Abstract
Purpose
What are reported definitions of HAP in trauma patient research?
Methods
A systematic review was performed using the PubMed/MEDLINE database. We included all English, Dutch, and German original research papers in adult trauma patients reporting diagnostic criteria for hospital-acquired pneumonia diagnosis. The risk of bias was assessed using the MINORS criteria.
Results
Forty-six out of 5749 non-duplicate studies were included. Forty-seven unique criteria were reported and divided into five categories: clinical, laboratory, microbiological, radiologic, and miscellaneous. Eighteen studies used 33 unique guideline criteria; 28 studies used 36 unique non-guideline criteria.
Conclusion
Clinical criteria for diagnosing HAP—both guideline and non-guideline—are widespread with no clear consensus, leading to restrictions in adequately comparing the available literature on HAP in trauma patients. Studies should at least report how a diagnosis was made, but preferably, they would use pre-defined guideline criteria for pneumonia diagnosis in a research setting. Ideally, one internationally accepted set of criteria is used to diagnose hospital-acquired pneumonia.
Level of evidence
Level III.
Similar content being viewed by others
Background
Nosocomial pneumonia is among the most frequent complications in trauma patients and is associated with increased mortality and poor prognosis [1,2,3]. The incidence of nosocomial pneumonia ranges from 4.3 to 38.3% in the literature, and this wide variety may cast doubt on the individual studies’ comparability [4, 5].
Several types of nosocomial pneumonia have been described in the literature [6]. Most guidelines on nosocomial pneumonia create a distinction between hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) [7,8,9]. Although VAP essentially is a particular type of HAP, the etiology is not the same. In VAP, endotracheal intubation enables upper respiratory tract colonization by inserting a foreign body; therefore, the two pneumonia types should not be considered equivalent [10]. Nonetheless, the diagnostic criteria are similar for HAP and VAP in most guidelines, though they differ in the exact duration of mechanical ventilation and the time between mechanical ventilation and pneumonia onset to distinguish VAP from HAP [7,8,9].
To diagnose hospital-acquired pneumonia, microbiologic diagnostics are superior to clinical symptoms or radiologic examination [10]. Collecting sputum or tracheal secretions has high sensitivity but low specificity, while bronchoalveolar lavage and comparable methods have both high sensitivity and specificity. However, as fluid is introduced into the lungs, bronchoalveolar lavage is generally unsuitable for non-mechanically ventilated patients and is, therefore, mainly used to diagnose VAP [11]. Thus, HAP diagnosis is reliant on clinical criteria.
The combination of varying incidence and diagnostic criteria reliance raises the question of what criteria have been previously used to diagnose HAP in trauma patient research [12, 13]. Potentially, HAP incidence varies because of the use of different diagnostic criteria. Therefore, this systematic review was conducted to create an overview of reported definitions of hospital-acquired pneumonia in trauma research.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic research and Meta-Analysis (PRISMA) checklist and registered on PROSPERO (review identification number CRD42022350131) [14].
Search strategy and execution
A literature search was performed in PubMed/MEDLINE. The search syntax was constructed to identify studies that stated a definition for pneumonia (Supplemental Table 1) from initiation to September 2019. The search syntax included the following: the MeSH terms and subheadings “Wounds and Injuries,” “Injuries,” “Pneumonia,” “Incidence,” “Prevalence,” “Risk Factors,” and “Prevention and Control”; keywords derived from the MeSH terms and subheadings; and additional keywords on trauma patients, clinical criteria, definitions, prediction, and prophylaxis. Animal studies were excluded from the syntax.
Review process
The search results were imported into Rayyan for processing [15]. Rayyan is a free online tool that helps researchers conduct systematic reviews. Studies in trauma patients with a reported definition of HAP were included, with no limitations set on the type of trauma. We excluded certain study populations (pediatric, burns, (near-)drowning, non-traumatic fractures, postmortem), other entities of pneumonia or pulmonary complications (solely as an outcome or mixed with HAP), non-original research papers, and studies in a language other than English, Dutch, or German. We assumed that all Intensive Care Unit admitted patients were at risk for VAP unless stated differently. Subsequently, we excluded studies that did not use clinical criteria to diagnose HAP but presented references to these studies separately in Supplemental Table 2.
