Abstract
Alzheimer’s disease (AD) is a multifactorial incurable neurodegenerative disorder. To date, cholinesterase inhibitors (ChEI) are the mainstay line of treatment to ameliorate the symptoms of AD. Tacrine and donepezil are considered two important cornerstones of anti-dementia drugs. Accordingly, novel series of hexahydrobenzothienocyclopentapyridines, octahydrobenzo-thienoquinolines, hexahydrocyclopenta(thienoquinoline/thienodipyridine), and octahydropyrido-thienoquinolines were efficiently synthesized from readily available reagent, e.g. cyclohexanones, cyclopentanone, and 1-methyl-piperidin-4-one to afford 14 new compounds. All new compounds were screened against their acetylcholinesterase, butyrylcholinesterase, and β-amyloid protein inhibition. In AChE inhibition assay, compound 3,7-dimethyl-1,2,3,4,7,8,9,10-octahydrobenzo[4,5]thieno[2,3-b]quinolin-11-amine (2h) showed IC50 value 9.24 ± 0.01 μM × 10−2 excelling tacrine. Compound 1,7-dimethyl-1,2,3,4,7,8,9,10-octahydrobenzo[4,5]thieno[2,3-b]quinolin-11-amine (2e) possess excellent IC50 values 0.58 ± 0.02 μM × 10−2 and 0.51 ± 0.001 μM × 10−4 for both butyrylcholinesterase and β-amyloid protein inhibition assays, sequentially. In silico ADME studies were investigated for the promising members (octahydrobenzo-thienoquinolines 2c, 2d, 2e, 2h, 2i, and octahydropyrido-thienoquinolines 4e) and all the results were illustrated. A comparative docking study was conducted between the promising members and both tacrine and donepezil in both acetyl and butyryl choline active sites. The results revealed extra binding patterns and good agreement with the biological results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
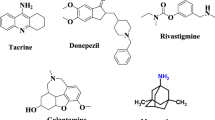
Alzheimer’s disease (AD) is an irreversible progressive neurodegenerative multi-hitting disease. It affects mostly elderly people leading to a gradual loss of memory, impairment of cognitive abilities, speech difficulties, severe behavioral disturbance, and ultimately death. It is estimated that over 50 million people worldwide are living with dementia with the expected increase in the number up to 152 million by 2050 [1]. The consequent high social and economic complications are demonstrated in Morbidity and Mortality 2022 Report [2]. Consequently, the current attention was directed towards the development of new members able to treat or at least slow down the progression of such a multifactorial disease [3]. Nevertheless, the uncertainty of the definite cause of (AD) entangles the scientists’ mission [4, 5]. For this reason, several hypotheses including the cholinergic hypothesis, amyloid cascade hypothesis, tau protein hypothesis, oxidative stress hypothesis, bio-metals dyshomeostasis hypothesis, innate immune hypothesis, Osaka mutation, and others have been proposed in an attempt to understand and explain the disease real pathogenesis [6,7,8]. From the cholinergic hypothesis point of view [9, 10], elevating the essential neurotransmitter acetylcholine (ACh) levels through simultaneous inhibition of both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) has great benefits in dealing with the disease [11, 12]. The AChE and BuChE are two hydrolytic enzymes responsible for the termination of ACh action at the synapse by cleaving Ach to choline and acetate moiety. The two enzymes are very similar in structure and showing 65 identities in amino acids sequence [13]. AChE is found in many types of conducting tissue: nerve and muscle, central and peripheral tissues, motor and sensory fibers. Meanwhile, BuChE, also known as plasma cholinesterase, is found primarily in the liver. Different from AChE, BuChE hydrolyzes butyrylcholine more quickly than ACh [14]. The activity of AChE is higher in motor neurons than in sensory neurons. Accordingly, acetylcholinesterase inhibitors (AChEI) were the pioneer hitting tools for the management of AD by inhibiting AChE. Thus, an increase in ACh bioavailability at neurons synapse causes an improvement in the neuronal transmission in the brain and terminally enhancing memory functions of AD patients [15]. On the other hand, the amyloid hypothesis deals with the abnormal aggregation and deposition of amyloid-β-proteins (AβP) in the brain as the causative agent of AD pathogenesis [7, 16]. The genetic mutations in the membrane amyloid precursor protein (APP) gene results in a variation of the APP sequence cleavage producing neurotoxic insoluble amyloid β peptides [17,18,19]. These insoluble peptides aggregate to form polymorphic oligomers and ultimately macroscopic plaques that deposit at neurons causing inflammation, neuron injury, disruption of Ca2+ homeostasis, disturbances of synapse signaling, neuron loss, and terminal neuron death [20]. Accordingly, a series of anti-Aβ immunotherapies have been conducted to clear the cerebral Aβ deposition, including active Aβ immunization with target antigens and passive immunization via direct administration of Aβ-specific antibodies [21]. More recently, in 2021, the FDA approved Aduhelm® (Aducanumab), the first putative disease-modifying therapy (DMT) approved for AD. Aducanumab is an amyloid β directed human monoclonal antibody able to reduce brain Aβ plaques as measured by PET-imaging studies [22]. Up to the present moment, no effective treatment for AD disease for curing and arresting the disease progression [23, 24]. Tacrine or 9-amino-1,2,3,4-tetrahydroaminoacridine (THA) was the first AChEI approved by the Food and Drug Administration (FDA) in 1993 and was launched on the market under the brand name of cognex® [25]. THA is a centrally acting non-selective competitive inhibitor of both AChE and BChE and also modulates nicotinic receptors [4, 26]. Unfortunately, it was withdrawn in 2013 shortly after its release due to its hepatotoxicity [27, 28]. In 2006, Donepezil (Aricept®) has been licensed by the US Food and Drug Administration (FDA) for the symptomatic treatment of AD. It is worth mentioning that donepezil (Aricept®) is sighted as a potent and selective piperidine AChEI derivative possessing a dual-acting anti-Alzheimer mode with safer margins [29,30,31].
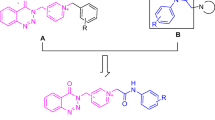
Although relying only on the cholinergic hypothesis is not logical [29], both tacrine and donepezil are still considered cornerstones in the development of new, effective and multi-targeting members [32,33,34,35,36]. Accordingly, many tacrine analogs have been introduced (Fig. 1). The 7-methoxy tacrine I (7-MEOTA) exhibited a good AChE inhibitory profile with limited hepatotoxicity due to its different metabolic clearance pathway compared to THA [37]. Furthermore, introducing different substituents in the 6 and/or 7 positions as in the case of 6-chlorotacrine II improved both the AChE inhibitory profile and selectivity [38]. Meanwhile, replacing the benzene ring in tacrine structure with different heterocyclic fused ring systems to afford tetracyclic structure, III [39], IV, V [40], and VI [41] has proven to be a promising direction. In continuation to modify and uncover new tacrine analogs, our team sketched this piece of work. More specifically, the thiophene nucleus is considered an important pharmacophore in variant bioactive molecules where thiophene derivatives show a wide range of biological activities, such as anti-Alzheimer, anti-inflammatory, anti-fungal, anti-depressant, anti-malarial, anti-cancer, anti-hyperglycemic, and more [42]. Depending on the above-mentioned facts, the sight was drawn toward discovering new thiophene derivatives as anti-Alzheimer agents. Taking into our consideration that thiophene is bioisosteric of benzene; we planned to synthesize tacrine analogs where the benzene ring in tacrine structure (ring A) is replaced by different cycloalkyl thiophenes while keeping intact the central key-binding core “4-amino pyridine”. Besides, the cyclohexane fused ring in tacrine structure (ring B) is preserved or replaced either by five-membered or six-membered cycloalkanes (Fig. 2). The newly designed compounds were successfully synthesized. Their acetylcholinesterase, butyrylcholinesterase and β-amyloid protein IC50 inhibition assays were also investigated and compared with both tacrine and donepezil as reference standards.
Results and discussion
Chemistry
Novel series of hexahydrobenzothienocyclopentapyridines, octahydrobenzothienoquinolines, hexahydrocyclopenta(thienoquinoline/thienodipyridine), and octahydropyridothienoquinolines were efficiently synthesized and illustrated in Schemes 1 and 2. The synthesis of target tacrine derivatives 2a–i and 4a–e was prepared in two steps. Initially, preparation of substituted thiophene intermediates 1a–c, 3a, and 3b via one-pot Gewald’s reaction of starting cycloalkanone a–e, with malononitrile, morpholine, and sulfur in absolute ethanol as a solvent. The target structures 2a–i and 4a–e were prepared through Lewis’s acid-catalyzed Friedlander reaction between the obtained thiophene derivatives 1a–c or 3a,b, and different cyclic ketones. It is worth mentioning that anhydrous zinc chloride was used as a Lewis acid [43]. Friedlander reaction [44] provides high yield and rapid access to synthesize the target tacrine products via Lewis acid-mediated cyclodehydration of various aminocyano derivatives with various cyclic ketones [45].
