Abstract
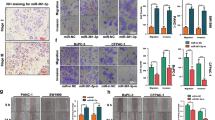
The aim of this study was to investigate the role of miR-208 in the invasion and metastasis of pancreatic cancer cells and the underlying molecular mechanism. miR-208 mimic, miR-208 inhibitor and NC were transfected into pancreatic cancer cell line Bxpc3 using liposome. Transwell invasion and scratch assays were used to test cell migratory and invasive abilities. Western blotting and quantitative PCR methods were used to detect E-cadherin, fibronectin and vimentin protein and mRNA expression in pancreatic cancer cell line BxPC3 after transfection by miR-208 mimic, miR-208 inhibitor and NC. Transwell invasion and scratch assays showed that after overexpressing miR-208, pancreatic cancer cell line BxPC3 exhibited enhanced in vitro migratory and invasive abilities, while after downregulating miR-208 expression, cell migratory and invasive abilities were decreased. Western blotting and quantitative PCR showed that after overexpressing miR-208, expression of E-cadherin, an epithelial cell marker, was decreased and expression of fibronectin and vimentin, interstitial cell markers, was increased in pancreatic cancer cell line BxPC3; however, after inhibiting miR-208, increased E-cadherin expression and decreased fibronectin and vimentin expression were observed in pancreatic cancer cell line BxPC3. After overexpressing miR-208, p-AKT and p-GSK-3β expression was altered by activating AKT/GSK-3β/snail signaling pathway. miR-208 induces epithelial to mesenchymal transition of pancreatic cancer cell line BxPC3 by activating AKT/GSK-3β/snail signaling pathway and thereby promotes cell metastasis and invasion.




Similar content being viewed by others
References
Maitra, A., & Hruban, R. H. (2008). Pancreatic cancer. Annual Review of Pathology, 3, 157–188.
Hidalgo, M. (2010). Pancreatic cancer. New England Journal of Medicine, 362(17), 1605–1617.
Cai, X., et al. (2006). Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathogens, 2(3), e23.
Bartel, D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116(2), 281–297.
Wu, L., Fan, J., & Belasco, J. G. (2006). MicroRNAs direct rapid deadenylation of mRNA. Proceedings of the National Academy of Sciences of the United States of America, 103(11), 4034–4039.
Llave, C., et al. (2002). Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science, 297(5589), 2053–2056.
Cui, Q., et al. (2006). Principles of microRNA regulation of a human cellular signaling network. Molecular Biology Organization, 2, 46.
Calin, G. A., et al. (2002). Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences USA, 99(24), 15524–15529.
Michael, M. Z., et al. (2003). Reduced accumulation of specific microRNAs in colorectal neoplasia. Molecular Cancer Research, 1(12), 882–891.
Johnson, S. M., et al. (2005). RAS Is Regulated by the let-7 MicroRNA Family. Cell, 120(5), 635–647.
Zhang, Y., et al. (2011). Insulin promotes vascular smooth muscle cell proliferation via microRNA-208-mediated downregulation of p21. Journal of Hypertension, 29(8), 1560–1568.
Xin, M., Olson, E. N., & Bassel-Duby, R. (2013). Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nature Reviews Molecular Cell Biology, 14(8), 529–541.
Itoh, T., Takeda, S., & Akao, Y. (2010). MicroRNA-208 modulates BMP-2-stimulated mouse preosteoblast differentiation by directly targeting V-ets erythroblastosis virus E26 oncogene homolog 1. Journal of Biological Chemistry, 285(36), 27745–27752.
Jin, J. C., et al. (2013). Effect of OSW-1 on microRNA expression profiles of hepatoma cells and functions of novel microRNAs. Molecular Medicine Reports, 7(6), 1831–1837.
Meyer, T., & Hart, I. (1998). Mechanisms of tumour metastasis. European Journal of Cancer, 34(2), 8.
Woodhouse, E. C., Chuaqui, R. F., & Liotta, L. A. (1997). General mechanisms of metastasis. Cancer, 80(8 Suppl), 1529–1537.
Horikawa, T., et al. (2011). Epstein-Barr Virus latent membrane protein 1 induces Snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. British Journal of Cancer, 104(7), 1160–1167.
Thiery, J. P. (2002). Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer, 2(6), 442–454.
Lee, J. M., et al. (2006). The epithelial-mesenchymal transition: new insights in signaling, development, and disease. Journal of Cell Biology, 172(7), 973–981.
Cano, A., et al. (2000). The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biology, 2(2), 76–83.
Nieto, M. A. (2002). The snail superfamily of zinc-finger transcription factors. Nature Reviews Molecular Cell Biology, 3(3), 155–166.
Eger, A., et al. (2005). DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast. Cancer cells, 24(14), 2375–2385.
Huber, M. A., Kraut, N., & Beug, H. (2005). Molecular requirements for epithelial–mesenchymal transition during tumor progression. Current Opinion in Cell Biology, 17(5), 548–558.
Perez-Moreno, M. A., et al. (2001). A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. Journal of Biological Chemistry, 276(29), 27424–27431.
Chang, F., et al. (2003). Involvement of PI3 K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia, 17(3), 590–603.
Yuan, Z. Q., et al. (2000). Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene, 19(19), 2324–2330.
Tanno, S., et al. (2001). AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Research, 61(2), 589–593.
Grille, S. J., et al. (2003). The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Research, 63(9), 2172–2178.
Lester, R. D., et al. (2007). uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. Journal of Cell Biology, 178(3), 425–436.
Julien, S., et al. (2007). Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene, 26(53), 7445–7456.
Wang, X., et al. (2007). NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell, 128(1), 129–139.
Larue, L., & Bellacosa, A. (2005). Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene, 24(50), 7443–7454.
Muraoka, R. S., et al. (2002). Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. Journal of Clinical Investigation, 109(12), 1551–1559.
Bakin, A. V., et al. (2000). Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. Journal of Biological Chemistry, 275(47), 36803–36810.
Bachelder, R. E., et al. (2005). Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. Journal of Cell Biology, 168(1), 29–33.
Zhou, B. P., et al. (2004). Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nature Cell Biology, 6(10), 931–940.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, A., Shao, C., Jin, G. et al. miR-208-Induced Epithelial to Mesenchymal Transition of Pancreatic Cancer Cells Promotes Cell Metastasis and Invasion. Cell Biochem Biophys 69, 341–346 (2014). https://doi.org/10.1007/s12013-013-9805-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9805-3