Abstract
Purpose
The objectives of this study were to analyse the clinical value of protein S100b (S100b) in association with clinical findings and anticoagulation therapy in predicting traumatic intracranial haemorrhage (tICH) and unfavourable outcomes in elderly individuals with low-energy falls (LEF).
Methods
We conducted a retrospective study in the emergency department (ED) of the LMU University Hospital, Munich by consecutively including all patients aged ≥ 65 years presenting to the ED following a LEF between September 2014 and December 2016 and receiving an emergency cranial computed tomography (cCT) examination. Primary endpoint was the prevalence of tICH. Multivariate logistic regression models and receiver operating characteristics were used to measure the association between clinical findings, anticoagulation therapy and S100b and tICH.
Results
We included 2687 patients, median age was 81 years (60.4% women). Prevalence of tICH was 6.7% (180/2687) and in-hospital mortality was 6.1% (11/180). Skull fractures were highly associated with tICH (odds ratio OR 46.3; 95% confidence interval CI 19.3–123.8, p < 0.001). Neither anticoagulation therapy nor S100b values were significantly associated with tICH (OR 1.14; 95% CI 0.71–1.86; OR 1.08; 95% CI 0.90–1.25, respectively). Sensitivity of S100b (cut-off: 0.1 ng/ml) was 91.6% (CI 95% 85.1–95.9), specificity was 17.8% (CI 95% 16–19.6), and the area under the curve value was 0.59 (95% CI 0.54 − 0.64) for predicting tICH.
Conclusion
In conclusion, under real ED conditions, neither clinical findings nor protein S100b concentrations or presence of anticoagulation therapy was sufficient to decide with certainty whether a cCT scan can be bypassed in elderly patients with LEF. Further prospective validation is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Low-energy falls (LEF) (e.g. from standing height, chair, bed or less than 5 steps from stair) are amongst the most frequent causes for presentation to a primary care physician or the emergency department (ED) in elderly adults. Head injury, including traumatic intracranial haemorrhage (tICH), is considered to be the most common fall-related injury in this population [1]. Both morbidity and mortality after falls appear to increase with age [2]. Trauma registry analysis reveals that severe head injuries are common in elderly trauma victims, mainly due to falls from low levels [3]. In agreement with other authors, we found a prevalence for tICH in elderly adults with LEF presenting to the ED between 5 and 7% [4,5,6], whilst observed in-hospital mortality in patients with tICH due to LEF ranged from 8% [4] to 18% [6]. Although anticoagulation and antiplatelet therapies are considered to increase the risk for tICH [7,8,9], more recent studies do not support such an association [4, 6, 10, 11].
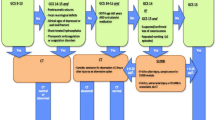
Consensus guidelines and clinical decision rules either lack specific recommendations for performing cranial computed tomography (cCT) in elderly adults with LEF and signs of head trauma [12,13,14] or recommend a cCT in every head trauma patient aged 65 years or older [15]. In addition, biomarkers like the calcium-binding protein S100b, which is largely but not exclusively expressed in brain astrocytes and released upon head injury [16, 17], have been shown to be correlated with CT abnormalities in trauma patients with mild traumatic brain injury, when sampled within 3–12 h after the injury [16,17,18,19,20]. The serum biomarker protein S100b has been added to the Scandinavian Guidelines for Initial Management of Minimal, Mild and Moderate Head Injuries for determination of need for cCT imaging in the low-risk group of patients [18, 21]. It accurately predicts normal cCT findings after minor head injury in elderly adults [22]. The determination of serum levels of protein S100b with a cut-off threshold of 0.1 ng/ml is an integral part in guidelines [18] and local protocols for management [16] of adult minor head trauma. However, it remains unclear from previous data whether protein S100b is a reliable negative prediction tool for tICH in elderly adults with LEF in real-life ED circumstances, when in the majority of patients’ protein S100b, sampling cannot be provided in the 3 to 6 h interval from the index fall.
Therefore, the objective of this study was to analyse the clinical value of protein S100b in association with clinical findings and anticoagulation/antiplatelet medication in predicting tICH and unfavourable outcomes in elderly individuals with LEF.
