Abstract
Introduction
While numerous randomized controlled trials (RCTs) have been conducted in the field of trauma, a substantial portion of them are yielding negative results. One potential contributing factor to this trend could be the lack of agreement regarding the chosen definitions across different trials. The primary objective was to identify the terminology and definitions utilized for the characterization of multiple trauma patients within randomized controlled trials (RCTs).
Methods
A systematic review of the literature was performed in MEDLINE, EMBASE and clinicaltrials.gov between January 1, 2002, and July 31, 2022. RCTs or RTCs protocols were eligible if they included multiple trauma patients. The terms employed to characterize patient populations were identified, and the corresponding definitions for these terms were extracted. The subsequent impact on the population recruited was then documented to expose clinical heterogeneity.
Results
Fifty RCTs were included, and 12 different terms identified. Among these terms, the most frequently used were “multiple trauma” (n = 21, 42%), "severe trauma" (n = 8, 16%), "major trauma" (n = 4, 8%), and trauma with hemorrhagic shock" (n = 4, 8%). Only 62% of RCTs (n = 31) provided a definition for the terms used, resulting a total of 21 different definitions. These definitions primarily relied on the injury severity score (ISS) (n = 15, 30%), displaying an important underlying heterogeneity. The choice of the terms had an impact on the study population, affecting both the ISS and in-hospital mortality. Eleven protocols were included, featuring five different terms, with "severe trauma" being the most frequent, occurring six times (55%).
Conclusion
This systematic review uncovers an important heterogeneity both in the terms and in the definitions employed to recruit trauma patients within RCTs. These findings underscore the imperative of promoting the use of a unique and consistent definition.
Similar content being viewed by others
Introduction
Trauma is an important health issue worldwide, with an estimated 50 million individuals injured every year. It is a leading cause of death and disability especially in people under 40 years old [1, 2]. For these reasons, many randomized controlled trials have been conducted aiming to improve health outcomes after trauma. However, despite high expectancies, many of these trials have yielded negative results [3,4,5,6]. The impact of negative trials is an important issue for clinicians, patients and funders [7, 8]. Different reasons have been suggested to explain negative results in trials, such as lack of power, issues related with complex interventions or population heterogeneity [9,10,11]. Another potential reason is the lack of an accurate definition of the medical condition under investigation within a trial, especially in time-sensitive situations like trauma where early inclusions may be required despite uncertain or incomplete information.
In 2009, Butcher et al. suggested in a systematic review that at least 47 different definitions were coexisting in the published literature for multiple trauma patients [12]. Yet, among all trauma populations, those with the most severe injuries require a clear definition to ensure consistency across studies. In 2014, an international consensus proposed a unique definition and identified “polytrauma” patients as those with significant injuries of three or more points in two or more different anatomic Abbreviated Injury Score (AIS) regions in conjunction with one or more additional variables such as a systolic blood pressure inferior or equal to 90 mmHg, a Glasgow score inferior or equal to 8, a base excess inferior or equal to -6 mmol/L, an international normalized ratio superior or equal to 1.4 or an age superior or equal to 70 year [13]. Despite the involvement of international experts and a rigorous methodological process, this definition remains a challenge, particularly in studies that focus on prehospital care or early in-hospital care, where Injury Severity Score (ISS) cannot be determined until CT scan is performed and interpreted [14].
We hypothesized that varying definitions continue to be employed, which can result in heterogeneity across trauma randomized controlled trials (RCTs). The primary objective was to perform a systematic review to identify the terminology and definitions utilized for the characterization of multiple trauma patients within published and ongoing randomized controlled trials (RCTs).
Methods
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [15] (Additional file 1: Material 1).
Terms and definition
We defined as the terms all the synonyms used in RCTs to name these most severely injured patients (e.g., “multiple trauma”, “polytrauma” or “severe trauma”) and as the definition as the criteria reported to circumscribe each term (e.g., “Injury Severity Score (ISS) > 15”).
To ensure consistency in this systematic review, we adopted a single term and chose to use “multiple trauma” to align with the existing Mesh Terms thesaurus that have been utilized in PubMed since 1988 [16].
