Abstract
Purpose
We aimed to evaluate and compare effects of photobiomodulation (PBM) and low-magnitude high-frequency mechanical vibration (HFMV) on orthodontic retention.
Methods
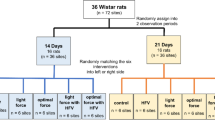
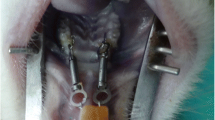
Sixty-four female Wistar albino rats were divided into 9 groups (2 negative and positive controls each, 3 PBM and 2 HFMV groups) and studied for 25 days. In the experimental groups, closed nickel–titanium closed coil springs with a 50 cN force were placed for 10 days between the maxillary incisor and molar. PBM and HFMV were applied daily over long- (15 days) and short-term (7 days) retention periods. The PBM groups received PBM with a single wavelength (650 nm) or higher wavelengths (532, 650, 940 nm) for 9 min per day. HFMV groups received HFMV of 10, 20, and 30 Hz for 10 min per day. Right and left maxilla were assessed using micro-computed tomography imaging and real-time polymerase chain reaction. The amount of tooth movement during the retention period, expression levels of cyclooxygenase‑2 (COX-2), osteoprotegerin (OPG), and receptor activator of nuclear factor-kappa B ligand (RANKL) mRNA gene expression levels, OPG/RANKL ratios, alveolar bone trabecular thickness (Tb.Th), trabecular number (Tb.N), and structure model index were analyzed. Kruskal–Wallis and Mann–Whitney U tests were used for multiple comparisons of the nonparametric distributed data and binary comparisons, respectively.
Results
When using the long-term retention protocol, PBM and HFMV treatment increased Tb.N (p < 0.05) and decreased COX‑2 mRNA gene expression levels (p < 0.05) and Tb.Th (p < 0.05) compared to controls. For short-term retention, PBM and HFMV decreased the amount of relapse tooth movement compared to controls. In addition, Tb.Th (p < 0.05) and the mRNA gene expression levels of COX‑2 and RANKL (p < 0.05) were decreased.
Conclusion
PBM and HFMV might be able to support retention after orthodontic tooth movement by reducing bone resorption and increasing bone quality.
Zusammenfassung
Zielsetzung
Unser Ziel war es, die Auswirkungen von Photobiomodulation (PBM) und schwacher hochfrequenter mechanischer Vibration (HFMV) auf die kieferorthopädische Retention zu untersuchen und zu vergleichen.
Methoden
Vierundsechzig weibliche Wistar-Albino-Ratten wurden in 9 Gruppen aufgeteilt (je 2 Negativ- und Positivkontrollen, 3 PBM- und 2 HFMV-Gruppen) und 25 Tage lang untersucht. In den Versuchsgruppen wurden 10 Tage lang geschlossene Nickel-Titan-Schraubenfedern mit einer Kraft von 50 cN zwischen den oberen Schneidezähnen und Molaren platziert. PBM und HFMV wurden täglich über lange (15 Tage) und kurze (7 Tage) Retentionszeiten appliziert. Die PBM-Gruppen erhielten PBM mit einer einzigen Wellenlänge (650 nm) oder höheren Wellenlängen (532, 650, 940 nm) für 9 min pro Tag. Die HFMV-Gruppen erhielten HFMV von 10, 20 und 30 Hz für 10 min pro Tag. Der rechte und linke Oberkiefer wurde mittels Mikro-Computertomographie-Bildgebung und Real-time-Polymerasekettenreaktion untersucht. Das Ausmaß der Zahnbewegung während der Retentionszeit, das mRNA-Expressionsniveau von Zyklooxygenase‑2 (COX-2), Osteoprotegerin (OPG) und Rezeptoraktivator des Nuklearfaktor-κ-B-Liganden (RANKL), das OPG/RANKL-Verhältnis, die trabekuläre Dicke des Alveolarknochens (Tb.Th), die trabekuläre Anzahl (Tb.N) und der Strukturmodellindex wurden analysiert. Für multiple Vergleiche der nichtparametrisch verteilten Daten bzw. für binäre Vergleiche wurden der Kruskal-Wallis- und der Mann-Whitney-U-Test verwendet.
