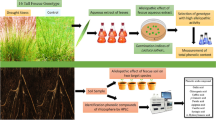
Abstract
Allelopathic plants release secondary compounds into the soil that then suppress the growth of nearby plants. Allelopathy has been shown for the invasive Japanese knotweed (Fallopia japonica) and Bohemian knotweed (F. × bohemica). The aggressive and dominant invaders represent a serious threat to the local plant communities outside their native range. Here, we analysed the phenols in the knotweed rhizomes using nuclear magnetic resonance. We also evaluated the allelopathic potential of methanol extracts of F. japonica and F. × bohemica rhizomes and compared these with the effects of the individual knotweed phenols resveratrol, epicatechin and emodin, and their mixture. Rhizomes of both knotweeds contained similar amounts of epicatechin and emodin, with 24% higher resveratrol in F. × bohemica. Only the F. × bohemica methanol extract inhibited radish (Raphanus sativus) seed germination. After 3 days of treatments with 10% (w/v) extracts of both knotweeds, radish seedlings showed up to 70% shorter roots. In contrast, root growth of seedlings treated with the individual phenols resveratrol, epicatechin and emodin, and their mixture, was inhibited by up to 30%, similar to the 1% knotweed extracts. Biochemical parameters of oxidative stress also increased in the roots of treated seedlings, with high levels of malondialdehyde in particular indicating lipid peroxidation. Total antioxidative capacity was also increased in seedlings exposed to 0.6 mg/mL resveratrol and emodin. This study shows higher allelopathic potential of the knotweed methanol extracts compared to the individual phenols and their mixture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Japanese knotweed (Fallopia japonica var. japonica (Houtt.) Ronse Decr.) was introduced into Europe and North America as an ornamental plant from Asia in the nineteenth century (Bailey et al. 2009). Since then, this robust perennial herb has spread rapidly and extensively, and is today ranked among the 100 most invasive plants in the world (Lowe et al. 2000). Japanese knotweed is not only a serious threat to local plant communities, but also it can hybridise with giant knotweed (Fallopia sachalinensis (F. Schmidt) Ronse Decr.), to produce the genetically variable and even more invasive Bohemian knotweed (Fallopia × bohemica (Chrtek and Chrtková) J. P. Bailey) (Bailey et al. 2009; Siemens and Blossey 2007). In Slovenia, invasive Japanese and Bohemian knotweeds cause ecological problems especially along rivers, railways and roads, and for some other ruderal habitats (Strgulc Krajšek and Dolenc Koce 2015).
The rapid regeneration of these knotweeds (Bímová et al. 2003) contributes to their spread in new environments and reduces local biodiversity. Knotweeds develop dense stands of more than 3 m in height (Bailey et al. 2009), and they produce large amounts of both aboveground and underground biomass, with an extensive rhizome system (Frantík et al. 2013). The shadow from their dense canopy has strong negative influence on nearby plants (Moravcová et al. 2011). However, this rapid growth is not the only factor that determines the success of knotweeds in the competition among plants for natural resources. Knotweeds also reduce species richness through the phenomenon known as allelopathy (Murrell et al. 2011).
The term allelopathy refers to chemically mediated interactions between plants. Allelochemicals are secondary metabolites, and especially phenols. These metabolites can have more than one biological role in the plant, but once released into the environment, they can also suppress growth and development of nearby plants (Latif et al. 2017) which can be considered as the strategy of invasiveness called the Novel Weapon Hypothesis (Callaway and Aschehoug 2000). Allelopathic compounds can be found in leaves, bark, roots, flowers and fruits (Weir et al. 2004). In knotweeds, most secondary compounds are stored in the underground rhizomes (Chen et al. 2013; Frantík et al. 2013). Knotweeds contain several biologically active compounds, among which the most common are resveratrol, emodin, (–)-epicatechin, (–)-catechin, piceid, resveratroloside, piceatannol glucoside and emodin-8-O-glucoside (Fan et al. 2009). Their amounts in knotweeds vary depending on the geographical area (Fan et al. 2010) and the growing season (Frantík et al. 2013), and increase with plant age (Chen et al. 2013).
Allelopathic compounds can alter the growth of neighbouring plants by targeting their vitally important metabolic processes, including photosynthesis, mitochondrial respiration, nucleic acid formation and mineral uptake (Latif et al. 2017). Furthermore, allelochemicals can also trigger uncontrolled production and accumulation of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) (Gechev et al. 2006). At lower concentrations, ROS act as signalling molecules that are involved in plant growth and have important roles in developmental processes, including root development (Yamada et al. 2020). On the other hand, environmental stressors can stimulate excessive production of ROS, which can lead to oxidative stress and cause damage to proteins, lipids and nucleic acids. ROS can also trigger lipid peroxidation, and thereby cause cell membrane damage (Gechev et al. 2006).
The plant defence system against ROS consists of enzymatic antioxidants such as catalase and peroxidase, which detoxify H2O2, and non-enzymatic antioxidants like α-tocopherol and ascorbate. The contents of antioxidants in plants change during stress exposure (Sánchez-Moreno et al. 1998). Our previous studies have shown that in radish seedlings, aqueous extracts of F. japonica and F. × bohemica rhizomes can increase the levels of biochemical parameters of oxidative stress, such as malondialdehyde (MDA) and total antioxidative capacity (TAC) (Šoln et al. 2021a), which leads to various ultrastructural changes in the cells of the root tip (Šoln et al. 2021b). These changes indicate as strong suppression of root elongation after 7 days of exposure to these knotweed extracts.