One reviewer (TK) assessed the in- and exclusion stepwise: first, the patient population; second, the pneumonia outcome; and lastly, other remaining criteria. The same reviewer assessed the methodological quality using the MINORS criteria: a clarification of used criteria can be found in Supplemental Table 3 [16]. The possible score on the MINORS criteria ranges from 0 (lowest) to 24 (highest) for comparative studies. In non-comparative studies, 16 is the highest possible score. Any borderline cases were discussed with a second reviewer (DS) before definitive in-/exclusion or quality scoring.
For each study, the following data were obtained: first author, year of publication, study period, study design, cohort size, and the applied diagnostic criteria. All data extraction was conducted by one reviewer (TK).
Results
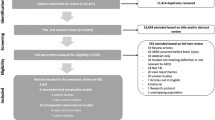
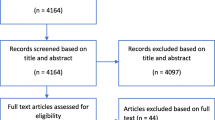
The PubMed/MEDLINE database search resulted in 5758 studies. One hundred and sixteen studies were eligible for the qualitative comparison; seventy studies (60%) did not use clinical criteria to diagnose HAP (e.g., medical records or ICD-codes; Supplemental Table 2). The remaining 46 studies were included in the qualitative analysis [12, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. The study selection process is summarized in the PRISMA flowchart (Fig. 1). The included studies were performed retrospectively (21/46) and prospectively (25/46). Table 1 shows the baseline characteristics of the included studies.
Diagnostic criteria
Forty-eight unique criteria were described in the included studies. We divided the criteria into five main categories: clinical (pulmonary symptoms and vital signs), laboratory (e.g., C-reactive protein, leukocytes), microbiologic (cultures or pathology), radiologic (X-ray or computed tomography), and miscellaneous (prescribed antibiotics and diagnosis in the medical health record). Radiologic criteria were most commonly used in the included studies (45/46) [12, 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. Clinical, laboratory, and microbiologic criteria were applied in 72, 28, and 39 percent of the included studies, respectively. Miscellaneous criteria were present in eight studies: four studies with only non-guideline criteria [12, 28, 30, 61] and as an addition to guideline criteria in the other four other studies [26, 27, 45, 48].
Guideline criteria were used to diagnose HAP in 18 out of 46 studies (Table 2). The five guidelines that were used originated from the United States of America or Europe: the Centers for Disease Control and Prevention (CDC), the European Center for Disease Prevention and Control (ECDC), the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA), the Swedish Intensive Care Registry (SIR), and the British Society for Antimicrobial Chemotherapy (BSAC). The CDC criteria were cited in 13 out of 18 studies, whereas the ATS/IDSA, ECDC, SIR, and BSAC guidelines were used in the remaining four studies. Two studies applied the criteria of two different guidelines: Djuric et al. used the CDC and ECDC guidelines, and Ewan et al. used the ATS and BSAC guidelines [23, 61]. In the studies that used guideline criteria, 33 unique criteria were observed. The remaining 28 out of 46 studies described 37 non-guideline criteria to diagnose HAP (Table 3).
A detailed overview of the used criteria in all included studies was added in Supplemental Table 4.
Methodological quality of included studies
The MINORS score for comparative studies ranged from 9 to 22 on a potential maximum score of 24. For non-comparative studies, the range was 4 to 9 out of 16. The minimum (9 vs. 4) and maximum scores (22 vs. 21) were not considerably different for studies with guideline and non-guideline criteria, respectively (Table 1; Supplemental Table 5).
Discussion
This systematic review provides a general overview of criteria utilized in trauma patient research to diagnose hospital-acquired pneumonia. In only 46 out of 5749 original studies, well-defined criteria were reported, either pre-defined by published guidelines or clear non-guideline criteria. Forty-eight unique criteria were presented and clustered into five categories: clinical, laboratory, microbiological, radiological, and miscellaneous.
In the 28 studies without pre-defined guideline criteria to diagnose HAP, 37 unique criteria were reported. The heterogeneity in the applied criteria can mainly be attributed to the vast diversity in clinical, laboratory, and microbiological thresholds. For example, when considering leukocyte count as an indicator of HAP, up to five different thresholds were reported, describing both an elevated and decreased leukocyte count as indicative of HAP. One could imagine that a lower cut-off point of leukocytosis (e.g., 10 × 109/L versus 13 × 109/L) may lead to a higher estimate of HAP cases in a research population. Similar threshold differences were observed for body temperature, including “fever” or “febrile” as subjective criteria.