Methods
Study design and setting
This retrospective single-centre observational study represents a subset of a published larger patient cohort [23], including patients from one university tertiary care hospital in Germany (University Hospital of Ludwig-Maximilians-University Munich) using electronic health records (EHR). The study is in accordance with the declaration of Helsinki and was conducted using STROBE guidelines. Ethics approval was obtained from the local ethics committee (EK LMU 17-217, approved May 10, 2017).
Study population
Parts of the methods used in this study have been previously described [24]. In short, patients aged 65 years and older, who suffered from a LEF (as previously defined [24]: standing height, low level furniture, low level ≤ 1 m) and underwent cCT examination within 48 h of the index visit to the ED from September 1st 2014, to December 31st 2016, were consecutively included. Patients referred from another hospital with preceding imaging, patients who required trauma team activation, as well as patients with a delayed presentation (≥ 8 days after the fall) were excluded from this study. A subgroup of patients presenting from January 1, 2016, to December 31, 2016 has been previously analysed and published [4].
Data collection
The radiology information system (RIS) of the study centre was screened for patients aged 65 years and older, receiving a CT examination within 48 h of their index visit to the ED during the above mentioned 28-months-period [24]. EHR of retrieved cases were manually screened for documented LEF by two trained non-blinded reviewers (R.W., A.L. S.N. and V.P.). Baseline demographics, in-hospital mortality, Emergency Severity Index (ESI), Glasgow Coma Scale (GCS) on initial presentation, loss of consciousness (LOC), visible signs of injuries above the clavicles (e.g. hematoma, bruises, wounds), symptoms of head injury (amnesia, headache, vertigo, nausea, vomiting), final injury diagnosis, polypharmacy (more than five medications), laboratory values of the initial routine blood sampling upon presentation to the ED (serum calcium-binding protein S100b, serum albumin, serum sodium, serum urea, serum creatinine, thrombocytes in cell blood count, activated partial thromboplastin time, aPTT and international normalised ratio INR in citrate blood measurement) and anticoagulation/antiplatelet therapy were extracted from the EHR. Anticoagulation therapy was distinguished as previously described [4]: oral anticoagulants (OACs; phenprocoumon or acenocoumarol), direct oral anticoagulants (DOACs; dabigatran, rivaroxaban, apixaban or edoxaban), heparins (liquemin or low-molecular-weight heparins), antiplatelet agents (acetylsalicylic acid, clopidogrel, ticagrelor or prasugrel), single or dual therapy and no anticoagulation/antiplatelet therapy. A tICH was classified as epidural hematoma, subdural hematoma, traumatic subarachnoid haemorrhage or intraparenchymal haemorrhage. Fractures of the skull and maxillofacial or cervical spine fractures were extracted from the final radiology report. Chart abstraction for all parameters was performed for all patients by two trained observers (R.W., S.N., A.L. and V.P.) in every case. Disagreement or equivocalness was decided upon by a third observer (R.W. and V.P.).
The Injury Severity Score (ISS; range: 1–75) was calculated based on the Abbreviated Injury Scale (AIS) [25], extracted from the final radiology and discharge report or directly from cCT images, performed by a senior trauma surgeon (V.P.). Using the highest AIS (range: 1–6) of the three (AISA, AISB, AISC) most severely injured body regions, the ISS was determined as: ISS = AISA2 + AISB2 + AISC2. Head-specific AIS (hAIS) was defined by the most severe cranial/intracranial injury.
Screening and chart review abstraction were conducted in accordance with the recommendations for medical chart review [26, 27], which were fulfilled for 11 of 12 guidelines (abstractors were not blinded to the hypothesis). Double-data entry was performed in a Microsoft Access 2010/2016 database (Microsoft, Redmond, Washington, USA).
Key outcome measures
The primary endpoint of the study was the endpoint of a tICH. Secondary endpoints were head-specific AIS in case of an intracranial haemorrhage, in-hospital mortality and neurosurgical intervention in case of tICH.