Data source and search strategy
A comprehensive search was conducted in MEDLINE and EMBASE using standardized vocabulary and free text to identify RCTs including multiple trauma patients published between January 1, 2002, and July 31, 2022. To ensure the broader recruitment among randomized controlled studies including multiple trauma patients, we used in the search equation a wide range of terms such as multiple trauma, polytrauma, severe trauma or multiple injuries. In addition, we also searched ClinicalTrials.gov to identify protocols of ongoing trials including multiple trauma patients. Details of the search strategy are provided in (Additional file 1: Material 2).
Inclusion and exclusion criteria
Eligibility criteria were RCTs or protocol for RCTs that included or aimed to include adult patients with multiple trauma. We excluded RCTs with less than 20 patients in the intervention arm and considered the arm with the smallest number of patients for RCTs with more than one intervention arm. We excluded RCTs recruiting only traumatic brain injury patients, post hoc analysis of previously published RCTs and publications in language other than English.
After identification and exclusion of duplicates, two reviewers (T.J & A.J) independently examined titles and abstracts to assessed eligibility of retrieved reports. All disagreements were resolved by discussions.
Data extraction
A standardized data collection form was used to extract the following information from full-text reports: study characteristics (name, first author, country of the first authors, year of publication, name of the journal, multicentric, inclusion criteria, verbatim description of both arms [intervention(s) and control] and main outcome), characteristics of the population included (number of patients, age, sex ratio, ISS), outcomes (mortality, main outcome significance as reported by the p-value) and finally the terms and definitions used to describe multiple trauma patients in these studies.
Risk of bias assessment
One reviewer (T.J) extracted the risk of bias from each full text included using the revised risk of bias assessment tool for randomized trial (RoB2) [17] and thus focused on five categories: the randomization process, the potential deviations from intended interventions, missing outcome data management, outcomes measurement process and the selection in reported results. The overall risk was thus determined from these five categories and reported for each published study.
Statistical analysis
The quantitative data were reported either as mean and standard deviation, or as minimum and maximum values. When the mean value was not available in full-text article, we use a method developed by Luo et al. to estimate the mean and standard deviation from median and quantiles [18]. Categorical data were described as counts and percentage. The thresholds used to qualify the included RCT as significant are those determined by the authors of the studies. We did not contact the trials authors for missing information. Statistical analyses and figures were performed with Python (v3.10.7).
Results
Search results
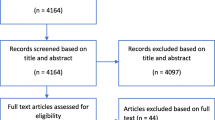
For published RCTs, after removing duplicates, 1699 studies were retrieved from the search. Of these, 1621 were excluded after title and abstract assessment. Following the initial screening process, the full texts of the 78 remaining studies were evaluated for eligibility. Of these, 10 studies were excluded due to insufficient sample size, eight studies did not involve multiple trauma patients, five studies involved post hoc analysis of other RCTs, four studies were not available in English language, and one study was identified as a duplicate. Consequently, 50 RCTs met inclusion criteria after rigorous full-text examination.
For protocols, 117 reports were obtained from clinicaltrials.gov for potential inclusion in the study protocols. After conducting a comprehensive screening process, 103 reports that did not target multiple trauma patients were excluded. Of the 14 remaining reports, three were found to be related to previously published RCTs, leaving a total of 11 which met inclusion criteria (Fig. 1).
Characteristics of the RCTs
Table 1 provides a summary of the key characteristics of the included studies, with detailed information available in (Additional file 1: Material 3). Among these studies, 46% were multicentric (n = 23) and 56% (n = 28) involved a pharmacological intervention, while others were targeting a non-pharmacological treatment. In pharmacological RCTs, the control arm involved a placebo in 39% of the studies (n = 11/28).
The number of participants included ranged from 41 to 1629 with a mean number of 233 patients. Included studies demonstrated a wide variation in baseline patient characteristics. The mean age ranged from 31 to 70 years old, and the percentage of male patients ranged from 27 to 94%. Additionally, the ISS ranged from 11 to 37 points, highlighting the variability in the severity of injuries among the studies. Out of the 41 studies that reported a result for the predefined primary outcome, 13 studies (32%) reported statistically significant results.
