Abstract
Background
Endovascular thrombectomy (ET) efficacy and safety in stroke with a large ischemic core is still inconclusive as this population has been underrepresented in ET randomized controlled trials (RCTs).
Methods
We conducted a systematic review and meta-analysis synthesizing RCTs, which were retrieved by systematically searching: PubMed, Web of Science, SCOPUS, and Cochrane through February 18th, 2023. Our primary outcome was neurological disability measured by the modified Rankin scale (mRS). Dichotomous outcomes were pooled using risk ratio (RR) along with confidence interval (CI) using Revman V. 5.4 software.
Results
Three RCTs with a total of 1010 patients were included in our analysis. ET significantly increased the rates of functional independence (mRS ≤ 2) (RR: 2.54 with 95% CI [1.85, 3.48]), independent ambulation (mRS ≤ 3) (RR: 1.78 with 95% CI [1.28, 2.48]), and early neurological improvement (RR: 2.46 with 95% CI [1.60, 3.79]). However, there was no difference between endovascular thrombectomy and medical care in excellent neurological recovery (mRS ≤ 1) (RR: 1.35 with 95% CI [0.88, 2.08]). Also, ET significantly reduced the rate of poor neurological recovery (mRS 4–6) (RR: 0.79 with 95% CI [0.72, 0.86]). However, endovascular thrombectomy was associated with more incidence of any intracranial hemorrhage (RR: 2.40 with 95% CI [1.90, 3.01] [0.72, 0.86]).
Conclusion
ET combined with medical care was associated with better functional outcomes compared with medical care alone. However, ET was associated with a higher rate of intracranial hemorrhage. This can support extending ET indication in the management of stroke with a large ischemic core.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endovascular thrombectomy (ET) is shifting paradigms in the therapy regimens for acute ischemic stroke (AIS) caused by large vessel occlusion (LVO). Selected patients with LVO have shown better outcomes with ET as compared to medical therapy alone [1,2,3,4,5]. Current guidelines state that ET is considered when the terminal section of the internal carotid artery or the main stem of the middle cerebral artery is blocked [6,7,8], with ischemic cores on imaging no greater than 70 ml and Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) greater than six, or when there is a disparity between the volume of perfusion delay area and the ischemic core volume [9, 10]. Current guidelines do not recommend ET for patients with low Alberta Stroke Program Early Computed Tomographic Score (ASPECTS < 6) or large ischemic cores (> 70 ml) on imaging because they have been historically underrepresented in thrombectomy trials; hence, evidence in this regard is still limited [11,12,13,14].
However, more recently, several meta-analyses based primarily on observational studies have suggested better neurological outcomes and lower death rates in (ASPECTS 0–5) and infarct-core volumes from ≥ 50 mL or greater on CT perfusion or diffusion-weighted magnetic resonance imaging (MRI) [15,16,17,18]. Furthermore, the RESCUE-Japan LIMIT randomized controlled trial (RCT) (Recovery by Endovascular Salvage for Cerebral Ultra-Acute Embolism-Japan Large Ischemic Core Trial) from Japan demonstrated that individuals with an ASPECTS value of 3 to 5 had better functional outcomes with endovascular therapy than with medical care. Still, they also experienced more intracranial hemorrhages [19]. This was furtherly supported by the most recent findings from the Chinese ANGEL-ASPECT RCT [20] and the international SELECT‑2 RCT [21], which showed that patients with acute LVO in the anterior circulation and an ASPECTS score of 3–5 and an infarct-core volume of 70 to 100 ml [20]/≥ 50 mL [21] had better outcomes with endovascular therapy administered within 24 h than with medical management alone.
Therefore, the latest evidence mandates an up-to-date review. Our meta-analysis aims to investigate the safety and efficacy of ET & medical therapy versus medical therapy alone in patients with AIS and ASPECTS (3–5).
Methodology
Protocol Registration
Our systematic review and meta-analysis adhered to the guidelines provided by the PRISMA statement [22] and the Cochrane handbook for systematic reviews and meta-analyses [23]. The protocol for this review has been registered and published in PROSPERO with the following ID: CRD42023407277.