Some studies cited established guidelines as a basis for diagnosis, but the authors added new criteria or deleted pre-defined criteria, thus introducing (potential) aggregate bias. For instance, four studies added “medical record documentation” or “start of antibiotic treatment” as a criterion to diagnose pneumonia in addition to guideline criteria [12, 28, 30, 61]. Also, several studies added specific criteria (e.g., hypothermia, worsening gas exchange, leukopenia, and bronchoalveolar lavage) to the ATS/IDSA criteria [12, 22, 34, 61, 64]. Though all are clinically relevant criteria, adjusting pre-defined criteria complicates the comparison of studies that use the same guidelines and increases bias.
Eighteen studies applied existing guideline criteria to diagnose HAP. However, five different guidelines were encountered, leading to a further decrease in uniformity. We encountered a similar variation in body temperature and leukocyte count cut-offs (Table 2) [7,8,9, 65,66,67]. However, the number of variations was lower for the pre-defined guideline criteria: three versus five cut-offs for body temperature and leukocytosis. The 2015 ATS/IDSA guideline contained no distinct thresholds for hyperthermia and leukocytosis, resulting in differences between studies that used this guideline (Table 2) [67]. Despite the attempts to generate uniformity in diagnosing HAP by creating and using guideline criteria, the abovementioned differences make it difficult to compare the available literature completely. Only five studies diagnosed HAP based on the exact same criteria.
Guidelines are continuously updated based on new insights and available literature. The earliest CDC guideline dates from 1988, and the most recent from 2018. During these 30 years, the criteria for pneumonia diagnosis have changed substantially. For example, the 1988 CDC criteria for pneumonia diagnosis were clinical, radiologic, or microbiologic, while body temperature was not included as a criterion [68]. However, the 2018 guideline provides a more elaborate set of clinical, radiological, microbiological, and laboratory criteria [7]. As a result, it is more difficult to compare older data sets to more recent studies. Full implementation of or compliance with guideline criteria in clinical practice is hardly feasible for two reasons: patient care and study design. Study subjects are patients; therefore, clinical examination and experience remain decisive in starting pneumonia treatment. The authors understand that an inconclusive or negative X-ray should not delay antibiotic treatment, and awaiting a microbial culture is not mandatory or necessary in seriously ill patients. Using guideline criteria for pneumonia diagnosis in retrospective studies might be impracticable. Also, uniform diagnostic criteria for pneumonia are hard to accomplish in database or registry studies. Nonetheless, these limitations should be mentioned when encountered.
Previously, review studies have been issued on lacking definitions in trauma research, such as fracture-related infections and non-unions of long bones [69,70,71]. To resolve a lack of definition, these review studies provide a basis for a consensus definition. Subsequently, Delphi method studies can be helpful in reaching consensus. Our study displays the wide variety of clinical criteria for HAP diagnosis in trauma research and exhibits how studies ought to be compared with caution. The comparison of results is essential in trauma patient research and for guidelines. Guideline issuers pursue workable and representable guidelines to help clinicians in decision-making. Continuous improvement is established with the results of clinical studies, for which comparability of results is necessary. Our results emphasize this importance. Though our study addressed a scientific problem rather than a clinical one, it can still impact day-to-day practice.
One established definition of HAP would improve the comparability of trauma research; expert consensus could be a solid foundation to start with. Given the complexity of trauma patients, any diagnostic definition should address potential issues and pitfalls to avoid overdiagnosis. Currently, hyperthermia is incorporated in all guidelines, and sputum and dyspnea (with or without worsening gas exchange) in all but one. Leukocytosis, a common marker for infection, is included in all guidelines. Microbiologic information and evidence of infection aid in diagnosis and treatment; the CDC and ECDC describe several types of respiratory cultures. Radiologic evidence of pneumonia—either radiographic or CT imaging—also supports a diagnosis. Expert consensus should incorporate these criteria. Nonetheless, hypo- or hyperthermia, dyspnea, and leukocytosis are also signs of the systemic inflammatory response syndrome, commonly observed in trauma patients and potentially complicating the diagnostic process [72]. Also, posttraumatic fever may have a non-infectious origin, such as neurogenic fever, and trauma is associated with an increased immune response, adding to the need for a dedicated leukocytosis threshold [73, 74]. Lastly, sputum can result from (severe) pulmonary contusion, though unlikely to be purulent [75]. We propose that a decision-making algorithm includes hyperthermia (≥ 38.5 ℃) and leukocytosis (> 12 × 109/L) as major criteria, in addition to microbiologic and radiologic evidence. Dyspnea and (purulent) sputum should be considered minor criteria. Our considerations and recommendations serve as a basis for expert consensus.
