Abstract
Purpose
The biochemical relapse-free survival (bRFS) rate after treatment with permanent iodine-125 seed implantation (PSI) or combined seeds and external beam radiotherapy (COMB) for clinical stage T1–T2 localized prostate cancer is a clinically relevant endpoint. The goal of this work was to evaluate the influence of relevant patient- and treatment-related factors.
Materials and methods
The study population comprised 312 consecutive patients treated with permanent seed implantation. All patients were evaluable for analysis of overall survival (OS) and disease-specific survival (DSS), 230 for bRFS, of which 192 were in the PSI group and 38 in the COMB group. The prescribed minimum peripheral dose was 145 Gy for PSI, for COMB 110 Gy implant and external beam radiotherapy of 45 Gy. The median follow-up time was 33 months (range 8–66 months). bRFS was defined as a serum prostate-specific antigen (PSA) level ≤ 0.2 ng/ml at last follow-up.
Results
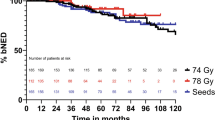
Overall, the actuarial bRFS at 50 months was 88.4 %. The 50-month bRFS rate for PSI and COMB was 90.9 %, and 77.2 %, respectively. In the univariate analysis, age in the categories ≤ 63 and > 63 years (p < 0.00), PSA nadir (≤ 0.5 ng/ml and > 0.5 ng\ml) and PSA bounce (yes/no) were the significant predicting factors for bRFS. None of the other patient and treatment variables (treatment modality, stage, PSA, Gleason score, risk group, number of risk factors, D90 and various other dose parameters) were found to be a statistically significant predictor of 50-month bRFS.
Conclusion
The biochemical failure rates were low in this study. As a proof of principle, our large monocenteric analysis shows that low-dose-rate brachytherapy is an effective and safe procedure for patients with early stage prostate cancer.
Zusammenfassung
Hintergrund und Ziel
Das biochemisch rezidivfreie Überleben (bRFS) nach der Brachytherapie mit permanenter Iod-125-Seed-Implantation (PSI) oder in Kombination mit externer Radiotherapie (COMB) ist beim Patienten mit frühem Prostatakarzinom (T1/T2) ein relevanter klinischer Endpunkt. Ziel der Arbeit war es, Langzeitergebnisse und prognostische Faktoren einer großen Kohorte zu analysieren.
Patienten und Methoden
Eine Kohorte von 312 Patienten wurde mit PSI allein oder in Kombination mit einer externen Radiotherapie behandelt. Alle Patienten konnten zur Analyse der Gesamtüberlebensrate (OS) und der krankheitsfreien Überlebensrate (DFS) herangezogen werden; in 230 Fällen wurde das bRFS kalkuliert, davon 192 in der PSI- und 38 in der COMB-Gruppe. Die Dosis der Brachytherapie betrug im Monotherapie-Arm 145 Gy und bei der Kombinationstherapie 110 Gy plus 45 Gy externe Radiotherapie. Die mediane Nachbeobachtungszeit betrug 33 Monate (Spanne 8–66 Monate). Das bRFS war definiert über ein prostataspezifisches Antigen-(PSA-)Level im Serum ≤ 0,2 ng/ml beim letzten Follow-up.
Ergebnisse
Das bRFS nach 50 Monaten lag bei 88,4 %, wobei das bRFS im Monotherapie-Arm 90,9 % und im Kombinationsarm 77,2 % betrug. Die univariate Analyse ergab, dass Alter (≤ 63 vs. > 63 Jahre; p < 0,00), PSA-Nadir (≤ 0,5 ng/ml vs. > 0,5 ng/ml) und PSA-Wiederanstieg (ja/nein) signifikant den primären Studienendpunkt beeinflussten.
Schlussfolgerung
Die biochemischen Fehlerraten in dieser Studie waren gering und das bRFS ist somit gut und vergleichbar Unsere monozentrische Studie mit ausreichender Patientenzahl zeigt, dass sich die Low-Dose-Rate-Brachytherapie als eine effektive und sichere Methode für diese Patientengruppe erwiesen hat.