Data Sources and Search Strategy
(B.A. and M.A.) performed a thorough electronic search for relevant literature by utilizing several databases, including PubMed (MEDLINE), Web of Science, SCOPUS, and the Cochrane Central Register of Controlled Trials (CENTRAL) until February 18th, 2023. They did not use any limitations on their search. Further details about the search strategy, including the keywords and search terms, as well as the results of the search, can be found in (Table S1).
Eligibility Criteria
A PICO criterion was used to include RCTs: population (P): patients with AIS with a large infarct size defined as large vessel occlusion with ASPECTS score 3 to 5; intervention (I): endovascular thrombectomy plus medical therapy; control (C): medical therapy alone; outcome (O): primary outcomes of this review are the efficacy outcomes: early neurological improvement assessed by ≥ 4 points reduction in the National Institutes of Health Stroke Scale (NIHSS), excellent neurological recovery (modified Rankin Scale (mRS) 0–1), functional Independence (mRS 0–2), and independent ambulation (mRS 0–3). The secondary outcomes included safety outcomes: (any-cause mortality at 90 days, poor neurological recovery (mRS 4–6), any intracerebral hemorrhage, symptomatic intracerebral hemorrhage, and decompressive craniectomy).
We did not consider a range of research designs in our analysis. Specifically, we excluded non-human studies, preliminary reports, various forms of observational studies, single-arm clinical trials, in vitro experiments conducted on tissues and cultures, book chapters, editorial, and press articles, publications that only contain abstracts or posters, unpublished study protocols, and studies that were conducted in languages other than English.
Study Selection
The review process was carried out using the Covidence online tool. (H.A.S. and A.S.) reviewed the retrieved records independently after eliminating any duplicated records. The full-texts of the records that met the initial eligibility criteria were examined through full-text screening. Any discrepancy was resolved by consensual discussion and agreement.
Data Extraction
Two reviewers (H.A.S. and A.S.) independently extracted all data using a standardized electronic spreadsheet: study characteristics (country, study design, total participants, main inclusion criteria, intervention, and comparison methods, ASPECTS, timing after symptoms onset (time window), and baseline imaging); baseline characteristics (age, sex, number of patients in each group, ASPECTS score, NIHSS, infarct core volume, and occlusion location); efficacy outcomes data (early neurological improvement, excellent neurological recovery (mRS 0–1), functional Independence (mRS 0–2), and independent ambulation (mRS 0–3)), and safety outcomes (any-cause mortality at 90 days, poor neurological recovery (mRS 4–6), any intracerebral hemorrhage, symptomatic intracerebral hemorrhage, and decompressive craniectomy). Any discrepancy was resolved by consensual discussion and agreement.
Risk of Bias and Quality Assessment
Two reviewers (H.A.S. and A.S.) assessed the quality of the studies included in the research independently using the Cochrane RoB2 tool [24]. The domains that were evaluated included the risk of bias resulting from the randomization process, the risk of bias due to deviation from the intended intervention, the risk of bias due to missing outcome data, the risk of bias in measuring the outcome, and the risk of bias in selecting the reported results. In the event of any disagreements, the reviewers discussed and resolved them through consensus.
To appraise the quality of evidence, two reviewers (M.A. and B.A.) utilized the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines [25, 26]. The evaluation was carried out for each outcome, and the decisions were justified and documented. Any discrepancy was resolved by consensual discussion and agreement.
Statistical Analysis
The RevMan v5.3 software [27] was used for statistical analysis. To combine the outcomes for dichotomous outcomes, the risk ratio was used, while the mean difference (MD) was used for continuous outcomes. Both were calculated with a 95% confidence interval (CI) using the fixed-effects model. However, the random-effects model was used in case of significant heterogeneity. The presence and extent of heterogeneity were evaluated using the Chi-square and I‑square tests, respectively. Following the Cochrane Handbook (chapter nine) [28], heterogeneity was considered significant if the alpha level for the Chi-square test was below 0.1, while the I‑square test results were interpreted as follows: not significant for 0–40%, moderate heterogeneity for 30–60%, and substantial heterogeneity for 50–90%. On significant heterogeneity, a leave-one-out sensitivity analysis was conducted.