Some limitations of this study should be considered. Firstly, PubMed/MEDLINE was the only search engine used in this study, which could result in an incomplete overview of applied clinical criteria for HAP diagnosis. However, the wide variety of clinical criteria and the difficult comparison between studies are evident in the current number of included studies. Secondly, our overview of reported diagnostic criteria did not consider the recommended combinations of these criteria. Not doing so resulted in a more comprehensible overview of used criteria and did not diminish the conclusion of this study.
Conclusion
As few studies in trauma patient research report a clear, clinical definition of hospital-acquired pneumonia, results cannot be adequately compared. Moreover, the wide variety of non-guideline criteria and diversity in pre-defined guideline criteria do not facilitate proper comparison. Studies should at least report how a diagnosis was made, but preferably, they would use pre-defined guideline criteria for pneumonia diagnosis in a research setting. Ideally, one internationally accepted set of criteria is used to diagnose hospital-acquired pneumonia.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ATS:
-
American Thoracic Society
- BSAC:
-
British Society for Antimicrobial Chemotherapy
- CDC:
-
Centers for Disease Control and Prevention
- ECDC:
-
European Center for Disease Prevention and Control
- HAP:
-
Hospital-acquired pneumonia
- IDSA:
-
Infectious Disease Society of America
- SIR:
-
Swedish Intensive Care Registry
- VAP:
-
Ventilator-associated pneumonia
References
Glance LG, Stone PW, Mukamel DB, Dick AW. Increases in mortality, length of stay, and cost associated with hospital-acquired infections in trauma patients. Arch Surg. 2011;146(7):794–801. https://doi.org/10.1001/archsurg.2011.41.
Esnault P, Nguyen C, Bordes J, D’Aranda E, Montcriol A, Contargyris C, et al. Early-onset ventilator-associated pneumonia in patients with severe traumatic brain injury: incidence, risk factors, and consequences in cerebral oxygenation and outcome. Neurocrit Care. 2017;27(2):187–98. https://doi.org/10.1007/s12028-017-0397-4.
Major JS, Welbourne J. Nosocomial infection in trauma intensive care. J Intensive Care Soc. 2015;16(3):193–8. https://doi.org/10.1177/1751143715579076.
Andermahr J, Greb A, Hensler T, Helling HJ, Bouillon B, Sauerland S, et al. Pneumonia in multiple injured patients: a prospective controlled trial on early prediction using clinical and immunological parameters. Inflamm Res. 2002;51(5):265–72.
Byun JH, Kim HY. Factors affecting pneumonia occurring to patients with multiple rib fractures. Korean J Thorac Cardiovasc Surg. 2013;46(2):130–4. https://doi.org/10.5090/kjtcs.2013.46.2.130.
Anand N, Kollef MH. The alphabet soup of pneumonia: CAP, HAP, HCAP, NHAP, and VAP. Semin Respir Crit Care Med. 2009;30(1):3–9. https://doi.org/10.1055/s-0028-1119803.
Network NHS. Pneumonia (ventilator-associated [VAP] and nonventilator-associated pneumonia [PNEU]) event: Centre for Disease Control (CDC); 2021 [Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf. Accessed 15 Dec 2020.
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–111. https://doi.org/10.1093/cid/ciw353.
European Center for Disease Prevention and Control (ECDC). Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals: full-scale survey and codebook. Stockholm: ECDC; 2012.
Rotstein C, Evans G, Born A, Grossman R, Light RB, Magder S, et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol. 2008;19(1):19–53. https://doi.org/10.1155/2008/593289.
Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19(4):637–57. https://doi.org/10.1128/CMR.00051-05.
Yang Y, Young JB, Schermer CR, Utter GH. Use of ketorolac is associated with decreased pneumonia following rib fractures. Am J Surg. 2014;207(4):566–72. https://doi.org/10.1016/j.amjsurg.2013.05.011.
