Abstract
Although recognition using cuticular chemistry is important for host–parasite interactions within aculeate Hymenoptera, cuticular hydrocarbon (CHC) profiles of only a few host–parasite pairs were characterized and compared. One largely neglected family in this context is the Mutillidae (velvet ants), whose species are ectoparasitoids of bees and wasps. In our study, we characterized and compared the CHC profiles of five species of Mutillidae and seven host species. The CHC profile of velvet ants differed among species and included large proportions of n-alkanes and methyl-branched alkanes. Alkenes were much less abundant in the CHC profiles of three species of velvet ants compared with their hosts, while the other two species possess a much lower abundance of methyl-branched alkanes than their hosts. Both the number of peaks and compound diversity were generally higher in velvet ants compared with their hosts. Thus, CHC profiles of parasitoids did not show signs of mimicry when compared with their hosts. In dyadic encounters between one species of velvet ant and its host bee species, the parasitoid mainly avoided interacting, while aggression by the host was rare. Our results suggest that velvet ants did not evolve chemical mimicry, perhaps in accordance with their wide host spectrum which would limit chemical specialization. However, the reduction of alkenes in social bee-attacking species and the reduction of methyl-branched alkanes in social wasp-attacking species may favour host nest invasion, since these two CHC classes are known to be important in nestmate recognition for social bees and wasps, respectively. A larger, phylogeny-corrected comparison of Mutillidae and hosts may help clarifying the evolution of the CHC profile of these parasitoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brood parasitism, i.e. invasion of a host’s nest to exploit its resources, is widespread in insects and fascinated natural historians for a long time (Buschinger 2009; Cini et al. 2019; Litman 2019). Brood parasites can be subdivided into three following categories: parasitoids (whose brood feeds on the host immatures) (O’Neill 2001), cleptoparasites (whose brood feeds on the host’s food stores) (Michener 2007), and social parasites (reproductive females occupy and exploit the worker force of the host colony to raise their own offspring) (Lorenzi 2006). These brood parasites have to sneak into the host nests without being detected by the adult hosts. In doing so, females of insect brood parasites easily leave chemical traces in the nest during invasion, potentially provoking a defensive response by the host, including the destruction of the parasites’ egg or by attacking the adult parasite (Rosenheim 1988; Ballesteros et al. 2012; Polidori et al. 2009). Thus, brood parasites evolved strategies to reduce the probability of being detected by their hosts or, alternatively, to reduce damages in case of direct fights. In turn, hosts evolved mechanisms to detect brood parasites, to avoid parasitism, or to physically defend themselves better in case of direct fight (Poulin et al. 2000). This leads to an evolutionary arms race between hosts and parasites (Dawkins and Krebs 1979; Schmid-Hempel 1998) involving reciprocal adaptations in behaviour (e.g. Polidori et al. 2010; Foitzik et al. 2003), morphology (e.g. Ortolani and Cervo 2010) or physiology (e.g. Brandt et al. 2005; Wurdack et al. 2015; Barbero et al. 2009). The latter often alter communication channels that allow parasites to enter the host’s nest undetected.
Among such physiological adaptations in brood parasitic insects, many are linked to strategies that prevent recognition by hosts via chemical deception and are especially studied in aculeate Hymenoptera (bees, wasps and ants), a diverse insect group in which parasitism evolved independently several times (O’Neill 2001; Lorenzi 2006; Michener 2007). Chemical deception exploits chemical recognition and communication in intra- and interspecific interactions in insects (Howard and Blomquist 2005). Three strategies are known, all of them are essentially based on modifications of the cuticular hydrocarbon (CHC) profile, i.e. a thin layer of non-polar substances that covers the entire surface of insects. Besides their primary function in reducing desiccation, abrasion, and infection (e.g. Menzel et al. 2017), CHCs act as semiochemicals in various contexts of communication (Howard and Blomquist 2005; Blomquist and Bagnères 2010). One strategy is chemical mimicry, where the parasite synthetises de novo a CHC profile matching that of the host (Strohm et al. 2008; Wurdack et al. 2015; Polidori et al. 2020a). A second strategy is chemical insignificance, where the parasites have a quantitatively reduced CHC profile and/or a simpler composition of the profile, so that they are poorly perceived by the hosts (Johnson et al. 2001; Kroiss et al. 2009; Polidori et al. 2020b). A third strategy is chemical camouflage, which seems to be restricted to social parasites, where the parasite’s CHC profile is acquired from the host through extended contacts with nest material or the hosts themselves (Lenoir et al. 2001; Cini et al. 2011; Johnson et al. 2001; Lorenzi 2006). For chemical camouflage, the selective pressure acts on the behavioural traits of the parasite (i.e. fear response, social behaviour) while for the de novo synthesis of CHC profile (mimicry and insignificance), the selection pressure acts on the biosynthetic pathway of the CHCs.
CHC profiles and their relation with those of the hosts were characterized for species belonging to only few families within aculeate Hymenoptera (Vespidae, Apidae, Halictidae, Formicidae, Chrysididae) and, among them, most often in social parasites compared with solitary parasitoids or kleptoparasites. For the latter two groups, data are available mainly for cuckoo wasps (Chrysididae) (Strohm et al. 2008; Wurdack et al. 2015; Polidori et al. 2020a; Soon et al. 2021; Fröhlich et al. 2022; Castillo et al. 2022) and for cuckoo bees (Halictidae) (Polidori et al. 2020b). However, brood parasitism evolved in many other aculeate families, such as Sapygidae, Pompilidae, Scoliidae, Apidae, and Megachilidae (Danforth et al. 2019; O’Neill 2001; Branstetter et al. 2017). Hence, we are just at the beginning to understand the diversity and evolution of CHC profiles in solitary aculeates and to evaluate if natural enemies employ chemical deception strategies to increase the success of host attack. One of the neglected families whose CHC profile remains largely not studied are the Mutillidae. Investigations were made only on Mutilla europea L., a parasitoid of bumblebees and a kleptoparasite of the eusocial paper wasp Polistes biglumis L. (Uboni et al. 2012, Uboni and Lorenzi 2013) and recently on Myrmilla capitata (Lucas), a parasitoid of digger bees (Ronchetti et al. 2023). In M. europea, the CHC profile includes more hydrocarbons compared with its host, but the total amount of CHCs on the cuticle is significantly lower than the amount found on the Polistes host, suggesting an insignificance strategy. The M. capitata CHC profile also diverges from that of the host bees and shows a complex composition.
Mutillidae is a large wasp family including around 4600 species from 220 genera (Brothers and Lelej 2017; Pagliano et al. 2019). They are known as velvet ants because of their well-visible and often dense pilosity all over their body, and because their females are apterous (Mickel 1928). These wasps are ectoparasitoids and attack post-defecated larvae or pupae of other insects, most often other aculeate Hymenoptera, both solitary and social and both ground-nesters and aerial-nesters (reviewed in Ronchetti and Polidori 2020). Despite being elusive insects, velvet ants can be still observed patrolling the nesting areas of their hosts, especially in the early morning and late afternoon (Manley and Spangler 1983; Schmidt and Buchmann 1986; Polidori et al. 2009, 2010; Lienhard et al. 2010). Host use, probably known for only about 2–3% of all described mutillid species (Brothers 1989; Ronchetti and Polidori 2020), varies across mutillid lineages. Mutillids specialize on hosts with different ecological traits (nest type, larval diet and sociality). Therefore, they can be defined as ecological specialists rather than taxonomically specialist in their host use (Ronchetti and Polidori 2020). Velvet ants have remarkably interested zoologists for a long time because of the wealth of their defence strategies. These include the ability to stridulate by rubbing a scraper on a file on their gaster (Polidori et al. 2013), a variation of aposematic coloration which might lead to large Müllerian mimicry complexes (Wilson et al. 2015; Hines et al. 2017), the longest sting relative to body size among aculeate Hymenoptera (Sadler et al. 2018), a remarkably strong exoskeleton (Schmidt and Blum 1977), Zn-enriched mandibles (Jorge et al. 2017), and a powerful venom (Starr 1985; Schmidt 1990; Jensen et al. 2021). All these traits were shown or at least strongly suggested to primarily serve as deterrent to the attack of predators (Schmidt and Blum 1977; Masters 1979; Gall et al. 2018; Schmidt et al. 2021), but some of them may also favour host nest invasions (e.g. cuticle robustness, metals in mandibles).