Results
Search Results and Study Selection
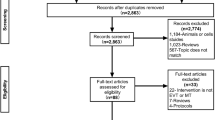
A total of 1995 studies were identified and evaluated for screening based on their titles and abstracts. After removing 969 duplicates and 1012 studies that did not match the inclusion criteria, fourteen full-text articles were assessed. Out of these, eleven were found to be irrelevant and excluded, leaving three RCTs to be included in the qualitative and quantitative analysis (Fig. 1).
Characteristics of Included Studies
We included a total of three RCTs [19,20,21]. Detailed summary characteristics of the included studies are outlined in (Table 1). A total of 1010 patients were included, of which 509 were allocated to the ET group and 501 patients to the medical management group. Most patients were men, including 297 (58.3%) in the ET group and 302 (60.3%) in the medical management group. Further baseline characteristics are highlighted in (Table 2).
Risk of Bias and Quality of Evidence
Using the Cochrane RoB2 tool’s five domains, we evaluated each outcome included in the quantitative synthesis’s risk of bias (Fig. 2). All of the included RCTs showed an overall high risk of bias, mainly attributed to the performance bias due to the lack of blinding.
Quality assessment of the risk of bias in the included trials. The upper panel presents a schematic representation of risks (low = red, unclear = yellow, and high = red) for specific types of biases of each of the studies in the review. The lower panel presents risks (low = red, unclear = yellow, and high = red) for the subtypes of biases of the combination of studies included in this review
Efficacy Outcomes
Endovascular thrombectomy significantly increased the rates of functional independence (mRS ≤ 2) (RR: 2.54 with 95% CI [1.85, 3.48], P = 0.00001) (low-quality evidence) (Fig. 3a; Table 3), independent ambulation (mRS ≤ 3) (low-quality evidence) (RR: 1.78 with 95% CI [1.28, 2.48], P = 0.0006) (low-quality evidence) (Fig. 3b; Table 3), and early neurological improvement (RR: 2.46 with 95% CI [1.60, 3.79], P = 0.0001) (low-quality evidence) (Fig. 3c; Table 3). However, there was no difference between endovascular thrombectomy and medical care in excellent neurological recovery (mRS ≤ 1) (RR: 1.35 with 95% CI [0.88, 2.08], P = 0.17) (low-quality evidence) (Fig. 3d; Table 3).
Pooled studies were homogenous in functional independence (mRS ≤ 2) (P = 0.64, I2 = 0%), early neurological improvement (P = 0.20, I2 = 38%), and excellent neurological recovery (mRS ≤ 1) (P = 0.19, I2 = 40%). However, studies were heterogenous in independent ambulation (mRS ≤ 3) (P = 0.09, I2 = 58%). Therefore, we performed a sensitivity analysis, and heterogeneity was best resolved by excluding ANGEL-ASPECT RCT (P = 0.60, I2 = 0%) (Table S2).
Safety Outcomes
Endovascular thrombectomy significantly reduced the rate of poor neurological recovery (mRS 4–6) (RR: 0.79 with 95% CI [0.72, 0.86], P = 0.00001) (low-quality evidence) (Fig. 4a; Table 3), with no difference regarding all-cause mortality (RR: 0.95 with 95% CI [0.78, 1.16], P = 0.61) (low-quality evidence) (Fig. 4b; Table 3), symptomatic intracranial hemorrhage (RR: 1.83 with 95% CI [0.95, 3.55], P = 0.07) (low-quality evidence) (Fig. 4c; Table 3), and decompressive craniectomy (RR: 1.22 with 95% CI [0.43, 3.41], P = 0.71) (very low-quality evidence) (Fig. 4d; Table 3). However, endovascular thrombectomy was associated with more incidence of any intracranial hemorrhage (RR: 2.30 with 95% CI [1.50, 3.51], P = 0.0001) (very low-quality evidence) (Fig. 4e; Table 3).
Pooled studies were homogenous in poor neurological recovery (mRS 4–6) (P = 0.96, I2 = 0%), all-cause mortality (P = 0.55, I2 = 0%), and symptomatic intracranial hemorrhage (P = 0.50, I2 = 0%). However, studies were heterogenous in decompressive craniectomy (P = 0.07, I2 = 70%) and any intracranial hemorrhage (P = 0.06, I2 = 71%), and sensitivity analysis was not applicable.
