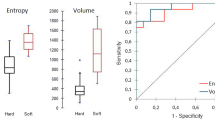
Abstract
Purpose
To develop and validate a combined model incorporating conventional clinical and imaging characteristics and radiomics signatures based on head and neck computed tomography angiography (CTA) to assess plaque vulnerability.
Methods
We retrospectively analyzed 167 patients with carotid atherosclerosis who underwent head and neck CTA and brain magnetic resonance imaging (MRI) within 1 month. Clinical risk factors and conventional plaque characteristics were evaluated, and radiomic features were extracted from the carotid plaques. The conventional, radiomics and combined models were developed using fivefold cross-validation. Model performance was evaluated using receiver operating characteristic (ROC), calibration, and decision curve analyses.
Results
Patients were divided into symptomatic (n = 70) and asymptomatic (n = 97) groups based on MRI results. Homocysteine (odds ratio, OR 1.057; 95% confidence interval, CI 1.001–1.116), plaque ulceration (OR 6.106; 95% CI 1.933–19.287), and carotid rim sign (OR 3.285; 95% CI 1.203–8.969) were independently associated with symptomatic status and were used to construct the conventional model and s radiomic features were retained to establish the radiomics model. Radiomics scores incorporated with conventional characteristics were used to establish the combined model. The area under the ROC curve (AUC) of the combined model was 0.832, which outperformed the conventional (AUC = 0.767) and radiomics (AUC = 0.797) models. Calibration and decision curves analysis showed that the combined model was clinically useful.
Conclusion
Radiomics signatures of carotid plaque on CTA can well predict plaque vulnerability, which may provide additional value to identify high-risk patients and improve outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a leading cause of death and disability, with a majority of ischemic stroke of all stroke types [1]. Carotid atherosclerosis accounts for approximately 18–25% of ischemic strokes [2]. High-resolution magnetic resonance imaging (MRI) can detect carotid plaque characteristics, including intraplaque hemorrhage (IPH), lipid-rich necrotic cores, and fibrous caps, which have been shown to be associated with plaque vulnerability [3]; however, due to the long acquisition time and patient contraindications of carotid MRI, acute stroke decision-making is often based on computed tomography (CT) and CT angiography (CTA) as a quick, easy and widely used imaging modality to identify cerebral hemorrhage or artery occlusion [4]. For patients with suspected carotid atherosclerosis, CTA provides a substitute for patients without access to MRI, and therefore a better understanding of carotid plaque characteristics on CTA is imperative.
Although the measurement of carotid artery stenosis is an important means to stratify patients with treatment, other plaque characteristics on CTA are helpful in estimating plaque vulnerability [5]. Plaque features, such as length [6], thickness [7], ulceration [8], and texture [4, 9] are substantially related to the onset of ischemic stroke. Furthermore, a large-sample study (n = 19,804) conducted by Martins et al. found that morphological abnormalities of the internal carotid artery (ICA) were associated with hypertension, hyperlipidemia, diabetes, and heart disease, and ICA tortuosity was associated with ipsilateral cerebral ischemia [10]. Therefore, plaque composition and biomechanics were critical factors for carotid artery plaque risk assessment [11].
Compared with conventional assessment, radiomics provides more comprehensive information by converting images to higher dimensional data and allowing high-throughput extraction of quantitative image features to provide decision support and accurately phenotype diseases in vivo [12,13,14,15]. Previous studies revealed that radiomic features improved the ability to detect vulnerable plaques on coronary CTA [16, 17] and identify symptomatic plaques in the carotid [18] and basilar arteries [19] on MRI. Recently, CTA-based radiomics analysis also showed promising results in carotid plaques analysis [20]. In this study, we aimed to investigate conventional and radiomic features of carotid atherosclerotic plaques on CTA associated with symptomatic plaque status. We also integrated critical characteristics to build a high-risk plaque model and evaluate its performance in differentiating symptomatic from asymptomatic carotid plaques.