Ergebnisse
Bei Anwendung des Langzeit-Retentionsprotokolls erhöhte die PBM- und HFMV-Behandlung die Tb.N (p < 0,05) und verringerte die COX-2-mRNA-Genexpressionslevel (p < 0,05) und die Tb.Th (p < 0,05) im Vergleich zu den Kontrollen. Bei der Kurzzeitretention verringerten PBM und HFMV im Vergleich zu den Kontrollen das Ausmaß der Rezidivzahnbewegung. Darüber hinaus wurden Tb.Th (p < 0,05) und die mRNA-Genexpressionsniveaus von COX‑2 und RANKL (p < 0,05) verringert.
Schlussfolgerung
PBM und HFMV könnten in der Lage sein, die Retention nach kieferorthopädischer Zahnbewegung zu unterstützen, indem sie die Knochenresorption reduzieren und die Knochenqualität erhöhen.









Similar content being viewed by others
References
Reitan K (1969) Principles of retention and avoidance of posttreatment relapse. Am J Orthod Dentofacial Orthop 55:776–790. https://doi.org/10.1016/0002-9416(69)90050-5
Reitan K (1959) Tissue rearrangement during retention of orthodontically rotated teeth. Angle Orthod 29:105–113. https://doi.org/10.1043/0003-3219(1959)029%3C0105:TRDROO%3E2.0.CO;2
Andriekute A, Vasiliauskas A, Sidlauskas A (2017) A survey of protocols and trends in orthodontic retention. Prog Orthod 18:31. https://doi.org/10.1186/s40510-017-0185-x
Kalajzic Z, Peluso EB, Utreja A, Dyment N, Nihara J, Xu M et al (2013) Effect of cyclical forces on the periodontal ligament and alveolar bone remodeling during orthodontic tooth movement. Angle Orthod 84:297–303. https://doi.org/10.2319/032213-234.1
Kim S‑J, Kang Y‑G, Park J‑H, Kim E‑C, Park Y‑G (2013) Effects of low-intensity laser therapy on periodontal tissue remodeling during relapse and retention of orthodontically moved teeth. Lasers Med Sci 28:325–333. https://doi.org/10.1007/s10103-012-1146-8
Yadav S, Assefnia A, Gupta H, Vishwanath M, Kalajzic Z, Allareddy V et al (2015) The effect of low-frequency mechanical vibration on retention in an orthodontic relapse model. Eur J Orthod 38:44–50. https://doi.org/10.1093/ejo/cjv006
Han G, Chen Y, Hou J, Liu C, Chen C, Zhuang J, Meng W (2010) Effects of simvastatin on relapse and remodeling of periodontal tissues after tooth movement in rats. Am J Orthod Dentofacial Orthop 138:550.e1–550.e7. https://doi.org/10.1016/j.ajodo.2010.04.026
Liu Y, Zhang T, Zhang C, Jin S, Yang R, Wang X, Jiang N, Gan YH, Kou XX, Zhou YH (2017) Aspirin blocks orthodontic relapse via inhibition of CD4+ T lymphocytes. J Dent Res 96:586–594. https://doi.org/10.1177/0022034516685527
Dolci GS, Portela LV, de Souza DO, Fossati ACM (2017) Atorvastatin-induced osteoclast inhibition reduces orthodontic relapse. Am J Orthod Dentofacial Orthop 151:528–538. https://doi.org/10.1016/j.ajodo.2016.08.026
Pandeshwar P, Roa MD, Das R, Shastry SP, Kaul R, Srinivasreddy MB (2016) Photobiomodulation in oral medicine: a review. J Investig Clin Dent 7:114–126. https://doi.org/10.1111/jicd.12148
Celebi F, Turk T, Bicakci AA (2019) Effects of low-level laser therapy and mechanical vibration on orthodontic pain caused by initial archwire. Am J Orthod Dentofacial Orthop 156:87–93. https://doi.org/10.1016/j.ajodo.2018.08.021
Saito S, Shimizu N (1997) Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop 111:525–532. https://doi.org/10.1016/s0889-5406(97)70152-5
Altan BA, Sokucu O, Ozkut MM, Inan S (2012) Metrical and histological investigation of the effects of low-level laser therapy on orthodontic tooth movement. Lasers Med Sci 27:131–140. https://doi.org/10.1007/s10103-010-0853-2