The proposed mechanism [46, 47] for the Friedlander reaction is illustrated in Fig. 3. The reaction mechanism for compound 2d synthesis is taken as a good example where the thiophene intermediate (1b) reacted with the cyclohexanone (1) in the presence of ZnCl2 as a Lewis acid This mechanism involves three steps: (i) The initial addition of the amino group of compound (1b) to the carbonyl carbon of cyclohexanone (1) forming the amino alcohol intermediate A. (ii) Water loss and formation of the imine intermediate B, which tautomerizes to the enamino form C. (iii) The intramolecular aldol-like cyclo condensation with the cyano group forming compound 2d through intermediate D. Accordingly, the large Friedlander annulation is the simplest rapid access method for the synthesis of target tacrine analogs. Meanwhile, the changes in the physical properties of new compounds from the start were considered a good sign of the success of the reaction. This includes melting points, altered behavior towards crystallization solvents, and physical appearance. Although the required reaction time to synthesize all new target compounds was 8–10 h, their yield varied from 60% to 84% according to the position of substituents and ring size. For example, octahydrobenzo-thienoquinolines as in compounds 2g, 2h, and 2i achieved a higher yield than that of octahydrobenzothienoquinolines as in compounds 2c, 2d, 2e, and 2f. So, the yield was improved by the presence of methyl substituent in the ring (A) at position 3 than that presented at position 1. Also, hexahydrobenzothienocyclopentapyridines as in compound 2a achieved the highest yield (84%) may be due to the small ring size of cyclopentanone. Finally, octahydropyrido-thienoquinolines as in compounds 4c, 4d, and 4e achieved a high yield may be due to the presence of N-CH3 substituent in the ring (A). Meanwhile, the IR spectrum of the newly synthesized compounds revealed the absence of the cyano group absorption band at 2188–2195 cm−1. Regarding the 1H NMR spectra, both series 2 and 4 showed a singlet signal of (NH2) protons with accurate integration and D2O-exchangeable behavior in their spectra. Moreover, the presence of seven peaks in the aromatic region of their 13C NMR spectra confirms the formation of thienopyridine core. Regarding the 1H NMR spectra of compounds 2a, 2b, 2d, and 2g, they all exhibited significant doublet peaks at the range of δ 1.03–1.21 ppm corresponding to the 3 protons of (CH3) group. On the other hand, compounds 2e, 2f, 2h, and 2i showed a two doublet/or multiplet peaks equivalent to two (CH3) groups at the range of δ 1.04–1.27 ppm. Nevertheless, the change in the environment around the protons easily affects the 1H NMR shifts. Compounds 4b, 4c, 4d, and 4e revealed the presence of a singlet peak corresponding to the (N-CH3) protons at δ 2.89, 2.36, and 2.35 ppm, respectively. Both 4d and 4e revealed the presence of an additional doublet peak of (CH3) protons at δ 1.25 and 1.24 ppm, sequentially.