As the radish roots showed relatively severe signs of stress, we here have further investigated the allelopathic potential of these knotweed extracts against radish seedlings after 3 days of germination, to detect the first signs of inhibition. Although various short-term exposure scenarios to knotweed extracts (Vrchotová and Šerá 2008; Moravcová et al. 2011; Dolenc Koce and Šoln 2018; Šoln et al. 2021a, 2021b) and applications of individual phytochemicals (Fan et al. 2010; Tucker Serniak 2016) have been studied previously, their influence has never been compared nor analysed in the same study. Therefore, we compared the effects of these knotweed extracts to those of their most abundant phenols, i.e. resveratrol, epicatechin and emodin. The same concentrations of extracts and phenols were used as in previous experiments of Šoln et al. (2021a, b) and Tucker Serniak (2016) to allow for comparisons of the studies.
The aims of the present study were therefore: (i) to quantify the amounts of resveratrol, epicatechin and emodin in the rhizomes of F. japonica and F. × bohemica; (ii) to compare the allelopathic potential of methanol extracts from F. japonica and F. × bohemica rhizomes with that of the individual knotweed phenols resveratrol, epicatechin and emodin, and their mixture; and (iii) to analyse the morphological and biochemical changes in radish seedlings after treatments with these F. japonica and F. × bohemica methanol extracts and the selected phenols.
Materials and methods
Plant material
Rhizomes of F. japonica and F. × bohemica were collected in October 2018 in Ljubljana, Slovenia (46° 2′ 33.98″ N, 14° 27′ 0.91″ E; 46° 3′ 0.3″ N, 14° 28′ 44″ E; respectively). The rhizomes were rinsed with tap water to remove any soil, then dried, lyophilised, ground to a powder with a cutting mill (SM 200; Retsch, Germany), and stored in the dark at room temperature until the nuclear magnetic resonance (NMR) analysis and the preparation of the extracts.
NMR analysis of Fallopia rhizomes
To determine the contents of the three main allelochemicals in the F. japonica and F. × bohemica rhizomes, the ground material was resuspended in ethanol (12 g rhizome material in 100 mL 96% ethanol). The mixtures were left stirring at 400 rpm for 24 h at room temperature on a magnetic stirrer (Rotamix 550MMH; Tehtnica, Slovenia). After the extraction, mixtures were vacuum filtered and the solvent was evaporated under reduced pressure at 30 °C using a rotary evaporator (RV 10 digital V; IKA-Werke, Germany). The extraction was independently repeated three times (N = 3).
For NMR analysis, 6 mg of the individual standards of resveratrol, (–)-epicatechin and emodin (all Sigma-Aldrich, USA) and 50 mg dried F. japonica or F. × bohemica extracts were dissolved in 0.7 mL d-dimethylsulphoxide (DMSO; Fluorochem, UK). 1H NMR spectra of standards and the F. japonica and F. × bohemica ethanol extracts were recorded using an Avance III 500 NMR spectrometer (Bruker, USA) at 296 K.
Identification of these three allelochemicals in the F. japonica and F. × bohemica ethanol extracts was achieved by comparison of the 1H NMR spectra of standard compounds with the spectra of the extracts, using the MestReNova software. The quantification of resveratrol, epicatechin and emodin in these extracts was obtained from the integral of their 1H NMR signals in the spectra using the known amount of standard 1-bromo-4-nitrobenzene (Sigma-Aldrich, USA) added to the NMR sample.
1H NMR of 1-bromo-4-nitrobenzene: 1H NMR (500 MHz, DMSO) δ 8.15 (d, J = 9.0 Hz, 2H), 7.88 (d, J = 9.0 Hz, 2H) ppm.
Preparation of methanol extracts and selection of phenols for germination test
The ground rhizome material (10 g) was resuspended in 33% methanol (100 mL), and this 10% (w/v) suspension was shaken at 175 rpm for 24 h at room temperature. The suspension was then vacuum filtered (filter paper Grade 520A; Whatman, Maidstone, UK) to obtain the final methanol extracts that were used undiluted as the 10% extract, and that were diluted with 33% methanol to the final concentration of the 1% extract. The methanol extracts were prepared fresh before the experiment.
The most frequent secondary compounds in knotweeds are resveratrol, (–)-epicatechin and emodin, and these were selected for this study. Each of these phenols (Sigma-Aldrich, USA) was dissolved in 33% methanol (VWR Chemicals, USA) to prepare the 0.2 mg/mL and 0.6 mg/mL treatments. Additionally, a mixture of these phenols was prepared according to Tucker Serniak (2016) with the following concentrations: 0.2 mg/mL resveratrol, 0.6 mg/mL (–)-epicatechin and 0.2 mg/mL emodin.
Seed germination and early seedling growth
The germination tests were performed in Petri dishes (2r = 14 cm) on filter paper. First, 10 mL methanol extracts or phenols were applied to each individual filter paper, with 33% methanol used as the control. The wet filter papers were dried at 40 °C for approx. 20 min to ensure evaporation of the methanol. Each dry filter paper was then placed in a Petri dish. Fifty seeds of radish (Raphanus sativus L. cv. Saxa 2) were laid on the filter paper and irrigated with 10 mL distilled water. The seeds were left to germinate under laboratory conditions for 3 days (22 ± 2 °C, 15-h light/9-h dark photoperiod). Five independent replicates were performed for each treatment (N = 250).
After 3 days, the germinating seeds were counted to determine the germination rate. The resulting seedlings were photographed using a digital camera (EOS 1000D; Canon, Japan) with an EF-S 60 mm f/2.8 Macro USM objective (Canon, Japan). The lengths of the roots and shoots were measured on the digital photos using the ImageJ 1.x software (Schneider et al. 2012). The roots were separated from the shoots, weighed, frozen in liquid nitrogen and stored at − 20 °C until biochemical analysis.