Terms & definitions used in RCTs
Among RTCs included, 12 different terms were used. The most frequently used was “multiple trauma” that occurred in 42% studies (n = 21) [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. The second, “severe trauma”, occurred in 16% studies (n = 8) [4, 5, 40,41,42,43,44,45] and was followed by “major trauma” [46,47,48,49] and “trauma with hemorrhagic shock” (8%, n = 4) [3, 50,51,52]. Other terms used were “multiple injuries”[53,54,55], “severely injured patients”[6, 56, 57], “polytrauma” [58, 59], “traumatic hypovolemic shock” [60], “seriously injured patients”[61], “trauma patients at risk for hemorrhagic shock”[62], “multisystem trauma patients” [63] and “hypotensive trauma” [64] (Additional file 1: Material 4). The terms were found at least once among the inclusion criteria in XX% of the included RCT (n = XX).
Associated with these terms, 21 different definitions were identified. Moreover, 38% of RCTs did not report any definition of the term used (n = 19). When a definition was reported, the ISS was the most commonly used criteria (30%, n = 15), with a wide range of possible thresholds ranging from 9 to 20, the most frequently used being superior to 15 (16%, n = 8). Other definitions included the involvement of at least two body regions in eight studies (16%) or physiological parameters such as systolic blood pressure or heart rate in six studies (12%). Figure 2 reports the broad distribution across terms and related definition, highlighting that a given term can be associated with several definitions, and conversely, that a given definition can be related with several terms. Moreover, among RCTs that reported a definition, only 6% supported the choice with a citation (n = 2). Finally, despite the publication of the Berlin definition in 2014, none of the 23 included RCTs published after its release used this definition.
Definitions according to terms used *: major vascular injury or > 6 rib fracture or complex pelvic fracture or > 20% blood loss or AIS > 4 for thorax/abdo or > 3 regions with AIS > 3. $: Patient with suspected trauma & respiratory rate > 30/min, pulse > 120/min, systolic blood pressure < 100 mmHg, Glasgow coma scale < 13, estimated exterior blood loss > 500 mL, abnormal pupillary reaction OR patient with a clinical suspicion of one of the following diagnoses: fractures from at least two long bones, flail chest, open chest or multiple rib fractures, severe abdominal injury, pelvic fracture, unstable vertebral fractures OR fall from a high height, ejection from a vehicle, death of occupant in same vehicle, wedged or trapped chest / abdomen. For clarity, terms used were grouped by similarity in three overarching categories with “injuries severity”, “hemorrhagic shock” and “blood pressure”-related definitions
Impact of the terms on baseline characteristics and outcomes in RCTs
The choice of terms used did not appear to affect the demographic characteristics of included patients. When grouped by terms used, mean age ranged from 34 to 45, and the percentage of male subjects ranged from 70 to 85%. However, the types and severity of injuries did differ according to the terms chosen. The percentage of included patients suffering from traumatic brain injury ranged from 10 to 84, and the mean ISS ranged from 11 to 32. Furthermore, outcomes varied according to the terms used, as illustrated by the mortality rate, which could range from 9 to 31% (Fig. 3).
Risk of bias in RCTs
The overall risk of bias was moderate with 68% of studies (34/50) considered at low risk and 12% considered with high risk (6/50) and with some concerns for remaining studies (10/50–20%) (Additional file 1: Material 5). The risk of bias was found to originate from protocol deviation in 12% (n = 6/50), from imprecision outcomes measurement in 10% (n = 5/50), from selection bias in 10% (n = 5/50), from randomization in 8% (n = 4/50) and from missing data bias in 6% (n = 3/50).
Terms and definitions used in clinical trial protocols
Among the 11 protocols included, five different terms were used. The most frequently used was “severe trauma”, with six occurrences (55%), followed by “multiple trauma” (18%, n = 2) while other terms were “severely injured patient”, “polytrauma” and “multiple injuries” (9%, n = 1). Of these 11 protocols, 45% (n = 5) did not provide a definition of the term used.
When a definition was provided, 27% (n = 3) relied on the Injury Severity Score (ISS) with thresholds of either > 15 (n = 2) or > 18, while other definitions were used only once, such as “at least one Vittel criteria”, “systolic blood pressure inferior to 70 mmHg and heart rate HR superior to 108 bpm” and “simultaneous injuries in two or more organs”.