In our study, we analysed the composition of CHC profiles of velvet ants and their hosts. Because velvet ant species are often generalist in their host choice, we hypothesize that chemical mimicry did not evolve in these parasitoids. Indeed, chemical mimicry was essentially associated with hymenopteran brood parasites with a narrow host specialization (Strohm et al. 2008; Wurdack et al. 2015; Polidori et al. 2020a). However, velvet ants may still have evolved chemical traits favouring host nest invasion, such as reduction of CHC profile complexity, or reduction in the abundance of CHC classes involved in host chemical communication, that would make them less detectable by the hosts.
Material and methods
Sample collection and species synopsis
The collection of velvet ants was performed between 1st and 30th of June 2018 at three localities in Southern Europe: Alberese (Tuscany, Italy: 42°40′5″N, 11°6′23″E), El Saler (Valencia, Spain: 39°22′57″N 0°19′57″W) and Almarail (Soria, Spain: (41°34′ 50″N, 2°22′52″W). Five mutillid species were collected (Fig. 1A–B). Hosts were collected either at the nesting sites where their associated mutillids were found or in other locations (see below). Only female velvet ants and females of their host species were collected. Myrmilla capitata (n = 10) (Myrmillinae) and workers of its host bee Lasioglossum malachurum (Kirby) (n = 10) (Halictidae) were sampled in Alberese, at a nesting site of the latter, located in a trail bounded in part by cultivated fields (wheat or alfa-alfa, depending on the year), nearby the Ombrone River. Myrmilla calva (n = 6) and its host bee L. malachurum were collected at the same location, while a second host bee species, Lasioglossum calceatum (Scopoli) (n = 7), was collected at southern German localities in Baden-Württemberg (Freiburg: 47°59′41’’N, 7°50′59’’E). Nemka viduata (Pallas) (n = 7) (Smicromyrmini) and its host wasps Stizus continuus (Klug) (n = 13) and Bembecinus tridens (Fabricius) (n = 5) (Crabronidae) were collected at El Saler, at one nesting site of both hosts. Dasylabris maura (L.) (n = 4) (Dasylabrini) and its host wasp Ammophila laevicollis (Ed. André) (n = 6) (Sphecidae) were collected at a host nesting site at Almarail, while a second host wasp species, Philanthus triangulum (Fabricius) (n = 10), was collected at a nesting site in Würzburg (Germany: 49°47′40″N 9°55′46″E). Tropidotilla litoralis (Petagna) (n = 11) (Mutillini) was collected at Alberese, while its host wasp Polistes gallicus (L.) (n = 3) (Vespidae) was collected on Pag, an island of Croatia (44°30′27″N 14°56′26″E). Stizus continuus, B. tridens, P. triangulum and A. laevicollis are solitary species specialized in hunting grasshoppers, homopterans, honeybees and lepidopteran larvae, respectively, to feed the brood (O’Neill 2001; Evans and O’Neill 2007), while L. malachurum, L. calceatum and P. gallicus are eusocial species with a generalist pollen (the former two) or arthropod (the latter) larval diet (Michener 2007; Hunt 2007). All hosts are ground-nesting species except P. gallicus, which builds aerial paper nests (Hunt 2007).
A phylogenetic relationships among the five studied mutillid species, following Brothers and Lelej (2017). B pictures taken on field of adult females of three of the studied species of velvet ants (left to right: T. litoralis, N. viduata, M. capitata). C representative chromatograms of the CHC profiles of three species of velvet ants (above, coloured) and their respective hosts (below, in grey)
In all cases, both the velvet ant and host individuals were collected on sunny days, at hours of their foraging activity peak in the morning (9.00–12.00) (Polidori, et al. 2009, 2010). Female mutillid wasps were sampled using large plastic tubes with sponge caps directly on the ground, while hosts were sampled with an entomological net while approaching their nests or while exiting from their nests or while foraging on flowers. Once collected, the specimens were frozen at – 20 °C. The morphology of the individuals was observed under a stereomicroscope to confirm identification of species and sex.
Chemical analysis
The samples were immerged in hexane in glass vials for 10 min to extract their cuticular profile. The extracts were stored at – 20 °C. The specimens were stored in 99% ethanol to preserve the DNA. By using a gentle stream of nitrogen, the volume of the extracts was reduced to about 200 µl. The gas chromatography/mass spectrometry analysis was performed using an Agilent 6890 gas chromatograph (GC) coupled with an Agilent 5975 Mass Selective Detector (MS) (Agilent, Waldbronn, Germany). The GC (split/splitless injector in splitless mode for 1 min, injected volume: 1 µl at 300 °C) was equipped with a DB-5 Fused Silica capillary column (30 m × 0.25 mm ID, df = 0.25 µm, J&W Scientific, Folsom, USA). We used helium as carrier gas at constant flow of 1 ml per minute. The following temperature program was used: start temperature 60 °C, temperature increase by 5 °C per minute up to 300 °C and isotherm at 300 °C for 10 min. The electron ionization mass spectra (EIMS) were acquired at an ionization voltage of 70 eV (source temperature: 230 °C). For recording and the analysis of chromatograms and mass spectra (Fig. 1C), we used the software HP Enhanced ChemStation G1701AA Version A.03.00. The compounds were identified by using retention indices and diagnostic ions. The retention index of each peak was calculated according to the formula of McNaught and Wilkinson (1997).
Behavioural assays
Behavioural interactions were studied between females of M. capitata and females (workers) of its host bee L. malachurum at their nesting site in Alberese in July 2021 (9.00–15.00). Staged dyadic encounters in a circle-tube apparatus (e.g. Pabalan et al. 2000; Boesi and Polidori 2011; Polidori et al. 2020b) were used to test if a host bee reacts differently when encounters a velvet ant or a conspecific. We recorded the interactions between M. capitata and L. malachurum; data on conspecific interactions in L. malachurum from the same nest aggregations come from a previous work (Polidori et al. 2020b). The circle-tube apparatus consisted of a 22 cm-long piece of clear sterile plastic tube of 0.7 cm inner diameter fashioned into circles. Pairs of M. capitata and L. malachurum were collected at the host nest aggregation and kept for a maximum of 15 min in a shaded place before to be used for the experiments. We first introduced the host bee in the circle-tube. After two minutes we introduced the parasitoid to resemble the situation of a nest invasion attempt. Each trial lasted 10 min, which was sufficient to detect behavioural differences (Pabalan et al. 2000). After each experiment, the used arena was discarded to avoid odour contaminations in subsequent trials (Smith and Weller 1989). A total of 26 trials, 19 host–host and 7 mutillid–host trails, were performed.
The recorded behaviours were classified as one of the three categories following previous circle-tube studies: tolerant, aggressive, and avoidant interactions (Pabalan et al. 2000; Packer 2006; Boesi and Polidori 2011; Polidori et al. 2020b). Tolerant behaviours include “mutual passing” (individuals accommodate each other while they pass in opposite directions), “following” (a forward movement by an individual toward the other which walks backward through the circle tube), and “stop in contact” (individuals in a frontal encounter stop in contact and touch each other slowly with antennae and mandibles). Aggressive behaviours include “C-posture” (an individual curls her abdomen under the thorax with the intention to sting the other one), “mandibular hold” (an individual clamps the mandibles around the neck, limbs or antenna of the other one), “mandibular flare” (an individual quickly approaches the other one with open mandibles) and “push and lunge” (an individual lunges forward, often with a short forceful push, against the other one, usually with mandibles open). Avoidant behaviours include “withdrawing” (an individual makes a 180° ± turn away from the other one and backs moves away from it) and “stop without contact” (the two individuals stop in front to each other but without touching) (Supplementary file BehavDATA).