Goulart CS, Nouer PRA, Mouramartins L, Garbin IU, Lizarelli RDFZ (2006) Photoradiation and orthodontic movement: experimental study with canines. Photomed Laser Surg 24:192–196. https://doi.org/10.1089/pho.2006.24.192
Seifi M, Shafeei HA, Daneshdoost S, Mir M (2007) Effects of two types of low-level laser wave lengths (850 and 630 nm) on the orthodontic tooth movements in rabbits. Lasers Med Sci 22:261–264. https://doi.org/10.1007/s10103-007-0447-9
Franzen TJ, Zahra SE, El-Kadi A, Vandevska-Radunovic V (2014) The influence of low-level laser on orthodontic relapse in rats. Eur J Orthod 37:111–117. https://doi.org/10.1093/ejo/cju053
Rubin C, Judex S, Qin YX (2006) Low-level mechanical signals and their potential as a non-pharmacological intervention for osteoporosis. Age Ageing 35(2):ii32–ii36. https://doi.org/10.1093/ageing/afl082
Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K (2004) Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 19:343–351. https://doi.org/10.1359/jbmr.0301251
Rubin C, Turner AS, Müller R, Mittra E, McLeod K, Lin W, Qin Y‑X (2002) Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res 17:349–357. https://doi.org/10.1359/jbmr.2002.17.2.349
Zhang C, Zhang L, Xu X, Duan P, Wu H (2014) Mechanical vibration may be a novel adjuvant approach to promoting stability and retention following orthodontic treatment. Dent Hypotheses 5:98–102. https://doi.org/10.4103/2155-8213.136751
Zhang C, Li J, Zhang L, Zhou Y, Hou W, Quan H, Li X, Chen Y, Yu H (2012) Effects of mechanical vibration on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Arch Oral Biol 57:1395–1407. https://doi.org/10.1016/j.archoralbio.2012.04.010
Rody WJ Jr, Wheeler TT (2017) Retention management decisions: a review of current evidence and emerging trends. Semin Orthod 23:221–228. https://doi.org/10.1053/j.sodo.2016.12.009
Faul F, Erdfelder E, Lang A‑G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Ren Y, Maltha JC, Kuijpers-Jagtman AM (2004) The rat as a model for orthodontic tooth movement—a critical review and a proposed solution. Eur J Orthod 26:483–490. https://doi.org/10.1093/ejo/26.5.483
Zhao N, Liu Y, Kanzaki H, Liang W, Ni J, Lin J (2012) Effects of local osteoprotegerin gene transfection on orthodontic root resorption during retention: an in vivo micro-CT analysis. Orthod Craniofac Res 15:10–20. https://doi.org/10.1111/j.1601-6343.2011.01532.x
Misawa Y, Kageyama T, Moriyama K, Kurihara S, Yagasaki H, Deguchi T, Ozawa H, Sahara N (2007) Effect of age on alveolar bone turnover adjacent to maxillary molar roots in male rats: a histomorphometric study. Arch Oral Biol 52:44–50. https://doi.org/10.1016/j.archoralbio.2006.06.012
Tuner J, Hode L (2002) Laser therapy: clinical practice scientific background. Prima Books, Grangesberg
Judex S, Boyd S, Qin YX, Turner S, Ye K, Muller R, Rubin C (2003) Adaptations of trabecular bone to low magnitude vibrations result in more uniform stress and strain under load. Ann Biomed Eng 31:12–20. https://doi.org/10.1114/1.1535414
Hildebrand T, Ruegsegger P (1997) Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin 1:15–23. https://doi.org/10.1080/01495739708936692
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486. https://doi.org/10.1002/jbmr.141
Hasegawa T, Yoshimura Y, Kikuiri T, Yawaka Y, Takeyama S, Matsumoto A et al (2002) Expression of receptor activator of NF-kappa B ligand and osteoprotegerin in culture of human periodontal ligament cells. J Periodontal Res 37:405–411. https://doi.org/10.1034/j.1600-0765.2002.01603.x