Pharmacology
The target compounds in Schemes 1 and 2 were evaluated for their AChE, BuChE, and Aβ inhibitory activity. Tacrine and donepezil were assigned as the reference drugs of choice. Table 1 discloses the in-vivo activity results in terms of IC50 ± standard deviation (SD). In light of our findings, the synthesized compounds showed a wide range of AChE inhibition (IC50 = 9.20 ± 0.02–18.43 ± 0.02 μM × 10−2) compared to tacrine and donepezil as reference standards (IC50 = 18.46 ± 0.03 μM × 10−2 and 12.94 ± 0.02 μM × 10−2), respectively. All Compounds except 2a, 2b, 2f, 2g, 4a, 4b, and 4c displayed an inhibitory effect on the AChE enzyme better than donepezil, while all compounds showed better inhibitory effects on the same enzyme than tacrine. Accordingly, compounds 2h, 2i, 4d, and 4e showed the best inhibitory effect on the AChE enzyme and excelled both tacrine and donepezil as a reference standard. Furthermore, the synthesized compounds showed a wide range of BuChE inhibition (IC50 = 0.58 ± 0.02–2.02 ± 0.01 μM × 10−2) compared to tacrine and donepezil (IC50 = 2.50 ± 0.03 μM × 10−2 and 0.93 ± 0.01 μM × 10−2), respectively. All compounds except 2a, 2b, 2f, 2g, 4a, 4b, and 4c displayed an inhibitory effect on the BuChE enzyme than donepezil whereas all compounds tested showed a better effect on the same enzyme than tacrine. The concentration of 2i required to inhibit 50% of the BuChE enzyme is almost like that of donepezil (IC50 = 0.93 ± 0.01 μM). Accordingly, compounds 2c, 2d, 2e, 2h, 2i, and 4e showed the best inhibitory effect on the BuChE enzyme and excelled both tacrine and donepezil as reference standards. Besides, the synthesized compounds showed a wide range of Aβ inhibition (IC50 = 0.51 ± 0.001–1.04 ± 0.002 μM × 10−4) compared to tacrine and donepezil as reference standards (IC50 = 0.95 ± 0.003 μM × 10−4 and 0.61 ± 0.002 μM × 10−4), respectively. Compounds 2c, 2d, 2e, 2h, 2i, and 4e resulted in more decrement of Aβ compared to both tacrine and donepezil, as the concentration of these compounds required to decrease 50% of Aβ was less than that of both reference drugs. Concisely, Compounds 2c, 2d, 2e, 2h, 2i, and 4e excelled tacrine and donepezil against all the activities as illustrated in Fig. 4.
Structure–activity relationship (SAR)
As illustrated (Fig. 4), Compounds 2c, 2d, 2e, 2h, 2i, and 4e excel tacrine and donepezil against all the activities. The close analysis of the structures of these potent compounds revealed that all potent compounds possessing cyclohexyl rings in both rings A and B (Fig. 2). So, the six-membered ring in both rings A and B is the optimum ring size. Moreover, the replacement of either benzene ring or cyclohexyl moieties of tacrine by a smaller size ring (cyclopentyl ring) led to less inhibitory activity in all assays as in compounds 2a, 2b, 4a, and 4b. Besides, the replacement of benzene ring with thiophene one is not only maintaining the activity but also improving the IC50 values. Regarding the substituents effect, it was found that substitution on both rings A and B was also beneficial to the activity regardless of their position as in compounds 2e, 2h, 2i, and 4e. On the other hand, the monosubstitution on either one of the rings did not show better selective activity as in case of 2a, 2b, 4a, 4b, and 4c. For more evidence on our previously mentioned SAR, Saeedi et al. reported the synthesis of similar tacrine analogs and concluded that cycloalkyl substituents apparently played a more important role in AChE inhibition as mostly synthesized compounds possessing cyclohexyl moiety depicted a better inhibitory activity.
Physicochemical in silico ADME study and pharmacokinetic properties’ prediction
The results from the Swiss ADME predictor showed that compounds (2c, 2d, 2e, 2h, 2i, and 4e) exhibited an anticipated log P value of 4.00, 4.00, 4.28, 4.25, 4.18, and 3.06, respectively. Moreover, they all exhibited moderate water solubility, and high GIT absorption with expected high oral bioavailability. The good values of blood–brain barrier (BBB) permeability assign their efficient ability to reach and bind to their central receptor active site which is quite essential for their biological efficacy. In accordance, the Swiss ADME web tool simply predicts the drugs bioavailability through the bioavailability radar graph where six physicochemical properties are taken into consideration: lipophilicity (LIPO), size (SIZE), polarity (POLAR), solubility (INSOLU), flexibility (FLEX), and saturation (INSATU). The pink colored zone indicates the suitable physicochemical space for oral bioavailability and in which the radar plot of the drug must fall entirely to be considered a bioavailable drug. All six compounds demonstrated a promising bioavailability radar where each compound radar plot entirely falls in the pink-colored zone that indicates good bioavailability. Furthermore, the BOILED-Egg model produces a rapid way to predict two crucial pharmacokinetic parameters namely human gastrointestinal absorption (HIA) and blood–brain barrier (BBB) permeability as a function of the position of the small molecules in the WLOGP (lipophilicity)-versus-TPSA (polarity) graph. The Boiled-Egg model demonstrates two regions, the white region is the space of the molecules with a greater extent of GIT absorption and the yellow region (yolk) is the space for highly probable BBB permeation. Yellow and white regions are not mutually exclusive. It is worthy to note that all six compounds investigated were classified in the physiochemical area closely like that of the tacrine reference drug (Fig. 5). They showed high gastrointestinal absorption and easily permeated blood–brain barrier. All compounds were found to be a PGP substrate means there would be an issue in the excretion of the drug. The bioavailability radar graph and boiled egg model for six compounds are illustrated in the supplementary data file (Figs. Bio. S1–Bio. S4). On the other hand, the drug-likeness generated in accordance with the major pharmaceutical companies; Lipinski (Pfizer) [48], Ghose’s (Amgen) [49], Veber’s (GSK) [50], Egan’s (Pharmacia) [51], and Muegge’s (Bayer) [52] filters support their promising bioavailability. Briefly, the six potent compounds exhibited a promising biological efficiency with hopeful pharmacokinetic properties towards their computational results of the physicochemical and pharmacokinetic properties study. The results obtained from the Swiss ADME tool are all summarized in Table 2.
