Abstract
Polygraphus proximus, a four-eyed fir bark beetle, is an invasive bark beetle species which has caused extensive damage to forests of Abies sibirica in southern and western Siberia and to Abies species in the European part of Russia. There is a high risk that the pest insect will spread to areas where it is currently not considered present, such as the European Union. In these areas, it threatens to attack conifer forests of various species which may result in major environmental and economic impact. The aim of this study was to identify pheromone components of P. proximus that can be used as pheromone baits. Males and females of P. proximus were allowed to bore into the bark of stem sections of Abies sibirica at the laboratory, and volatiles were collected with solid-phase microextraction (SPME). Analyses of these extracts with gas chromatography-mass spectrometry (GC–MS) revealed several sex-specific compounds. In total, twelve male-specific compounds and one female-specific compound were identified. The major male-specific compound determined by GC peak area was (Z)‐2‐(3,3‐dimethylcyclohexylidene)‐ethanol [(Z)-DMCHE] and the minor male-specific compounds were 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butenal, benzyl alcohol, fragranol, 7-methyl-3-methylene-6-octen-1-ol, (Z)- and (E)-2-(3,3-dimethylcyclohexylidene)-acetaldehyde, geraniol, geranial and papayanol. The only female-specific compound was identified as 1-hexanol. Two of the male-specific compounds, (Z)‐DMCHE and 3-methyl-2-buten-1-ol were shown to attract males and females of P. proximus in field studies. Thus, we now for the first time can present the structures of two male-specific components that are biologically active parts of P. proximus aggregation pheromone. However, some chemical communication overlap between P. proximus and P. subopacus needs to be further investigated as (Z)‐DMCHE also attracted males and females of P. subopacus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large bark beetle outbreaks are a major cause of forest damage in different parts of the world. Some bark beetle species which can cause considerable tree mortality are the mountain pine beetle Dendroctonus ponderosae in the US and Canada (Grégoire et al. 2015), the spruce bark beetle Ips typographus in Europe and the four-eyed fir bark beetle Polygraphus proximus in Russia (Kononov et al. 2016; Krivets et al. 2019; Pavlov et al. 2020). P. proximus is an invasive species which has infested more than 660,000 km2 of forest in southern and western Siberia, mainly Abies sibirica (De la Peña et al. 2020). It originates from the Russian Far East and neighboring countries, from where it seems to have spread along the Trans-Siberian Railway into Siberia and the European part of Russia (Kerchev 2014a). First discovered in Siberia 13 years ago, it is now considered the most aggressive bark beetle ever found on Siberian fir trees (Baranchikov et al. 2010). There is a high risk that P. proximus will eventually spread to the European Union and if it does, a major economic and environmental impact can be expected, as has already happened in Russia. P. proximus threatens to attack species of the genera Abies, Pinus, Picea, Larix and Tsuga (EPPO 2014; De la Peña et al. 2020). Efficient pheromone traps would facilitate early detection of P. proximus if they were available but, up to now, the pheromone of P. proximus is not known.
In 2016, it was suggested that females of P. proximus produce an aggregation pheromone based on olfactometer studies, however, no specific pheromone compounds were identified (Kerchev and Pousheva 2016). Other studies have indicated that the males produce a pheromone which attracts females (Kerchev 2014b) or that both males and females of P. proximus can secrete aggregation pheromones (Krivets et al. 2019). P. proximus is a monogamous species, unlike most other species in the Polygraphus genus (Kerchev 2014b; Köbayashi and Takagi 2020). In four other Polygraphus species, male-specific aggregation pheromones have been found. Polygraphus rufipennis has been shown to use 3-methyl-3-buten-1-ol as their aggregation pheromone (Bowers et al. 1991), Polygraphus poligraphus use (−)-(R)-terpinen-4-ol (Schurig et al. 1985; Rahmani et al. 2015), Polygraphus punctifrons use (+)-(1R,2S)-grandisol and (–)-(R)-terpinen-4-ol as the main components of their aggregation pheromone (Rahmani et al. 2019) and Polygraphus subopacus use (Z)-2-(3,3-dimethylcyclohexylidene)-ethanol as their main aggregation pheromone component (Viklund et al. 2021). It has been shown that bark beetles of the same genus often have overlapping pheromone components (Blomquist et al. 2010).
In addition to pheromones, beetles of P. proximus use acoustic signals to communicate with each other. Males of P. proximus have been shown to produce acoustic signals which are species-specific when compared to the signals produced by P. subopacus males and Polygraphus nigrielytris males (Kerchev 2020). Similarly to other bark beetles, P. proximus has been associated with phytopathogenic fungi, mainly Grosmannia aoshimae, Ophiostoma subalpinum and O. nikkoense (Pashenova et al. 2018), and infestation of fir logs with G. aoshimae increases their colonization by P. proximus in the field (Baranchikov et al. 2017). Previously it has been shown that fungal symbionts of bark beetles can produce volatiles which increase the attraction of the beetles to attacked trees (Kandasamy et al. 2016). P. proximus has been caught in traps baited with synthetic pheromones of Ips sexdentatus and Ips acuminatus, although the number of caught P. proximus was not reported (Kerchev et al. 2019). Pheromone baits for I. sexdentatus and I. acuminatus often contain ipsenol, ipsdienol, cis-verbenol and 2-methyl-3-buten-2-ol, as these are produced by the males of these species or have been shown to increase the beetles’ attraction to the baits (Knizek et al. 2022).
The aim of this work was to identify pheromone components of P. proximus using solid-phase micro extraction together with gas chromatography and mass spectrometry (SPME–GC–MS). These methods have previously been shown to be useful for pheromone identification in Polygraphus bark beetles (Rahmani et al. 2015, 2019; Viklund et al. 2021).
Materials and methods
In this study, volatiles released by boring P. proximus were collected with SPME at the Sukachev Institute of Forest in Krasnoyarsk, Russia by a researcher from the Eco-Chemistry group, Mid Sweden University, Sundsvall Sweden. GC–MS-analyses of the collected samples were then conducted at the Mid Sweden University in Sundsvall, Sweden.