Discussion
Main findings
This systematic review of contemporary RCTs recruiting multiple trauma patients found 12 different terms used to describe this population and that nearly 40% of the RCTs did not report any definition of the term used. Where definitions were included, there were more than 20 variations. These results expose that despite an international consensus in 2014 [13], a substantial heterogeneity remains in the terms and definitions used in RCTs involving multiple trauma patients.
This diversity implies a significant between-trials heterogeneity regarding both baseline characteristics (such as the mean ISS that ranged from 11 to 32) and outcomes (such as mortality ranging between 9 to 31%). The lack of consistency in the terminology used could explain some differences in the required co-interventions, variations in the observed treatment effects and finally the low rate of significant outcomes observed in our study and in the existing literature.
Discussion with existing literature
In 2009, a systematic review including any type of studies identified 47 possible definitions of multiple trauma [12]. Almost fifteen years later, our review underpins the persistence of this heterogeneity both in the terms used and, in the definition associated with these terms. This issue of variability in defining a specific clinical conditions for trials has been previously highlighted in various contexts, including ARDS [65], traumatic hemorrhagic shock [66] or refractory septic shock [67].
The time-sensitive nature of trauma care leads to the challenge of specifying a consistent and unique definition. As an example, the selection of variables used in the definition involves a balance between the availability of variables and the timing of the intervention being evaluated. For instance, variables such as AIS or ISS are strongly associated to patient severity, making them suitable for inclusion in a definition, as proposed by the Pape et al. [13]. However, these variables rely on the completion and interpretation of a full-body CT scan which limits the use of this definition for research projects carried out prior to scanning. Furthermore, it restricts the use of this definition in low-income settings with limited resources such as CT scans. On the other hand, some variables may be available very early after the trauma, such as blood pressure or heart rate [3, 62], but these variables, if only considered at one time point are also likely to be less specific, potentially failing to include the population of interest.
Nevertheless, standardized consistent definitions are possible. Time-sensitive conditions have been defined as demonstrated with the Berlin definition for ARDS [68] or SEPSIS-III definition [69]. The strengths of these two definitions rely on objective, easily measurable and accurate clinical criteria that can be promptly measured and capture essential criteria of each syndrome. These characteristics allow for simple use and offer consistency, whether applied prospectively or retrospectively.
Limitations
First, defining the scope of a systematic review involves defining a population. This was a methodological challenge as analyzing this definition was the main aim of this systematic review. Thus, we chose to use a spectrum of synonyms of multiple trauma in the search equation and to include after titles and abstracts screening those RCTs that reported authors commitment to include trauma with a certain severity. This choice was guided by systematic reviews that have had a similar focus in other clinical situations such as polypharmacy [70], community health workers [71] or labor [72]. It nevertheless leads to the exclusion of RCTs such as CRASH II that reported in their abstract the intention to include “adult trauma patients with, or at risk of, significant bleeding” and that are not indexed in PubMed under the Mesh “multiple trauma” [73].
Second, due to the broad diversity of populations, interventions and outcomes included in this systematic review, it was not possible to evaluate the impact of the choice of terms or definition on the effectiveness of the interventions. This study only reports that providing a clear definition of the term used did not seems to be associated with an increased proportion of significantly positive primary outcomes.
Third, there is a possibility that some terms or definitions may have been missed, especially if the trial that used them was not within the scope of the systematic review. As a result, an additional term or definition could emerge, or another occurrence of a term or definition already included in the systematic review. Such an event would not alter the message of the review but would only emphasize the importance of the existing heterogeneity.
Fourth, this study exclusively focuses on the terms employed to denote the, though it can be argued that the true determinant of the recruited population lies within inclusion criteria. Such a statement might downplay the importance of the heterogeneity exposed in this study. Nonetheless, our findings also expose that terms such as “multiple trauma” or “severe trauma” are frequently used within the included manuscripts inclusion criteria section. Such utilization overall strengthens the problematic exposed as all these terms convey a certain degree of ambiguity. Indeed, even if widely acknowledged that, irrespective of the specific term employed, these patients are at a heightened risk of poor outcomes, an incredible diversity of potential clinical presentations exists, and this diversity can ultimately lead to the categorization of markedly distinct patients under the same generic overarching term.
Finally, it is not possible, within the context of this work, to recommend the use of one term or definition over another. The sole aim of this systematic review was to determine which terms were commonly used in the literature and which definitions were associated with these terms. Therefore, the purpose of this work was not to identify a consensus definition or to determine whether a given definition was more often associated with a significant outcome.