Houston WJ (1983) The analysis of errors in orthodontic measurements. Am J Orthod 83:382–390. https://doi.org/10.1016/0002-9416(83)90322-6
de Souza Galvão MC, Sato JR, Coelho EC (2012) Dahlberg formula: a novel approach for its evaluation. Dental Press J Orthod 17:115–124. https://doi.org/10.1590/S2176-94512012000100015
Michaeli Y, Steigman S, Harari D (1985) Recovery of the dental and periodontal tissues of the rat incisor following application of continuous intrusive loads: a long-term study. Am J Orthod 87:135–143. https://doi.org/10.1016/0002-9416(85)90023-5
Wolf M, Schulte U, Küpper K, Bourauel C, Keilig L, Papageorgiou SN, Dirk C, Kirschneck C, Daratsianos N, Jäger A (2016) Post-treatment changes in permanent retention. J Orofac Orthop 77:446–453. https://doi.org/10.1007/s00056-016-0054-0
Schneider D, Smith S, Campbell C, Hayami T, Kapila S, Hatch N (2015) Locally limited inhibition of bone resorption and orthodontic relapse by recombinant osteoprotegerin protein. Orthod Craniofac Res 18:187–195. https://doi.org/10.1111/ocr.12086
Tunér J, Beck-Kristensen PH, Ross G, Ross A (2015) Photobiomodulationin dentistry. In: Convissar RA (ed) Principles and practice of laser dentistry, 2nd edn. Elsevier, St. Louis, pp 251–274
Keklikci HB, Yagci A, Yay AH, Goktepe O (2020) Effects of 405-, 532-, 650-, and 940-nm wavelengths of low-level laser therapies on orthodontic tooth movement in rats. Prog Orthod 21:1–14. https://doi.org/10.1186/s40510-020-00343-3
Ekizer A, Turker G, Uysal T, Guray E, Tasdemir Z (2016) Light emitting diode mediated photobiomodulation therapy improves orthodontic tooth movement and miniscrew stability: a randomized controlled clinical trial. Lasers Surg Med 48:936–943. https://doi.org/10.1002/lsm.22516
Fonseca PD, de Lima FM, Higashi DT, Koyama DFV, Toginho DD, Dias IFL, de Paula Ramos S (2013) Effects of light emitting diode (LED) therapy at 940 nm on inflammatory root resorption in rats. Lasers Med Sci 28:49–55. https://doi.org/10.1007/s10103-012-1061-z
da Silva APRB, Petri AD, Crippa GE, Stuani AS, Stuani AS, Rosa AL, Stuani MBS (2012) Effect of low-level laser therapy after rapid maxillary expansion on proliferation and differentiation of osteoblastic cells. Lasers Med Sci 27:777–783. https://doi.org/10.1007/s10103-011-0968-0
Glinkowski W (2001) Low-level energy laser therapy in the musculoskeletal system lasers in the musculoskeletal system. Springer, Berlin, pp 188–198
Glinkowski W, Pokora L (2001) Lasers in therapy: laser instruments. Centrum Techniki Laserowe, Warszawa
Salehi P, Heidari S, Tanideh N, Torkan S (2015) Effect of low-level laser irradiation on the rate and short-term stability of rotational tooth movement in dogs. Am J Orthod Dentofacial Orthop 147:578–586. https://doi.org/10.1016/j.ajodo.2014.12.024
Xu M, Deng T, Mo F, Deng B, Lam W, Deng P, Zhang X, Liu S (2009) Low-intensity pulsed laser irradiation affects RANKL and OPG mRNA expression in rat calvarial cells. Photomed Laser Surg 27:309–315. https://doi.org/10.1089/pho.2008.2283
Liu JG (2013) REMOTE SENSING I passive sensors. Reference module in earth systems and environmental sciences. Encyclopedia of geology, pp 431–439
Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR (2013) Low level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Sem Cutan Med Surg 1:41