Biochemical parameters associated with oxidative stress
Lipid peroxidation and TAC were measured spectrophotometrically as previously described by Dolenc Koce and Šoln (2018) and Šoln et al. (2021a). The protocols are described briefly below.
Lipid peroxidation was measured via the content of MDA. Samples of 100 mg roots were homogenised in 1.5 mL potassium phosphate buffer (100 mM, pH 7.0) and centrifuged at 15,339×g for 20 min at 4 °C. Next, 200 µL supernatant from the samples was added to 800 µL acetic acid reagent (0.5% [w/v] thiobarbituric acid in 20% [w/v] trichloroacetic acid), incubated for 30 min at 95 °C, and cooled to stop the reaction. The MDA content was determined spectrophotometrically (UV-1800; Shimadzu, Kyoto, Japan) at 532 nm and 600 nm.
Total antioxidative capacity was measured as the content of 2,2-diphenyl-1-picryl-hydrazyl (DPPH). Root samples (100 mg) were homogenised in 2 mL methanol and centrifuged at 15,339×g for 5 min at 4 °C. Then 30 µL supernatant from the samples was added to 2 mL 12 µM DPPH and incubated in the dark for 15 min. The DPPH content was measured spectrophotometrically at 515 nm. The TAC was calculated using the calibration curve of Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) at the following concentrations: 0.2, 0.4, 0.6, 0.8, 1.5 and 2 mM.
Statistical analysis
For the statistical analysis, all data were normalised to the mean control values. Mean values and standard errors were calculated. The means of the controls and treatments within the experiment were compared using one-way ANOVA followed by Dunnett two-sided comparison test, with a confidence interval of 95%, using the XLSTAT version 2020.3.1 statistical software for Excel (Addinsoft, Paris, France). To enable comparison of three separate experiments, the data were normalised to the control value (set at 100% and shown in figures), and the treatments were compared by one-way ANOVA followed by Tukey multiple comparisons test with 95% family-wise confidence level using the Prism version 9.0.0 for Windows software package (GraphPad Software, San Diego, California, USA). The level for statistical significance was set at P < 0.05.
The graphs and heat map were designed with the Prism version 9.0.0 for Windows software package (GraphPad Software, San Diego, CA, USA).
Results and discussion
One of the mechanisms of invasiveness of Japanese and Bohemian knotweeds is a chemical weapon, allelopathy (Murrell et al. 2011), as these plants release various bioactive compounds into the soil that can negatively affect the growth of nearby plants (Latif et al. 2017). Knotweeds are known to contain many biologically active compounds, especially in their rhizomes (Frantík et al. 2013). The main phytochemicals are resveratrol, emodin, (–)-epicatechin, (–)-catechin, piceid and resveratroloside (Fan et al. 2009). In this study, we compared the effects of methanol extracts of knotweed rhizomes and the selected phenols resveratrol, epicatechin and emodin, and their mixture, on radish seeds and seedlings. In contrast to 7 days of treatment and growth in our previous studies (Šoln et al. 2021a, b), in the present study, 3-day-old seedlings were analysed to detect the very first changes following germination.
NMR analysis of knotweed rhizomes
Conventional metrological quantification techniques are mostly faced with challenges such as specific response factors and chemicals, as well as mechanistic considerations. On the other hand, quantitative NMR (qNMR) is a direct method that only requires complete solubility and the presence of detectable nuclei; e.g. hydrogen. Nowadays, qNMR has found wide recognition in pharmaceutical analysis, natural product chemistry, metrology and forensics (Jaki et al. 2020). This method is a powerful non-destructive analytical tool that allows identification of compounds also in complex samples and quantification without the need for calibration curves of internal or external standards (Pauli et al. 2005; Simmler et al. 2014).
Secondary compounds in knotweed rhizomes have been mainly analysed using various chromatographic techniques (Fan et al. 2009; Chen et al. 2013; Frantík et al. 2013; Šoln et al. 2021a) and mass spectrometry (Fan et al. 2009). Xiao et al. (2002) used NMR to structurally identify some new compounds from rhizomes of F. japonica from China. Here, we used a NMR to quantify secondary metabolites in the rhizomes of the invasive F. japonica and F. × bohemica from Europe for the first time.
The characteristic signals of the standard NMR spectra were 7.39 ppm (H-2′, H-6′), 6.37 ppm (H-2, H-6) and 6.11 ppm (H-4) for resveratrol (Fig. 1A), 5.89 ppm (H-8) and 5.71 ppm (H-6) for epicatechin (Fig. 1B), and 7.45 ppm (H-5) for emodin (Fig. 1C). The signals for these compounds were confirmed in the rhizomes of F. japonica and F. × bohemica, as shown in Fig. 1D,E, respectively. Of these signals, those with the best compromise between intensity and overlap were selected for the quantitative analysis.
The total yields of knotweed ethanol extracts were 58.50 mg/g dry mass for F. japonica rhizome and 62.08 mg/g dry mass for F. × bohemica rhizome. The predominant phenol in these extracts was epicatechin, which accounted for about half of the content, followed by resveratrol at about one-third, and emodin with about one-fifth (Table 1). In this dry matter, the F. japonica and F. × bohemica extracts contained ~ 36 mg/g epicatechin each, 21 mg/g and 26 mg/g resveratrol, respectively, and ~ 14 mg/g emodin (Table 1). In F. japonica the ratio of resveratrol:epicatechin:emodin was 1.5:2.6:1.0, and in F. × bohemica, this ratio was 1.9:2.6:1.0, whereby F. × bohemica contained 5 mg/g more resveratrol than F. japonica.