Sampling of volatiles from boring beetles
Overwintering beetles were reared from the bark of Siberian fir (Abies sibirica), collected in the mixed coniferous taiga forest near the city of Krasnoyarsk, Siberia. 43 males and 34 females of P. proximus beetles were placed on 50 cm long stem sections of A. sibirica (10–15 cm diameter) and were allowed to bore into the bark. Eight stem sections were placed horizontally at the laboratory in room temperature (20–22 °C). Each insect was placed in an Eppendorf tube which had been pinned to the stem and where the end had been cut off and covered with aluminium foil. Insects were placed in the Eppendorf tube on the stem sections between Jan 19–24, 2017. Out of the tested beetles 26 females and 36 males started boring into the bark within 0–4 days. Volatiles from the beetles were sampled with SPME 3–7 days after the beetles had been placed on the bark. The SPME fiber was introduced into the cut end of the Eppendorf tube and the opening was sealed with aluminium foil. Sampling time was 1 h, except for two red fibers which were left over night to sample one male and one female. The most active beetles were chosen for sampling based on the amount of frass they produced. In total, 15 females and 15 males were sampled and 11 samples were taken from the fir background where a hole had been drilled manually 0–2, 24 or 48 h before sampling. Males, females and the fir background were sampled individually in separate Eppendorf tubes. As sex-specific compounds were in focus, males and females also served as background references for each other, to account for compounds which may have been produced by the tree in response to herbivorous activity. 17 red fibers, 6 yellow fibers, 6 black fibers, 6 orange fibers and 6 pink fibers were used. 2 males, 2 females and 2 fir backgrounds were sampled with each type of fiber, except for the red fibers where 7 males, 7 females and 3 backgrounds were sampled.
The stationary phases of the SPME fibers were PDMS (polydimethylsiloxane) 100 µm for red fibers and 30 µm for yellow fibers, PDMS/DVB (divinylbenzene) 65 µm for pink fibers, carboxen/PDMS 75 µm for black fibers and carbowax/DVB 65 µm for orange fibers. All fibers were from Supelco (Bellefonte, PA, USA). The SPME fibers were conditioned in Sweden in the GC inlet at 250 or 280 ºC until no peaks were seen on the chromatograms. Directly after conditioning the fibers were placed through septa into 2 ml glass vials which had been prefilled with argon and were then transported to Russia. After sampling of volatiles between Jan 24 and 30, 2017 each SPME fiber was again placed into glass vials with argon and transported to the Mid Sweden University. They were kept in the vials at room temperature until GC–MS analyses, which were conducted between Feb 1 and March 23, 2017.
Analysis of collected volatiles
A mid-polar HP5-MS GC column (30 m × 0.25 mm × 0.25 μm, Agilent J&W Scientific, Folsom, USA) was used and the temperature was set to 50 °C for 2 min, then increased by 10 °C/min up to 230 °C and then increased by 15 °C/min to 280 °C, where it was held for 5 min. A polar VF23-MS column was also used (30 m × 0.25 mm × 0.25 μm, Agilent J&W Scientific, Folsom, USA) and the temperature was set to 50 °C for 2 min and then increased by 5 °C/min up to 250 °C and held there for 10 min.
GC peaks which were found in at least three samples from either males or females, but not in samples from both sexes or from the fir background, were identified by comparing retention times and MS spectra to synthetic references. The synthetic references were chosen based on top suggestions from the NIST 14 MS library, except for one case where no good match could be found in the MS library and where the compound was eventually identified based on a mass spectrum published in the literature.
The gas chromatograph was a Hewlett-Packard 6890 from Agilent Technologies (Santa Clara, CA, USA) and the mass spectrometer was a HP 5973 N, operating in electron impact ionization mode (EI, 70 eV). Helium was used as mobile phase (flow rate 1 mL/min) and the injector was set to 250 °C, splitless mode. Transfer line temperature was set to 230 °C. Desorption time for each SPME fiber was 5 min. The software used for analysis of the raw MS data was Workstation 7.0.0 (Agilent) together with NIST 14 mass spectral library.
EAG studies of P. subopacus
Many of the sex-specific compounds which were identified in P. proximus have previously been tested for activity on P. subopacus antennae (Viklund et al. 2021). Three additional compounds, fragranol, geraniol and 1-hexanol were also tested on P. subopacus, using the same methodology as described by Viklund et al (2021). Unfortunately, P. proximus was not available for EAG studies as it is a quarantine species in the European Union (De la Peña et al. 2020) and could not be brought to Sweden, where the EAG studies were conducted.
Chemicals
3-Methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butenal, benzaldehyde and benzyl alcohol were purchased from Sigma-Aldrich (Schnelldorf, Germany). (Z)- and (E)-2-(3,3-dimethylcyclohexylidene)-ethanol (Grandlure II and (E)-isomer of Grandlure II), racemic grandisol (Grandlure I) and a 1:1 mixture of (Z)- and (E)- 2-(3,3-dimethylcyclohexylidene)-acetaldehyde (Grandlure III and IV) were from Bedoukian Research (Danbury, CT, USA). Citral was purchased from Acros Organics (Geel, Belgium). Racemic fragranol and 7-methyl-3-methylene-6-octen-1-ol (γ-isogeraniol) were synthesized at our laboratory. These syntheses have been described previously (Rahmani et al. 2019; Viklund et al. 2021; Yong et al. 2001). Papayanol was synthesized at our laboratory starting from racemic grandisol, according to the method described by Zarbin et al. (2010). (Z)- and (E)-2-(3,3-dimethylcyclohexylidene)-acetaldehyde were separated by flash chromatography (straight-phase silica gel, 60 Å, 230–400 mesh) using a gradient technique with an increasing concentration of ether (0–100%) in pentane. The (Z)- and (E)-isomers could be easily identified after separation based on their mass spectra. Mass spectra for these compounds have been published previously in Viklund et al. (2021).
Field experiments
Three field studies were conducted in 2017, 2018 and 2019 in an outbreak area of P. proximus in Siberian fir (Abies sibirica) dominated mixed coniferous forest near Krasnoyarsk, Russia. According to dendrochronological analysis, fir dieback from P. proximus attack at this locality has started in the middle of 1970’s (Baranchikov et al. 2014). Until 2019, approximately 60% of firs were killed there although spruce (Picea abovata) and pines (Pinus sibirica) were not attacked.