Predictor variables
Definition of predictor variables was carried out as previously described [4]. In short, any anticoagulation or antiplatelet therapy, age, sex, ESI level, GCS, presence of polypharmacy, reported LOC, symptoms of head injury, injury signs above the clavicles, newly diagnosed fractures of the skull, maxillofacial or cervical spine and laboratory values (S100b, serum albumin, serum sodium, serum urea, serum creatinine and INR) were predictor variables for tICH. In a further step, anticoagulation/antiplatelet therapy at three different levels of granularity was the predefined predictor variables. First, any anticoagulation or antiplatelet therapy vs. no anticoagulation or antiplatelet therapy. Second, antiplatelet therapy exclusively, anticoagulation therapy exclusively and combination of anticoagulation and antiplatelet vs. no anticoagulation/antiplatelet therapy. Third, single antiplatelet therapy exclusively, dual antiplatelet therapy exclusively, OAC exclusively, DOACs exclusively, combination of OAC and antiplatelet, and combination of direct-acting oral anticoagulants and antiplatelet vs no anticoagulation/antiplatelet therapy. Group sizes for heparins (n = 19) and for combinations of more than one anticoagulation (n = 3) or combinations of heparins with any antiplatelet agent (n = 17) or combination of more than one anticoagulation with an antiplatelet agent (n = 0) were small; therefore, these groups were excluded from further analysis (see Table 1).
Statistics
For descriptive statistics, the median and interquartile ranges (IQRs) were used to report continuous and ordinal data, where applicable. The Kruskal–Wallis test was used to compare central tendency for continuous and ordinal data. The Pearson’s chi-squared test with Yates’s continuity correction or the Fisher exact test was used for the comparison of categorical data. A first multivariable logistic regression, including all predictor variables, was calculated to identify risk factors for the primary outcome tICH. Of all identified statistically significant risk factors, ESI level below 5, GCS below 15, and injury signs above the clavicles were chosen as covariates for the subsequent regression models because they are easily assessable at triage. For the binary outcomes tICH and in-hospital mortality, multivariable logistic regression models were adjusted for ESI level, GCS and injury signs above the clavicles to assess the association with anticoagulation/antiplatelet therapy. For the outcome of in-hospital mortality, the multivariable model was additionally adjusted for age and sex. Multivariable quasi-Poisson regression models adjusted for ESI level, GCS and injury signs above the clavicles were calculated to assess the association between anticoagulation/antiplatelet therapy and continuous ordinal outcomes, respectively (head-specific injury severity, hAIS).
The receiver operating characteristic (ROC) analysis was performed to determine whether S100b can be used as a diagnostic tool for predicting tICH. The area under the ROC curve (AUC) was calculated with 95% confidence intervals (CI). Partial AUC (pAUC) was used to compare only a proportion of the AUC curve, which was set to the clinically relevant range of 90 to 100% sensitivity. The P values < 0.05 were considered significant. R software v.4.1.2 (https://www.R-project.org/) and RStudio 2022.07.1 Build 554 were used to carry out the statistical analysis.
Results
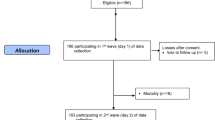
We included 2687 patients in the analysis (Fig. 1). Median age was 81 years (IQR 75 − 88) and 60.4% of included patients were female. Table 1 shows baseline demographic information. Overall in-hospital mortality rate was 2.0% (55/2687), admission rate to intensive care unit (ICU) was 4.0% (107/2687) (Table 1).
Traumatic intracranial haemorrhage was detected in 180 of 2687 patients (6.7%). Of these 180 patients with tICH, 127 (70.6%) had a severe tICH (hAIS ≥ 3), 24 were admitted to the ICU (14.0%). Of 180 patients with tICH, 150 (83.3%) had an isolated head injury, 22 received a neurosurgical intervention (12.2%) and 11 (6.1%) died in the hospital (Table 1). Additional detailed information about patients with tICH receiving neurosurgical intervention or none is given in Supplementary Table 1.
Anticoagulation/antiplatelet therapy was documented in 1386 of 2687 (51.6%) patients, with a prevalence for tICH of 93 of 1386 (6.7%) vs. no anticoagulation/antiplatelet therapy in 1084 of 2687 (40.3%) patients, with a prevalence of 72 of 1084 (6.6%). In 217 of 2687 (8.1%) patients, the documentation about anticoagulation/antiplatelet therapy was missing, of which 15 of 217 (6.9%) patients were diagnosed with a tICH.