Material and Methods
Study Population
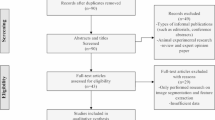
This retrospective study was approved by the local institutional review committee (No. Y [2022]114), and the requirement for informed consent was waived. We included 1063 consecutive patients with clinically suspected stroke who underwent head and neck CTA and brain MRI within 1 month between October 2019 and July 2022. The exclusion criteria were as follows: (1) insufficient clinical data; (2) negative findings on carotid CTA; (3) presence of cerebral hemorrhage, tumor, trauma or previous brain surgery; (4) posterior circulation stroke; (5) suspected cardioembolic sources; (6) carotid artery dissection; (7) accompanied by intracranial vessel diseases, such as atherosclerotic stenosis, aneurysm or moyamoya disease; (8) previous carotid stenting and endarterectomy; (9) insufficient image quality for plaque analysis and radiomics extraction (see Fig. 1 for the flow chart of patient selection).
Demographic, clinical and laboratory data were collected from all patients, including age, sex, smoking status, hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, medication history, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, lipoprotein(a) and homocysteine (Hcy).
Imaging Acquisition
Head and neck CTA was performed using a 256-slice CT scanner (Brilliance iCT; Philips Medical Systems, Cleveland, OH, USA). The aortic arch to the skull vertex was covered by CTA examination in the helical scanning mode. The scanning parameters were as follows: detector collimation, 128 × 0.625 mm; tube voltage, 120 kV; tube current, 250 mA; rotation time, 0.33 s; pitch, 0.763; section increment, 0.45 mm; and slice thickness, 0.9 mm. Approximately 50–60 mL of nonionic iodine contrast agent was injected at a flow rate of 4.0–5.0 mL/s via a high-pressure injector, followed by a 20–30 mL flush of saline at the same rate.
The MRI examinations were performed on a 3.0T MR scanner (Discovery 750; GE Healthcare, Chicago, IL, USA) with a 20-channel head coil using standardized protocols, including T1 and T2 weighted imaging, T2 fluid-attenuated inversion recovery (FLAIR) and diffusion weighted imaging (DWI). Ischemic lesions were assessed on DWI with the following parameters: TR 4000 ms, TE 68 ms, FOV 24 cm×24 cm, slice thickness 6 mm, matrix 128 × 128, intersection gap 1.2 mm, number of excitations 1, b‑values of 0 and 1000 s/mm2.
Imaging Analysis
Carotid plaque characteristics were assessed on CTA using GE Healthcare Advantage Workstation (AW4.4, USA). Carotid stenosis, plaque features and carotid artery tortuosity were measured and evaluated as follows: (1) the degree of carotid stenosis was defined as mild (< 50%) and moderate to severe (50–99.9%) stenosis using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria [21]; (2) the maximum plaque thickness: measured on the cross-section of the plaque perpendicular to the long axis of the vessel, (3) the soft plaque thickness: a plaque with a mean attenuation of 40–50 HU was considered soft [5]; (4) plaque ulceration: defined as at least 1 mm of contrast agent entering the plaque in any one plane [22]; (5) carotid rim sign: a positive carotid rim sign was defined as adventitial calcification (< 2 mm thickness) with interior soft plaque (> 2 mm thickness), whereas a negative carotid rim sign was defined as adventitial calcification (< 2 mm thickness) with little interior soft plaque (< 2 mm thickness) ([23]; Fig. 2); (6) positive remodelling (PR): characterized as an outside vessel diameter that was 10% larger than the mean diameter of the segments that were both proximal and distal to the plaque [24] and (7) common carotid tortuosity index (CCTI) and internal carotid tortuosity index (ICTI): calculated using the following formula: ([actual distance/linear distance] − 1) × 100 [25]. The actual distance of the vessel was measured along the arterial center line from the proximal to the distal point in the curved projection reformation. The linear distance of the vessel was determined as the shortest distance between the same two points in the three-dimensional volume rendering image. Proximal vessel points were uniformly placed at the arterial origin. The common carotid artery was placed distally at the carotid bifurcation, and the ICA was placed distally at the entrance of the outer carotid canal (Fig. 2).