Statistical analysis
We first deleted all the compounds which added less than 0.01% to the overall relative amount within each group. If a compound shows up to more than 0.01% in a single group, we kept it in all investigated groups for the comparative analysis. In a second step we eliminated all compounds which did not occur at least in 50% of all individuals within a group. The final matrix, including both velvet ants and hosts, included a total of 153 peaks (Tables S1–S2). Prior to the statistical analysis, since relative peak areas of a sample are not statistically independent, we transformed all the % peak values following the modified Aitchison (1986)’s method used by Strohm et al. (2008). With such transformation (log10((% peak area/geometric mean of % peak area) + 1)), we did not exclude compounds that do not occur in all samples, which is more adequate in case of parasite-host CHC comparisons (since the exclusion of peaks that are not present in all samples may erroneously increase the similarity between the groups). Transformed relative values used in the subsequent statistical analyses are provided in the Supplementary file ChemDATA.
We tested for chemical differences between groups by performing non-parametric comparisons and multivariate analyses. We tested for differences in the abundances of CHC classes between parasitoids and their hosts with Mann–Whitney test in case of systems composed by one parasitoid and one host or with Kruskal–Wallis test followed by Dunn’s paired comparisons in case of systems composed by one parasitoid and two hosts. Bonferroni correction for multiple comparisons was applied to P-values in the latter case. We compared the total number of peaks and the peak diversity among groups as an overall indication of CHC profile complexity (Torres et al. 2018, and references therein). The peak diversity was estimated with the Shannon–Wiener index, with higher values indicating greater chemical diversity; this index depends on both the number of substances and on the relative abundances of the substances. We used permutation tests (2000 permutations) to test for differences in peak numbers and Shannon–Wiener diversity in parasitoid–host paired comparisons. In addition, we measured the chain-length as the number of carbon atoms in the backbone of each molecule, and we calculated, for each species, a quantitative chain length distribution as the sum of CHC abundances separately for each chain-length (Menzel and Schmitt 2012). Then, we compared the distributions of compound abundances across chain-lengths between velvet ants and hosts, with a Kolmogorov–Smirnov test (one test per each of the parasitoid–host pair). We also repeated this latter analysis using only n-alkanes, since n-alkanes aggregate more tightly, especially molecules with higher chain-length, compared with methyl-branched alkanes and alkenes, thus making them less volatile (Gibbs and Pomonis 1995; Gibbs 2002).
The multivariate analyses were all based on Bray–Curtis dissimilarity matrices, which are suitable for zero-inflated datasets (Leyer and Wesche 2007). These analyses do not require a priori grouping of species, meaning that these methods allow pattern formation that are exclusively based on CHC similarities. We first performed an agglomerative cluster analysis based on the unweighted pair group method using arithmetic means of Bray–Curtis dissimilarities. Second, Bray–Curtis dissimilarities were used for ordinations using non-metric multidimensional scaling analysis (NMDS), which is a non-parametric method that avoids assuming linearity among variables (McCune et al. 2002) and whose resulting plot shows the spatial distances between individuals (i.e. their chemical distances). In the NMDS, deviations are expressed in terms of “stress”, for which values ≤ 0.15 indicate a good fit of ordination (Kruskal and Carroll 1969).
PERMANOVA (Non-Parametric MANOVA) was employed to test for differences among the studied species (Anderson 2001). The significance is computed by permutation of group membership (9999 replicates). Pairwise PERMANOVA between all pairs of groups was also computed as a post-hoc test. To correct error rates for multiple comparisons, we used the Bonferroni—Holm procedure (Holm 1979) (or sequential Bonferroni significance), a procedure which adjusts the rejection criteria for each hypothesis (Giacalone et al. 2018). Similarity percentages (SIMPER) were calculated to identify the compounds that predominantly contributed to the Bray–Curtis dissimilarities among pairs of species (Clarke 1993). We used the Kruskal–Wallis test to compare the average SIMPER dissimilarity among host–parasitoid, parasitoid–parasitoid, and host–host species pairs.
Count data from the behavioural experiments were not always normally distributed (Shapiro–Wilk test: W = 0.46–0.93, 0.00001 < P < 0.6) and contain many zeros. Thus, as in previous studies with similar behavioural data (e.g. Legendre et al. 2008; Smith et al. 2019; Polidori et al. 2020a, b,) we used the non-parametric Kruskal–Wallis test (followed by paired comparisons through Dunn’s procedure) to test for differences in the total number of interactions and in the number of aggressive, tolerant, and avoidance interactions observed across the three groups of heterospecific and conspecific trials (M. capitata towards L. malachurum, L. malachurum towards M. capitata and in L. malachurum-L. malachurum pairs).
In the text and tables, mean values are expressed with standard error. The statistical analyses were performed in PAST 3.04 (Paleontological Statistics Software Package) (Hammer et al. 2001).
Results
Cuticular hydrocarbon profiles
The CHC profile of the velvet ants was composed of 37–59 substances (median = 49) and showed a Shannon–Wiener diversity ranging from 2.55 to 3.12 (median = 2.87), depending on the species (Table 1). The CHC profile of the hosts was composed of 22 to 60 substances (median = 41) and showed a Shannon–Wiener diversity ranging from 1.99 to 2.99 (median = 2.42), depending on the species (Table 1). In paired permutation tests, parasitoids had both, higher compound richness and diversity than their hosts in four cases, while richness only was higher in a parasitoid than in its host in an additional case. In the remaining paired comparisons, parasitoids and hosts either did not differ in both, compound richness and diversity (2 cases), or parasitoids had both, lower compound richness and diversity than their hosts (1 case: N. viduata vs. S. continuus) (Table 1).
The CHC profiles of velvet ants spanned the following five substance classes: n-alkanes, monomethyl-branched alkanes, dimethyl-branched alkanes, trimethyl-branched alkanes, and alkenes (Fig. 2A, Table S1–S2). All substance classes were represented in all species, except for trimethyl-branched alkanes, which lacked in N. viduata. n-Alkanes were abundant in all species (38.5%–72.4%), followed by monomethyl-branched alkanes (9.8%–45.3%). Dimethyl-branched alkanes were less abundant, reaching maximum 16.1% and in one case being < 1%. Trimethyl-branched alkanes, when present, never exceeded 2% in abundance. Alkenes were abundant only in N. viduata (33.3%), being rarely represented (0.1–4.2%) in the other species. Overall, the relative abundances of the substance classes differed across species (Fig. 2A, Table S1–S2). Host species also show significant variation in the relative abundance of the CHC classes. Hosts possessed substances belonging to the same substance classes detected in velvet ants, plus alkadiens, which were rare (0.5%) and only present in L. calceatum (Fig. 2A, Table S1–S2). n-Alkanes were the most abundant substance class in the hosts, while methyl-branched alkanes were abundant in S. continuus and P. gallicus (> 61%) but much rarer in other host species (< 6%); similar patterns appeared while considering monomethyl- and dimethyl-branched alkanes separately. Alkenes were abundant in all hosts (> 12%) apart from S. continuus and P. gallicus, in which this compound class is much rarer (< 4%) (Fig. 2A, Table S1–S2). Trimethyl-branched alkanes only occurred in P. gallicus (Fig. 2A, Table S1).