Andermahr J, Hensler T, Sauerland S, Greb A, Helling HJ, Prokop A, et al. Risk factors for the development of pneumonia in multiple injured patients. Results of a prospective clinical trial. Unfallchirurg. 2003;106(5):392–7. https://doi.org/10.1007/s00113-003-0592-y.
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, P-DTAG, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–96. https://doi.org/10.1001/jama.2017.19163.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6. https://doi.org/10.1046/j.1445-2197.2003.02748.x.
Wutzler S, Blasius FM, Stormann P, Lustenberger T, Frink M, Maegele M, et al. Pneumonia in severely injured patients with thoracic trauma: results of a retrospective observational multi-centre study. Scand J Trauma Resusc Emerg Med. 2019;27(1):31. https://doi.org/10.1186/s13049-019-0608-4.
Warren C, Medei MK, Wood B, Schutte D. A nurse-driven oral care protocol to reduce hospital-acquired pneumonia. Am J Nurs. 2019;119(2):44–51. https://doi.org/10.1097/01.NAJ.0000553204.21342.01.
Seok J, Cho HM, Kim HH, Kim JH, Huh U, Kim HB, et al. Chest trauma scoring systems for predicting respiratory complications in isolated rib fracture. J Surg Res. 2019;244:84–90. https://doi.org/10.1016/j.jss.2019.06.009.
Conradsson D, Phillips J, Nizeyimana E, Hilliar C, Joseph C. Risk indicators of length of acute hospital stay after traumatic spinal cord injury in South Africa: a prospective, population-based study. Spinal Cord. 2019;57(9):763–9. https://doi.org/10.1038/s41393-019-0286-0.
Yadollahi M, Kashkooe A, Feyzi M, Bornapour S. Risk factors of mortality in nosocomial infected traumatic patients in a trauma referral center in south of Iran. Chin J Traumatol. 2018;21(5):267–72. https://doi.org/10.1016/j.cjtee.2018.03.002.
Guo C, Lei M, Wang Y, Hua L, Xue S, Yu D, et al. Oral administration of probiotic Lactobacillus casei Shirota decreases pneumonia and increases pulmonary functions after single rib fracture: a randomized double-blind, placebo-controlled clinical trial. J Food Sci. 2018;83(8):2222–6. https://doi.org/10.1111/1750-3841.14220.
Djuric O, Markovic-Denic L, Jovanovic B, Bumbasirevic V. Agreement between CDC/NHSN surveillance definitions and ECDC criteria in diagnosis of healthcare-associated infections in Serbian trauma patients. PLoS One. 2018;13(10):e0204893. https://doi.org/10.1371/journal.pone.0204893.
Denis AR, Feldman D, Thompson C, Mac-Thiong JM. Prediction of functional recovery six months following traumatic spinal cord injury during acute care hospitalization. J Spinal Cord Med. 2018;41(3):309–17. https://doi.org/10.1080/10790268.2017.1279818.
Yoo JH, Kim KT, Kim TY, Hwang JH, Chang JD. Postoperative fever after hemiarthroplasty in elderly patients over 70 years of age with displaced femoral neck fracture: necessity of routine workup? Injury. 2017;48(2):441–6. https://doi.org/10.1016/j.injury.2016.12.013.
Folbert EC, Hegeman JH, Gierveld R, van Netten JJ, Velde DV, Ten Duis HJ, et al. Complications during hospitalization and risk factors in elderly patients with hip fracture following integrated orthogeriatric treatment. Arch Orthop Trauma Surg. 2017;137(4):507–15. https://doi.org/10.1007/s00402-017-2646-6.
Curtis K, Asha SE, Unsworth A, Lam M, Goldsmith H, Langcake M, et al. ChIP: an early activation protocol for isolated blunt chest injury improves outcomes, a retrospective cohort study. Australas Emerg Nurs J. 2016;19(3):127–32. https://doi.org/10.1016/j.aenj.2016.06.002.
Yun HC, Weintrob AC, Conger NG, Li P, Lu D, Tribble DR, et al. Healthcare-associated pneumonia among U.S. combat casualties, 2009 to 2010. Mil Med. 2015;180(1):104–10. https://doi.org/10.7205/MILMED-D-14-00209.