Carotid computed tomography angiography (CTA) imaging markers and stroke work-up. This 73-year-old man presented with abrupt onset right-sided weakness and numbness. Axial diffusion-weighted imaging (a) showed an acute infarct (long arrows). Axial CTA of the carotid arteries (b) demonstrated a thick left proximal internal carotid artery plaque with a positive rim sign (long arrow). Multiplanar reformat of CTA images (c) demonstrated the ulcerated carotid plaque (long arrow). Three-dimensional volume rendering (d) showed the linear distance of the vessel was determined as the shortest distance between the same two points, the actual distance of the vessel was measured from the proximal to distal point along the arterial center line from the curved projection reformation
Patients were divided into symptomatic and asymptomatic groups according to the following criteria: (1) clinical symptoms continuing for at least 24 h within 2 weeks before imaging scans; and (2) acute/subacute stroke findings on DWI in the ipsilateral carotid artery territory [26, 27].
Radiomics Plaque Segmentation
Patient data were anonymized and transmitted to The Deepwise Multimodal Research Platform v2.0 (Beijing Deepwise & League of PHD Technology Co., Ltd, Beijing, China, https://keyan.deepwise.com), which can be used for labeling, region of interest (ROI) feature extraction, and model development [20, 28]. In the symptomatic group, we segmented the carotid plaque located ipsilateral to the infarct. For patients with bilateral plaques in the asymptomatic group, we segmented unilateral plaque with a larger volume. For patients with plaques involving both the internal and external carotid arteries, we only segmented the internal carotid plaques. A radiologist visually assessed the outer border of the arterial wall and segmented the plaque boundaries with an appropriate center level and window width.
Reproducibility Evaluation
In a subgroup of patients (n = 30) who were randomly chosen from the research population, two radiologists independently segmented the plaques for radiomic features extraction and assessed the conventional features 2 weeks after the first assessment. Intraclass correlation coefficient (ICC) and Cohen’s kappa coefficient were calculated for the intraobserver and interobserver consistency: < 0.40, poor agreement; 0.41–0.60, moderate; 0.61–0.80, substantial; and > 0.80, excellent.
Model Development
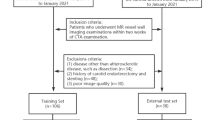
We selected clinical and conventional plaque characteristics with statistical differences in univariate and multivariable logistic regression analyses and built the conventional model. For radiomics model development, we first preserved radiomic features with intraobserver and interobserver ICC > 0.8 and eliminated redundant features with linear correlation coefficient values (threshold = 0.7). We then used the analysis of variance F‑value, mutual information, and linear models penalized with the L1 norm algorithm to form radiomics signature. Finally, a combined model was created by integrating selected conventional characteristics and radiomics signature. All models were trained using the fivefold cross-validation method. The workflow of the model development is shown in Fig. 3.
Statistical Analysis
All statistical analyses were performed using SPSS software (SPSS statistics, version 25.0; SPSS Inc., Armonk, NY, USA: IBM Corp.), statistical software packages R (version 3.3.2; http://www.R-project.org, The R Foundation), and Free Statistics software (version 1.7). The consistency of ROI segmenting, dimension reduction of features and ML model development were performed on the Deepwise Multimodal Research Platform v2.0. Categorical variables are reported as frequencies (%) and continuous variables as means ± standard deviation or median (interquartile range). Classified variables were compared using the χ2-test. Comparisons of continuous variables were performed using Student’s t-test or Mann–Whitney U test. Variables with statistical significance in the univariate analysis were used in the multivariate logistic analysis. Model performance was evaluated using receiver operating characteristic (ROC), calibration, and decision curve analyses. All statistical tests were two-sided. Statistical significance was set at p < 0.05.