Experiment 1
The major male-specific compound identified in the GC–MS analysis was used in a field experiment in 2017 to assess its attractiveness to P. proximus. Traps baited with (Z)-DMCHE in a solvent, n-nonane, were alternated with control traps containing only n-nonane. They were placed along lines with 20–25 m between the traps and the treatments were rotated (moved one trap position forward) each time the traps were emptied. Two sites were used with approximately 1–2 km between them. There were 25 traps per treatment, of which 15 were at the first site and 10 were at the second site. The experiment was conducted between June 18, 2017 and September 4, 2017. Traps were emptied at irregular intervals depending on the flight activity of the beetles.
Experiment 2
In 2018, nine treatments were tested in the field by the same methodological approach as presented above. There were 10 traps per treatment and one site was used. The tested treatments included (Z)-DMCHE, (E)-DMCHE, γ-isogeraniol and racemic grandisol, alone and in combinations. Dispensers with n-nonane and traps without dispensers were used as controls. Treatments were rotated when the traps were emptied. The experiment was conducted between May 27, 2018 and July 27, 2018.
Experiment 3
In 2019, 12 treatments were tested in the field by the same methodological approach as presented above and there were 5 traps per treatment. The tested compounds were (Z)-DMCHE, 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butenal, geraniol and citral (a 1:1 mixture of geranial and neral), alone and in combinations. n-Nonane and unbaited traps were used as controls. The experiment was conducted between May 27, 2019 and July 31, 2019.
Traps and dispensers
The traps used in all field experiments were standard pheromone traps used by forest and quarantine services in Russia (Fig. 1a). Each trap is made of plastic and has four wings (45 × 15 cm each), a funnel (25 cm high, upper diameter 30 cm) and a 500 ml collection jar (12 cm high). The dispensers used for the field experiments were wick baits (Fig. 1b; Birgersson et al. 2012). In experiment 1, 50 mg of (Z)-DMCHE was dissolved in 8 mL of n-nonane and contained in a 12 mL glass vial. The dissolved compound was released through a teflon tube, 8 cm × 15 mm i.d. which was lined with cotton yarn and inserted through a drilled hole in the lid of the vial. The solvent, n-nonane, was used to control the release rate. Release rates from the dispensers were not measured, but based on previous laboratory studies in Sweden with similar compounds, the expected release rate of (Z)-DMCHE in a fume hood (22–25 °C, air flow 0.5–0.6 m/s) was 0.8 mg/day (Viklund et al. 2019). In experiment 2 and 3, 50 mg of (Z)-DMCHE was combined with 5 mg of an additional compound in 8 mL of n–nonane. Each compound was also used alone. The additional compounds were (E)-DMCHE, γ-isogeraniol, rac-grandisol, 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butenal, geraniol and citral. Release rates were expected to be 0.08 mg/day for each of these compounds.
Statistical analyses
As treatment positions were changed when the traps were emptied, each rotation was considered a replicate. Relative catches were used in the statistical analyses since the beetles’ flight activity was expected to vary with time. The number of beetles caught by each treatment was converted to proportions of the total catch within that replicate. The arcsine square root transformation was used to better fit the assumption of normality. Data was analyzed with Welch’s analysis of variance (ANOVA) and a significance level of α = 0.05 together with Games-Howell post hoc test due to unequal variances (Welch, 1951). In Experiment 1, Welch’s two sample T test was used instead of ANOVA as only two treatments were compared. Missing data were handled using the average number of insects caught per trap for that treatment in that replicate (i.e., if there were ten traps baited with (Z)-DMCHE and insects could not be counted in two of the traps due to predators, the average number of insects per trap for that specific date was calculated from the other eight traps). Statistical tests were performed in R.
Results
Sampling and analysis of volatile organic compounds from boring bark beetles
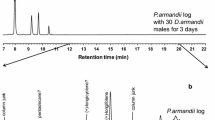
In total, twelve male-specific compounds and one female-specific compound were seen in the SPME extractions from P. proximus when the HP5-MS column was used (Fig. 2 and Supplementary Information, Figs. S1–S12).
GC chromatograms showing volatile organic compunds from a P. proximus male and female. Insects were sampled with orange SPME fibers which were kept in vials with argon for 5 days before analysis. The GC column was an HP5-MS and the temperature was set to 50 °C for 2 min, then increased by 10 °C/min until 230 °C and after that increased by 15 °C up to 280 °C where it was held for 5 min. The chromatograms have been zoomed in on 0–20 min
The major male-specific compound (GC-peak at 10.39 min in Fig. 2) was identified as (Z)-2-(3,3-dimethylcyclohexylidene)-ethanol [(Z)-DMCHE)]. This compound was found in SPME extracts from all males in which sex-specific compounds were observed (13 males), but not from any of the females or the background samples which were analyzed. The second largest male-specific compound was 7-methyl-3-methylene-6-octen-1-ol (γ-isogeraniol, GC peak at 10.25 min in Fig. 2). These two compounds were identified from all types of SPME fibers which were used in the analysis.
The other male-specific compounds were mainly seen when using the orange (Fig. 2), pink and black SPME fibers, and they were identified as 3-methyl-3-buten-1-ol (rt 2.64 min), 3-methyl-2-buten-1-ol (rt 3.17 min), 3-methyl-2-butenal (rt 3.32 min), benzyl alcohol (rt 7.36 min), fragranol (rt 10.22 min), (Z)-2-(3,3-dimethylcyclohexylidene)-acetaldehyde (rt 10.80 min, (Z)-DMCHA), (E)-2-(3,3-dimethylcyclohexylidene)-acetaldehyde (rt 10.92 min, (E)-DMCHA), geraniol (rt 10.75 min), geranial (rt 11.01 min) and papayanol (rt 10.97 min). The only female-specific compound was identified as 1-hexanol (rt 4.58 min).
Benzaldehyde (rt 6.15 min) was seen in samples from males of P. proximus, but also from the females and the fir background. As it coeluted with other compounds from the fir background, it was not possible to determine whether the GC peak of benzaldehyde was larger in males than in females.
The compounds with the shortest retention times, 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol and 3-methyl-2-butenal, generated very small GC peaks when the orange SPME fiber was used (Fig. 2), however, GC peaks were larger when the pink SPME fiber was used (Fig. 3), making the identification of these compounds easier.