A relative abundance of the hydrocarbons substance classes in the CHC profiles of the five studied species of velvet ants and of their seven host species. B box‐and‐whisker plots showing medians (horizontal lines within boxes), 1° and 3° quartiles (horizontal lines closing the boxes) and maximum and minimum values (ends of the whiskers) for relative abundance of the 17 detected n-alkanes. Outliers with a value more than 1.5 times the interquartile range are shown as circles, values with more than three times the interquartile range are shown as stars. C, contribution to abundance of the of the 17 detected n-alkanes in all the studied parasitoid-host systems
The abundance of n-alkanes differed in most host–parasitoid systems. Exceptions include M. calva-L. calceatum, N. viduata-B. tridens and T. litoralis-P. gallicus parasite-host pair (Table 2, Fig. S1). M. capitata and M. calva had lower abundances of n-alkanes than their host L. malachurum but did not differ from its host L. calceatum. Dasylabris maura had a higher abundance of n-alkanes than its host P. triangulum, but a similar abundance compared to the other host A. laevicollis. Nemka viduata had a higher abundance of n-alkanes than its host S. continuus, but a similar abundance compared to the other host B. tridens (Table 2, Fig. S1). Monomethyl-branched alkanes were always more abundant in parasitoids than in their hosts, with exception of N. viduata and T. litoralis, which had lower abundances than their hosts or a similar abundance in the case of N. viduata compared to B. tridens (Table 2, Fig. S1). Dimethyl-branched alkanes followed a similar pattern, being always more abundant in parasitoids than in their hosts, with exception of N. viduata and T. litoralis, which had lower (N. viduata to S. continuus) or similar (N. viduata to B. tridens and T. litoralis to P. gallicus) abundances than their hosts (Table 2, Fig. S1). Trimethyl-branched alkanes were much rarer, being absent in seven out of 12 studied species (Table 2, Fig. S1). At last, alkenes were always less abundant in parasitoids than in their hosts, with exception of N. viduata and T. litoralis, which had higher (N. viduata to S. continuus) or similar (N. viduata to B. tridens and T. litoralis to P. gallicus) abundances than their hosts (Table 2, Fig. S1).
The carbon chain length of the CHC profiles ranged from 19 to 35 C atoms, with highest abundances recorded in all species for compounds with 23–31 C atoms (Fig. 2B). Few differences appeared among species. For example, compounds with 19 C atoms were only found in one velvet ant and one host species, while compounds with 35 C atoms only occurred on the cuticle of two velvet ant and one host species (Table S1-S2). However, overall, velvet ants and their hosts had similar total chain lengths in their CHC profiles. This was true, considering all compounds (Kolmogorov–Smirnov test: 0.17 < D < 0.29, N = 17, P > 0.3) and n-alkanes alone (Kolmogorov–Smirnov test: 0.12 < D < 0.24, N = 17, P > 0.6) (Fig. 2C).
The CHC profiles of the five velvet ant species, as well as that of the seven host species, were species-specific. In the dendrogram produced by the hierarchical cluster analysis, individuals of each species formed clusters (Fig. 3A). The two main groups of clusters included six species each. In one, five species were hosts and one was a parasitoid, while, in the other one, four species were parasitoids and two were hosts (Fig. 3A). There may be preliminary indications for a possible effect of phylogeny on chemical distances in velvet ants: the two species of Myrmilla fall close to each other in a sub-cluster, while the other sub-cluster included N. viduata and T. litoralis, and the last species, D. maura, was more distant. This pattern (Dasylabris + ((Myrmilla) + (Nemka + Tropidotilla))) reasonably reflects the current known phylogenetic relationships in velvet ants (Fig. 1A).
A dendrogram based on the agglomerative cluster analysis (Bray‐Curtis distances) of all individual CHC profiles from the 12 studied species. Colours identify species as depicted in (B), grey arrows link each velvet ant species with its host(s). B non‐metric multidimensional scaling (NMDS) based on Bray‐Curtis distances of all individual CHC profiles from the 12 studied species. Triangles represent mutillids and circles represent host species
In accordance with the cluster analysis, the 12 studied species resulted in significantly separated clusters in the NMDS multivariate space (stress = 0.13, PERMANOVA: total sum of square = 13.39, within-group sum of square = 0.74, n = 92, F = 124.3, P = 0.0001), despite partial overlap of L. calceatum with three other host species. In all cases, all paired species differences were significant (Pairwise test: 0.0001 ≤ P ≤ 0.03) (Fig. 3B). The SIMPER analysis showed that the overall average chemical distance ranged between 30.8 and 74.5 and did not differ among host-parasitoid (median = 56.4), parasitoid-parasitoid (median = 58.6) and host-host (median = 48.0) species pairs (Kruskal–Wallis test: χ2 = 3.83, df = 2, P = 0.15). In host–parasitoid contrasts, the lowest values of distance were recorded between a parasitoid and its host only in the cases of D. maura vs. P. triangulum and N. viduata vs. S. continuus (Fig. S2). In all other cases, the velvet ant species showed the lowest distance to a non-host species (Fig. S2). Especially bee hosts (Lasioglossum spp.) had very high distances to their Myrmilla parasitoids (Fig. S2). The SIMPER analysis revealed that the compounds contributing to more than 1% of CHC distances among species (36 substances for a total of 46.2% of contribution) were monomethyl-branched alkanes (21 substances) and alkenes (9 substances). Alkenes were the substance class most contributing to chemical distances (9%–15%) in all cases except in T. litoralis-P. gallicus in which the most important substance class was dimethyl-branched alkanes (8.1%) (Fig. S3). Monomethyl-branched alkanes also importantly accounted for distances in all cases except in N. viduata-B. tridens and in M. calva-L. calceatum in which n-alkanes were important (Fig. S3).
Behaviour
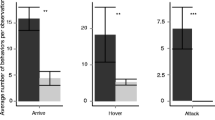
Individuals placed in the circle-tubes started to interact within 1 min from the start of the experiments. Overall, we have recorded 579 interactions, out of which most were tolerant (350), followed by avoidant (204) and by aggressive behaviours (25). Lasioglossum malachurum interacted much more with a conspecific (11–64 interactions/trial, median = 21) than with M. capitata (3–19 interactions/trial, median = 7), and the velvet ants also tended to weakly interact with the host bee (1–11 interactions/trial, median = 7) (Fig. 4A) (Kruskal–Wallis test: χ2 = 20.1, df = 2, P < 0.0001). Dunn’s test for paired comparison revealed that overall differences are due essentially to the behaviour recorded in the L. malachurum-L. malachurum trials. Tolerant behaviours, expressed mainly with “mutual passing” (58% of cases) were very common in host bee intra-specific trails (5–45 interactions/trial, median = 14) but they were very rare or null in the heterospecific experiments (L. malachurum-M. capitata: median = 3, M. capitata- L. malachurum: no records) (Kruskal–Wallis test: χ2 = 24.1, df = 2, P < 0.0001). Differences occurred only between conspecific trials and heterospecific trials (Dunn’s test: P < 0.001) (Fig. 4B). Aggressive interactions, most often expressed by “mandibular hold” and “push and lunge” (72% of cases) (Fig. 4B) were overall rarely recorded in the experiments: while the host bee performed 0–2 aggressions towards a conspecific (median = 0) and no aggressions were observed in M. capitata towards the host bee, the former showed a significantly higher number of aggressions towards the parasitoid (2–4 interactions/trial, median = 3) (Kruskal–Wallis test: χ2 = 15.9, df = 2, P < 0.0001; Dunn’s test: P < 0.001) (Fig. 4B). Avoidant behaviours, essentially expressed by “withdrawing” (92% of cases), were highly common in both host bee intraspecific trials (0–19 interactions/trial, median = 4) and in heterospecific trials (L. malachurum towards M. capitata: 0–11 interactions/trial, median = 3; M. capitata towards L. malachurum: 1–11 interactions/trial, median = 9) (Kruskal–Wallis test: χ2 = 3.8, df = 2, P = 0.15) (Fig. 4B).