Results
Clinical Characteristics
In total, 167 patients were included in the final analysis. Patients were mainly older (66.2 ± 7.7 years; 78.4% male), among whom smoking (54.5%) and hypertension (68.9%) accounted for a higher proportion. The clinical characteristics of patients in the symptomatic and asymptomatic groups are shown in Table 1. Smoking was more prevalent in the symptomatic group than in the asymptomatic group (65.7% vs. 46.4%, p = 0.013). Total cholesterol (4.7 vs. 4.2 mmol/L, p = 0.022), lipoprotein(a) (231.4 vs. 134.2 mg/L, p < 0.002), and Hcy (13.9 vs. 11.0 µmol/L, p < 0.001) were higher in the symptomatic group than the asymptomatic group. Multivariate logistic regression analysis revealed that Hcy (odds ratio, OR, 1.057; 95% confidence interval, CI, 1.001–1.116) was the only independent risk factor associated with symptomatic plaques (Table 2).
Plaque Features on CTA
Univariate analysis showed that the degree of carotid artery stenosis (OR 1.02; 95% CI 1.01–1.04), total plaque thickness (OR 1.44; 95% CI 1.16–1.79), soft plaque thickness (OR 1.64; 95% CI 1.31–2.04), plaque length (OR 1.06; 95% CI 1.01–1.10), plaque ulceration (OR 9.00; 95% CI 3.22–25.20), carotid rim sign (OR 6.57; 95% CI 2.75–15.7), and PR (OR 2.01; 95% CI 1.08–3.76) were related to plaque vulnerability. Further multivariate logistic regression analysis revealed that plaque ulceration (OR 6.106; 95% CI 1.933–19.287) and carotid rim sign (OR 3.285; 95% CI 1.203–8.969) were independent predictors of symptomatic plaques (Table 2). Intraobserver and interobserver reproducibility of conventional imaging features assessment was excellent (supplementary materials Table S1).
Radiomics Signature
A total of 2107 radiomic features were extracted and normalized using the Z‑score from each ROI. The ICC for calculating radiomic features is summarized in the supplementary material (Fig. S1). First, radiomic features with ICC ≤ 0.8 were removed by interobserver and intraobserver agreement, and the remaining 1916 features were included in the analysis. Another 112 features were removed because of low discrimination. Then 1760 features were removed according to the linear correlation value (threshold = 0.7). A mutual information algorithm was used to analyze all remaining features. Finally, six significant radiomic features were retained to construct the radiomics model by using the logistic regression algorithm provided by the Deepwise Multimodal Research Platform [20], including wavelet-HLL_gldm_GrayLevelNonUniformity, wavelet-LLH_firstorder_Mean, wavelet-HLL_glcm_Imc2, log-sigma-2-0-mm-3D_gldm_LargeDependenceLowGrayLevelEmphasis, square_glcm_InverseVariance, and the exponential_ngtdm_Strength.
Model Development and Performance
We developed three formulas based on independent risk factors and radiomics signature to calculate each patient’s risk score. They were presented as follows:
Table 3 shows the discriminative power of the three models. The radiomics model demonstrated a good diagnostic performance in the training (area under the curve, AUC 0.840; 95% CI 0.781–0.898) and validation cohorts (AUC 0.797; 95% CI 0.731–0.863). The conventional model had lower discrimination for symptomatic plaques in the training (AUC 0.789; 95% CI 0.715–0.863) and validation cohorts (AUC 0.767; 95% CI 0.689–0.845). The AUCs of the integrated model were the highest, with 0.856 (95% CI 0.800–0.913) and 0.832 (95% CI: 0.770–0.894) for the training and validation sets, respectively (Fig. 4). DeLong’s test showed that the integrated models significantly improved model performance compared to the conventional model in training (AUC 0.856 vs. 0.789, p = 0.005) and validation cohorts (AUC 0.832 vs. 0.767, p = 0.011). Although the diagnostic efficiency of the integrated model relatively outperformed the radiomics model, there was no significant difference in the training (AUC 0.856 vs. 0.840; p = 0.688) and validation cohorts (AUC 0.832 vs. 0.797; p = 0.452).
Nomogram Construction and Evaluation
On the basis of the combined model, a nomogram was constructed that incorporated three selected conventional features (Hcy, plaque ulceration, and carotid rim sign) and radiomics score for predicting plaque vulnerability (Fig. 5). The value of each of these variables was given a score on the point scale axis. A total score could be easily calculated by adding each single score to the total point scale to estimate the risk of plaque vulnerability.