Analysis of the serum protein S100b concentrations upon presentation to the ED was performed in 1851 of 2687 patients of our study population (68.9%). 2469 (91.9%) of these patients patients presented within 24 h delay after the fall. In 1532 of 1851 patients (82.8%), serum S100b concentrations were elevated above 0.1 ng/ml, which determines the cut-off value for screening of tICH [16, 18]. However, exact time of sampling after the fall was not determined due to imprecise given information.
The multivariable logistic regression model for risk factors of tICH indicated skull fracture as the strongest predictor (OR 46.3; 95% CI 19.3–123.8, p < 0.001). Facial bone fracture (OR 2.29; 95% CI 1.33–4.33, p = 0.01), male gender (OR 1.60; 95% CI 1.01–2.54, p = 0.04) and increased INR value (OR 1.36; 95% CI 1.03–1.77, p = 0.02) were further predictive for tICH. GCS level (OR 0.83; 95% CI 0.66–1.09, p = 0.14) and ESI level (OR 0.79; 95% CI 0.50–1.24, p = 0.3) were not predictive for tICH (Fig. 2). Any anticoagulation or antiplatelet medication was not predictive for tICH (OR 1.14; 95% CI 0.71–1.86, p = 0.6). The biomarker S100b was not predictive (OR 1.08; 95% CI 0.90–1.25, p = 0.35) for tICH. Supplementary Table 2 summarises the underlying univariate logistic regression analysis of potential risk factors for tICH and severe tICH.
Multivariable regression models demonstrated that medication with any anticoagulation or antiplatelet agent was not associated with the primary outcome of tICH (OR 0.97; 95% CI 0.68–1.37; p = 0.85), in-hospital mortality (OR 1.96; 95% CI 0.95–4.34; p = 0.08), or ISS (incident rate ratio IRR: 0.97; 95% CI 0.88–1.07; p = 0.60) (Supplementary Fig. 1). In patients with tICH (180/2687), any anticoagulation or antiplatelet therapy was not associated with head-specific injury severity (IRR: 0.75; 95% CI 0.36–1.55. p = 0.43) (Supplementary Fig. 1). Anticoagulation combined with an antiplatelet agent was associated with in-hospital mortality after a fall (OR 4.82; 95% CI 1.40–14.8; p = 0.008). Combined treatment of an oral anticoagulant with an antiplatelet agent (OR 5.23; 95% CI 0.72–23.875; 0 = 0.052) or combined treatment of a direct-acting oral anticoagulant with an antiplatelet agent (OR 6.16; 95% CI 1.29–22.38; p = 0.009) was also associated with in-hospital mortality.
With a cut-off threshold of 0.1 ng/ml, sensitivity was 91.6% (CI 95% 85.1–95.9), specificity was 17.8% (CI 95% 16–19.6), positive predictive value (PPV) was 7.4% (CI 95% 7.9–7.8) and negative predictive value (NPV) was 96.7% (CI 95% 94.2–98.2) for predicting tICH. The AUC values of S100b were 0.59 (95% CI 0.54 − 0.64) for predicting tICH. The ROC curve showing the performance of S100b for prediction of tICH is presented in Fig. 3. With a cut-off threshold of 0.305 pg/ml, sensitivity was 63%, specificity was 54%, PPV was 95% and negative predictive NPV was 9.2% for predicting tICH. In 10 patients with a tICH detected by cCT and presenting within 24 h after the index fall, serum protein S100b concentrations were below the defined cut-off threshold of 0.1 ng/ml upon initial measurement. Characteristics of these patients are presented in Supplementary Table 3.