Box‐and‐whisker plots showing medians (horizontal lines within boxes), 1° and 3° quartile (horizontal lines closing the boxes), and maximum and minimum values (ends of the whiskers) for the number of behavioural interactions recorded in circle‐tube experiments. A total number of interactions. B number of aggressive, tolerant and avoidant interactions. Outliers with a value more than 1.5 times, the interquartile range are shown as circles. *** means that differences among types of interactions are significant at p < 0.001; letters identify pairwise differences (Dunn’s procedure). Pictures on the left show a female L. malachurum “holding with mandibles” (above) and “pushing and lunging” (below) a female M. capitata (both aggressive behaviours)
Discussion
Enlarging the spectrum of studied lineages is fundamental to reconstruct the evolution of CHC in a large group of Hymenoptera, the Aculeata, spanning very diverse parasitism strategies. In this study, we explored some aspects of the chemical ecology of Mutillidae, by characterizing the CHC profiles of five species and evaluating the chemical differences between them and those of their bee and wasp hosts. Chemical strategies by parasitoid aculeates which attack bees and wasps were previously investigated only in chrysidid wasps, which are in general more specialized in host choice compared with velvet ants. Hence, we investigated whether the more generalist mutillids evolved chemical strategies to parasitize their hosts.
Species-specificity is a common feature of CHC profiles in insects, including Hymenoptera (e.g. Kather and Martin 2012; Caderón-Fernández et al. 2012; Moore et al. 2021) and was confirmed here for velvet ants. The inter-specific differences among the five studied velvet ant species seem to harbour a possible effect of common ancestry, since the placement of species in the clusters seem to follow the known phylogeny (Brothers and Lelej 2017, Waldren et al. 2023). However, we must investigate CHC profiles of more velvet ant species to further test this hypothesis. Indeed, since CHC profiles in Hymenoptera are usually under strong selection by intra- and inter-specific communication and/or abiotic factors, changes in CHC profiles generally do not show a phylogenetic signal (Hefetz 1993).
We found large chemical distances between CHC profiles of velvet ants and those of their hosts, except for D. maura which had the shortest chemical distance with its host P. triangulum and N. viduata which had the shortest distance with its host S. continuus. These two cases may suggest at first that weak chemical mimicry evolved in such systems. However, it is also true that both D. maura and N. viduata had larger chemical distances to other hosts, making this scenario unlikely. Not even a weak mimicry in these two cases are likely to be the result of local adaptation. Indeed, both hosts of N. viduata nested in close sympatry in a single mixed nesting area of about 100 m2, while D. maura was collected at the same nesting site of its more chemically distant host species (A. laevicollis) and around 2000 km from the nesting site of its chemically closest host. The same is true for P. gallicus, which exhibit a more similar CHC to its parasitoid T. litoralis, the latter having been collected thousands of km far from its host. CHC distances did not point to even a weak chemical mimicry in any other velvet ant-host systems. This is especially visible in the two Myrmilla species attacking bees, which had much different CHC profiles. Hence, overall, chemical mimicry seems to essentially lack in velvet ants, although most of the species of this family remain to be studied to generalize this conclusion with more confidence. In any case, our findings are in accordance with the hypothesis outlined before. Since these parasitoids are known to be taxonomically generalists in host use, each species often attacking host species from different genera or even families, it is not possible to match the CHC profiles of all of the exploited host species. Differences in the CHCs profile between mutillids and hosts were previously reported for M. europea and for previously studied individuals of M. capitata (Uboni et al. 2012; Ronchetti et al. 2023), and hence they are probably common for these parasitoid-host systems.
In previous studies on brood parasitic aculeate Hymenoptera, the evolution of chemical mimicry seems indeed associated with the degree of taxonomic host specialization. In chrysidid wasps, host specificity is often high (generally one cuckoo wasp species parasitizes one or two host species of a single genus) and accordingly chemical mimicry is more common (Strohm et al. 2008; Wurdack et al. 2015; Castillo et al. 2022; Polidori et al. 2020a). On the other hand, Sphecodes cuckoo bees, which are more generalist (each species attack host bee species from different genera and sometimes families) do not show chemical mimicry at all. Instead, these cuckoo bees evolved an alternative chemical insignificance strategy by reducing the CHC profile complexity and lacking CHC classes relevant in host nestmate recognition (Polidori et al. 2020b). Also in the only velvet ant species studied before our investigation, which attacks disparate hosts (bumblebees and paper wasps), insignificance is achieved by reducing the number of compounds as well as through the reduction of the total amount of hydrocarbons on the cuticle (Uboni et al. 2012). Chemical insignificance based on CHC amount reduction and/or profile simplification was also previously reported among many socially parasitic Hymenoptera at the invasion stage, i.e., prior to colony integration (and hence camouflage) (Lorenzi 2006; Johnson et al. 2001; Dronnet et al. 2005). In our study, velvet ants showed a complex CHC profile, often more diverse and richer in compounds than their hosts. However, we did not study if velvet ants possessed smaller amounts of hydrocarbons than their hosts. This strategy has to be tested in a future study.
On the other hand, we found patterns of alkene and methyl-branched alkane abundances that could perhaps suggest an adaptation to decrease CHC profile detectability by the hosts. There is evidence that these two substance classes are more important than n-alkanes in the recognition process, though not in all Hymenoptera (review in van Zweden and d'Ettorre 2010, see also below). Concerning alkenes, they were found to be less abundant in velvet ants compared with their hosts in most of the studied systems. On the other hand, monomethyl-branched alkanes were linked to nest mate recognition in some eusocial wasps (Dani et al. 1996; Dapporto et al. 2006; Lorenzi et al. 2004; Ruther et al. 2002; Tannure-Nascimento et al. 2007), and they were found in lower abundance in a velvet ant species attacking Polistes social wasps. Interestingly, methyl-branched alkanes were also found in lower proportions in M. europea compared with its Polistes host (Uboni et al. 2012). In addition to these two substance classes, bee-attacking mutillids were the only species having lower abundances of n-alkanes compared with their hosts, and kin-based odour differences in L. malachurum were seen to be mainly based on n-alkanes (Soro et al. 2011). Chemically insignificant profiles have often a reduced number of substances or even lack entire substance classes important for host recognition (Johnson et al. 2001; Martin et al. 2008; Polidori et al. 2020b). This could be the case for velvet ants. However, the actual importance of these hydrocarbon classes in detecting velvet ants during nest invasion has to be evaluated in future studies. Furthermore, it may be important, to achieve chemical insignificance, to have both a simpler CHC profile and a reduction of CHCs amount, and this remains to be verified in our studied mutillid species.
The CHC profile’s chain length was similar between the parasitoids and their hosts. This is interesting, since mutillid females, being wingless and thus being constantly in contact with the high temperatures of soil in summer, would be expected to have greater abundances of compounds with long chain length, which are known to reduce the effects of desiccation by having higher melting temperatures (Gibbs 1998; Gibbs and Pomonis 1995; Gibbs et al. 2003). An increase of unsaturated compounds (alkenes), on the other hand, are known to decrease CHC profile melting temperatures and can cause increased rates of water loss (Gibbs 1998). Indeed, alkenes were found to be poorly represented in velvet ants, except for N. viduata. Also, methyl-branched alkanes seem to protect from water loss (Gibbs 1998), though to minor extent compared with n-alkanes, and were found to be generally abundant in velvet ants.