An integrated model nomogram was developed with homocysteine (Hcy), carotid rim sign, plaque ulceration and radiomics score of the selected radiomic features incorporated. Use the constructed nomogram to identify symptomatic plaques, carotid rim sign and plaque ulceration (0 indicates absent; 1 present)
The calibration curve of the combined model demonstrated a good fit between predicted and actual possibility of ischemic stroke in the training and validation cohorts (Fig. 6). The decision curves compare the net benefits at various threshold probabilities in the training and validation sets to demonstrate the clinical utility of the prediction model. The integrated model had an excellent overall net benefit when the risk threshold range was 0.15–0.82 (Fig. 7).
Decision curve analysis for the integrated model in the training and validation datasets. The y‑axis represented the net benefit, the x‑axis represented threshold probability. The gray curve line represented the assumption that all patients have symptomatic plaques, while the black horizontal line represented the assumption that no patients have symptomatic plaques. When the risk threshold range is 0.15–0.82, the model presents the benefit
Discussion
This study constructed an integrated predictive model that incorporated conventional clinical risk factors, plaque characteristics, and radiomics signature on CTA to identify symptomatic plaques, and validated its clinical utility using calibration and decision curves. The integrated model performed better than the conventional or radiomics model alone for plaque vulnerability assessment, which would assist in clinical management and improve patient outcomes.
The relationship between carotid plaque characteristics on CTA and ischemic stroke, such as plaque composition, vulnerability, and carotid artery tortuosity, has been extensively studied [4, 5, 9, 29]. According to the American Society of Neuroradiology and European Society of Cardiology guidelines, carotid plaque composition and the degree of stenosis are both factors that affect the risk of stroke [30, 31]. More attention should be focused on the comprehensive assessment of all risk factors rather than the degree of luminal stenosis alone. Our findings showed that plaque ulceration, carotid rim sign, and Hcy were independent risk factors. Plaque ulceration was speculated to be a risk factor for ipsilateral ischemic stroke [32]. A systematic review and meta-analysis found that plaque ulceration increased the risk of ipsilateral ischemia by 2.2 times [9]. There was a strong correlation between carotid rim sign and IPH, which was generally accepted as a strong predictor of cerebrovascular events [23, 33]. Nardi et al. found that IPH was often observed in mild carotid artery stenosis and suggested that IPH might associate with non-carotid artery occlusive stroke [34]. The plasma Hcy level would affect endothelial function, cell-cell adhesion, and oxidative response, which accelerates plaque formation and adds risk of acute cerebral infarction [35,36,37,38].
Different carotid plaque components present different CT attenuation values; however, due to the partial volume effect, it is difficult to visually differentiate the internal composition of plaques on CTA. Radiomics provides an objective, automated, and data-driven description of plaque features by measuring heterogeneity or shape that are mostly invisible to the human eye [15]. A proof-of-principle study found that radiomics was a feasible and reliable method to extract information beyond luminal stenosis in carotid CTA [39]. In our study, we chose six significant radiomic features and found that plaques with higher heterogeneity and lower strength features were more likely to be symptomatic, indicating that these plaques might contain more complicated components such as lipids or hemorrhage. Our results demonstrated that radiomic features of carotid plaques were strongly associated with ipsilateral ischemic stroke and could be used to predict plaque vulnerability. Radiomics signatures of carotid plaque on CTA may be a promising tool for identifying high-risk plaques and assessing stroke risk.
A recent case-control study of 24 patients demonstrated that CT texture characteristics could distinguish symptomatic and asymptomatic patients with carotid plaques [11]. Another study included 120 patients with moderate-to-severe carotid stenosis (> 50%).and revealed that the radiomics model improved the diagnostic performance of symptomatic plaques compared to conventional clinical assessment [20]. Our results were consistent with these findings that radiomic features could effectively identify symptomatic carotid plaques; however, Baradaran et al. found that there was also a high risk of ischemic stroke in patients with mild carotid stenosis [4]. In this study, we included patients with all degrees of atherosclerosis carotid stenosis (45.7% of patients in the symptomatic group with carotid stenosis < 50%). We further proposed a comprehensive model incorporating clinical risk factors, plaque features, and radiomic features, and achieved better performance than the conventional and radiomics models. Our work would add evidence to the literature which applied radiomics for plaque vulnerability assessment.
