toedebusch R, Mahmood A, Bajwa J, Carmack T, Kumar SA, Ardhanari S, DeMarco VG, Emter CA, Pulakat L (2017) Cardiovascular disease progression in female Zucker diabetic fatty rats occurs via unique mechanisms compared to males. Sci Rep 7(1):17823–17823
Jourkesh M, Soori R, Earnest CP, Mirheidari L, Ravasi AA, Stannard SR, Monsalves-Alvarez M (2018) Effects of six weeks of resistance-endurance training on microRNA-29 expression in the heart of ovariectomised rats. Przeglad Menopauzalny Menopause Rev 17(4):155–160
Habibi P, Alihemmati A, Nasirzadeh M, Yousefi H, Habibi M, Ahmadiasl N (2016) Involvement of microRNA-133 and -29 in cardiac disturbances in diabetic ovariectomized rats. Iran J Basic Med Sci 19(11):1177–1185
Li J et al (2015) MicroRNA-29b inhibits endometrial fibrosis by regulating the Sp1-TGF-β1/Smad-CTGF axis in a rat model. Reprod Sci 23(3):386–394
Hu H, Hu S, Xu S, Gao Y, Zeng F, Shui H (2018) miR-29b regulates Ang II-induced EMT of rat renal tubular epithelial cells via targeting PI3K/AKT signaling pathway. Int J Mol Med 42(1):453–460
Bernardo BC, Ooi JYY, Matsumoto A, Tham YK, Singla S, Kiriazis H, Patterson NL, Sadoshima J, Obad S, Lin RCY, McMullen JR (2016) Sex differences in response to miRNA-34a therapy in mouse models of cardiac disease: identification of sex-, disease- and treatment-regulated miRNAs. J Physiol 594(20):5959–5974
Y. Liu et al., 2019 “MicroRNA-34a promotes renal fibrosis by downregulation of Klotho in tubular epithelial cells,” Mol. Ther., Feb.
Song L et al (2019) Pterostilbene prevents hepatocyte EMT in fructose-induced liver fibrosis through suppressing miR-34a/Sirt1/p53 and TGF-β1/Smads signaling. Br J Pharmacol 0(ja)
Tang Y, Tang Y, Cheng Y (2017) miR-34a inhibits pancreatic cancer progression through Snail1-mediated epithelial–mesenchymal transition and the Notch signaling pathway. Sci Rep 7:38232
Kang J, Kim EG, Kim W, Seong KM, Youn HS, Kim JW, Kim J, Youn BH (2013) Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. J Biol Chem 288(38):27343–27357
Chen K-HE, Bustamante K, Nguyen V, Walker AM (2017) Involvement of miR-106b in tumorigenic actions of both prolactin and estradiol. Oncotarget 8(22):36368–36382
Habibi P, Alihemmati A, NourAzar A, Yousefi H, Mortazavi S, Ahmadiasl N (2016) Expression of the Mir-133 and Bcl-2 could be affected by swimming training in the heart of ovariectomized rats. Iran J Basic Med Sci 19(4):381–387
Muraoka N et al (2014) MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J 33(14):1565
Chen X-B, Li W, Chu A-X (2019) MicroRNA-133a inhibits gastric cancer cells growth, migration, and epithelial-mesenchymal transition process by targeting presenilin 1. J Cell Biochem 120(1):470–480
Liu A, Shao C, Jin G, Liu R, Hao J, Song B, Ouyang L, Hu X (2014) miR-208-induced epithelial to mesenchymal transition of pancreatic cancer cells promotes cell metastasis and invasion. Cell Biochem Biophys 69(2):341–346
van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 105(35):13027–13032
Ioannis K et al Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload–induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc 2(2):e000078
Tijsen AJ, van der Made I, van den Hoogenhof MM, Wijnen WJ, van Deel ED, de Groot NE, Alekseev S, Fluiter K, Schroen B, Goumans MJ, van der Velden J, Duncker DJ, Pinto YM, Creemers EE (2014) The microRNA-15 family inhibits the TGFβ-pathway in the heart. Cardiovasc Res 104(1):61–71
Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E (2011) Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 124(14):1537–1547
Sanchez-Ruderisch H, Queirós AM, Fliegner D, Eschen C, Kararigas G, Regitz-Zagrosek V (2019) Sex-specific regulation of cardiac microRNAs targeting mitochondrial proteins in pressure overload. Biol Sex Differ 10(1):8–8