Receiver operating characteristic curve (ROC) demonstrating performance of serum calcium-binding protein S100b (S100b) as biomarker for predicting traumatic intracranial haemorrhage (tICH) on cCT scan within 48 h of the index fall. Illustrated is the partial area under the curve (pAUC) (with 95% confidence interval), with specificity and sensitivity set to 90–100% and the cut-off value of 2.1 ng/ml at the particular level of specificity/sensitivity
Discussion
In this retrospective cohort analysis of a single-centre university tertiary care hospital, we found an overall prevalence of 6.7% for tICH in elderly patients with low-energy falls. Neither anticoagulation, antiplatelet therapy nor the combined treatment was relevant risk factors for tICH or severe intracranial haemorrhage. Conversely, concomitant skull fractures are highly associated with tICH. Although the calcium-binding protein S100b is valuable in determining the need for cCT in younger patients, the serum biomarker showed a poor performance in predicting tICH in this real-life cohort of elderly patients with exclusively low-energy falls. This study combines factors of clinical examination, the presence of an antiplatelet/anticoagulation therapy and the biomarker protein S100b for prediction of tICH in the elderly patient with LEF, where mild TBI is possible but not obvious in every case.
Comparable to our previously published analysis [4] and other recent results from a prospective observational study in a cohort of elderly adults with LEF [6], the prevalence for a tICH was 6.7%, confirming this substantial rate of partially relevant low-energy accident injury. In this present study cohort, an increased INR and concomitant skull fractures, detected by cCT scan, were associated with a tICH, whereas no other clinical finding or laboratory parameters proved to be significantly associated with tICH, in contrast to the prior published data [4, 6, 22]. However, the recent analysis adds more support to prior findings [4, 6, 10, 11, 22, 28, 29], that neither anticoagulation, antiplatelet therapy nor their combinations are relevant risk factors for tICH or severe tICH. Consequently, the absence of anticoagulation or antiplatelet therapy in patients’ medication does not allow for omission of cCT in elderly adults with LEF and suspected brain injury.
Elevated serum levels of the calcium-binding protein S100b, sampled within 3 to 12 h after the injury, have been shown to be correlated with CT abnormalities in trauma patients with mild traumatic brain injury [16,17,18,19,20]. The biomarker has been added to the Scandinavian Guidelines for Initial Management of Minimal, Mild and Moderate Head Injuries for determination of need for cCT imaging in the low-risk group of patients [18, 21]. Prior prospective studies, including all kinds of trauma mechanisms and all ages of trauma patients in the ED, analysed performance of protein S100b for the prediction of tICH [22, 30, 31] with cut-off levels of 0.1 ng/ml [31], 0.105 ng/ml [22, 32] or 0.2 ng/ml [30] and S100b sampling within 3 [22, 31, 32] to 4 [30] hours after the index accident. In older patients with mild traumatic brain injury, negative protein S100b levels sampled within 3 h after the impact predicted a normal cCT with an AUC of 0.73 (CI 0.67–0.79), a sensitivity of 98.0% (CI 89.5–99.7%), a specificity of 35.3% (CI 31.9–38.8%), a NPV of 99.6% (CI 97.9–99.9%) and a PPV of 9.4% (CI 7.2–12.2%) [22]. Accordingly, classification performance for detecting tICH in cCT yielded a sensitivity of 100% (95% CI 63–100), a specificity of 5% (95% CI 2–13), a NPV of 100% (CI 40–100%), and a PPV of 11% (CI 5–20%) with an AUC of 0.78 (CI 0.67–0.89) [30]. Elevated S100b levels were more common in the group of elderly adults [31, 32], aged 65 years or older, whilst specificity for CT findings was higher in the subgroup of younger adults (35%) vs. 30% in the entire population, with a sensitivity of 100% [31]. Mean S100b serum levels differ in different types of tICH when measured within 3 h after the injury, i.e. S100b is the highest in patients with cerebral oedema and lowest in patients with concussion symptoms and no traumatic findings in cCT [32]. The analysis of our study revealed no significant association of S100b levels with tICH detected in cCT, with poorer performing characteristics of the biomarker in the prediction of tICH (AUC: 0.59) and lower sensitivity and specificity than in prior studies [22, 30]. Noteworthy, 10 out of 180 patients with tICH (5 of them with severe tICH with a hAIS ≥ 3) had no elevated serum levels of protein S100b, although sampled within 24 h after the index fall. Half-life of protein S100b is known to be 2 to 6 h in mild traumatic brain injury and 24 h in severe brain injury [33, 34], with an optimal time interval for screening of 1–3 h after the head impact [35]. We interpret our findings mainly in the light of the imprecise information given about the exact time point of the index fall, which was rarely within the 1–3 h interval. Therefore, this might limit the clinical value of protein S100b as a screening biomarker in real-life cohorts of elderly adults with LEF and unknown or imprecise delay between injury and presentation to the ED.