Implications
Also, the lack of consistency in definitions and underlying clinical heterogeneity presents a challenge for integrating previously published evidence. Meta-analysis assumes that populations are similar enough to be pooled into a single measure of effect, but this assumption is undermined when authors fail to provide a clear (or any) definition. This incomplete reporting has been shown to significantly contribute to research waste [74].
The observed heterogeneity in definitions may also contribute to physicians' uncertainty at the bedside. In a prospective observational study, trauma surgeons have been reported to only reach a moderate agreement regarding whether a given patient should be qualified as a multiple trauma or not [75]. This finding challenges the common belief that caregivers base their health care diagnosis on rigorous definitions and emphasize the need of a standardization of these which encompass the complexity and time-sensitive nature of trauma care.
The heterogeneity in definitions used may also reflect the presence of several phenotypes within this population, as it has been advocated for ARDS [76]. It could indeed be argued that severe traumatic brain injury and hemorrhagic shock, as well as penetrating and non-penetrating trauma, are different diseases. In this light, it might be necessary to consensually delineate subgroups within the definition to acknowledge for these differences [77].
Finally, for stakeholders involved in the design of future RTCs, it may be important to acknowledge that the terms used can have a direct impact on critical outcomes, such as the mortality rate. This awareness can be particularly relevant when determining the appropriate number of patients to treat.
Conclusion
In conclusion, our systematic review has revealed significant heterogeneity in the terms and in definitions used to qualify multiple trauma patients in randomized controlled trials. This underscores the importance of further efforts to establish a unique and consistent definition of multiple trauma, taking into consideration the time-sensitive nature of this pathology.
Availability of data and materials
All data used during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ISS:
-
Injury Severity Score
- RCT:
-
Randomized Controlled Trial
- ARDS:
-
Acute Respiratory Distress Syndrome
- AIS:
-
Abbreviated Injury Scale
- HLA-DR:
-
Human Leukocyte Antigen-DR Isotype
- CT:
-
Computed Tomography scan
References
Haagsma J, Graetz N, Bolliger I, Murray C, Vos T. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev. 2016;22(1):3–18.
Murray C, Vos T, Lozano R, Memish Z. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223.
Crombie N, Doughty HA, Bishop JRB, Desai A, Dixon EF, Hancox JM, et al. Resuscitation with blood products in patients with trauma-related haemorrhagic shock receiving prehospital care (RePHILL): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Haematol. 2022;9(4):e250–61.
Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471.
Sierink JC, Treskes K, Edwards MJR, Beuker BJA, den Hartog D, Hohmann J, et al. Immediate total-body CT scanning versus conventional imaging and selective CT scanning in patients with severe trauma (REACT-2): a randomised controlled trial. The Lancet. 2016;388(10045):673–83.
Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. The Lancet. 2018;392(10144):283–91.
Ospina-Tascón GA, Büchele GL, Vincent JL. Multicenter, randomized, controlled trials evaluating mortality in intensive care: Doomed to fail? Crit Care Med. 2008;36(4):1311–22.
Tonelli AR, Zein J, Adams J, Ioannidis JPA. Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intensive Care Med. 2014;40(6):769–87.
Laffey JG, Kavanagh BP. Negative trials in critical care: why most research is probably wrong. Lancet Respir Med. 2018;6(9):659–60.
Legrand M. Negative trials in critical care medicine and the hurdles. Lancet Respir Med. 2018;6(10): e53.
Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;30: n2061.
Butcher N, Balogh ZJ. The definition of polytrauma: the need for international consensus. Injury. 2009;40:S12-22.
Pape HC, Lefering R, Butcher N, Peitzman A, Leenen L, Marzi I, et al. The definition of polytrauma revisited: An international consensus process and proposal of the new ‘Berlin definition.’ J Trauma Acute Care Surg. 2014;77(5):780–6.
Balogh ZJ. Polytrauma: it is a disease. Injury. 2022;53(6):1727–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021: n71.
National Library of Medicine. Multiple Trauma [MeSH term] [Internet]. 1988. Available from: https://www.ncbi.nlm.nih.gov/mesh/68009104
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;l4898.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805.