Our study has several limitations. First, we conducted a single-center retrospective study with a small sample size, and all enrolled patients underwent CTA examination using the same CT scanner and imaging protocol. We performed fivefold cross-validation and calculated a reliable estimate of diagnostic accuracy. Our results need further validation on external cohorts using data from different vendors and machines. Second, we included patients with large artery atherosclerosis stroke and excluded other etiologies as much as possible; however, we could not ascertain that all strokes were caused by the carotid atherosclerotic plaque. Third, there was inevitable subjectivity and variability when manually extracting carotid plaques. We removed radiomic features with interobserver and intraobserver ICC ≤ 0.8 to ensure the retained features were highly consistent and prevent subjective error. Future studies will be conducted on large-scale, prospective cohorts and integrate more clinical, imaging, and radiomics features to improve stroke risk stratification.
Conclusion
The CTA-derived radiomics signature of carotid plaques could effectively identify symptomatic plaques. Radiomic features combined with conventional clinical and imaging parameters may be a useful tool for improving risk stratification and guiding clinical decision-making in patients with carotid atherosclerotic plaques.
References
GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820.
Ooi YC, Gonzalez NR. Management of extracranial carotid artery disease. Cardiol Clin. 2015;33:1–35.
McNally JS, McLaughlin MS, Hinckley PJ, Treiman SM, Stoddard GJ, Parker DL, Treiman GS. Intraluminal thrombus, intraplaque hemorrhage, plaque thickness, and current smoking optimally predict carotid stroke. Stroke. 2015;46:84–90.
Baradaran H, Eisenmenger LB, Hinckley PJ, de Havenon AH, Stoddard GJ, Treiman LS, Treiman GS, Parker DL, Scott McNally J. Optimal carotid plaque features on computed tomography angiography associated with ischemic stroke. J Am Heart Assoc. 2021;10:e19462.
Baradaran H, Gupta A. Carotid vessel wall imaging on CTA. AJNR Am J Neuroradiol. 2020;41:380–6.
Zhao L, Zhao H, Xu Y, Zhang A, Zhang J, Tian C. Plaque length predicts the incidence of microembolic signals in acute anterior circulation stroke. Dis Markers. 2021;2021:2005369.
Gupta A, Baradaran H, Kamel H, Pandya A, Mangla A, Dunning A, Marshall RS, Sanelli PC. Evaluation of computed tomography angiography plaque thickness measurements in high-grade carotid artery stenosis. Stroke. 2014;45:740–5.
Homburg PJ, Rozie S, van Gils MJ, van den Bouwhuijsen QJ, Niessen WJ, Dippel DW, van der Lugt A. Association between carotid artery plaque ulceration and plaque composition evaluated with multidetector CT angiography. Stroke. 2011;42:367–72.
Baradaran H, Al-Dasuqi K, Knight-Greenfield A, Giambrone A, Delgado D, Ebani EJ, Kamel H, Gupta A. Association between carotid plaque features on CTA and cerebrovascular Ischemia: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38:2321–6.
Martins H, Mayer A, Batista P, Soares F, Almeida V, Pedro AJ, Oliveira V. Morphological changes of the internal carotid artery: prevalence and characteristics. A clinical and ultrasonographic study in a series of 19 804 patients over 25 years old. Eur J Neurol. 2018;25:171–7.
Zaccagna F, Ganeshan B, Arca M, Rengo M, Napoli A, Rundo L, Groves AM, Laghi A, Carbone I, Menezes LJ. CT texture-based radiomics analysis of carotid arteries identifies vulnerable patients: a preliminary outcome study. Neuroradiology. 2021;63:1043–52.
Kolossváry M, De Cecco CN, Feuchtner G, Maurovich-Horvat P. Advanced atherosclerosis imaging by CT: Radiomics, machine learning and deep learning. J Cardiovasc Comput Tomogr. 2019;13:274–80.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–77.