Our behavioural data revealed that at least in the studied system composed of M. capitata and the bee L. malachurum, velvet ants are poorly recognized as a foe by the host. Indeed, while aggressive behaviours were expressed by the host at slightly higher frequencies in heterospecific than in conspecific encounters, these behaviours remain rare, compared with avoidance behaviours. Such results were similar to what was observed in aculeate brood parasites that either employ chemical mimicry (e.g. Chrysididae: Strohm et al. 2008; Polidori et al. 2020a) or chemical insignificance (Polidori et al. 2020b; Uboni et al. 2012; Uboni and Lorenzi 2013) to reduce host aggression. In M. capitata-L. malachurum, perhaps the very low abundance of alkenes may help parasitoids to hide from their host bees. Interestingly, Sphecodes cuckoo bees attacking Lasioglossum hosts lack alkenes and are not attacked by the hosts in similar behavioural experiments (Polidori et al. 2020b). Despite the clearly aggressive behaviours recorded in our experiments (e.g. biting with mandibles), it was previous highlighted that true quantitative behavioural experiments should record the behavioural sequence and then use unbiased learning approaches to categorize similar pattern which then get interpreted, because the biological meaning of some behaviours (e.g. those falling in the “avoidance” category) may not necessarily be the same for all species (Dew et al. 2014). Hence, further experiments carried out with high-resolution cameras and machine learning tools will give more insights into the interactions between mutillid wasps and their hosts.
In conclusion, velvet ants did not evolve chemical mimicry. On the other hand, the possible role of some hydrocarbons-related chemical traits (reduced abundance of certain CHC classes), which may help them to invade the host nest by reducing host detection, need to be formally tested in future studies. However, morphological adaptations, including a robust, heavily sclerotized body, seem to be widespread as alternative/additional strategy to chemical strategies in brood parasitic insects (Kistner 1979; Cervo 1994; Tishechkin et al. 2017; von Beeren et al. 2021) and could be important traits favouring nest invasion in velvet ants.
Data availability
The authors declare the availability of data in the supplementary section of the manuscript.
References
Ballesteros Y, Tormos J, Gayubo SF, Asís JD (2012) Notes on the prey, nesting behaviour and natural enemies of three Bembix sand wasps (Hymenoptera: Crabronidae) in the Iberian Peninsula. Ann Soc Entomol Fr 48:281–288
Barbero F, Thomas JA, Bonelli S, Balletto E, Schönrogge K (2009) Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 323:782–785
Blomquist GJ, Bagnères A-G (2010) Insect hydrocarbons: biology, biochemistry and chemical ecology. Cambridge University Press, Cambridge
Boesi R, Polidori C (2011) Nest membership determines the levels of aggression and cooperation between females of a supposedly communal digger wasp. Aggress Behav 37:405–416
Brandt M, Heinze J, Schmitt T, Foitzik SA (2005) Chemical level in the coevolutionary arms race between an ant social parasite and its hosts. J Evol Biol 18:576–586
Branstetter MG, Danforth BN, Pitts JP, Faircloth BC, Ward PS, Buffington ML, Gates MW, Kula RR, Brady SG (2017) Phylogenomic insights into the evolution of stinging wasps and the origins of ants and bees. Curr Biol 27:1019–1025
Brothers DJ (1989) Alternative life-history styles of mutillid wasps (Insecta, Hymenoptera). In: Bruton MN (ed) Alternative life-history styles of animals. Perspectives in Vertebrate Science. Springer, Dordrecht, pp 279–291
Brothers DJ, Lelej AS (2017) Phylogeny and higher classification of Mutillidae (Hymenoptera) based on morphological reanalyses. J Hym Res 60:1–97
Buschinger A (2009) Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol News 12:219–235
Caderón-Fernández GM, Girotti JR, Juárez Cuticular MP (2012) Hydrocarbon pattern as a chemotaxomony marker to assess intraspecific variablility in Triatoma infestans, a major vector of Chagas’ disease. Med Vet Entomol 26:201–209
Castillo R, Wurdack M, Pauli T, Keller A, Feldhaar H, Polidori C, Niehuis O, Schmitt T (2022) Evidence for a chemical arms race between cuckoo wasps of the genus Hedychrum and their distantly related host apoid wasps. BMC Ecol Evol 22:138. https://doi.org/10.1186/s12862-022-02093-8
Cervo R (1994) Morphological adaptations to the parasitic life in Polistes sulcifer and P. atrimandibularis (Hymenoptera Vespidae). Ethol Ecol Evol 6:61–66
Cini A, Bruschini C, Signorotti L, Pontieri L, Turillazzi S, Cervo R (2011) The chemical basis of host nest detection and chemical integration in a cuckoo paper wasp. J Exp Biol 214:3698–3703
Cini A, Sumner S, Cervo R (2019) Inquiline social parasites as tools to unlock the secrets of insect sociality. Philos Trans R Soc B 374:20180193
Clarke KR (1993) Non-parametric multivariate analysis of changes in community structure. Aust J Ecol 18:117–143
Danforth BN, Minckley RL, Neff JL (2019) The solitary bees: biology, evolution, conservation. Princeton University Press, Princeton
Dani FR, Fratini S, Turillazzi S (1996) Behavioural evidence for the involvement of Dufour’s gland secretion in nestmate recognition in the social wasp Polistes dominulus (Hymenoptera: Vespidea). Behav Ecol Sociobiol 38:311–319
Dapporto L, Cini A, Palagi E, Morelli M, Simonti A, Turillazzi S (2006) Nestmate recognition and identification of cuticular hydrocarbons composition in the swarm founding paper wasp Ropalidia opifex. Biochem Syst Ecol 34:617–625
Dawkins R, Krebs JR (1979) Arms races between and within species. Proc Royal Soc B 205:489–511
Dew RM, Gardner MG, Schwarz MP (2014) The problems of a priori categorisation of agonism and cooperation: circle-tube interactions in two Allodapine bees. Ethology 120:551–562
Dronnet S, Simon X, Verhaeghe JC, Rasmont P, Errard C (2005) Bumblebee inquilinism in Bombus (Fernaldaepsithyrus) sylvestris (Hymenoptera, Apidae): behavioural and chemical analyses of host-parasite interactions. Apidologie 10:59–70
Evans HE, O’Neill KM (2007) The sand wasps: natural history and behaviour. Harvard University Press, Harvard
Foitzik S, Fischer B, Heinze J (2003) Arms races between social parasites and their hosts: geographic patterns of manipulation and resistance. Behav Ecol 14:80–88
Fröhlich D, Zangl L, Raspotnig G, Koblmuller S (2022) Inter and intrasexual variation in cuticular hydrocarbons in Trichrysis cyanea (Linnaeus, 1758) (Hymenoptera: Chrysididae). Insects 13:159. https://doi.org/10.3390/insects13020159
Gall BG, Spivey KL, Chapman TL, Delph RJ, Brodie ED, Wilson JS (2018) The indestructible insect: velvet ants from across the United States avoid predation by representatives from all major tetrapod clades. Ecol Evol 8:5852–5862
Giacalone M, Agata Z, Cozzucoli PC, Alibrandi A (2018) Bonferroni-Holm and permutation tests to compare health data: methodological and applicative issues. BMC Med Res Methodol 18:81. https://doi.org/10.1186/s12874-018-0540-8
Gibbs AG (1998) Waterproofing properties of cuticular lipids. Am Zool 38:471–482
Gibbs AG (2002) Lipid melting and cuticular permeability: new insights into an old problem. J Insect Physiol 48:391–400