Kamiya K, Koda M, Furuya T, Kato K, Takahashi H, Sakuma T, et al. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: a comparison with high-dose methylprednisolone as a historical control. Eur Spine J. 2015;24(5):963–7. https://doi.org/10.1007/s00586-014-3373-0.
Landeen C, Smith HL. Examination of pneumonia risks and risk levels in trauma patients with pulmonary contusion. J Trauma Nurs. 2014;21(2):41–9. https://doi.org/10.1097/JTN.0000000000000029.
Schirmer-Mikalsen K, Moen KG, Skandsen T, Vik A, Klepstad P. Intensive care and traumatic brain injury after the introduction of a treatment protocol: a prospective study. Acta Anaesthesiol Scand. 2013;57(1):46–55. https://doi.org/10.1111/j.1399-6576.2012.02785.x.
Mica L, Keller C, Vomela J, Trentz O, Plecko M, Keel MJ. Obesity and overweight as a risk factor for pneumonia in polytrauma patients: a retrospective cohort study. J Trauma Acute Care Surg. 2013;75(4):693–8. https://doi.org/10.1097/TA.0b013e31829a0bdd.
Hyllienmark P, Brattstrom O, Larsson E, Martling CR, Petersson J, Oldner A. High incidence of post-injury pneumonia in intensive care-treated trauma patients. Acta Anaesthesiol Scand. 2013;57(7):848–54. https://doi.org/10.1111/aas.12111.
Yeung L, Miraflor E, Strumwasser A, Sadeghi P, Victorino GP. Does gastric volume in trauma patients identify a population at risk for developing pneumonia and poor outcomes? J Surg Res. 2012;178(2):874–8. https://doi.org/10.1016/j.jss.2012.07.067.
Hakim SM, Latif FS, Anis SG. Comparison between lumbar and thoracic epidural morphine for severe isolated blunt chest wall trauma: a randomized open-label trial. J Anesth. 2012;26(6):836–44. https://doi.org/10.1007/s00540-012-1424-4.
Strumwasser A, Chu E, Yeung L, Miraflor E, Sadjadi J, Victorino GP. A novel CT volume index score correlates with outcomes in polytrauma patients with pulmonary contusion. J Surg Res. 2011;170(2):280–5. https://doi.org/10.1016/j.jss.2011.03.022.
Becher RD, Hoth JJ, Neff LP, Rebo JJ, Martin RS, Miller PR. Multidrug-resistant pathogens and pneumonia: comparing the trauma and surgical intensive care units. Surg Infect (Larchmt). 2011;12(4):267–72. https://doi.org/10.1089/sur.2010.052.
Karunakar MA, Staples KS. Does stress-induced hyperglycemia increase the risk of perioperative infectious complications in orthopaedic trauma patients? J Orthop Trauma. 2010;24(12):752–6. https://doi.org/10.1097/BOT.0b013e3181d7aba5.
Garcia-Alvarez F, Al-Ghanem R, Garcia-Alvarez I, Lopez-Baisson A, Bernal M. Risk factors for postoperative infections in patients with hip fracture treated by means of Thompson arthroplasty. Arch Gerontol Geriatr. 2010;50(1):51–5. https://doi.org/10.1016/j.archger.2009.01.009.
Friese RS, Sperry JL, Phelan HA, Gentilello LM. The use of leukoreduced red blood cell products is associated with fewer infectious complications in trauma patients. Am J Surg. 2008;196(1):56–61. https://doi.org/10.1016/j.amjsurg.2007.08.063.
Schlosser HG, Volk HD, Splettstosser G, Brock M, Woiciechowsky C. A new qualitative interleukin-6 bedside test can predict pneumonia in patients with severe head injury–comparison to the standard Immulite test and a semiquantitative bedside test. J Neurosurg Anesthesiol. 2007;19(1):5–9. https://doi.org/10.1097/01.ana.0000211026.18926.89.
Schirmer-Mikalsen K, Vik A, Gisvold SE, Skandsen T, Hynne H, Klepstad P. Severe head injury: control of physiological variables, organ failure and complications in the intensive care unit. Acta Anaesthesiol Scand. 2007;51(9):1194–201. https://doi.org/10.1111/j.1399-6576.2007.01372.x.