Similar content being viewed by others
References
Wust P, Postrach J, Kahmann F, Henkel T, Graf R, Cho CH, Budach V, Böhmer D (2008) Postimplantation analysis enables improvement of dose–volume histograms and reduction of toxicity for permanent seed implantation. Int J Radiat Oncol Biol Phys 71:28–35
Peinemann F, Grouven U, Bartel C, Sauerland S, Borchers H, Pinkawa M, Heidenreich A, Lange S (2011) Permanent interstitial low-dose rate brachytherapy for patients with localised prostate cancer: a systematic review of randomised and nonrandomised controlled clinical trials. Eur Urol 60:881–893
Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy CA, Reuther AM, Carlson TP, Klein EA (2004) Radical prostatectomy, external beam radiotherapy < 72 Gy, external beam radiotherapy >or = 72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int J Radiat Oncol Biol Phys 8:25–33
Crook JM, Gomez-Iturriaga A, Wallace K, Ma C, Fung S, Alibhai S, Jewett M, Fleshner N (2011) Comparison of health-related quality of life 5 years after SPIRIT: surgical prostatectomy versus interstitial radiation intervention trial. J Clin Oncol 29:362–368
Sylvester JE, Grimm PD, Wong J et al (2011) Fifteen-year biochemical relapse-free survival, cause-specific survival, and overall survival following I-125 prostate brachytherapy in clinically localized prostate cancer: Seattle experience. Int J Radiat Oncol Biol Phys 81:376–381
Machtens S, Baumann R, Hagemann J, Warszawski A, Meyer A, Karstens JH, Jonas U (2006) Long-term results of interstitial brachytherapy (LDR-brachytherapy) in the treatment of patients with prostate cancer. World J Urol 24:289–295
Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, Keyes M, Kupelian P, Lee WR, Machtens S, Mayadev J, Moran BJ, Merrick G, Millar J, Roach M, Stock R, Shinohara K, Scholz M, Weber E, Zietman A, Zelefsky M, Wong J, Wentworth S, Vera R, Langley S (2012) Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the prostate cancer results study group. BJU Int 109:22–29
Guckenberger M, Lawrenz I, Flentje M (2014) Moderately hypofractionated radiotherapy for localized prostate cancer: long-term outcome using IMRT and volumetric IGRT. Strahlenther Onkol 190:48–53
Lohm G, Lütcke J, Jamil B, Höcht S, Neumann K, Hinkelbein W, Wiegel T, Bottke D (2014) Salvage radiotherapy in patients with prostate cancer and biochemical relapse after radical prostatectomy: long-term follow-up of a single-center survey. Strahlenther Onkol 190:727–731
Yoshida K, Yamazaki H, Takenaka T, Kotsuma T, Yoshida M, Masui K, Yoshioka Y, Narumi Y, Oka T, Tanaka E (2014) High-dose-rate interstitial brachytherapy in combination with androgen deprivation therapy for prostate cancer: are high-risk patients good candidates? Strahlenther Onkol 2014 May 17
Merrick GS, Zelefsky M, Sylvester K (2013) American Brachytherapy Society Prostate Low-Dose Rate Task Group. http://www.americanbrachytherapy.org/guidelines/prostate_low-doseratetaskgroup.pdf. Accessed: 13 October 2014
Davis BJ, Horwitz EM, Lee WR, Crook JM, Stock RG, Merrick GS, Butler WM, Grimm PD, Stone NN, Potters L, Zietman AL, Zelefsky MJ (2012) American Brachytherapy Society. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy 11:6–19
Rosenthal SA, Bittner NH, Beyer DC, Demanes DJ, Goldsmith BJ, Horwitz EM, Ibbott GS, Lee WR, Nag S, Suh WW, Potters L (2011) American Society for Radiation Oncology; American College of Radiology. American Society for Radiation Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys 79:335–341
Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T, Zattoni F (2011) European Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 59:61–71
Wust P, von Borczyskowski DW, Henkel T, Rosner C, Graf R, Tilly W, Budach V, Felix R, Kahmann F (2004) Clinical and physical determinants for toxicity of 125-I seed prostate brachytherapy. Radiother Oncol 73:39–48
Giberti C, Chiono L, Gallo F, Schenone M, Gastaldi E (2009) Radical retropubic prostatectomy versus brachytherapy for low-risk prostatic cancer: a prospective study. World J Urol 27:607–612
Battermann JJ, Boon TA, Moerland MA (2004) Results of permanent prostate brachytherapy, 13 years of experience at a single institution. Radiother Oncol 71:23–28
Zelefsky MJ, Hollister T, Raben A, Matthews S, Wallner KE (2000) Five-year biochemical outcome and toxicity with transperineal CT-planned permanent I-125 prostate implantation for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 47:1261–1266
Blasko JC, Grimm PD, Sylsvester JE, Cavanagh W (2000) The role of external beam radiotherapy with I-125/Pd-103 brachytherapy for prostate carcinoma. Radiother Oncol 57:273–278
Dattoli M, Wallner K, True L, Cash J, Sorace R (2003) Long-term outcomes after treatment with external beam radiation therapy and palladium 103 for patientswith higher risk prostate carcinoma: influence of prostatic acid phosphatase. Cancer 97:979–983
Ho AY, Burri RJ, Cesaretti JA, Stone NN, Stock RG (2009) Radiation dose predicts for biochemical control in intermediate-risk prostate cancer patients treated with low-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 75:16–22
Aaltomaa SH, Kataja VV, Lahtinen T, Palmgren JE, Forsell T (2009) Eight years experience of local prostate cancer treatment with permanent I125 seed brachytherapy- morbidity and outcome results. Radiother Oncol 91:213–216
Kupelian PA, Elshaikh M, Reddy CA, Zippe C, Klein EA (2002) Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol 20:3376–3385
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC (1999) Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281:1591–1597
Iselin CE, Robertson JE, Paulson DF (1999) Radical perineal prostatectomy: oncological outcome during a 20-year period. J Urol 161:163–168
Le Fur E, Malhaire JP, Baverez D, Delage F, Perrouin-Verbe MA, Schlurmann F, Guerif S, Fournier G, Pradier O, Valeri A (2012) Impact of learning curve and technical changes on dosimetry in low-dose brachytherapy for prostate cancer. Strahlenther Onkol 188:1091–1095
Goldner G, Pötter R, Battermann JJ, Schmid MP, Kirisits C, Sljivic S, van Vulpen M (2012) Comparison of seed brachytherapy or external beam radiotherapy (70 Gy or 74 Gy) in 919 low-risk prostate cancer patients. Strahlenther Onkol 188:305–310
Compliance with ethical guidelines
Conflict of interest
H. Badakhshi, R. Graf, V. Budach, and P. Wust state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Funding
No funding to support the research in regard to this paper was received.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badakhshi, H., Graf, R., Budach, V. et al. Permanent interstitial low-dose-rate brachytherapy for patients with low risk prostate cancer. Strahlenther Onkol 191, 303–309 (2015). https://doi.org/10.1007/s00066-014-0762-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0762-6