Discussion
Our meta-analysis showed that ET increased rates of functional independence (mRS ≤ 2), independent ambulation (mRS ≤ 3), and early neurological improvement. However, excellent neurological recovery (mRS ≤ 1) did not differ significantly between the two groups. Similarly, among the safety outcomes ET significantly reduced the rate of poor neurological recovery (mRS 4–6). Other safety outcomes, such as all-cause mortality, symptomatic intracranial hemorrhage, and decompressive craniectomy, were similar between the two groups. Despite the encouraging results, ET significantly increased the risk of any intracranial hemorrhage incidence.
Our results are consistent with the previous evidence, reporting a decreased incidence of unfavorable outcomes in patients treated with ET. In an individual data pooled meta-analysis, Román et al. showed a comparable rise in functional independence rates (mRS-scores 0–2) with ET in patients with ASPECTS less than six or ischemic core volume greater than or equal to 50 ccs or both [17]. Kerleroux et al. performed a meta-analysis on patients who had a substantial ischemic volume at admission and were undergoing ET; the results showed a significant drop in mRS 3–6 [15]. Based on the culminating evidence, it is safe to suggest that endovascular thrombectomy is emerging as an effective intervention as opposed to medical therapy only, even in subjects having large ischemic core volumes.
Without clear guidelines, this opens new hopes for treatment in patients, especially with low ASPECT scores (< 5) and large ischemic cores that have been traditionally factored out from ET trials. Worth noting, previous RCTs intended to demonstrate large treatment effect size and therefore only enlisted patients with small infarct size or ASPECTS 6–10 rigidly defined by imaging techniques [12] and leaving a considerable population of patients who could have profited from the treatment but could not qualify for the imaging inclusion criteria.
Similarly, a higher incidence of mortality or symptomatic intracranial hemorrhage was not reported. Nonetheless, in patients receiving ET, the risk of any intracranial hemorrhage continues to be considerable. However, this outcome did not make ET inferior as a treatment option since symptomatic intracranial hemorrhage risk was not elevated, and it remained to be assessed whether it was the result of a procedural complication or the intervention (ET) itself.
Individual risk for cerebral hemorrhage is influenced by several variables, including age, race, ethnicity, blood pressure control during ET, and the existence of concomitant conditions. Also, it depends on the technique used to retrieve the stent, how long the surgery takes, and how many times it is attempted before successful recanalization. Hence, the safety profile of ET can be further enhanced in the future by using modern imaging techniques to forecast the risk for symptomatic intracranial hemorrhage, such as DW MRI, perfusion CT, and digital subtracted angiography (DSA) for each individual [29].
Strengths and Limitations
Our meta-analysis is based on data from the three most recent RCTs, with minimal statistical heterogeneity (any heterogeneity encountered was resolved by sensitivity analysis) among outcomes guaranteeing the relevance and reliability of our conclusions to be the gold standard evidence regarding this matter. Nevertheless, there are however certain limitations due to the inherent characteristics of the included studies: first, our results might not be generalizable to all patient populations due to two of the included studies being based in Asian geography (Japan & China) [19, 20]. Second, there is also variability in the time window the patients were enrolled, which can influence patient outcomes. As in ANGEL-ASPECT [20], 63.3% of the patients were enrolled in the 6‑to-24-hour time window, whereas in RESCUE-Japan LIMIT [19], 28.6% of the patients were enrolled in this late window. Third, due to the lack of agreement on the management between different centers, several confounding variables may have gone unreported & therefore impacted our results. Similarly, there may be institutional variability in assessing AIS depending on whether CT or diffusion-weighted MRI was used to calculate ASPECTS values. Fourth, another factor that can introduce bias and hence influence the validity is the difference in group sizes notably, the number of patients receiving IV thrombolysis remained smaller than ET, possibly due to eligibility limitations. Finally, the GRADE assessment yielded low to very low-quality evidence, limiting the clinical endorsement of our findings.