The similarities between these knotweeds can be attributed to F. × bohemica being a hybrid of F. japonica and F. sachalinensis, which show similar invasive potentials (Bailey et al. 2009). These knotweeds also inhabit ecologically similar habitats. In the present study, the plants of these F. japonica and F. × bohemica knotweeds were collected within 2 weeks of each other, but from different locations. The stand of F. japonica grew along the river, while that of F. × bohemica was along the road, and thus their microlocations might have contributed to the different resveratrol contents due to the different ecological conditions. It is known that taxonomically related species from the same environment do not necessarily produce similar concentrations of secondary metabolites (Imatomi et al. 2013). Their contents can vary also according to ploidy level, age of plants and geographical area. Plants of F. japonica from Switzerland contained more catechin and epicatechin, piceatannol glucoside and resveratroloside than the plants from China (Fan et al. 2009). Different F. × bohemica hybrids have shown less resveratroloside than the octoploid F. japonica, while the contents of resveratrol were similar (Frantík et al. 2013). Thus, the contents of these phenols and their ratios may differ according to environmental factors. In addition, different extraction and detection methods can also make direct comparisons difficult. While Frantík et al. (2013) used ethanol and HPLC, we at first used methanol and ethanol extraction in our preliminary analysis. As there was no difference in the compositions of these three secondary metabolites, we further used only the ethanol extraction, which provided for better identification with NMR.
Seed germination and early radish seedling growth
The germination rate of control seeds was 92.27% ± 1.45 and the results of treatments with knotweed extracts and phenols were normalised to this value (Fig. 2). The one-way ANOVA showed that germination was significantly affected by the treatments (P = 0.0199, df = 10, MS = 45.74, F = 2.45). Between the treatments, the only significant difference was between 0.2 mg/mL resveratrol and the mixture, with the resveratrol treatment causing by 9.8% smaller germination rate (P = 0.0306; ANOVA and Tukey test). In other treatments there is an evident trend that higher concentrations cause lower germination rates but the effect is not statistically significant. When compared to the control, the methanol extract of F. × bohemica rhizomes significantly inhibited germination not only at 10% (5.7% reduction; P = 0.0411; ANOVA and Dunnett test) but also at 1% (6.9% reduction; P = 0.0115; ANOVA and Dunnett test). In contrast, the methanol extract of F. japonica did not reduce the germination rate. The resveratrol contents in these two knotweeds might result in different effects on radish seed germination. Also, the germination rate of seeds that were treated with 0.2 mg/mL resveratrol was significantly reduced compared to the control (by 9.3%; P = 0.0066; ANOVA and Dunnett test), while epicatechin and emodin treatments had no effects on the seed germination rates. This finding supports previous observation that resveratrol content could be the factor that most affects radish seed germination.
Germination rates of radish seeds under treatments with knotweed phenols (0.2, 0.6 mg/mL), their mixture (Mix; 0.2 mg/mL of Re and Em, 0.6 mg/mL of Ep) and the F. japonica (FJ) and F. × bohemica (FB) rhizome extracts (1%, 10%). Normalised data are means ± standard error (N = 150). Different letters indicate statistically significant difference between treatments (P < 0.05; one-way ANOVA and Tukey test). Re resveratrol, Ep epicatechin, Em emodin
These relatively small differences in germination rates can be related to different chemical compositions of these methanol extracts, as seed germination is a very sensitive process that can be disrupted by allelopathic stress (Šerá 2012). Interestingly, the selected knotweed phenols predominantly had no effects on germination, while some allelochemicals, such as sorgoleone, have been reported to strongly inhibit seed germination of several weeds (Uddin et al. 2014). Responses to allelopathic stress vary among plant species, as some are more sensitive than others (Cruz-Ortega et al. 2002). For example, Šera (2012) reported a strong inhibitory effect of knotweed leaves on seed germination of mustard (Leucosinapis alba), while germination of cockspur (Echinochloa crus-galli) was not affected. These effects will also depend on the concentration and duration of the exposure to the allelochemicals. In our previous studies, radish seedlings were observed over 7 days of treatment with aqueous extracts of F. japonica and F. × bohemica rhizomes (Šoln et al. 2021a) and leaves (Dolenc Koce and Šoln 2018), and their germination was delayed rather than inhibited after this period.
During germination, the primary root emerges, and this is the plant organ that is most affected by conditions in the growth medium. The root length of control seedlings was 21.50 mm ± 0.85 and the results of treatments with knotweed extracts and phenols were normalised to this value. The one-way ANOVA showed that root length was significantly affected by the treatments (P < 0.0001, df = 10, MS = 3712, F = 18.1). Among the treatments, 10% knotweed extracts showed the highest inhibitory potential as the radish root, i.e. the roots treated with 10% F. × bohemica extract were more than 32% shorter compared to the roots treated with the most inhibitory phenol 0.6 mg/mL epicatechin (P = 0.0359; ANOVA and Tukey test), whereas 1% extracts were mainly on the level of 0.6 mg/mL phenols (Fig. 3A). When compared to the control, the radish roots were significantly shorter due to the knotweed extracts. The roots of the seedlings treated with the 1% extracts of F. japonica and F. × bohemica were 19.5% and 18.4% shorter than the control, respectively (both P < 0.001; ANOVA and Dunnett test). The 10% extracts resulted in greater inhibition of root growth, by 67.4% and 69.5%, respectively (both P < 0.001; ANOVA and Dunnett test). The differences between each of the extracts of these two knotweeds did not reach significance. In treatments with the selected knotweed phenols, only the treatments with 0.6 mg/mL epicatechin showed shorter root length than control (by 37.6%; P = 0.0092; ANOVA and Dunnett test) and with the mixture of all three (by 24.2%; P = 0.0447; ANOVA and Dunnett test).