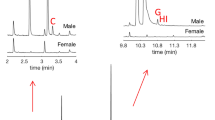
GC chromatograms showing male-specific compounds 3-methyl-3-buten-1-ol (rt 2.64 min), 3-methyl-2-buten-1-ol (rt 3.17 min) and 3-methyl-2-butenal (rt 3.32 min). These compounds were clearly seen when a pink SPME fiber was used. The GC column and temperature program was the same as in Fig. 2. The chromatograms have been zoomed in on 2.5–3.5 min
As the mass spectra of (Z)-DMCHE and (E)-DMCHE are quite similar (Fig. 4), and since the retention times of these compounds are close on an HP5-MS column, a VF23-MS column was also used to determine whether it was only the (Z)-isomer which was present in P. proximus males, or if there was also an amount of the (E)-isomer. A slower program was used, starting at 50 °C for 2 min and then increasing by 5 °C per minute until the temperature reached 250 °C where it was held for 10 min. These chromatograms showed that P. proximus males emit (Z)-DMCHE but not (E)-DMCHE (Fig. 5 and Supplementary Information, Fig. S13).
GC chromatograms showing a) the major male-specific compound in P. proximus, b the absence of this compound in the female, c a synthetic reference of (Z)-DMCHE and d a synthetic reference of (E)-DMCHE. Insects were sampled with pink SPME fibers and the GC column was a VF23-MS. The temperature was set to 50 °C for 2 min, then increased by 5 °C/min until 250 °C where it was held for 10 min. The chromatograms have been zoomed in on 17.5–18.3 min. The minor, male-specific GC peaks which elute after (Z)-DMCHE were identified as fragranol and geraniol based on mass spectral analysis as well as retention times
Another challenge was the identification of a GC-peak close to the GC-peak of γ-isogeraniol, showing a similar mass spectra to grandisol. The mass spectra of grandisol and fragranol are near identical, but retention times differ slightly on an HP5-MS column, as shown previously in our study of P. subopacus (Viklund et al. 2021). Grandisol and fragranol had previously been synthesized at our laboratory, and these references could be used to identify fragranol as the male-specific compound in P. proximus. Fragranol eluted just before γ-isogeraniol and was identified by comparing the unknown peak at retention time 10.22 min to a synthetic sample of fragranol, giving an overlapping retention time and identical mass spectrum (Figs. 6, 7).
GC chromatograms showing a male-specific compounds in P. proximus, b a synthetic reference of grandisol, c a synthetic reference of fragranol and d a synthetic reference of γ-isogeraniol. The insect was sampled with an orange SPME fiber and the GC column was an HP5-MS. The temperature started at 50 °C for 2 min, increased by 10 °C/min until 230 °C and then by 15 °C/min up to 280 °C where it was held for 5 min. The chromatograms have been zoomed in on 10.0–10.5 min
All compounds, except for papayanol, were identified using suggestions from the NIST14 MS library and then comparing the MS spectra and retention times to those of synthetic references. A compound was considered male-specific if it was found in at least three males and not in any of the females or background samples which were analyzed. For papayanol, the NIST14 MS library did not yield any good quality suggestions. Instead, a suggestion of the structure of this compound came up from a literature search where insects with similar pheromone systems were investigated. We found a compound produced by the papaya borer, Pseudopiazurus obesus (Zarbin et al. 2010) with an identical mass spectrum and when this compound (papayanol) was synthesized in our laboratory, its mass spectrum and retention time matched the male-specific compound in P. proximus. The stereochemistry of fragranol and papayanol in P. proximus was not determined in this study.
Field experiments
Experiment 1
(Z)-DMCHE caught significantly more P. proximus than the control traps with n-nonane (Welch’s T test, two-tailed, T = − 13.0; df = 14; P < 0.001). However, P. subopacus were also caught in these traps and in much larger numbers than in the control traps (T = − 122.8; df = 14; P < 0.001). Total catches of each species, as well as a mean per trap per rotation are presented in Table 1.
Experiment 2
Treatment effects were compared in two groups. First, trap catches by (Z)-DMCHE was compared to catches by (E)-DMCHE, γ-isogeraniol, grandisol, control traps with n-nonane and the unbaited control traps. According to Welch’s ANOVA, there were significant differences between at least two of these treatments (F = 9.6; df = 5; P < 0.001 for P. proximus and F = 37.0; df = 5; P < 0.001 for P. subopacus). Then, trap catches by (Z)-DMCHE was compared to the combinations of (Z)-DMCHE with (E)-DMCHE, γ-isogeraniol or grandisol, as well as to the control traps baited with n-nonane. There were significant differences between these treatments (F = 9.9; df = 4; P < 0.001 for P. proximus and F = 46.4; df = 4; P < 0.001 for P. subopacus).
According to Games-Howell’s post hoc test, (Z)-DMCHE caught significantly more P. proximus than the control traps with n-nonane (P = 0.003). (E)-DMCHE did not catch significantly more (or less) P. proximus than the control traps with n-nonane and neither did γ-isogeraniol or grandisol. The combination of (Z)- and (E)-DMCHE caught significantly fewer P. proximus than (Z)-DMCHE alone (P = 0.008) and the catches of the combination were not significantly different from the catches in control traps with n-nonane. γ-Isogeraniol or grandisol in combination with (Z)-DMCHE did not increase or decrease the catches compared to (Z)-DMCHE alone. P. proximus did not seem to be attracted to n-nonane, as the catches in control traps with n-nonane were not significantly different from the unbaited control traps. For P. subopacus, (Z)-DMCHE caught more beetles than the control traps with n-nonane (P < 0.001). Catches were not significantly altered when γ-isogeraniol, grandisol or (E)-DMCHE was added to (Z)-DMCHE. For both species, total catches as well as a mean per trap per rotation are presented in Table 2.
Experiment 3
Once again, treatment effects were compared in two groups where (Z)-DMCHE was first compared to 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butenal, geraniol, citral and the control traps, and then compared to the combinations of (Z)-DMCHE with these compounds. There were significant differences between treatments in both comparisons and for both species according to Welch’s ANOVA (F = 17.3; df = 6; P < 0.001 and F = 6.3; df = 6; P < 0.001 for P. proximus; F = 62.4; df = 6; P < 0.001 and F = 68.9; df = 6; P < 0.001 for P. subopacus). The ANOVAs were followed by Games-Howell’s post hoc test.