Noor Mohammad Arefian. Effect of Partial Parenteral Versus Enteral Nutritional Therapy on Serum Indices in Multiple Trauma Patients. Tanaffos. 2007;6(4):37–41.
Najafi A, Mojtahedzadeh M, Ahmadi KH, Abdollahi M, Mousavi M, Chelkeba L, et al. The immunological benefit of higher dose N-acetyl cysteine following mechanical ventilation in critically ill patients. DARU J Pharm Sci. 2014;22(1):57.
Stephen CM. Splanchnic hypoperfusion-directed therapies in trauma: a prospective. Randomized Trial Am Surg. 2005;71(3):252–60.
for the Working Group on Selective Decontamination of the Digestive Tract, Stoutenbeek CP, Van Saene HKF, Little RA, 2007 Whitehead A. The effect of selective decontamination of the digestive tract on mortality in multiple trauma patients: a multicenter randomized controlled trial. Intensive Care Med. 33(2):261–70.
Kagan I, Cohen J, Stein M, Bendavid I, Singer P. Preemptive enteral nutrition enriched with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in severe multiple trauma: a prospective, randomized, double-blind study. Intensive Care Med. 2015;41(3):460–9.
Velmahos GC. Electrostimulation for the prevention of deep venous thrombosis in patients with major trauma: A prospective randomized study. Surgery. 2005;137(5):493–8.
Grintescu IM, Vasiliu IL. The influence of parenteral glutamine supplementation on glucose homeostasis in critically ill polytrauma patients–A randomized-controlled clinical study. Clin Nutr. 2015;34(2):377–82.
Kagan I, Singer P. Effect of supplemental enteral fish oil on the development of psychological complications in critically ill multiple-trauma patients: 6 months’ follow-up. J Parenter Enter Nutr. 2020;45(7):1567–80.
Curry N, Rourke C, Davenport R, Beer S, Pankhurst L, Deary A, et al. Early cryoprecipitate for major haemorrhage in trauma: a randomised controlled feasibility trial. Br J Anaesth. 2015;115(1):76–83.
Kahn J, Grupp U. Computed tomography in trauma patients using iterative reconstruction: reducing radiation exposure without loss of image quality. Acta Radiol. 2016;57(3):362–9.
Akbari E, Safari S, Hatamabadi H. The effect of fibrinogen concentrate and fresh frozen plasma on the outcome of patients with acute traumatic coagulopathy: a quasi-experimental study. Am J Emerg Med. 2018;36(11):1947–50.
Habib T. Early probiotics in preventing ventilator-associated pneumonia after multiple trauma. Asian J Pharm Clin Res. 2020;13(10):83–5.
Cotae AM. The impact of monitoring depth of anesthesia and nociception on postoperative cognitive function in adult multiple trauma patients. Med Kaunas. 2021;57(5):408.
Pirente N, Blum C, Wortberg S. Quality of life after multiple trauma: the effect of early onset psychotherapy on quality of life in trauma patients. Langenbecks Arch Surg. 2007;392:739–45.
Pape HC, Rixen D, Morley J, EPOFF Study Group. (2007) Impact of the method of initial stabilization for femoral shaft fractures in patients with multiple injuries at risk for complications (borderline patients). Ann Surg. 246(3):491–9.
Pneumatikos I, Koulouras V, Nathanail C, Goe D. Selective decontamination of subglottic area in mechanically ventilated patients with multiple trauma. Intensive Care Med. 2002;28(4):432–7.
Roquilly A, Mahe PJ, Seguin P, Guitton C. Hydrocortisone therapy for patients with multiple trauma: the randomized controlled HYPOLYTE study. JAMA. 2011;305(12):1201–9.
Saltzherr T, Bakker FC, LF Beenen, Dijkgraaf M, (2012) REACT Study Group. Randomized clinical trial comparing the effect of computed tomography in the trauma room versus the radiology department on injury outcomes. Br J Surg. 99(Suppl 1):105–13.
Tsilika M, Thoma G, Aidoni Z, Tsaousi G, Fotiadis K, Stavrou G, et al. A four-probiotic preparation for ventilator-associated pneumonia in multi-trauma patients: results of a randomized clinical trial. Int J Antimicrob Agents. 2022;59(1): 106471.