Implications for Future Research
To address these limitations, First, large-scale RCTs should be conducted, including patients with diverse characteristics: demographic, comorbidities, and stroke risk factors. Studies should be designed to explore patient populations with low ASPECT scores < 5, baseline NIHSS score, large ischemic core, and optimal time window for ET. Further imaging protocols should be standardized by using a specific imaging modality, such as CT or DW-MRI, for stroke assessment. In this regard, results from ongoing RCTs are anticipated to provide promising results. In this regard, ongoing RCTs are committed to exploring the horizons of ET based on varying imaging modalities and inclusion criteria. Results from the European (TENSION, NCT03094715) trial investigating ASPECTS 3–5 at baseline in the extended time window of up to 12 h, the French (IN-EXTREMIS-LASTE, NCT03811769) trial exploring ET in a seven-hour time window, ASPECTS 0–5 on DWI-MRI or non-contrast CT, and the North American (TESLA, NCT03805308) assessing moderately large infarct volume NCCT ASPECTS 2–5 are likely to provide conclusive evidence.
Second, a patient-level & subgroup analysis is warranted for an in-depth exploration of patient factors to determine if patients with certain characteristics are more likely to benefit from ET. Additionally, a long-term follow-up duration, i.e., 12 months in contrast to the typical three months, should be conducted to better assess the impact of ET on patients’ quality of life vs. medical therapy alone. Finally, for ET to be adopted in widespread clinical practice, a cost-effectiveness analysis should be conducted between the two interventions. This is especially important in low and middle-income countries (LMIC) settings where the best evidence is required to convince policymakers and stakeholders to sponsor treatments.
Conclusion
Overall, ET with routine medical care in patients with AIS with a large ischemic core, defined as ASPECTS 3–5, was associated with better functional outcomes compared with medical care alone. Nonetheless, the rate of intracranial hemorrhage was significantly higher in patients undergoing ET; however, not symptomatic. This can support extending ET indication in the management of AIS with a large ischemic core.
References
Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. https://doi.org/10.1056/nejmoa1414792.
Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. https://doi.org/10.1056/nejmoa1414905.
Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. https://doi.org/10.1056/nejmoa1503780.
Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t‑PA vs. t‑PA alone in stroke. N Engl J Med. 2015;372:2285–95. https://doi.org/10.1056/nejmoa1415061.
Olvert A. Berkhemer, M.D., Puck S.S. Fransen, M.D., Debbie Beumer, M.D., Lucie A. van den Berg, M.D., Hester F. Lingsma, Ph.D., Albert J. Yoo, M.D., Wouter J. Schonewille, M.D., Jan Albert Vos, M.D., Ph.D., Paul J. Nederkoorn, M.D., Ph.D., Marieke J.H. We et al. (2015) A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N Engl J Med 372:394–394. https://doi.org/10.1056/nejmx140064.
Pexman JHW, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. Am J Neuroradiol. 2001;22:1534–42.
Yamagami H, Hayakawa M, Inoue M, Iihara K, Ogasawara K, Toyoda K, Hasegawa Y, Ohata K, Shiokawa Y, Nozaki K, Ezura M, Iwama T. JSS/JNS/JSNET Joint Guideline Authoring Committee. Guidelines for Mechanical Thrombectomy in Japan, the Fourth Edition, March 2020: A Guideline from the Japan Stroke Society, the Japan Neurosurgical Society, and the Japanese Society for Neuroendovascular Therapy. Neurol Med Chir (Tokyo). 2021;61(3):163–192. https://doi.org/10.2176/nmc.nmc.st.2020-0357. Epub 2021.
Warner JJ, Harrington RA, Sacco RL, Elkind MSV. Guidelines for the early management of patients with acute ischemic stroke 2019 update to the 2018 guidelines for the early management of acute ischemic stroke. Stroke. 2019;50:3331–2. https://doi.org/10.1161/STROKEAHA.119.027708.
Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. https://doi.org/10.1056/nejmoa1713973.
Lindsay E. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. J Emerg Med. 2018;54:583–4. https://doi.org/10.1016/j.jemermed.2018.02.029.
Bracard S, Ducrocq X, Mas JL, et al (2016) Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 15:1138–1147. https://doi.org/10.1016/S1474-4422(16)30177‑6.
Goyal M, Menon BK, Van Zwam WH, et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 387:1723–1731. https://doi.org/10.1016/S0140-6736(16)00163‑X.