Also now (Z)-DMCHE caught significantly more P. proximus than the traps baited with only n-nonane and so did 3-methyl-2-buten-1-ol (P = 0.001 and P < 0.001). 3-Methyl-3-buten-1-ol, 3-methyl-2-butenal, geraniol and citral did not catch more beetles than n-nonane on its own. When 3-methyl-2-buten-1-ol, 3-methyl-3-buten-1-ol, 3-methyl-2-butenal, geraniol or citral were combined with (Z)-DMCHE, catches were not higher than when only (Z)-DMCHE was used. For P. subopacus, (Z)-DMCHE caught more beetles than n-nonane (P < 0.001) but combining (Z)-DMCHE with 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butenal, geraniol or citral did not increase or decrease the catches of P. subopacus as compared to only (Z)-DMCHE. A summary of the experiment is presented in Table 3.
The proportion of beetles caught by each treatment in each replicate were used for the statistical analysis (Supplementary Information, Figs. S14–S23). Before the analysis, these proportions were transformed with the arcsine square root transformation.
Comparison of insect emitted compounds from P. subopacus and P. proximus
In total, 12 male-specific compounds and one female-specific compound were identified in P. proximus. Of these 13 compounds, 10 are also present in P. subopacus although the amounts appear to differ (Supplementary Information, Figs. S24–S27), a notable example was γ-isogeraniol which generated a large GC peak in P. proximus but a barley noticeable GC peak in P. subopacus. For fragranol and papayanol, the stereochemistry was not determined in our study. Thus, it is possible that P. proximus and P. subopacus use different enantiomers of fragranol. Two compounds which appeared to be specific for P. proximus, geraniol and 1-hexanol, were tested for activity on P. subopacus antennae with EAG. However, none of these compounds generated a strong response. Papayanol was also specific to P. proximus, but could unfortunately not be tested on P. subopacus antennae due to a lack of insects when the compound was available. The compounds which were specific to P. subopacus were (E)-DMCHE and grandisol. Both compounds elicited a strong to medium response in P. subopacus antennae. The method used for the EAG analysis of P. subopacus has been described previously (Viklund et al. 2021). As EAG studies were conducted in Sweden, P. proximus antennae could not be used since restrictions prevented us from bringing live insects to Sweden. P. proximus is a quarantine species in the European Union (De la Peña et al. 2020). All compounds which were detected in P. proximus and P. subopacus are presented in Table 4. Most of the EAG results relating to P. subopacus have been published previously (Viklund et al. 2021).
Discussion
In our study, twelve male-specific compounds were identified in the SPME collections from P. proximus; (Z)-DMCHE, γ-isogeraniol, 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butenal, benzyl alcohol, fragranol, (Z)- and (E)-2-(3,3-dimethylcyclohexylidene)-acetaldehyde, geraniol, geranial and papayanol. In all males, (Z)-DMCHE was identified as the largest male-specific GC peak. In the females, only one sex-specific compound was found and it was identified as 1-hexanol. Two of the male-specific compounds appeared to attract males and females of P. proximus in the field; (Z)-DMCHE and 3-methyl-2-buten-1-ol. These two compounds are, however, not likely to make up the complete pheromone, as catches were smaller than expected and not species-specific. The main male-specific compound (Z)-DMCHE has also recently been identified as the main male pheromone component of a closely related species, P. subopacus (Viklund et al. 2021). The composition of P. proximus’ pheromone appears to be strikingly similar to the pheromone of P. subopacus, as most of the male-specific compounds were found in both species; 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butenal, benzyl alcohol, fragranol, γ-isogeraniol, (Z)-DMCHE, (Z)- and (E)-DMCHA and geranial. But, geraniol and papayanol in males and 1-hexanol in females appeared to be specific to P. proximus, while grandisol and (E)-DMCHE were specific to P. subopacus. Another notable difference was the GC peak size of γ-isogeraniol. This compound generated the second largest GC peak in P. proximus males whereas it was barely noticeable in P. subopacus males. Although several of these compounds were tested in field experiments, no species-specific composition was identified for P. proximus. However, the combination of (Z)- and (E)-DMCHE at a ratio of 10:1 caught significantly fewer P. proximus than traps baited with only (Z)-DMCHE. In fact, adding the (E)-isomer to (Z)-DMCHE appeared to reduce catches of P. proximus to the same level as in the control traps with n-nonane (Experiment 2). It is thus possible that (E)-DMCHE is part of P. subopacus’ pheromone.
Several species of Anthonomus weevils also use (Z)-DMCHE, (Z)-DMCHA and (E)-DMCHA as parts of their aggregation pheromones. In these beetles, species-specificity seems to be achieved by different relative abundances of the pheromone components or by species-specific compounds such as grandisol, (E)-DMCHE or lavandulol, which are necessary for attraction in some species and repellants in other species (Tumlinson et al. 1969; Eller et al. 1994; Szendrei et al. 2011; Innocenzi et al. 2001; Rodriguez-Saona et al. 2020). A similar situation is seen in the bark beetles Pityogenes quadridens and Pityogenes bidentatus, whose pheromone blends are similar but where (E)-DMCHE, chalcogran and grandisol are species-specific pheromone components which repel one species from the pheromone blend of the other (Byers et al. 2013). In the pecan weevil Curculio caryae, the stereochemistry of grandisol may play a role in the species-specificity of their pheromone. These beetles emit two isomers of grandisol, although it is not clearly described whether these are enantiomers of grandisol, or if they are grandisol and its trans-isomer fragranol (Hedin et al. 1997). In P. proximus and P. subopacus, the stereochemistry of fragranol was not investigated but if these two species use different enantiomers of fragranol, it may be a species-specific component of their pheromone blends. Geraniol and γ-isogeraniol were seen among the volatiles from P. proximus in our studies, but were not active on P. subopacus antennae in EAG studies and had no significant effect in our field studies. It has been suggested by other authors that γ-isogeraniol and geraniol are biosynthetic precursors of (Z)-DMCHE and grandisol (Byers et al. 2013; Thompson and Mitlin 1979). The compound γ-isogeraniol was identified in our study as the second largest GC peak emitted from boring P. proximus males. Earlier it has been found among the volatiles from several other beetle species, but it is not known to be behaviorally active in bark beetles (Ambrogi et al. 2012; Byers et al. 2013).