Aerts HJ. The potential of radiomic-based phenotyping in precision medicine: a review. JAMA Oncol. 2016;2:1636–42.
Kolossváry M, Kellermayer M, Merkely B, Maurovich-Horvat P. Cardiac computed tomography radiomics: a comprehensive review on radiomic techniques. J Thorac Imaging. 2018;33:26–34.
Kolossváry M, Park J, Bang JI, Zhang J, Lee JM, Paeng JC, Merkely B, Narula J, Kubo T, Akasaka T, Koo BK, Maurovich-Horvat P. Identification of invasive and radionuclide imaging markers of coronary plaque vulnerability using radiomic analysis of coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2019;20:1250–8.
Kolossváry M, Karády J, Kikuchi Y, Ivanov A, Schlett CL, Lu MT, Foldyna B, Merkely B, Aerts HJ, Hoffmann U, Maurovich-Horvat P. Radiomics versus visual and histogram-based assessment to identify atheromatous lesions at coronary CT angiography: an ex vivo study. Radiology. 2019;293:89–96.
Zhang R, Zhang Q, Ji A, Lv P, Zhang J, Fu C, Lin J. Identification of high-risk carotid plaque with MRI-based radiomics and machine learning. Eur Radiol. 2021;31:3116–26.
Shi Z, Zhu C, Degnan AJ, Tian X, Li J, Chen L, Zhang X, Peng W, Chen C, Lu J, Jiang T, Saloner D, Liu Q. Identification of high-risk plaque features in intracranial atherosclerosis: initial experience using a radiomic approach. Eur Radiol. 2018;28:3912–21.
Dong Z, Zhou C, Li H, Shi J, Liu J, Liu Q, Su X, Zhang F, Cheng X, Lu G. Radiomics versus conventional assessment to identify symptomatic participants at carotid computed tomography angiography. Cerebrovasc Dis. 2022;51:647–654.
Barnett H, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, Eliasziw M. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53.
Saba L, Saam T, Jäger HR, Yuan C, Hatsukami TS, Saloner D, Wasserman BA, Bonati LH, Wintermark M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18:559–72.
Eisenmenger LB, Aldred BW, Kim SE, Stoddard GJ, de Havenon A, Treiman GS, Parker DL, McNally JS. Prediction of carotid intraplaque hemorrhage using adventitial calcification and plaque thickness on CTA. AJNR Am J Neuroradiol. 2016;37:1496–503.
Mosleh W, Adib K, Natdanai P, Carmona-Rubio A, Karki R, Paily J, Ahmed MA, Vakkalanka S, Madam N, Gudleski GD, Chung C, Sharma UC. High-risk carotid plaques identified by CT-angiogram can predict acute myocardial infarction. Int J Cardiovasc Imaging. 2017;33:561–8.
Morris SA, Orbach DB, Geva T, Singh MN, Gauvreau K, Lacro RV. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation. 2011;124:388–96.
Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89.
Kopczak A, Schindler A, Sepp D, Bayer-Karpinska A, Malik R, Koch ML, Zeller J, Strecker C, Janowitz D, Wollenweber FA, Hempel JM, Boeckh-Behrens T, Cyran CC, Helck A, Harloff A, Ziemann U, Poli S, Poppert H, Saam T, Dichgans M. Complicated carotid artery plaques and risk of recurrent Ischemic stroke or TIA. J Am Coll Cardiol. 2022;79:2189–99.
Qi LL, Wu BT, Tang W, Zhou LN, Huang Y, Zhao SJ, Liu L, Li M, Zhang L, Feng SC, Hou DH, Zhou Z, Li XL, Wang YZ, Wu N, Wang JW. Long-term follow-up of persistent pulmonary pure ground-glass nodules with deep learning-assisted nodule segmentation. Eur Radiol. 2020;30:744–55.
Saba L, Sanfilippo R, Suri JS, Cademartiri F, Corrias G, Mannelli L, Zucca S, Senis I, Montisci R, Wintermark M. Does carotid artery tortuosity play a role in stroke. Can Assoc Radiol J. 2021;72:789–96.