Considering protein S100b as a screening tool in the ED setting to reduce the number of CT scans and associated costs, two prior studies analysed the economic impact of protein S100b as pre-cCT screening tests [36, 37]. Inclusion of protein S100b in the Scandinavian guidelines [18, 21] with strict adherence during decision making, resulted in a cost-saving of up to 71 € per patient with suspected mild traumatic brain injury [36]. Waiting time for blood test or final CT scans were not considered in this study. Protein S100b becomes cost-lowering when the proportion of patients undergoing a cCT scan is high (> 78%) or the waiting time for CT scan results exceeds the waiting time for blood test results by 96 min [37]. Despite its ability to reduce costs, the performance of protein S100b to predict tICH is limited. Our data support the consideration, that protein S100b determination can be dispensed in the ED work-up of elderly adults with LEF and risk for tICH. However, as there are no reliable alternative clinical risk factors identified recently, no recommendations can be made to reduce cCT examinations in these patients.
The main strength of our study is the observational design with a representative cohort of a local population of elderly adults and meaningful sample sizes. This allowed the calculation of multivariable regression models for the primary outcome and subgroups of anticoagulation and antiplatelet therapies. Nevertheless, the study is limited by its retrospective design and by initial patient selection. The first limitation is that the main clinical findings were extracted from EHR, which makes the study dependent on documentation quality and granularity. Second, data are derived from a single centre. Thus, no conclusions can be drawn on the overall population of elderly adults with LEF. Third, the number of neurosurgical interventions, defining a clinically important brain injury, was too small to allow for a statistical meaningful analysis of risk and prediction factors. Despite in-hospital mortality of patients with tICH having increased more than threefold, the majority of these patients died from reasons other than acute tICH. This further limits the significance of the prediction model with regard to unfavourable outcomes due to tICH.
In conclusion, we found a prevalence of 6.7% for tICH in elderly adults presenting to the ED after a LEF. Determination of serum concentrations of the calcium-binding protein S100b does not allow reliable prediction of tICH if the exact time point of the trauma is unclear. Our data suggest that screening for clinical findings or biomarkers is not sufficient to safely decide for omission of cCT scan in elderly patients after LEF, neither is the prevalence of anticoagulation nor antiplatelet therapy. These findings merit further prospective validation.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gelbard R, Inaba K, Okoye OT, Morrell M, Saadi Z, Lam L, Talving P, Demetriades D. Falls in the elderly: a modern look at an old problem. Am J Surg. 2014;208(2):249–53.
Spaniolas K, Cheng JD, Gestring ML, Sangosanya A, Stassen NA, Bankey PE. Ground level falls are associated with significant mortality in elderly patients. J Trauma. 2010;69(4):821–5.
Spering C, Lefering R, Bouillon B, Lehmann W, von Eckardstein K, Dresing K, Sehmisch S. It is time for a change in the management of elderly severely injured patients! An analysis of 126,015 patient from the TraumaRegister DGU((R)). Eur J Trauma Emerg Surg. 2019;46(3):487–97.
Lampart A, Kuster T, Nickel CH, Bingisser R, Pedersen V. Prevalence and severity of traumatic intracranial hemorrhage in older adults with low-energy falls. J Am Geriatr Soc. 2020;68(5):977–82.
de Wit K, Merali Z, Kagoma YK, Mercier E. Incidence of intracranial bleeding in seniors presenting to the emergency department after a fall: a systematic review. Injury. 2020;51(2):157–63.
de Wit K, Parpia S, Varner C, Worster A, McLeod S, Clayton N, Kearon C, Mercuri M. Clinical predictors of intracranial bleeding in older adults who have fallen: a cohort study. J Am Geriatr Soc. 2020;68(5):970–6.
Grandhi R, Harrison G, Voronovich Z, Bauer J, Chen SH, Nicholas D, Alarcon LH, Okonkwo DO. Preinjury warfarin, but not antiplatelet medications, increases mortality in elderly traumatic brain injury patients. J Trauma Acute Care Surg. 2015;78(3):614–21.