Molecular docking study
In the aim of unwrapping the various ligand interactions with the specific enzymes’ active sites and to identify the molecular features responsible for the recorded biological activity, a brief in silico investigation was conducted. It is worth mentioning that the key interactions reproduced by the co-crystallized ligand tacrine with both AChE and BChE active sites are illustrated in the supplementary data file (refer to Fig. Bio.S5). Accordingly, the interaction between the ligand tacrine with the active-site gorge of AChE showed π–π interaction of its phenyl and pyridine rings with the phenyl ring of Phe-330. Also, both rings showed interaction with the five-membered ring of indole of Trp-84. Furthermore, the amino group of tacrine displayed a water-mediated H-bond with Asp-72, Ser-81, and Ser-122. On the other hand, the interaction between ligand tacrine with the active site of BChE showed π–π interaction of its phenyl and pyridine rings with Trp-A82. The amino group of tacrine displayed a water-mediated H-bond with Asp-A70, Ser-A79, and Thr-A120. Meanwhile, a molecular docking study using tacrine as a reference ligand was performed for the most potent newly synthesized compounds (2c, 2d, 2e, 2h, 2i, and 4e) to explore their possible binding modes with both targets (AChE and BChE) and to justify their biological potency. Default docking parameters were set and 13 different docking conformations were carried out for each compound/target.
AChE and BuChE molecular docking simulation study adopting tacrine as a reference ligand
AChE molecular docking findings
Compounds 2c and 2d showed a comparable binding pattern in the binding site of AChE with a predicted docking energy score of −12.5225 and −12.2435 kcal/mol, respectively (cf. −10.8740 kcal/mol for tacrine). Docking conformation of both 2c and 2d showed π–π interaction of their pyridine ring with the phenyl ring of Phe-330. In addition, their thiophene and pyridine rings showed interaction with the five-membered ring of indole of Trp-84. The amino group of compounds 2c and 2d displayed water-mediated H-bond with Ser-81, Asp-72, and Ser-122. Furthermore, the combined conformational superposition of tacrine with compounds 2c and 2d showed that the hydrophilic and hydrophobic parts nearly overlapped with each other. Thus, both compounds are bound to the active-site gorge of AChE with orientation and position like that seen from the x-ray crystal structure of the tacrine–AChE complex as illustrated in the supplementary data file (Fig. Bio.S6). Regarding 2e and 2h, their docking energy scores were −12.9183 and −12.8109 kcal/mol, respectively. The binding interaction patterns of both compounds in the binding site of AChE were almost like that of compounds 2c and 2d.
Finally, the docking energy score for compound 2i was −12.4504 kcal/mol with a similar binding interactions pattern with AChE active site gorge as the previously mentioned docked compounds. On the other hand, the molecular docking study of 4e exhibited an extra different cation–π interaction between the protonated nitrogen of its tetrahydrothienopyridine ring and the indole ring of Trp-432 in the AChE active site gorge with a docking energy score −12.8568 kcal/mol. To sum up, the main common feature is the interaction of the aromatic rings of tacrine and the newly synthesized compounds with the phenyl ring of Phe-330 and the indole ring of Tyr-84 of the AChE active site. The amino pyridine core is an important pharmacophore since the amino group forms water-mediated H-bond with AChE active site. The absence of this amino group leads to a loss of biological activity. The replacement of the benzene ring by the tetrahydrothienopyridine ring as in compound 4e is a promising direction due to its ability to form an extra cation–π interaction with AChE active site that agreed with the biological results. Finally, molecular-docking simulation of the six compounds in the AChE active site showed good agreement with the obtained pharmaco-biological results. In accordance, the superimposition of both compounds 2e and 4e with the ligand tacrine were demonstrated in Fig. 6 as an illustrative example of the mode of docking explained above.