Saba L, Yuan C, Hatsukami TS, Balu N, Qiao Y, DeMarco JK, Saam T, Moody AR, Li D, Matouk CC, Johnson MH, Jäger HR, Mossa-Basha M, Kooi ME, Fan Z, Saloner D, Wintermark M, Mikulis DJ, Wasserman BA. Carotid artery wall imaging: perspective and guidelines from the ASNR vessel wall imaging study group and expert consensus recommendations of the American society of Neuroradiology. AJNR Am J Neuroradiol. 2018;39:E9–E31.
Aboyans V, Ricco JB, Bartelink M, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816.
Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–25.
Altaf N, MacSweeney ST, Gladman J, Auer DP. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke. 2007;38:1633–5.
Nardi V, Benson J, Bois MC, Saba L, Larson AS, Özcan I, Ahmad A, Morse DW, Meyer FB, Brinjikji W, Lanzino G, Lerman LO, Savastano LE, Lerman A. Carotid plaques from symptomatic patients with mild stenosis is associated with Intraplaque hemorrhage. Hypertension. 2022;79:271–82.
Bertoia ML, Pai JK, Cooke JP, Joosten MM, Mittleman MA, Rimm EB, Mukamal KJ. Plasma homocysteine, dietary B vitamins, betaine, and choline and risk of peripheral artery disease. Atherosclerosis. 2014;235:94–101.
Leach NV, Dronca E, Vesa SC, Sampelean DP, Craciun EC, Lupsor M, Crisan D, Tarau R, Rusu R, Para I, Grigorescu M. Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur J Intern Med. 2014;25:762–7.
Wu W, Guan Y, Xu K, Fu XJ, Lei XF, Lei LJ, Zhang ZQ, Cheng Y, Li YQ. Plasma homocysteine levels predict the risk of acute cerebral infarction in patients with carotid artery lesions. Mol Neurobiol. 2016;53:2510–7.
Alsulaimani S, Gardener H, Elkind MS, Cheung K, Sacco RL, Rundek T. Elevated homocysteine and carotid plaque area and densitometry in the Northern Manhattan Study. Stroke. 2013;44:457–61.
Le E, Rundo L, Tarkin JM, Evans NR, Chowdhury MM, Coughlin PA, Pavey H, Wall C, Zaccagna F, Gallagher FA, Huang Y, Sriranjan R, Le A, Weir-McCall JR, Roberts M, Gilbert FJ, Warburton EA, Schönlieb CB, Sala E, Rudd J. Assessing robustness of carotid artery CT angiography radiomics in the identification of culprit lesions in cerebrovascular events. Sci Rep. 2021;11:3499.
Funding
This study was supported by the Key Research and Development Project of Liaoning Province of China (No. 2020JH2/10300119) and Liaoning Provincial “Selecting the Best Candidates by Opening Competition Mechanism” Science and Technology Program (No. 2022JH1/10400004).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by Yu Sun and Jie Hou. Data collection was performed by Rongrong Zhang, Hongrui You, Zhenguo Wang, Anxiaonan Zhang, Jianhua Zhang, Qiuyue Jin and Lianlian Zhao. Data analysis was performed by Xiaogang Li, Jitao Fan and Libo Zhang. The first draft of the manuscript was written by Jinglong Shi and revised by Benqiang Yang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
J. Fan is an employee of Beijing Deepwise & League of PHD Technology Co. Ltd. J. Shi, Y. Sun, J. Hou, X. Li, L. Zhang, R. Zhang, H. You, Z. Wang, A. Zhang, J. Zhang, Q. Jin, L. Zhao and B. Yang declare that they have no competing interests.
Ethical standards
This retrospective study was performed after consultation with the institutional ethics committee and in accordance with national legal requirements.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, J., Sun, Y., Hou, J. et al. Radiomics Signatures of Carotid Plaque on Computed Tomography Angiography. Clin Neuroradiol 33, 931–941 (2023). https://doi.org/10.1007/s00062-023-01289-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-023-01289-9