Minhas H, Welsher A, Turcotte M, Eventov M, Mason S, Nishijima DK, Versmee G, Li M, de Wit K. Incidence of intracranial bleeding in anticoagulated patients with minor head injury: a systematic review and meta-analysis of prospective studies. Br J Haematol. 2018;183(1):119–26.
van den Brand CL, Tolido T, Rambach AH, Hunink MG, Patka P, Jellema K. Systematic review and meta-analysis: is pre-injury antiplatelet therapy associated with traumatic intracranial hemorrhage? J Neurotrauma. 2017;34(1):1–7.
Hamden K, Agresti D, Jeanmonod R, Woods D, Reiter M, Jeanmonod D. Characteristics of elderly fall patients with baseline mental status: high-risk features for intracranial injury. Am J Emerg Med. 2014;32(8):890–4.
Nishijima DK, Gaona S, Elms AR, Farrales RD, Montoya J, Gilbert M, Trajano RP, Hatchel KM, Vinson DR, Ballard DW, et al. The incidence of traumatic intracranial hemorrhage in head-injured older adults transported by emergency medical services with and without anticoagulant or antiplatelet use. J Neurotrauma. 2018;35(5):750–9.
Stiell IG, Wells GA, Vandemheen K, Clement C, Lesiuk H, Laupacis A, McKnight RD, Verbeek R, Brison R, Cass D. The Canadian CT head rule for patients with minor head injury. Lancet. 2001;357(9266):1391–6.
Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. NEJM. 2000;343(2):100–5.
Smits M, Dippel DW, Steyerberg EW, de Haan GG, Dekker HM, Vos PE, Kool DR, Nederkoorn PJ, Hofman PA, Twijnstra A, et al. Predicting intracranial traumatic findings on computed tomography in patients with minor head injury: the CHIP prediction rule. Ann Intern Med. 2007;146(6):397–405.
Jagoda AS, Bazarian JJ, Bruns JJ Jr, Cantrill SV, Gean AD, Howard PK, Ghajar J, Riggio S, Wright DW, Wears RL, et al. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52(6):714–48.
Biberthaler P, Linsenmeier U, Pfeifer KJ, Kroetz M, Mussack T, Kanz KG, Hoecherl EF, Jonas F, Marzi I, Leucht P, et al. Serum S-100B concentration provides additional information fot the indication of computed tomography in patients after minor head injury: a prospective multicenter study. Shock. 2006;25(5):446–53.
Müller K, Townend W, Biasca N, Undén J, Waterloo K, Romner B, Ingebrigtsen T. S100B serum level predicts computed tomography findings after minor head injury. J Trauma. 2007;62(6):1452–6.
Undén J, Ingebrigtsen T, Romner B. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. BMC Med. 2013;11(1):50.
Undén J, Romner B. Can low serum levels of S100B predict normal CT findings after minor head injury in adults?: an evidence-based review and meta-analysis. Head Trauma Rehab. 2010;25(4):228–40.
Undén L, Calcagnile O, Undén J, Reinstrup P, Bazarian J. Validation of the Scandinavian guidelines for initial management of minimal, mild and moderate traumatic brain injury in adults. BMC Med. 2015;13:292.
Minkkinen M, Iverson GL, Kotilainen AK, Pauniaho SL, Mattila VM, Lehtimäki T, Berghem K, Posti JP, Luoto TM. Prospective validation of the scandinavian guidelines for initial management of minimal, mild, and moderate head injuries in adults. J Neurotrauma. 2019;36(20):2904–12.
Thaler HW, Schmidsfeld J, Pusch M, Pienaar S, Wunderer J, Pittermann P, Valenta R, Gleiss A, Fialka C, Mousavi M. Evaluation of S100B in the diagnosis of suspected intracranial hemorrhage after minor head injury in patients who are receiving platelet aggregation inhibitors and in patients 65 years of age and older. J Neurosurg. 2015;123(5):1202–8.
Pedersen V, Lampart A, Kammerlander C, Boecker W, Nickel CH, Bingisser R. Older patients with low-energy falls presenting to the emergency department: characteristics and outcomes. J Am Geriatr Soc. 2019;67(7):1527–9.