We found that for P. proximus, 3-methyl-2-buten-1-ol was attractive in the field even though a small amount of this compound was collected from males of P. proximus in our SPME–GC–MS study. The combination of this compound and (Z)-DMCHE did not increase the catches of P. proximus, nor decrease the number of P. subopacus caught in the traps as compared to only (Z)-DMCHE (Experiment 3). The effect of 3-methyl-2-buten-1-ol on P. proximus should be further examined in future field studies, but it is not likely that the compund will repel P. subopacus from traps baited with (Z)-DMCHE as P. subopacus antennae could not detect this compound in our EAG studies. This despite the fact that males of P. subopacus appears to produce 3-methyl-2-buten-1-ol themselves. However, it is possible that 3-methyl-2-buten-1-ol is part of P. proximus pheromone since a similar compound, 3-methyl-3-buten-1-ol, is the aggregation pheromone of the closely related species Polygraphus rufipennis (Bowers et al. 1991) and as 3-methyl-2-buten-1-ol is a major aggregation pheromone component in the walnut twig beetle, Pityophtorus juglandis (Seybold et al. 2015). The results of our field studies indicate that 3-methyl-2-buten-1-ol can be used in itself to monitor P. proximus and in this case, without interference of P. subopacus caught in the traps (Table 3).
In our first field experiment, Experiment 1, the catches of P. subopacus on (Z)-DMCHE were nearly three times larger than the catches of P. proximus, although the latter species was expected to be present in larger numbers at the sites used for the field studies since the chosen locations were outbreak areas of P. proximus. The high catches of P. proximus (but not P. subopacus) in control traps also confirms that P. proximus was far more common than P. subopacus at these locations. P. poligraphus was not present in the area where field studies were conducted, but we know from previous studies that they are also attracted to (Z)-DMCHE (Viklund et al. 2021). Thus, it seems clear that the complete compostition of P. proximus pheromone has yet to be determined. The difference in catches of P. proximus by (Z)-DMCHE compared to the control traps was also relatively small, since the baited traps caught only 5–14 times more P. proximus than the control traps with n-nonane. In field experiments in Sweden using pheromone traps with similar release rates of aggregation pheromones for P. poligraphus and P. punctifrons, we caught at least 250 times more beetles than the unbaited control traps (Viklund et al. 2019; Rahmani et al. 2019). Thus, we think that there should be other compound(s) in P. proximus pheromone which would increase catches and at the same time make the pheromone species-specific and for that EAG studies of P. proximus would be useful along with more field trials. However, such EAG studies are difficult to conduct at our laboratory in Sweden as P. proximus is a quarantine pest species in the European Union (De la Peña et al. 2020). Our EAG studies of P. subopacus can partly serve as replacement as there should be compound(s) in P. proximus pheromone which repels P. subopacus. Based on these EAG studies, it would be interesting to test (Z)- and (E)-DMCHE but also the stereoisomers of grandisol and fragranol, (Z)-DMCHA, (E)-DMCHA and the female-specific compound 1-hexanol on the antennae of P. proximus in the future. Papayanol and its stereoisomers may also be of interest, as it was not tested in our EAG studies and as it is a pheromone in other species of beetles such as the Papaya borer Pseudopazurus obesus (Zarbin et al. 2010) and the guava weevil, Conotrachelus psidii (Palacio-Cortés et al. 2015).
If species-specific pheromone traps were developed, they could be used to detect P. proximus at an early stage, delimit the current distribution area and demonstrate further spread. They could also be used in containment efforts, together with sanitation cuttings of attacked trees, as a way of reducing populations in outbreak areas by mass trapping or by transmission of insect pathogens (Kreutz et al. 2004). Without species-specific traps, a major challenge will be to differentiate between the different Polygraphus species which may be caught in the traps. Many Polygraphus species are very similar and can only be distinguished under a microscope, making identification difficult and time-consuming if catches consist of more than one species and large numbers of native species.
This study has, in addition to giving us new insights relating to sex-specific compounds in P. proximus, also shown that SPME–GC–MS can be used with a delay between SPME sampling and GC–MS analysis and that compounds are retained on the fiber when they are stored inert under argon. The pink, orange and black SPME fibers were considered most useful in our experiment as they collect the largest number of compounds. Red fibers could collect approximately half of the sex-specific compounds in P. proximus whereas the yellow SPME fibers only collected three of the male-specific compounds. These results gives that it is of utmost importance to use several fiber-types when looking for new substances using SPME.
To summarize, this work provides evidence of several male-specific compounds which are emitted from the four-eyed fir bark beetle P. proximus as well as one female-specific compound. (Z)-DMCHE was found to be the main compound released by boring P. proximus males according to the GC peak areas in our SPME–GC–MS analyses, and we have shown that (Z)-DMCHE attracts both males and females of P. proximus in the field. Thus, we suggest that (Z)-DMCHE is a component of P. proximus aggregation pheromone. The compound can be used as bait to catch P. proximus but then together with P. subopacus in the traps. However, 3-methyl-2-buten-1-ol can also be used as bait to catch only P. proximus, without interference of P. subopacus caught in the traps.