Cucchiara B, Kasner SE, Tanne D, et al. Factors Associated With Intracerebral Hemorrhage After Thrombolytic Therapy for Ischemic. Stroke Stroke. 2009;40:3067–72. https://doi.org/10.1161/strokeaha.109.554386.
Sarraj A, Hassan AE, Savitz S, et al. Outcomes of Endovascular Thrombectomy vs Medical Management Alone in Patients With Large Ischemic Cores. JAMA Neurol. 2019;76:1147. https://doi.org/10.1001/jamaneurol.2019.2109.
Kerleroux B, Janot K, Hak JF, et al. Mechanical thrombectomy in patients with a large ischemic volume at presentation: Systematic review and meta-analysis. J Stroke. 2021;23:358–66. https://doi.org/10.5853/jos.2021.00724.
Campbell BCV, Majoie CBLM, Albers GW, et al (2019) Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol 18:46–55. https://doi.org/10.1016/S1474-4422(18)30314‑4.
Román LS, Menon BK, Blasco J, et al (2018) Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol 17:895–904. https://doi.org/10.1016/S1474-4422(18)30242‑4.
Sarraj A, Grotta JC, Pujara DK, et al. Triage imaging and outcome measures for large core stroke thrombectomy—A systematic review and meta-analysis. J Neurointerv Surg. 2020;12:1172–9. https://doi.org/10.1136/neurintsurg-2019-015509.
Yoshimura S, Sakai N, Yamagami H, et al. Endovascular Therapy for Acute Stroke with a Large Ischemic Region. N Engl J Med. 2022;386:1303–13. https://doi.org/10.1056/nejmoa2118191.
Huo X, Ma G, Tong X, et al. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N Engl J Med. 2023; https://doi.org/10.1056/NEJMoa2213379.
Sarraj A, Hassan AE, Abraham MG, et al. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. N Engl J Med. 2023; https://doi.org/10.1056/NEJMoa2214403.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; https://doi.org/10.1136/BMJ.N71.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Cochrane Handb Syst Rev Interv. 2019. pp. 1–694. https://doi.org/10.1002/9781119536604.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; https://doi.org/10.1136/BMJ.L4898.
Guyatt GH, Oxman AD, Kunz R, et al (2008) Rating Quality of Evidence and Strength of Recommendations: What is “quality of evidence” and why is it important to clinicians? BMJ Br Med J 336:995. https://doi.org/10.1136/BMJ.39490.551019.BE.
Guyatt GH, Oxman AD, Vist GE, et al (2008) Rating Quality of Evidence and Strength of Recommendations: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ Br Med J 336:924. https://doi.org/10.1136/BMJ.39489.470347.AD.
RevMan | Cochrane Training. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman. Accessed 3 Aug 2021.
Cochrane Handbook for Systematic Reviews. Intervention. 2022. https://doi.org/10.1002/9780470712184.
Charbonnier G, Bonnet L, Biondi A, Moulin T. Intracranial Bleeding After Reperfusion Therapy in Acute Ischemic Stroke. Front Neurol. 2021; https://doi.org/10.3389/fneur.2020.629920.
Funding
We received no funding for this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.A. and B.A. conceived the idea. B.A. and M.A. designed the research workflow. B.A. and M.A. searched the databases. H.A.S. and A.S. screened the retrieved records, extracted relevant data, assessed the quality of evidence, and B.A. resolved the conflicts. M.A. and A.M. performed the analysis. U.S., M.A., and A.M. wrote the final manuscript. M.A. supervised the project. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
M. Abuelazm, U. Ahmad, H. Abu Suilik, A. Seri, A. Mahmoud and B. Abdelazeem declare that they have no competing interests.
Additional information
The authors Mohamed Abuelazm and Unaiza Ahmad have equal contributions and are co-first authors.
Availability of data and materials
Not applicable.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abuelazm, M., Ahmad, U., Abu Suilik, H. et al. Endovascular Thrombectomy for Acute Stroke with a Large Ischemic Core: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin Neuroradiol 33, 625–634 (2023). https://doi.org/10.1007/s00062-023-01306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-023-01306-x