Gibbs A, Pomonis JG (1995) Physical properties of insect cuticular hydrocarbons: the effects of chain length, methyl-branching and unsaturation. Comp Biochem Physiol 112:243–249
Gibbs AG, Fukuzato F, Matzkin LM (2003) Evolution of water conservation mechanisms in desert Drosophila. J Exp Biol 206:1183–1192
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Version 2.15. Paleontol Electron 4:9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
Hefetz A (1993) Hymenopteran exocrine secretions as a tool for chemosystematic analysis. Biochem Syst Ecol 21:163–169
Hines HM, Witkowski P, Wilson JS, Wakamatsu K (2017) Melanic variation underlies aposematic color variation in two hymenopteran mimicry systems. PLoS One 12:e0182135. https://doi.org/10.1371/journal.pone.0182135
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Hunt JH (2007) The evolution of social wasps. Oxford University Press, Oxford
Jensen T, Walker AA, Nguyen SH, Jin AH, Deuis JR, Vetter I, King GF, Schmidt JO, Robinson SD (2021) Venom chemistry underlying the painful stings of velvet ants (Hymenoptera: Mutillidae). Cell Mol Life Sci 78:5163–5177
Johnson CA, Vander Meer RK, Lavine B (2001) Changes in the cuticular hydrocarbon profile of the slave-maker ant queen, Polyergus breviceps Emery, after killing a Formica host queen (Hymenoptera: Formicidae). J Chem Ecol 27:1787–1804
Jorge A, Polidori C, Garcia-Guinea J, Nieves-Aldrey J (2017) Spectral cathodoluminescence analysis of hymenopteran mandibles with different levels of zinc enrichment in their teeth. Arthropod Struct Dev 46(1):39–48
Kather R, Martin SJ (2012) Cuticular hydrocarbon profiles as a taxonomic tool: advantages, limitations and technical aspects. Physiol Entomol 37:25–32
Kistner DH (1979) Social and evolutionary significance of social insect symbionts. In: Hermann HR (ed) Social insects. Academic Press, New York, pp 339–413
Kroiss J, Schmitt T, Strohm E (2009) Low level of cuticular hydrocarbons in a parasitoid of a solitary digger wasp and its potential for concealment. Entomol Sci 12:9–16
Kruskal J, Carroll JD (1969) Geometrical models and badness-of-fit functions. In: Krishnaiah PR (ed) Multivariate analysis. Academic Press, New York, pp 639–671
Legendre F, Pellens R, Grandcolas P (2008) A comparison of behavioral interactions in solitary and presocial Zetoborinae cockroaches (Blattaria, Blaberidae). J Insect Behav 21:351
Lenoir A, d’Ettorre P, Errard C, Hefetz A (2001) Chemical ecology and social parasitism in ants. Annu Rev Entomol 46:573–599
Leyer I, Wesche K (2007) Multivariate statistik in der Ökologie. Springer, Berlin
Lienhard A, Mirwald L, Hötzl T, Kranner I, Kastberger G (2010) Trade-off between foraging activity and infestation by nest parasites in the primitively eusocial bee Halictus scabiosae. Psyche 2010:707501
Litman JR (2019) Under the radar: detection avoidance in brood parasitic bees. Philos Trans R Soc B 374:20180196
Lorenzi MC (2006) The result of an arms race: the chemical strategies of Polistes social parasites. Ann Zool Fenn 43:550–563
Lorenzi MC, Cervo R, Zacchi F, Turillazzi S, Bagnères AG (2004) Dynamics of chemical mimicry in the social parasite wasp Polistes semenowi (Hymenoptera Vespidae). Parasitology 129:643–651
Manley DF, Spangler HG (1983) Observations on daily activity patterns of mutillid wasps of the genus Dasymutilla. J Georgia Entomol Soc 18:234–239
Martin S, Drijfhout F (2009) A review of ant cuticular hydrocarbons. J Chem Ecol 35:1151–1161
Martin SJ, Takahashi J, Ono M, Drijfhout FP (2008) Is the social parasite Vespa dybowskii using chemical transparency to get her eggs accepted? J Insect Physiol 54:700–707
Masters WM (1979) Insect disturbance stridulation: its defense role. Behav Ecol Sociobiol 5:187–200
McCune B, Grace J, Urban D (2002) Analysis of Ecological Communities. MjM Software Design, Gleneden Beach, Oregon
McNaught AD, Wilkinson A (1997) IUPAC compendium of chemical terminology, 2nd edn. Wiley-Blackwell, Oxford
Menzel F, Schmitt T (2012) Tolerance requires the right smell: first evidence for interspecific selection on chemical recognition cues. Evolution 66:896–904
Menzel F, Blaimer BB, Schmitt T (2017) How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc Royal Soc B 284:1850
Michener CD (2007) The bees of the world. The Johns Hopkins University Press, Baltimore
Mickel CE (1928) Biological and taxonomic investigations on the mutillid wasps. Bull Am Mus Nat Hist 143:1–351
Moore HE, Hall MJR, Drijfhout FP, Cody RB, Whitmore D (2021) Cuticular hydrocarbons for identifying Sarcophagidae (Diptera). Sci Rep 11:7732. https://doi.org/10.1038/s41598-021-87221-y
O’Neill KM (2001) Solitary wasps: natural history and behavior. Cornell University Press, Ithaca
Ortolani I, Cervo R (2010) Intraspecific body size variation in Polistes paper wasps as a response to social parasitism pressure. Ecol Entomol 35:352–359
Pabalan N, Davey KG, Packer L (2000) Escalation of aggressive interactions during staged encounters in Halictus ligatus say (Hymenoptera: Halictidae), with a comparison of circle tube behaviors with other halictine species. J Insect Behav 13:627–650
Packer L (2006) Use of artificial arenas to predict the social organization of halictine bees: data for fourteen species from Chile. Insectes Soc 53:307–315
Pagliano G, Brothers DJ, Cambra R, Lelej AS, Lo Cascio P, Matteini Palmerini M, Scaramozzino PL, Williams KA, Romano M (2019) Checklist of names in Mutillidae (Hymenoptera), with illustrations of selected species. Boll Mus Regionale Sci Nat Torino 36:5–425
Polidori C, Borruso L, Boesi R, Andrietti F (2009) Segregation of temporal and spatial distribution between kleptoparasites and parasitoids of the eusocial sweat bee, Lasioglossum malachurum (Hymenoptera: Halictidae). Entomol Sci 12:116–129
Polidori C, Mendiola P, Asís JD, Tormos J, Selfa J (2010) Temporal asynchrony and spatial co-occurrence with the host: the foraging patterns of Nemka viduata, a parasitoid of digger wasps (Hymenoptera: Mutillidae and Crabronidae). J Ethol 28:353–361
Polidori C, Pavan G, Ruffato G, Asís JD, Tormos J (2013) Common features and species-specific differences in stridulatory organs and stridulation patterns of velvet ants (Hymenoptera: Mutillidae). Zool Anz 252:457–468
Polidori C, Ballesteros Y, Wurdack M, Asís JD, Tormos J, Baños-Picón L, Schmitt T (2020a) Low host specialization in the cuckoo wasp, Parnopes grandior, weakens chemical mimicry but does not lead to local adaption. Insects 11:136. https://doi.org/10.3390/insects11020136
Polidori C, Geyer M, Schmitt T (2020b) Do Sphecodes cuckoo bees use chemical insignificance to invade the nests of their social Lasioglossum bee hosts? Apidologie 51:147–162
Poulin R, Morand S, Skorping A (2000) Evolutionary biology of host–parasite relationships: theory meets reality. Elsevier, Amsterdam and New York
Ronchetti F, Polidori C (2020) A sting affair: a global quantitative exploration of bee, wasp and ant hosts of velvet ants. PLoS ONE 15:e0238888. https://doi.org/10.1371/journal.pone.0238888
Ronchetti F, Schmitt T, Negri A, Epis S, Gabrieli P, Bandi C, Romano M, Polidori C (2023) Evidence of cuticular hydrocarbon profile alterations by Wolbachia in females, but not males, of an aculeate parasitoid wasp. Entomol Gen. https://doi.org/10.1127/entomologia/2022/1735