References
Ambrogi BG, Palacio-Cortés AM, Zarbin PHG (2012) Identification of male-produced aggregation pheromone of the curculionid beetle Sternechus subsignatus. J Chem Ecol 38:272–277. https://doi.org/10.1007/s10886-012-0080-3
Baranchikov Y, Akulov E, Astapenko S (2010) Bark beetle Polygraphus proximus: a new aggressive far eastern invader on Abies species in Siberia and European Russia. In: McManus KA, Gottschalk KW (eds) Proc. 21st US Department of agriculture interagency research forum on invasive species 2010; Gen Tech Rep NRS-P-75. Annapolis, MD. US Dep of Agric, Forest Service, North Res Stat: 64–65
Baranchikov YN, Demidko DA, Laptev AV, Petko VM (2014) Dynamics of Siberian fir dieback in the outbreak area of the four-eyed fir bark beetle Lesnoy Vestnik. Moscow State For Univ Bull 18(6):132–138 (in Russian)
Baranchikov YN, Demidko DA, Pashenova NV, Pertsovaya AA, Petko VM (2017) Ophiostomal fungi in the trunk of Siberian fir increase its attractiveness for bark beetles. Current Mycology in Russia. Moscow Nat Mycol Acad 6:355–357. https://doi.org/10.14427/cmr.2017.vi.10 (in Russian)
Birgersson G, Dalusky MJ, Espelie KE, Berisford CW (2012) Pheromone production, attraction, and interspecific inhibition among four species of Ips bark beetles in the southeastern USA. Psyche A Journal of Entomology 6:1–14. https://doi.org/10.1155/2012/532652
Blomquist GJ, Figueroa-Teran R, Aw M, Song M, Gorzalski A, Abbott NL, Chang E, Tittiger C (2010) Pheromone production in bark beetles. Insect Biochem Molec 40:699–712. https://doi.org/10.1016/j.ibmb.2010.07.013
Bowers WW, Gries G, Borden JH, Pierce HD Jr (1991) 3-methyl-3-buten-1-ol: An aggregation pheromone of the four-eyed spruce bark beetle, Polygraphus rufipennis (Kirby) (Coleoptera: Scolytidae). J Chem Ecol 17:1989–2002. https://doi.org/10.1007/BF00992583
Byers JA, Birgersson G, Francke W (2013) Aggregation pheromones of bark beetles, Pityogenes quadridens and P. bidentatus, colonizing Scotch pine: olfactory avoidance of interspecic mating and competition. Chemoecology 23:251–261. https://doi.org/10.1007/s00049-013-0139-9
De la Peña E, Kinkar M, Vos S (2020) Pest survey card on Polygraphus proximus. EFSA supporting publications. European Food Safety Authority, Parma. https://doi.org/10.2903/sp.efsa.2020.EN-1780. Accessed 29 Apr 2022
Eller FJ, Bartelt RJ, Shasha BS, Schuster DJ, Riley DG, Stansly PA, Mueller TF, Shuler K, Johnson B, Davis JH, Sutherland CA (1994) Aggregation pheromone for the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae): identification and field activity. J Chem Ecol 20:1537–1555. https://doi.org/10.1007/BF02059879
EPPO (2014) Pest risk analysis for Polygraphus proximus. EPPO, Paris. https://gd.eppo.int/taxon/POLGPR/documents. Accessed 29 Apr 2022
Grégoire J-C, Raffa KF, Lindgren BS (2015) Economics and politics of bark beetles. In: Vega FE, Hofstetter RW (eds) Bark beetles. Biology and ecology of native and invasive species, 1st edn. Academic Press, London, pp 585–613. https://doi.org/10.1016/B978-0-12-417156-5.00015-0
Hedin PA, Dollar DA, Collins JK, Dubois JG, Mulder PG, Hedger GH, Smith MW, Eikenbary RD (1997) Identification of male pecan weevil pheromone. J Chem Ecol 23:965–977. https://doi.org/10.1023/B:JOEC.0000006382.70034.66
Innocenzi PJ, Hall DR, Cross JV (2001) Components of male aggregation pheromone of strawberry blossom weevil, Anthonomus rubi Herbst. (Coleoptera: Curculionidae). J Chem Ecol 27:1203–1218. https://doi.org/10.1023/A:1010320130073
Kandasamy D, Gershenzon J, Hammerbacher A (2016) Volatile organic compounds emitted by fungal associates of conifer bark beetles and their potential in bark beetle control. J Chem Ecol 42:952–969. https://doi.org/10.1007/s10886-016-0768-x
Kerchev IA (2014a) Ecology of four-eyed fir bark beetle Polygraphus proximus Blandford (Coleoptera; Curculionidae, Scolytinae) in the West Siberian Region of Invasion. Russ J Biol Invasions 5:176–185. https://doi.org/10.1134/S2075111714030072
Kerchev IA (2014b) On monogyny of the four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Curculionidae: Scolytinae) and its reproductive behavior. Entomol Rev 94(8):1059–1066. https://doi.org/10.1134/S0013873814080028
Kerchev IA (2020) Interspecific differences of stridulatory signals in three species of bark beetles from the genus Polygraphus Er. (Coleoptera: Curculionidae, Scolytinae) inhabiting the island of Sakhalin. Peer J 8:8281. https://doi.org/10.7717/peerj.8281
Kerchev IA, Pousheva MS (2016) Olfactometric evidence for aggregation pheromone production by females of the four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Curculionidae: Scolytinae). Entomol Rev 96:821–825. https://doi.org/10.1134/S0013873816070010
Kerchev IA, Krivets SA, Mandelshtam MY. (2019) 3.3. Ips amitinus (Eichhoff, 1872) (Coleoptera, Curculionidae: Scolytinae)–A new pest of Pinus sibirica in Western Siberia. In: Gninenko YI (ed) Invasive Dendrophilous Organisms: Challenges and Protection Operations. All-Russian Research Institute of Silviculture and Mechanization of Forestry, Pushkino, pp 110–118
Knizek M, Liska J, Véle A (2022) Efficacy of synthetic lures for pine beetle monitoring. J For Sci 68:19–25. https://doi.org/10.17221/139/2021-JFS
Köbayashi K, Takagi E (2020) Mating systems of the tree-killing bark beetle Polygraphus proximus (Coleoptera: Curculionidae: Scolytinae). J Insect Sci 20(6):1–4. https://doi.org/10.1093/jisesa/ieaa140
Kononov A, Ustyantsev K, Blinov A, Fet V, Baranchikov YN (2016) Genetic diversity of aboriginal and invasive populations of four-eyed fir bark beetle Polygraphus proximus Blandford (Coleoptera, Curculionidae, Scolytinae). Agric For Entomol 18:294–301. https://doi.org/10.1111/afe.12161
Kreutz J, Zimmermann G, Vaupel O (2004) Horizontal transmission of the entomopathogenic fungus Beauveria bassiana among the spruce bark beetle, Ips typographus (Col, Scolytidae) in the laboratory and under field conditions. Biocontrol Sci Technol 14(8):837–884. https://doi.org/10.1080/788222844