Chytra I, Pradl R, Bosman R, Pelnář P, Kasal E, Židková A. Esophageal Doppler-guided fluid management decreases blood lactate levels in multiple-trauma patients: a randomized controlled trial. Crit Care. 2007;11(1):R24.
Zhang X. (2022) Effects of Targeted Intervention plus Comprehensive Nursing on the Quality of Life and Nursing Satisfaction in Multiple Traumas. Xi X, editor. Evid Based Complement Alternat Med. 2022:1–5.
Nascimento B, Callum J, Tien H, Rubenfeld G, Pinto R, Lin Y, et al. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results–guided transfusion in patients with severe trauma: a randomized feasibility trial. Can Med Assoc J. 2013;185(12):E583–9.
Nascimento B, Callum J, Tien H, Peng H, Rizoli S, Karanicolas P, et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): a randomized feasibility trial. Br J Anaesth. 2016;117(6):775–82.
James MFM, Michell WL, Joubert IA, Nicol AJ, Navsaria PH, Gillespie RS. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: the FIRST trial (Fluids in Resuscitation of Severe Trauma). Br J Anaesth. 2011;107(5):693–702.
Spinella PC, Thomas KA, Turnbull IR, Fuchs A, Bochicchio K, Schuerer D, et al. The immunologic effect of early intravenous two and four gram bolus dosing of tranexamic acid compared to placebo in patients with severe traumatic bleeding (TAMPITI): A randomized, double-blind, placebo-controlled. Single-Center Trial Front Immunol. 2020;11:2085.
Marjanovic N, Boisson M, Asehnoune K, Foucrier A, Lasocki S, Ichai C, et al. Continuous pneumatic regulation of tracheal cuff pressure to decrease ventilator-associated pneumonia in trauma patients who were mechanically ventilated. Chest. 2021;160(2):499–508.
Zhao Xd, Qin Yh, Jx Ma. Influence of intensive insulin therapy on vascular endothelial growth factor in patients with severe trauma. J Huazhong Univ Sci Technol. 2018;33:107–10.
Holmes A, Hodgins G, Adey S, Menzel S. Trial of interpersonal counselling after major physical trauma. Aust N Z J Psychiatry. 2007;41(11):926–33.
Helm M, Schuster R, Hauke J, Lampl L. Tight control of prehospital ventilation by capnography in major trauma victims. Br J Anaesth. 2003;90(3):327–32.
Costa ML, Achten J, Knight R, Bruce J, Trial WHIST, Collaborators. Effect of incisional negative pressure wound therapy vs standard wound dressing on deep surgical site infection after surgery for lower limb fractures associated with major trauma: the Whist randomized clinical trial. JAMA. 2020;323(6):519–26.
Baksaas-Aasen K, Gall LS, Stensballe J, Juffermans NP. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med. 2021;47(1):49–59.
Hauser CJ, Boffard K, Dutton R, Bernard GR. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69(3):489–500.
Curry N, Foley C, Wong H, Mora A. Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): results from a UK multi-centre, randomised, double blind, placebo-controlled pilot trial. Crit Care. 2018;22(1):164.
Morrison CA, Carrick MM, Norman MA, Scott BG. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma. 2011;70(3):652–63.
Lu Y, Liu L, Wang J, Cui L. Controlled blood pressure elevation and limited fluid resuscitation in the treatment of multiple injuries in combination with shock. Pak J Med Sci. 2018;34(5):1120–4.
Ma J, Han S, Liu X. Sodium bicarbonated Ringer’s solution effectively improves coagulation function and lactic acid metabolism in patients with severe multiple injuries and traumatic shock. Am J Transl Res. 2021;13(5):5043–50.
Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enter Nutr. 2007;31(2):119–26.
Bible LE, Pasupuleti LV, Alzate WD, Gore AV. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg. 2014;77(1):54–50.
Ho KM, Rao S, Honeybul S, Zellweger R, Wibrow B. A Multicenter Trial of Vena Cava Filters in Severely Injured Patient. N Engl J Med. 2019;381(4):328–37.
Liu T, Liu P, Chen J, Xie J. A randomized controlled trial of surgical rib fixation in polytrauma patients with flail chest. J Surg Res. 2019;242:223–30.
Innerhofer P, Fries D, Mittermayr M, Innerhofer N. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017;4(6):e258–71.