Root (A) and shoot (B) lengths of radish seedlings after 3 days of treatments with knotweed phenols (0.2, 0.6 mg/mL), their mixture (Mix; 0.2 mg/mL of Re and Em, 0.6 mg/mL of Ep) and the F. japonica (FJ) and F. × bohemica (FB) rhizome extracts (1%, 10%). Normalised data are means ± standard error (N = 150). Different letters indicate statistically significant difference between treatments (P < 0.05; one-way ANOVA and Tukey test). Re resveratrol, Ep epicatechin, Em emodin
Suppression of root length is commonly observed in short-term allelopathic studies, including treatments with diverse allelochemicals (Fan et al. 2010; Soltys et al. 2011; Tucker Serniak 2016; Yan et al. 2016; Araniti et al. 2018) and plant extracts (Vrchotová and Šerá 2008; Moravcová et al. 2011; Dolenc Koce and Šoln 2018; Šoln et al. 2021a, b). Compared to mature plants, seedlings are more sensitive to allelopathic stress, as their root system is not yet fully developed. Therefore, many potentially phytotoxic compounds can have strong impact on the root redox homeostasis (Lara-Nuñez et al. 2006; Yan et al. 2016), hormone regulation (Lupini et al. 2014), morphology and ultrastructure (Šoln et al. 2021b).
These data show that the greatest inhibition of root growth was not caused by the individual phenols or the mixture of them, although resveratrol, epicatechin (Fan et al. 2010; Tucker Serniak 2016) and emodin (Tucker Serniak 2016) have been reported to inhibit root growth. Here, we demonstrate that knotweed methanol extracts have greater allelopathic potential than any of the single selected phenols or of their mixture. Similar responses have been described for an ethanol extract of peach root (Prunus persica) bark, which inhibited shoot and root growth of peach seedlings more than benzoic acid in a 6-week study (Zhu et al. 2017). We therefore assume that also other phytochemicals in these knotweed rhizomes will contribute significantly to their allelopathic potential. Moreover, the combination of these different compounds in the rhizomes can provide synergism of their activity, as was suggested by Tucker Serniak (2016).
However, this inhibition of root growth in the radish seedlings might only be temporary, as root growth is very dynamic and has the potential to regenerate once the stressor is no longer present (Soltys et al. 2011). This was demonstrated in our previous study (Šoln et al. 2021a), where the root lengths of the radish seedlings treated with low concentrations (up to 2%) of aqueous extracts of knotweed rhizomes remained within the control range after 7 days, despite weaker growth in the first few days after germination. Higher extract concentrations (i.e. 5%, 10%) appeared to prevent this regeneration process, as these roots remained short (Šoln et al. 2021a).
The shoot lengths of the seedlings here were less affected by treatments with knotweed extracts and phenols. The control seedlings had 7.82 mm ± 0.48 long shoots and the results of treatments with knotweed extracts and phenols were normalised to this value (Fig. 3B). The one-way ANOVA showed that shoot length was affected by the treatments (P = 0.0025, df = 10, MS = 262, F = 3.4), but the only significant difference was between the mixture and F. japonica extracts of 1% (P = 0.0231) and 10% concentration (P = 0.0215; ANOVA and Tukey test), with the mixture treatment causing by 18.2% smaller shoots (Fig. 3B). The treatment with mixture was also the only one that significantly reduced the shoot length compared to the control (P = 0.0323; ANOVA and Dunnett test). Similarly, Tucker Serniak (2016) showed that radish seedlings exposed for 14 days to epicatechin, emodin and polydatin showed no inhibition of shoot growth compared to their control; indeed, they even reported a stimulatory effect on shoot growth with resveratrol treatment. Unlike roots, shoots are less affected by allelochemicals, as they are not in direct contact with them (Gniazdowska and Bogatek 2005). The shoot responses might also depend on the plant species and type of allelochemicals, as some studies have reported strong inhibition of shoot growth. For example, shoot growth of cucumber seedlings (Cucumber sativus) treated with 2-benzoxazolinone was strongly inhibited, while root growth was not inhibited to the same extent. The I50 value for the inhibition of shoot elongation was 0.11 mg/mL, while for the suppression of the root growth higher concentration (0.48 mg/mL) was needed (Burgos et al. 2004). In addition, plants also differ in their sensitivities to allelochemicals, as shown by Shixing et al. (2021), where an essential oil of Buffalo bur (Solanum rostratum) inhibited shoot and root growth of red-root amaranth (Amaranthus retroflexus) more than that of annual meadow grass (Poa annua).
Biochemical parameters associated with oxidative stress
At the cellular level, treatments with allelochemicals often lead to a burst of ROS in target plants (Yan et al. 2016; Araniti et al. 2018; Šoln et al. 2021a). ROS interact with lipids in the plasma membrane and initiate a radical chain reaction known as lipid peroxidation, which can subsequently lead to destruction of the cell membranes (Das and Roychoudhury 2014). Therefore, one of the well-used markers of oxidative stress is the content of MDA, a by-product of membrane degradation (Esterbauer et al. 1991). In the present study, the MDA contents in the roots of the seedlings treated with the F. japonica and F. × bohemica methanol extracts were significantly increased at both concentrations examined (Fig. 4A). MDA content in the control seedlings was 4.12 nM/g ± 0.33 and the results of treatments with knotweed extracts and phenols were normalised to this value (Fig. 4A). The one-way ANOVA showed that MDA content was affected by the treatments (P < 0.0001, df = 10, MS = 5146, F = 13.4). Similar as by the root length, 10% F. japonica and F. × bohemica extracts caused the highest effects as the level of MDA more than doubled compared to 0.6 mg/mL emodin treatment (for both P < 0.001; ANOVA and Tukey test). The effect was visible also with 1% extracts of F. japonica (P = 0.0071) and F. × bohemica (P = 0.0004; ANOVA and Tukey test). When compared to the control, 1% F. japonica extract increased MDA content by 23% (P = 0.0599), and the F. × bohemica extract by 34% (P = 0.0035; ANOVA and Dunnett test), while the 10% treatments increased the MDA contents by 66% and 79% (both P < 0.0001; ANOVA and Dunnett test), respectively. On the other hand, the individual knotweed phenols did not affect the MDA contents, except for a significant decrease with 0.6 mg/mL emodin (by 28%; P = 0.0556; ANOVA and Dunnett test).