Pérez-Cremades D, Mompeón A, Vidal-Gómez X, Hermenegildo C, Novella S (2018) Role of miRNA in the regulatory mechanisms of estrogens in cardiovascular ageing. Oxidative Med Cell Longev 2018:1–16
Zhang Y, Huang X-R, Wei L-H, Chung AC, Yu C-M, Lan H-Y (2014) miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol Ther J Am Soc Gene Ther 22(5):974–985
Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE (2009) miR-133 and miR-30 regulate connective tissue growth factor. Circ Res 104(2):170–178
von Gise A, Pu William T (2012) Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res 110(12):1628–1645
Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, Tomic-Canic M (2016) Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 365(3):495–506
Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM (2014) Resident fibroblast lineages mediate pressure overload–induced cardiac fibrosis. J Clin Invest 124(7):2921–2934
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13:952–961
Russell JL, Goetsch SC, Gaiano NR, Hill JA, Olson EN, Schneider JW (2011) A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res 108(1):51–59
Zou M, Wang F, Gao R, Wu J, Ou Y, Chen X, Wang T, Zhou X, Zhu W, Li P, Qi LW, Jiang T, Wang W, Li C, Chen J, He Q, Chen Y (2016) Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-β R II during TGF-β1-induced fibrogenesis in human cardiac fibroblasts. Sci Rep 6:24747
Feng B, Cao Y, Chen S, Chu X, Chu Y, Chakrabarti S (2016) miR-200b mediates endothelial-to-mesenchymal transition in diabetic cardiomyopathy. Diabetes 65(3):768
Liang C, Gao L, Liu Y, Liu Y, Yao R, Li Y, Xiao L, Wu L, du B, Huang Z, Zhang Y (2019) MiR-451 antagonist protects against cardiac fibrosis in streptozotocin-induced diabetic mouse heart. Life Sci 224:12–22
Geng H, Guan J (Sep. 2017) MiR-18a-5p inhibits endothelial–mesenchymal transition and cardiac fibrosis through the Notch2 pathway. Biochem Biophys Res Commun 491(2):329–336
Guttilla IK, Adams BD, White BA (2012) ERα, microRNAs, and the epithelial–mesenchymal transition in breast cancer. Trends Endocrinol Metab 23(2):73–82
Kent CN, Guttilla Reed IK (2016) Regulation of epithelial–mesenchymal transition in endometrial cancer: connecting PI3K, estrogen signaling, and microRNAs. Clin Transl Oncol 18(11):1056–1061
De Francesco EM, Maggiolini M, Musti AM (2018) Crosstalk between Notch, HIF-1α and GPER in breast cancer EMT. Int J Mol Sci 19(7):2011
Lobo RA (2016) Hormone-replacement therapy: current thinking. Nat Rev Endocrinol 13:220
Chung C-C, Kao Y-H, Chen Y-J, Chen Y-J (2013) Androgen modulates cardiac fibrosis contributing to gender differences on heart failure. Aging Male 16(1):22–27
Wang Y, Ma W, Lu S, Yan LH, Hu F, Wang ZH, Cheng B (2018) Androgen receptor regulates cardiac fibrosis in mice with experimental autoimmune myocarditis by increasing microRNA-125b expression. Biochem Biophys Res Commun 506(1):130–136
Drummond CA et al (2013) Gender differences in the development of uremic cardiomyopathy following partial nephrectomy: role of progesterone. J Hypertens Open Access 2. https://doi.org/10.4172/2167-1095.1000109
Dubey RK, Gillespie DG, Jackson EK, Keller PJ (1998) 17β-Estradiol, its metabolites, and progesterone inhibit cardiac fibroblast growth. Hypertension 31(1):522–528
Chung C-C, Hsu R-C, Kao Y-H, Liou J-P, Lu Y-Y, Chen Y-J (2014) Androgen attenuates cardiac fibroblasts activations through modulations of transforming growth factor-β and angiotensin II signaling. Int J Cardiol 176(2):386–393
Funding
This work was supported by National Institutes of Health grant R01HL131182 (ME).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Medzikovic, L., Aryan, L. & Eghbali, M. Connecting sex differences, estrogen signaling, and microRNAs in cardiac fibrosis. J Mol Med 97, 1385–1398 (2019). https://doi.org/10.1007/s00109-019-01833-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01833-6