Kamel HK, Iqbal MA, Mogallapu R, Maas D, Hoffmann RG. Time to ambulation after hip fracture surgery: relation to hospitalization outcomes. J Gerontol A Biol Sci Med Sci. 2003;58(11):1042–5. https://doi.org/10.1093/gerona/58.11.m1042.
McKinley WO, Tewksbury MA, Godbout CJ. Comparison of medical complications following nontraumatic and traumatic spinal cord injury. J Spinal Cord Med. 2002;25(2):88–93. https://doi.org/10.1080/10790268.2002.11753607.
Carson JL, Altman DG, Duff A, Noveck H, Weinstein MP, Sonnenberg FA, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39(7):694–700. https://doi.org/10.1046/j.1537-2995.1999.39070694.x.
Gonzalez RP, Holevar MR. Role of prophylactic antibiotics for tube thoracostomy in chest trauma. Am Surg. 1998;64(7):617–20 (discussion 20-1).
Claxton AR, Wong DT, Chung F, Fehlings MG. Predictors of hospital mortality and mechanical ventilation in patients with cervical spinal cord injury. Can J Anaesth. 1998;45(2):144–9. https://doi.org/10.1007/BF03013253.
Morrison JE, Wisner DH, Bodai BI. Complications after negative laparotomy for trauma: long-term follow-up in a health maintenance organization. J Trauma. 1996;41(3):509–13. https://doi.org/10.1097/00005373-199609000-00021.
Renz BM, Feliciano DV. Unnecessary laparotomies for trauma: a prospective study of morbidity. J Trauma. 1995;38(3):350–6. https://doi.org/10.1097/00005373-199503000-00007.
Nichols RL, Smith JW, Muzik AC, Love EJ, McSwain NE, Timberlake G, et al. Preventive antibiotic usage in traumatic thoracic injuries requiring closed tube thoracostomy. Chest. 1994;106(5):1493–8. https://doi.org/10.1378/chest.106.5.1493.
Beraldo PS, Neves EG, Alves CM, Khan P, Cirilo AC, Alencar MR. Pyrexia in hospitalised spinal cord injury patients. Paraplegia. 1993;31(3):186–91. https://doi.org/10.1038/sc.1993.35.
Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma–reduced septic morbidity. J Trauma. 1989;29(7):916–22. https://doi.org/10.1097/00005373-198907000-00003. (discussion 22-3).
Moore FA, Moore EE, Ammons LA, McCroskey BL. Presumptive antibiotics for penetrating abdominal wounds. Surg Gynecol Obstet. 1989;169(2):99–103.
LoCurto JJ Jr, Tischler CD, Swan KG, Rocko JM, Blackwood JM, Griffin CC, et al. Tube thoracostomy and trauma–antibiotics or not? J Trauma. 1986;26(12):1067–72. https://doi.org/10.1097/00005373-198612000-00001.
Grover FL, Richardson JD, Fewel JG, Arom KV, Webb GE, Trinkle JK. Prophylactic antibiotics in the treatment of penetrating chest wounds. A prospective double-blind study. J Thorac Cardiovasc Surg. 1977;74(4):528–36.
Allen GS, Cox CS Jr, Moore FA, Duke JH, Andrassy RJ. Pulmonary contusion: are children different? J Am Coll Surg. 1997;185(3):229–33.
Becher RD, Hoth JJ, Rebo JJ, Kendall JL, Miller PR. Locally derived versus guideline-based approach to treatment of hospital-acquired pneumonia in the trauma intensive care unit. Surg Infect (Larchmt). 2012;13(6):352–9. https://doi.org/10.1089/sur.2011.056.
Beghi G, De Tanti A, Serafini P, Bertolino C, Celentano A, Taormina G. Monitoring of hospital acquired pneumonia in patients with severe brain injury on first access to intensive neurological rehabilitation: first year of observation. Monaldi Arch Chest Dis. 2018;88(1):888. https://doi.org/10.4081/monaldi.2018.888.
Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM. A time-dependent analysis of intensive care unit pneumonia in trauma patients. J Trauma. 2004;56(2):296–301. https://doi.org/10.1097/01.TA.0000109857.22312.DF. (discussion -3).
Bozorgzadeh A, Pizzi WF, Barie PS, Khaneja SC, LaMaute HR, Mandava N, et al. The duration of antibiotic administration in penetrating abdominal trauma. Am J Surg. 1999;177(2):125–31. https://doi.org/10.1016/s0002-9610(98)00317-1.