Han J, Ren HQ, Zhao QB, Wu YL. Comparison of 3% and 7.5% hypertonic saline in resuscitation after traumatic hypovolemic shock. Shock. 2015;43(3):244–9.
Khan FA, Ledgerwood AM, Lucas CE. The role of pharmacological steroid therapy in preservation of renal function in severely injured patients requiring massive transfusion. Eur J Trauma Emerg Surg. 2016;42(4):477–81.
Sperry J, Guyette F, Brown J, Zenati M, PAMPer Study Group. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315–26.
Boelens PG, Houdijk AP, Fonk JC, Nijveldt RJ. Glutamine-enriched enteral nutrition increases HLA-DR expression on monocytes of trauma patients. J Nutr. 2002;132(9):2580–6.
Schreiber MA, Meier EN, Tisherman SA, Kerby JD, Newgard C. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687–95.
Juschten J, Tuinman PR, Guo T, Juffermans NP, Schultz MJ, Loer SA, et al. Between-trial heterogeneity in ARDS research. Intensive Care Med. 2021;47(4):422–34.
James A, Abback PS, Pasquier P, Ausset S, Duranteau J, Hoffmann C, et al. The conundrum of the definition of haemorrhagic shock: a pragmatic exploration based on a scoping review, experts’ survey and a cohort analysis. Eur J Trauma Emerg Surg. 2022. https://doi.org/10.1007/s00068-022-01998-9.
Antonucci E, Polo T, Giovini M, Girardis M, Martin-Loeches I, Nielsen ND, et al. Refractory septic shock and alternative wordings: a systematic review of literature. J Crit Care. 2023;75: 154258.
The ARDS Definition Task Force. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307(23):2526–33.
Singer M, Deutschman C, Seymour C, Vincent J, Angus D. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
Olaniran A, Smith H, Unkels R, Bar-Zeev S, Van Den Broek N. Who is a community health worker? – a systematic review of definitions. Glob Health Action. 2017;10(1):1272223.
Hanley GE, Munro S, Greyson D, Gross MM, Hundley V, Spiby H, et al. Diagnosing onset of labor: a systematic review of definitions in the research literature. BMC Pregnancy Childbirth. 2016;16(1):71.
CRASH-2 trial collaborators. (2010) Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. The Lancet. 376:10
Yordanov Y, Dechartres A, Porcher R, Boutron I, Altman DG, Ravaud P. Avoidable waste of research related to inadequate methods in clinical trials. BMJ. 2015;350:h809–h809.
Butcher NE, Enninghorst N, Sisak K, Balogh ZJ. The definition of polytrauma: Variable interrater versus intrarater agreement: A prospective international study among trauma surgeons. J Trauma Acute Care Surg. 2013;74(3):884–9.
Wilson J, Calfee C. ARDS Subphenotypes: Understanding a Heterogeneous Syndrome. Crit Care. 2020;24:102.
Tachino J, Matsumoto H, Sugihara F, Seno S, Okuzaki D, Kitamura T, et al. Development of clinical phenotypes and biological profiles via proteomic analysis of trauma patients. Crit Care. 2022;26(1):241.
Funding
This work was supported by a grant from the French Society of Anaestesiology, Critical Care and Peri Operative Medicine “Bourse d’aide à la mobilité”, 2022). The funder had no role in the study design, data collection and analysis, the preparation or approval of the manuscript, or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
TJ was contributed to conceptualization, methodology, formal analysis, validation, investigation, writing original draft and editing, visualization and supervision; EC was contributed to validation, investigation, editing, visualization and supervision; BD was contributed to validation and editing; MR was contributed to validation, editing and supervision; AJ was contributed to conceptualization, methodology, formal analysis, validation, investigation, writing original draft and editing, visualization and supervision; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no funding or conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Material 1: Check list PRISMA. Supplementary Material 2: Research Algorithm for RCT & Protocols. Supplementary Material 3: Characteristics of included studies. Supplementary Material 4: Distribution of terms used. Supplementary Material 5: Risk of bias assessment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jeanmougin, T., Cole, E., Duceau, B. et al. Heterogeneity in defining multiple trauma: a systematic review of randomized controlled trials. Crit Care 27, 363 (2023). https://doi.org/10.1186/s13054-023-04637-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04637-w