Krivets SA, Kerchev IA, Bisirova EM, Debkov NM, Chernova NA, Pats EN. (2019) 3.2.1. Four-eyed fir bark beetle Polygraphus proximus Blandford, 1894 (Coleoptera, Curculionidae: Scolytinae) in Western Siberia: review of a ten years of research of the invasion. In: Gninenko YI (ed) Invasive dendrophilous organisms: challenges and protection operations. All-Russian Research Institute of Silviculture and Mechanization of Forestry, Pushkino, pp 87–100
Palacio-Cortés AM, Valente F, Saad EB, Tröger A, Francke W, Zarbin PHG (2015) (1R,2S,6R)-Papayanol, aggregation pheromone of the guava weevil, Contrachelus psidii. J Braz Chem Soc 26:784–789. https://doi.org/10.5935/0103-5053.20150040
Pashenova NV, Kononov AV, Ustyantsev KV, Blinov AG, Pertsovaya AA, Baranchikov YN (2018) Ophiostomatoid fungi associated with the four-eyed fir bark beetle on the territory of Russia. Russ J Biol Invasions 9:63–74. https://doi.org/10.1134/S2075111718010137
Pavlov IN, Litovka YA, Golubev DV, Chromogin PV, Usoltseva YV, Makolova PV, Petrenko SM (2020) Mass reproduction of Polygraphus proximus Blandford in fir forests of Siberia infected with root and stem pathogens: monitoring, patterns, and biological control. Contemp Probl Ecol 13:71–84. https://doi.org/10.1134/S1995425520010060
Rahmani R, Hedenström E, Schroeder M (2015) SPME collection and GC-MS analysis of volatiles emitted during the attack of male Polygraphus poligraphus (Coleoptera, Curcolionidae) on Norway spruce. Z Naturforsch C 70:265–273. https://doi.org/10.1515/znc-2015-5035
Rahmani R, Wallin EA, Viklund L, Schroeder M, Hedenström E (2019) Identification and field assay of two aggregation pheromone components emitted by males of the bark beetle Polygraphus punctifrons (Coleoptera: Curculionidae). J Chem Ecol 45:356–365. https://doi.org/10.1007/s10886-019-01056-6
Rodriguez-Saona C, Alborn HT, Oehlschlager C, Calco C, Kyryczenko-Roth V, Tewari S, Sylvia MM, Averill AL (2020) Fine-tuning the composition of the cranberry weevil (Coleoptera: Curculionidae) aggregation pheromone. J Appl Entomol 144:417–421. https://doi.org/10.1111/jen.12752
Schurig V, Leyrer U, Kohnle U (1985) Enantiomer composition and absolute configuration of terpinen-4-ol from the bark beetle Polygraphus poligraphus. Naturwissenschaften 72:211. https://doi.org/10.1007/BF01195767
Seybold SJ, Dallara PL, Nelson LJ, Graves AD, Hishinuma SM, Gries R. (2015) Methods of monitoring and controlling the walnut twig beetle, Pityophthorus juglandis US 9,137,990 B2
Szendrei Z, Averill A, Alborn H, Rodriguez-Saona C (2011) Identification and evaluation of attractants for the cranberry weevil, Anthonomus musculus Say. J Chem Ecol 37:387–397. https://doi.org/10.1007/s10886-011-9938-z
Thompson AC, Mitlin N (1979) Biosynthesis of the sex pheromone of the male boll weevil from monoterpene precursors. Insect Biochem 9:293–294. https://doi.org/10.1016/0020-1790(79)90008-8
Tumlinson JH, Hardee DD, Gueldner RC, Thompson AC, Hedin PA, Minyard JP (1969) Sex pheromones produced by male boll weevil: Isolation, identification, and synthesis. Science 166:1010–1012. https://doi.org/10.1126/science.166.3908.1010
Viklund L, Rahmani R, Bång J, Schroeder M, Hedenström E (2019) Optimizing the attractiveness of pheromone baits used for trapping the four-eyed spruce bark beetle Polygraphus poligraphus. J Appl Entomol 143(7):721–730. https://doi.org/10.1111/jen.12641
Viklund L, Bång J, Schroeder M, Hedenström E (2021) Identification of male produced compounds in the bark beetle Polygraphus subopacus and establishment of (Z)-2-(3,3-dimethylcyclohexylidene)-ethanol as an aggregation pheromone component. Chemoecology 31:367–376. https://doi.org/10.1007/s00049-021-00358-0
Welch BL (1951) On the comparison of several mean values: an alternative approach. Biometrika 38:330–336. https://doi.org/10.2307/2332579
Yong KH, Lotoski JA, Chong M (2001) Studies on the alkylation of 3-methyl-3-buten-1-ol dianion: an efficient synthesis of 3-methylene-1-alkanols including a San Jose scale sex pheromone. J Org Chem 66:8248–8251. https://doi.org/10.1021/jo015940w
Zarbin PHG, Moreira MAB, Haftmann J, Tröger A, Franke S, Kopf J, Mori K, Francke W (2010) (1R,2S,6R)-2-Hydroxymethyl-2,6-dimethyl-3- oxabicyclo[4.2.0]octane, a new volatile released by males of the papaya borer Pseudopiazurus obesus (Col.: Curculionidae). Org Lett 12:2447–2449. https://doi.org/10.1021/ol100074q
Acknowledgements
Fredrik Andersson at the Mid Sweden University contributed with the synthesis of papayanol. Joakim Bång at the Mid Sweden University conducted EAG analyses. Vladimir Petko and Nikita Babichev at the Sukachev Institute of Forest took part in the field and laboratory experiments in Russia.
Funding
Open access funding provided by Mid Sweden University. This study was funded by the County Board of Västernorrland, the Region of Västernorrland, the Region of Jämtland and Härjedalen, the European Development Funds, Brattåsstiftelsen för skogsvetenskaplig forskning, Carl Tryggers Stiftelse för Vetenskaplig Forskning and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) (grant no. 239-2013‐669).
Author information
Authors and Affiliations
Contributions
LV, YB, MS and EH contributed to the study conception and design. SPME–GC–MS analyses, material preparation and statistical analysis were performed by LV. YB contributed with insects and stem sections for the SPME-analysis. AE and DD conducted the field studies in Russia. The first draft of the manuscript was written by LV. EH, YB and MS commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Günther Raspotnig.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viklund, L., Baranchikov, Y., Schroeder, M. et al. Identification of sex-specific compounds in the invasive four-eyed fir bark beetle Polygraphus proximus. Chemoecology 32, 183–195 (2022). https://doi.org/10.1007/s00049-022-00377-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-022-00377-5