Ewan VC, Sails AD, Walls AW, Rushton S, Newton JL. Dental and microbiological risk factors for hospital-acquired pneumonia in non-ventilated older patients. PLoS One. 2015;10(4):e0123622. https://doi.org/10.1371/journal.pone.0123622.
Giamberardino HI, Cesario EP, Carmes ER, Mulinari RA. Risk factors for nosocomial infection in trauma patients. Braz J Infect Dis. 2007;11(2):285–9. https://doi.org/10.1590/s1413-86702007000200024.
Rello J, Ausina V, Castella J, Net A, Prats G. Nosocomial respiratory tract infections in multiple trauma patients. Influence of level of consciousness with implications for therapy. Chest. 1992;102(2):525–9. https://doi.org/10.1378/chest.102.2.525.
Worrall CL, Anger BP, Simpson KN, Leon SM. Impact of a hospital-acquired/ventilator-associated/healthcare-associated pneumonia practice guideline on outcomes in surgical trauma patients. J Trauma. 2010;68(2):382–6. https://doi.org/10.1097/TA.0b013e318197bc74.
(SIR) SICR. [SIR's guideline for registration of adverse events and complications in intensive care in Sweden] 2019 [21.0:[Available from: https://www.icuregswe.org/globalassets/riktlinjer/komplikationer_21.pdf. Accessed 15 Dec 2020.
Masterton RG, Galloway A, French G, Street M, Armstrong J, Brown E, et al. Guidelines for the management of hospital-acquired pneumonia in the UK: report of the working party on hospital-acquired pneumonia of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2008;62(1):5–34. https://doi.org/10.1093/jac/dkn162.
Society AT, America IDSo. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. https://doi.org/10.1164/rccm.200405-644ST.
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–40. https://doi.org/10.1016/0196-6553(88)90053-3.
Metsemakers WJ, Kuehl R, Moriarty TF, Richards RG, Verhofstad MHJ, Borens O, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury. 2018;49(3):511–22. https://doi.org/10.1016/j.injury.2016.09.019.
Wittauer M, Burch MA, McNally M, Vandendriessche T, Clauss M, Della Rocca GJ, et al. Definition of long-bone nonunion: a scoping review of prospective clinical trials to evaluate current practice. Injury. 2021;52(11):3200–5. https://doi.org/10.1016/j.injury.2021.09.008.
Metsemakers WJ, Morgenstern M, McNally MA, Moriarty TF, McFadyen I, Scarborough M, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49(3):505–10. https://doi.org/10.1016/j.injury.2017.08.040.
Chakraborty RK, Burns B. Systemic inflammatory response syndrome. Treasure Island (FL): StatPearls Publisher; 2022.
Thompson HJ, Pinto-Martin J, Bullock MR. Neurogenic fever after traumatic brain injury: an epidemiological study. J Neurol Neurosurg Psychiatry. 2003;74(5):614–9. https://doi.org/10.1136/jnnp.74.5.614.
Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384(9952):1455–65. https://doi.org/10.1016/S0140-6736(14)60687-5.
Ganie FA, Lone H, Lone GN, Wani ML, Singh S, Dar AM, et al. Lung contusion: a clinico-pathological entity with unpredictable clinical course. Bull Emerg Trauma. 2013;1(1):7–16.
Author information
Authors and Affiliations
Contributions
TK: Conceptualization, Methodology, Investigation, Visualization, Writing - Original Draft; DS: Conceptualization, Methodology, Investigation, Writing - Original Draft; FH: Conceptualization, Resources, Writing - Review & Editing; KB: Conceptualization, Visualization, Writing - Review & Editing; RH: Conceptualization, Supervision, Writing - Review & Editing; MvB: Conceptualization, Methodology, Supervision, Writing - Review & Editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Prior abstract publication/presentation: Poster presentation at the 2022 European Congress of Trauma and Emergency Surgery in Oslo, Norway.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kobes, T., Smeeing, D.P.J., Hietbrink, F. et al. Definitions of hospital-acquired pneumonia in trauma research: a systematic review. Eur J Trauma Emerg Surg (2024). https://doi.org/10.1007/s00068-024-02509-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00068-024-02509-8