Rosenheim JA (1988) Parasite presence acts as a proximate cue in the nest-site selection process of the solitary digger wasp, Ammophila dysmica (Hymenoptera: Sphecidae). J Insect Behav 1:333–342
Ruther J, Sieben S, Schricker B (2002) Nestmate recognition in social wasps: Manipulation of hydrocarbon profiles induces aggression in the European hornet. Naturwissenschaften 89:111–114
Sadler EA, Pitts JP, Wilson JS (2018) Stinging wasps (Hymenoptera: Aculeata), which species have the longest sting? PeerJ 6:e4743
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton
Schmidt JO (1990) Hymenopteran venoms: Striving toward the ultimate defense against vertebrates. In: Evans DL, Schmidt JO (eds) Insect defenses: Adaptive mechanisms and strategies of prey and predators. State University of New York Press, New York, pp 387–419
Schmidt JO, Blum MS (1977) Adaptions and responses of Dasymutilla occidentalis (Hymenoptera, Mutillidae) to predators. Entomol Exp Appl 21:99–111
Schmidt JO, Buchmann SL (1986) Are mutillid scarce? (Hymenoptera: Mutillidae). Pan-Pac Entomolo 62:103–104
Schmidt JO, Schmidt LS, Schmidt DK (2021) The paradox of the velvet-ant (Hymenoptera, Mutillidae). J Hym Res 84:327–337
Smith BH, Weller C (1989) Social competition among gynes in Halictine bees – the influence of bee size and pheromones on behavior. J Insect Behav 2:397–411
Smith A, Simons M, Bazarko V, Seid M (2019) The influence of sociality, caste, and size on behavior in a facultatively eusocial bee. Insect Soc 66:153–163
Soon V, Castillo-Cajas RF, Johansson N, Paukkunen J, Rosa P, Ødegaard F, Schmitt T, Niehuis O (2021) Cuticular hydrocarbon profile analyses help clarify the species identity of dry-mounted cuckoo wasps (Hymenoptera: Chrysididae), including type material, and reveal evidence for a cryptic species. Insect Syst Div 5:3. https://doi.org/10.1093/isd/ixab002
Soro A, Ayasse M, Zobel MU, Paxton RJ (2011) Kin discriminators in the eusocial sweat bee Lasioglossum malachurum: the reliability of cuticular and Dufour’s gland odours. Behav Ecol Sociobiol 65:641–653
Starr CK (1985) A simple pain scale for field comparison of hymenopteran stings. J Entomol Sci 20:225–232
Strohm E, Kroiss J, Herzner G, Laurien-Kehnen C, Boland W, Schreier P, Schmitt T (2008) A cuckoo in wolves’ clothing? Chemical mimicry in a specialized cuckoo wasp of the European beewolf (Hymenoptera, Chrysididae and Crabronidae). Front Zool 5:2
Tannure-Nascimento IC, Nascimento FS, Turatti LC, Lopes NP, Trigo JR, Zucchi R (2007) Colony membership is reflected by variations in cuticular hydrocarbon profile in a Neotropical paper wasp Polistes satan (Hymenoptera, Vespidae). Gen Mol Res 6:390–396
Tishechkin AK, Kronauer DJC, von Beeren C (2017) Taxonomic review and natural history notes of the army ant-associated beetle genus Ecclisister Reichensperger (Coleoptera: Histeridae: Haeterinae). Coleopt Bull 71:279–288
Torres CW, Tonione MA, Ramírez SR, Sapp JR, Tsutsui ND (2018) Genetic and chemical divergence among host races of a socially parasitic ant. Ecol Evol 8:11385–11398
Uboni A, Lorenzi MC (2013) Poor odors, strength, and persistence give their rewards to Mutilla europaea visiting dangerous wasp nests. J Insect Behav 26:246–252
Uboni A, Bagnères AG, Christidès JP, Lorenzi CM (2012) Cleptoparasites, social parasites and a common host: chemical insignificance for visiting host nests, chemical mimicry for living in. J Insect Physiol 58:1259–1264
von Beeren C, Brückner A, Hoenle PO, Ospina-Jara B, Kronauer DJC, Blüthgen N (2021) Multiple phenotypic traits as triggers of host attacks towards ant symbionts: body size, morphological gestalt, and chemical mimicry accuracy. Front Zool 18:46. https://doi.org/10.1186/s12983-021-00427-8
van Zweden JS, d’Ettorre P (2010) The role of hydrocarbons in nestmate recognition. In: Blomquist GJ, Bagnères A-G (eds) Insect hydrocarbons: biology, biochemistry and chemical ecology. Cambridge University Press, Cambridge, pp 222–243
Waldren GC, Sadler EA, Murray EA, Bossert S, Danforth BN, Pitts JP (2023) Phylogenomic inference of the higher classification of velvet ants (Hymenoptera: Mutillidae). Syst Entomol. https://doi.org/10.1111/syen.12588
Wilson JS, Jahner JP, Forister ML, Sheehan ES, Williams KA, Pitts JP (2015) North American velvet ants form one of the world’s largest known Müllerian mimicry complexes. Curr Biol 25:R704–R706
Wurdack M, Herbertz S, Dowling D, Kroiss J, Strohm E, Baur H, Niehuis O, Schmitt T (2015) Striking cuticular hydrocarbon dimorphism in the mason wasp Odynerus spinipes and its possible evolutionary cause (Hymenoptera: Chrysididae, Vespidae). Proc R Soc Lond B 282:20151777. https://doi.org/10.1098/rspb.2015.1777
Acknowledgements
We thank the technicians at the Maremma Regional Park (Grosseto, Italty), the Albufera Natural Park (Valencia, Spain) and the Junta de Medio Ambiente of the Autonomous Region of Castilla y Leon for supporting our field study in their respective areas and to provide the permits to collect the bee and wasp individuals. The students J. M. Nicotra, D. Porceddu and E. Landoni helped in collecting the behavioural data. Thanks are due to Olena Riabinina (Durham University, UK) for revising the English.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. The study was funded by the project CGL2017- 83046-P from Ministerio de Ciencia e Innovación (Spain) (to CP).
Author information
Authors and Affiliations
Contributions
Conceptualization: CP, TS; Methodology: CP, TS, FR; Formal analysis and investigation: FR, CP, TS, MR; Writing—original draft preparation: FR, CP; Writing—review and editing: CP, FR, TS, MR; Funding acquisition: CP; Resources: CP, TS, FR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Günther Raspotnig.
Supplementary Information
Below is the link to the electronic supplementary material.
49_2023_382_MOESM1_ESM.tif
Fig. S1 Paired parasitoid-host differences in the abundance of the five CHC classes found in the cuticle of the studied species. A, n-alkanes. B, monomethyl-branched alkanes. C, dimethyl-branched alkanes. D, trimethyl-branched alkanes. E, alkenes. Alkadiens were only found in small proportions in L. calceatum (see text) and are not shown. Note that linear and methyl-branched alkanes were often more abundant in the mutillids, while alkenes were often more abundant in the hosts. Supplementary file1 (TIF 1157 KB)
49_2023_382_MOESM2_ESM.tif
Fig. S2 Bray–Curtis distances of each velvet ant species with all host species. In red, the actual host(s) for each species is shown. Note that the Bray–Curtis distance between a mutillid and its host was the shortest for D. maura-P. triangulum and for N. viduata-S. continuus. Bee hosts showed the greatest chemical distance from their mutillid parasitoids. Supplementary file2 (TIF 1329 KB)
49_2023_382_MOESM3_ESM.tif
Fig. S3 SIMPER contributions to dissimilarity of the 10 most important CHC compounds, pooled by CHC classes. Note that linear alkanes seem to contribute less to dissimilarity, compared with methyl-branched alkanes and alkenes. Supplementary file3 (TIF 926 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ronchetti, F., Schmitt, T., Romano, M. et al. Cuticular hydrocarbon profiles in velvet ants (Hymenoptera: Mutillidae) are highly complex and do not chemically mimic their hosts. Chemoecology 33, 29–43 (2023). https://doi.org/10.1007/s00049-023-00382-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-023-00382-2