Malondialdehyde (MDA) contents (A) and total antioxidative capacities (TAC) (B) in the roots of radish seedlings after 3 days of treatments with knotweed phenols (0.2, 0.6 mg/mL), their mixture (Mix; 0.2 mg/mL of Re and Em, 0.6 mg/mL of Ep) and the F. japonica (FJ) and F. × bohemica (FB) rhizome extracts (1%, 10%). Normalised data are means ± standard error (N = 150). Different letters indicate statistically significant difference between treatments (P < 0.05; one-way ANOVA and Tukey test). Re resveratrol, Ep epicatechin, Em emodin
As shown for cucumber roots exposed to cinnamic acid, increased membrane peroxidation decreases plasma-membrane H+-ATPase activity, which can lead to cell death (Ding et al. 2007). Therefore, it appears that oxidative stress is involved during growth after germination to, thus, inhibit the root growth of these radish seedlings treated with knotweed methanol extracts. As can be seen in the heat map in Fig. 5, the increase in MDA content and suppression of root growth are evident especially for the extract treatments. Similarly, exposure to coffee senna (Senna occidentalis) extracts (da Silva and Vieira 2019) and the coumarins umbelliferone and daphnoretin (Yan et al. 2016) increased lipid peroxidation while suppressing root growth. On the other hand, the present study showed that treatments with the individual phenols (except for 0.6 mg/mL emodin) and their mixture did not affect the MDA levels in these radish seedling roots, again suggesting higher allelopathic potential of the knotweed methanol extracts.
Heat map representing the changes in the different variables in radish seedlings after 3 days of treatments with knotweed phenols (0.2, 0.6 mg/mL), their mixture (Mix; 0.2 mg/mL of Re and Em, 0.6 mg/mL of Ep) and the F. japonica (FJ) and F. × bohemica (FB) rhizome extracts (1%, 10%). Presented are means of normalised data (% of control). Colour code: magenta, decreased value of variable; blue, increased value of variable; white, control value. MDA malondialdehyde, TAC total antioxidative capacity, Re resveratrol, Ep epicatechin, Em emodin
Antioxidants are cellular defence mechanisms against ROS (Das and Roychoudhury 2014). Here, we also monitored TAC as a measure of the levels of non-enzymatic antioxidants in the treated seedlings (Sanchez-Moreno et al., 1998). TAC was indeed a more sensitive marker than MDA (Fig. 4B). Control roots had TAC of 0.54 mM ± 0.03 and the results of treatments with knotweed extracts and phenols were normalised to this value (Fig. 4B). The one-way ANOVA showed that TAC was affected by the treatments (P < 0.0001, df = 10, MS = 297, F = 30.2) and concentration-dependent increase of TAC was observed in all treatments except epicatechin. When compared to the control, 10% extracts of F. japonica and F. × bohemica increased TAC, by 12% and 16% (both P < 0.0001; ANOVA and Dunnett test), respectively. The 1% F. × bohemica extract also significantly increased TAC by 6% (P = 0.0010; ANOVA and Dunnett test), with no significant effect for the 1% F. japonica extract. The greatest increase in TAC was shown in roots treated with 0.6 mg/mL resveratrol (by 22%; P < 0.0001; ANOVA and Dunnett test), with significantly increased TAC also seen for 0.6 mg/mL emodin (by 10%; P = 0.0050; ANOVA and Dunnett test) and the mixture (by 18%; P < 0.0001; ANOVA and Dunnett test).
These increased TAC indicate that these individual phenols can also stimulate some responses in these radish seedlings, although with lower effects, such that the plants can detoxify these negative effects, and thus avoid inhibition of root growth compared to that seen after the treatments with the knotweed methanol extracts. There are also other reports of increased antioxidant activities in plants exposed to allelopathic stress, such as 2-benzoxazolinone (Batish et al. 2006), cinnamic acid (Ding et al. 2007) and the garlic allelochemical diallyl disulphide (Cheng et al. 2016). As an example, an extract of peppermint (Mentha × piperita) increased the activity of the antioxidative enzymes catalase, ascorbate peroxidase and peroxidase in radish seedlings, which are involved in the detoxification of H2O2 (Mahdavikia et al. 2017). In the present study, we show that these ROS accumulated mainly in the roots of the treated seedlings, and especially in the root tips (see below). This is in agreement with our previous findings that aqueous extracts of knotweed rhizomes severely damage the root cap cells of 7-day-old radish seedlings (Šoln et al. 2021b). Therefore, H2O2 as a damaging agent might also be the reason for the shorter roots of the radish seedlings treated with these knotweed methanol extracts and the phenols.