Lampart A, Arnold I, Mader N, Niedermeier S, Escher A, Stahl R, Trumm C, Kammerlander C, Bocker W, Nickel C, et al. Prevalence of fractures and diagnostic accuracy of emergency x-ray in older adults sustaining a low-energy fall: a retrospective study. J Clin Med. 2020;9(1):97.
Gennarelli TA, Wodzin E, Association for the Advancement of Automotive Medicine. Abbreviated Injury Scale 2005. update 2008
Worster A, Bledsoe RD, Cleve P, Fernandes CM, Upadhye S, Eva K. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. 2005;45(4):448–51.
Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27(3):305–8.
Galliazzo S, Bianchi MD, Virano A, Trucchi A, Donadini MP, Dentali F, Bertu L, Grandi AM, Ageno W. Intracranial bleeding risk after minor traumatic brain injury in patients on antithrombotic drugs. Thromb Res. 2019;174:113–20.
Spektor S, Agus S, Merkin V, Constantini S. Low-dose aspirin prophylaxis and risk of intracranial hemorrhage in patients older than 60 years of age with mild or moderate head injury: a prospective study. J Neurosurg. 2003;99(4):661–5.
Papa L, Silvestri S, Brophy GM, Giordano P, Falk JL, Braga CF, Tan CN, Ameli NJ, Demery JA, Dixit NK, et al. GFAP out-performs S100β in detecting traumatic intracranial lesions on computed tomography in trauma patients with mild traumatic brain injury and those with extracranial lesions. J Neurotrauma. 2014;31(22):1815–22.
Calcagnile O, Holmén A, Chew M, Undén J. S100B levels are affected by older age but not by alcohol intoxication following mild traumatic brain injury. Scand J Trauma Resusc Emerg Med. 2013;21:52.
Wolf H, Frantal S, Pajenda G, Leitgeb J, Sarahrudi K, Hajdu S. Analysis of S100 calcium binding protein B serum levels in different types of traumatic intracranial lesions. J Neurotrauma. 2015;32(1):23–7.
Hicks C, Dhiman A, Barrymore C, Goswami T. Traumatic brain injury biomarkers, simulations and kinetics. Bioengineering. 2022;9(11):612.
Kawata K, Liu CY, Merkel SF, Ramirez SH, Tierney RT, Langford D. Blood biomarkers for brain injury: what are we measuring? Neurosci Biobehav Rev. 2016;68:460–73.
Hier DB, Obafemi-Ajayi T, Thimgan MS, Olbricht GR, Azizi S, Allen B, Hadi BA, Wunsch DC 2nd. Blood biomarkers for mild traumatic brain injury: a selective review of unresolved issues. Biomark Res. 2021;9(1):70.
Calcagnile O, Anell A, Unden J. The addition of S100B to guidelines for management of mild head injury is potentially cost saving. BMC Neurol. 2016;16(1):200.
Ruan S, Noyes K, Bazarian JJ. The economic impact of S-100B as a pre-head CT screening test on emergency department management of adult patients with mild traumatic brain injury. J Neurotrauma. 2009;26(10):1655–64.
Acknowledgements
To Tobias Kuster for assistance with R scripts and to Mary Conlon-Foerster for careful language editing.
Funding
Open Access funding enabled and organized by Projekt DEAL. RW obtained research funding from the LMU Munich. Publication fees are supported by the University Hospital, LMU Munich.
Author information
Authors and Affiliations
Contributions
RW, VP, AL, RS and CT designed the study. RW, AL, SN and VP performed the data collection. CT and RS provided raw data from RIS. RW and VP performed the statistical analysis of the data. RW and VP drafted the manuscript. All authors contributed to the interpretation of the data and writing of the manuscript. All authors revised the manuscript and approved it in the final form. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Ethics approval was obtained from the local ethic committee (EK LMU 17-217, approved May 10, 2017).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wania, R., Lampart, A., Niedermeier, S. et al. Diagnostic value of protein S100b as predictor of traumatic intracranial haemorrhage in elderly adults with low-energy falls: results from a retrospective observational study. Eur J Trauma Emerg Surg 50, 205–213 (2024). https://doi.org/10.1007/s00068-023-02324-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-023-02324-7