Li WT, Leu YC, Wu JL (2010) Red-light light-emitting diode irradiation increases the proliferation and osteogenic differentiation of rat bone marrow mesenchymal stem cells. Photomed Laser Surg 28:157–165. https://doi.org/10.1089/pho.2009.2540
Lim JH, Lee J, Choi J, Hong J, Jhun H, Han J, Kim S (2009) The effects of light-emitting diode irradiation at 610 nm and 710 nm on murine T‑cell subset populations. Photomed Laser Surg 27:813–818. https://doi.org/10.1089/pho.2008.2375
Turrioni APS, Basso FG, Alonso JRL, de Oliveira CF, Hebling J, Bagnato VS, de Souza Costa CA (2015) Transdentinal cell photobiomodulation using different wavelengths. Oper Dent 40:102–111. https://doi.org/10.2341/13-370-L
Kirschneck C, Wolf M, Reicheneder C, Wahlmann U, Proff P, Roemer P (2014) Strontium ranelate improved tooth anchorage and reduced root resorption in orthodontic treatment of rats. Eur J Pharmacol 744:67–75. https://doi.org/10.1016/j.ejphar.2014.09.039
Romão M, Marques MM, Cortes A, Horliana A, Moreira M, Lascala C (2015) Micro-computed tomography and histomorphometric analysis of human alveolar bone repair induced by laser phototherapy: a pilot study. Int J Oral Maxillofac Surg 44:1521–1528. https://doi.org/10.1016/j.ijom.2015.08.989
de Zepetnek JOT, Giangregorio LM, Craven BC (2009) Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis: a review. J Rehabil Res Dev 46:529–542. https://doi.org/10.1682/jrrd.2008.09.0136
Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L (2010) Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone 46:1508–1515. https://doi.org/10.1016/j.bone.2010.02.031
Christiansen BA, Silva MJ (2006) The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann Biomed Eng 34:1149–1156. https://doi.org/10.1007/s10439-006-9133-5
Alikhani M, Alansari S, Hamidaddin MA, Sangsuwon C, Alyami B, Thirumoorthy SN, Oliveira SM, Nervina JM, Teixeira CC (2018) Vibration paradox in orthodontics: anabolic and catabolic effects. PLoS ONE 13:e196540. https://doi.org/10.1371/journal.pone.0196540
Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K (2001) Low mechanical signals strengthen long bones. Nature 412:603–604. https://doi.org/10.1038/35088122
Dominguez A, Gomez C, Palma JC (2015) Effects of low-level laser therapy on orthodontics: rate of tooth movement, pain, and release of RANKL and OPG in GCF. Lasers Med Sci 30:915–923. https://doi.org/10.1007/s10103-013-1508-x
Funding
The research leading to these results received funding from Erciyes University Scientific Research Unit under Grant Agreement No TDH-2018-8304.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Öztürk and N. Gül Amuk declare that they have no competing interests.
Ethical standards
The Local Ethics Committee of Animal Experiments of the Erciyes University, Kayseri, Turkey (Approval code: 18/011) approved the study. During this study, it was declared that the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines and EU Directive 2010/63/EU criteria were complied with in animal experiments.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Öztürk, T., Gül Amuk, N. Three-dimensional imaging and molecular analysis of the effects of photobiomodulation and mechanical vibration on orthodontic retention treatment in rats. J Orofac Orthop 83 (Suppl 1), 24–41 (2022). https://doi.org/10.1007/s00056-021-00296-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00056-021-00296-0
Keywords
- Micro-computed tomography, X‑ray
- Retention after orthodontic treatment
- Relapse
- Polymerase chain reaction
- Animal model