In conclusion, our study shows that the rhizomes of F. japonica and F. × bohemica knotweed contain resveratrol, epicatechin and emodin, with 24% more resveratrol in F. × bohemica than F. japonica. The F. japonica and F. × bohemica methanol extracts and the knotweed phenols increase oxidative stress in the severely suppressed radish roots. The methanol extracts show higher phytotoxic activities against radish seedlings compared to the individual knotweed phenols or to their mixture. The effects of these treatments are mainly evident as reduced seed germination and strong suppression of root growth of the 3-day-old radish seedlings. Moreover, the biochemical parameters of oxidative stress, lipid peroxidation and antioxidants activity, increase in the roots of these treated seedlings. The observed differences between extracts of both knotweed species could be related to higher resveratrol content in F. × bohemica rhizomes. The results of laboratory experimental setup enable studies of the phytotoxic effects on cellular and anatomical level allowing insight to the mechanisms of allelopathy but have limited significance for allelopathic interactions in the field as these interactions are very complex and depend also on the environmental factors like soil composition. Therefore, for further analysis, we propose to conduct field experiments to investigate the effects of knotweed on other plants and rhizosphere microbiome.
References
Araniti F, Costas-Gil A, Cabeiras-Freijanes L, Lupini A, Sunseri F, Reigosa MJ, Abenavoli MR, Sánchez-Moreiras A (2018) Rosmarinic acid induces programmed cell death in Arabidopsis seedlings through reactive oxygen species and mitochondrial dysfunction. PLoS One 13(12):1–26. https://doi.org/10.1371/journal.pone.0208802
Bailey JP, Bímová K, Mandák B (2009) Asexual spread versus sexual reproduction and evolution in Japanese Knotweed s.l. sets the stage for the “battle of the Clones.” Biol Invasions 11(5):1189–1203. https://doi.org/10.1007/s10530-008-9381-4
Batish DR, Singh HP, Setia N, Kaur S, Kohli RK (2006) 2-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol Biochem 44:819–827
Bímová K, Mandák B, Pyšek P (2003) Experimental study of vegetative regeneration in four invasive Reynoutria taxa (Polygonaceae). Plant Ecol 166(1):1–11. https://doi.org/10.1023/A:1023299101998
Burgos NR, Talbert RE, Kim KS, Kuk YI (2004) Growth inhibition and root ultrastructure of cucumber seedlings exposed to allelochemicals from rye (Secale cereale). J Chem Ecol 30(3):671–689
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290(5491):521–523. https://doi.org/10.1126/science.290.5491.521
Chen H, Tuck T, Ji X, Zhou X, Kelly G, Cuerrier A, Zhang J (2013) Quality assessment of Japanese knotweed (Fallopia japonica) grown on Prince Edward Island as a source of resveratrol. J Agric Food Chem 61(62):6383–6392. https://doi.org/10.1021/jf4019239
Cheng F, Cheng ZH, Meng HW (2016) Transcriptomic insights into the allelopathic effects of the garlic allelochemical diallyl disulfide on tomato roots. Sci Rep 6:1–14. https://doi.org/10.1038/srep38902
Cruz-Ortega R, Ayala-Cordero G, Anaya AL (2002) Allelochemical stress produced by the aqueous leachate of Callicarpa acuminata: effects on roots of bean, maize, and tomato. Physiol Plant 116:20–27
da Silva F, Vieira EA (2019) Phytotoxic potential of Senna occidentalis (L.) Link extracts on seed germination and oxidative stress of Ipê seedlings. Plant Biol 21:770–779
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:1–13. https://doi.org/10.3389/fenvs.2014.00053
Ding J, Sun Y, Xiao CL, Shi K, Zhou YH, Yu JQ (2007) Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J Exp Bot 58:3765–3773
Dolenc Koce J, Šoln K (2018) Phytotoxic effects of Fallopia japonica and F. × bohemica leaves. Phyton-Ann Rei Bot 57(1–2):47–58. https://doi.org/10.12905/0380.phyton57-2018-0047
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Fan P, Hay AE, Marston A, Lou H, Hostettmann K (2009) Chemical variability of the invasive neophytes Polygonum cuspidatum Sieb. and Zucc. and Polygonum sachalinensis F. Schmidt ex Maxim. Biochem Syst Ecol 37(1):24–34. https://doi.org/10.1016/j.bse.2008.11.018
Fan P, Hostettmann K, Lou H (2010) Allelochemicals of the invasive neophyte Polygonum cuspidatum Sieb. & Zucc. (Polygonaceae). Chemoecology 20(3):223–227. https://doi.org/10.1007/s00049-010-0052-4
Frantík T, Kovářová M, Koblihová H, Bartůňková K, Nývltová Z, Vosátka M (2013) Production of medically valuable stilbenes and emodin in knotweed. Ind Crops Prod 50:237–243. https://doi.org/10.1016/j.indcrop.2013.07.017
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28(11):1091–1101. https://doi.org/10.1002/bies.20493
Gniazdowska A, Bogatek R (2005) Allelopathic interactions between plants. Multi site action of allelochemicals. Acta Physiol Plant 27:395–407
Imatomi M, Novaes P, Juliano Gualtieri SC (2013) Interspecific variation in the allelopathic potential of the family Myrtaceae. Acta Bot Bras 27(1):54–61
Jaki BU, Bzhelyansky A, Pauli GF (2020) Quantitative NMR (qNMR) for pharmaceutical analysis: The pioneering work of George Hanna at the US FDA. Magn Reson Chem 59(1):7–15. https://doi.org/10.1002/mrc.5099
Lara-Nuñez A, Romero-Romero T, Ventura JL, Blancas V, Anaya AL, Cruz-Ortega R (2006) Allelochemical stress causes inhibition of growth and oxidative damage in Lycopersicon esculentum Mill. Plant Cell Environ 29:2009–2016
Latif S, Chiapusio G, Weston LA (2017) Allelopathy and the role of allelochemicals in plant defence. Adv Bot Res. https://doi.org/10.1016/bs.abr.2016.12.001
Lowe S, Browne M, Boudjelas S, De Porter M (2000) 100 of the world’s worst invasive alien species. A selection from the global invasive species database. The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), New Zealand, p 12
Lupini A, Araniti F, Sunseri F, Abenavoli MR (2014) Coumarin interacts with auxin polar transport to modify root system architecture in Arabidopsis thaliana. Plant Growth Regul 74(1):23–31
Mahdavikia F, Saharkhiz MJ, Karami A (2017) Defensive response of radish seedlings to the oxidative stress arising from phenolic compounds in the extract of peppermint (Mentha × piperita L.). Sci Hortic 214:133–140
Moravcová L, Pyšek P, Jarošik V, Zákravský P (2011) Potential phytotoxic and shading effects of invasive Fallopia (Polygonaceae) taxa on the germination of native dominant species. NeoBiota 9:31–47. https://doi.org/10.3897/neobiota.9.1266
Murrell C, Gerber E, Krebs C, Parepa M, Schaffner U, Bossdorf O (2011) Invasive knotweed affects native plants through allelopathy. Am J Bot 98(1):38–43. https://doi.org/10.3732/ajb.1000135
Pauli GF, Jaki BU, Lankin DC (2005) Quantitative 1H NMR: development and potential of a method for natural products analysis. J Nat Prod 68(1):133–149. https://doi.org/10.1021/np0497301
Sánchez-Moreno C, Larrauri JA, Saura-Calixto F (1998) A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76(2):270–276
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Šerá B (2012) Effects of soil substrate contaminated by knotweed leaves on seed development. Pol J Environ Stud 21(3):713–717
Shixing Z, Xunzhi Z, Kai S, Caixia H, Kuchkarova N, Chi Z, Hua S (2021) Chemical composition and allelopathic potential of the invasive plant Solanum rostratum Dunal essential oil. Flora 274(151730):1–5. https://doi.org/10.1016/j.flora.2020.151730
Siemens TJ, Blossey B (2007) An evaluation of mechanisms preventing growth and survival of two native species in invasive Bohemian knotweed (Fallopia × bohemica, polygonaceae). Am J Bot 94(5):776–783
Simmler C, Napolitano JG, McAlpine JB, Chen S, Pauli GF (2014) Universal quantitative NMR analysis of complex natural samples. Curr Opin Biotechnol 25:51–59. https://doi.org/10.1016/j.copbio.2013.08.004
Šoln K, Likar M, Dolenc Koce J (2021a) Effects of rhizome extracts from invasive knotweed species Fallopia japonica and F. ×bohemica on radish seed germination and root growth of seedlings. Allelopathy J 52(1):103–118
Šoln K, Žnidaršič N, Dolenc Koce J (2021b) Root growth inhibition and ultrastructural changes in radish root tips after treatment with aqueous extracts of Fallopia japonica and F. ×bohemica rhizomes. Protoplasma 259:343–355. https://doi.org/10.1007/s00709-021-01668-4
Soltys D, Rudzińska-Langwald A, Kurek W, Gniazdowska A, Sliwinska E, Bogatek R (2011) Cyanamide mode of action during inhibition of onion (Allium cepa L.) root growth involves disturbances in cell division and cytoskeleton formation. Planta 234(3):609–621. https://doi.org/10.1007/s00425-011-1429-5
Strgulc Krajšek S, Dolenc Koce J (2015) Sexual reproduction of knotweed (Fallopia sect. Reynoutria) in Slovenia. Preslia 87:17–30
Tucker Serniak L (2016) Comparison of the allelopathic effects and uptake of Fallopia japonica phytochemicals by Raphanus sativus. Weed Res 56(2):97–101. https://doi.org/10.1111/wre.12199
Uddin MR, Park SU, Dayan FE, Pyon JY (2014) Herbicidal activity of formulated sorgoleone, a natural product of sorghum root exudate. Pest Manag Sci 70:252–257
Vrchotová N, Šerá B (2008) Allelopathic properties of knotweed rhizome extracts. Plant Soil Environ 54(7):301–303. https://doi.org/10.17221/420-pse
Weir TL, Park SW, Vivanco JM (2004) Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol 7(4):472–479. https://doi.org/10.1016/j.pbi.2004.05.007
Xiao K, Xuan L, Xu Y, Bai D, Zhong D (2002) Constituents from Polygonum cuspidatum. Chem Pharm Bull 50(5):605–608
Yamada M, Han X, Benfey PN (2020) RGF1 controls root meristem size through ROS signalling. Nature 50(5):605–608. https://doi.org/10.1038/s41586-019-1819-6
Yan Z, Wang D, Cui H et al (2016) Phytotoxicity mechanisms of two coumarin allelochemicals from Stellera chamaejasme in lettuce seedlings. Acta Physiol Plant 38(248):1–10. https://doi.org/10.1007/s11738-016-2270-z
Zhu W, Liu J, Ye J, Li G (2017) Effects of phytotoxic extracts from peach root bark and benzoic acid on peach seedlings growth, photosynthesis, antioxidance and ultrastructure properties. Sci Hortic 215:49–58
Acknowledgements
The authors are grateful to the staff of The Centre for Research Infrastructure at the Faculty of Chemistry and Chemical Technology (IC UL FCCT). The authors are also thankful to Dr. Maks Merela and his co-workers for rhizome grindings, and Dr. Chris Berrie for critical reading of the manuscript and English language editing.
Funding
This study was financially supported by the Slovenian Research Agency (Grant nos. P1-0212 and P1-0134).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Communicated by Günther Raspotnig.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Šoln, K., Horvat, M., Iskra, J. et al. Inhibitory effects of methanol extracts from Fallopia japonica and F. × bohemica rhizomes and selected phenolic compounds on radish germination and root growth. Chemoecology 32, 159–170 (2022). https://doi.org/10.1007/s00049-022-00375-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-022-00375-7