BuChE molecular modeling findings
The molecular docking study of the most potent newly synthesized compounds (2c, 2d, 2e, 2h, 2i, and 4e) was carried into the active site of the BChE target using tacrine as a reference ligand. Compounds (2c, 2d, 2e, and 2i) showed a comparable binding mode with an active site of BChE with docking energy scores of −11.6155, −11.4758, −11.0447, and −12.1545 kcal/mol, respectively in comparison to the tacrine binding score of −10.5068 kcal/mol. The similarity of the docking conformation of compounds 2c, 2d, 2e, and 2i with tacrine was seen in the π–π interactions of their phenyl and pyridine ring with Trp-A82. The amino groups of 2c, 2e, and 2i displayed water-mediated H-bond with Asp-A70, Ser-A79, and Thr-A120 while the amino group of 2d displayed water-mediated H-bond with only Asp-A70, Ser-A79 residues. The conformational superposition of tacrine with compounds 2c, 2d, 2e, and 2i showed comparable overlapping with orientation and position like that seen from the x-ray crystal structure of the tacrine–BuChE complex. Regarding compounds 2h and 4e, their docking energy score was −11.7471 and −15.0700 kcal/mol. The interaction of both compounds in the binding site of BuChE showed a binding pattern that differs from the other docked compounds. The amino group of 2h displayed a water-mediated H-bond with only Thr-A120. Besides, its phenyl and pyridine rings showed cation–π interaction with His-A438. Regarding compound 4e, its amino group showed interaction with His-A438 while its phenyl and pyridine rings showed π–π interaction with Trp-A82. Furthermore, compound 4e showed an extra different interaction between protonated nitrogen of thienopyridine ring and Glu-A197 The interaction of compound 2i is taken as a good example (Fig. 7) while the rest of the interactions are illustrated in the supplementary data file (Figs. Bio.S8 and Bio.S9).
Experimental
All reagents and solvents were used without further purification. All the recorded melting points were taken in an open glass capillary on a Griffin apparatus and the values given were uncorrected. Elemental analyses were carried out using FLASH 2000 CHNS analyzer, Thermo Scientific (USA) at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University. C, H, N, and S analysis values were accepted within a range of ±0.4% of theoretically calculated percentages. IR spectra were determined using potassium bromide disks and values were represented in cm−1. IR spectra were recorded on the Shimadzu IR 435 spectrophotometer (Shimadzu Corp., Kyoto, Japan) Faculty of Pharmacy, Cairo University. 1H NMR spectra were carried out on Bruker 400 MHz (Bruker Corp., Billerica, MA, USA) spectrophotometer, Faculty of Pharmacy, Cairo University. Chemical shifts were recorded in ppm on δ scale, coupling constants (J) were given in Hz and peak multiplicities are designed as follows: s, singlet; d, doublet; dd, doublet of doublet; t, triplet; q, quartet; qui, quintet; m, multiplet. 13C NMR spectra were carried out on Bruker 100 MHz spectrophotometer, Faculty of Pharmacy, Cairo University. The reaction progress was monitored by TLC using aluminum sheets precoated with UV fluorescent silica gel (MERCK 60F 254), and spots were visualized using UV Lamp. The solvent system used was methanol, ethyl acetate, and toluene with the ratio 1:2:3. The respectively used ketones (a–e) were commercially available from Acros Organic Company.
2-Amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbonitrile 1a
Yield: 86%; M.P. 147–148 °C. The compound was prepared according to the reported procedure [53].
2-Amino-4-methyl-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbonitrile 1b
Yield: 43%; M.P. 126–128 °C. The compound was prepared according to the reported procedure [54].
2-Amino-6-methyl-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbonitrile 1c
Yield: 81%; M.P. 117–188 °C. The compound was prepared according to the reported procedure [54].
General procedure for preparation of compounds 2a–i
The respective thiophenes 1a–c (0.01 mol) were mixed with the appropriate ketones (10 mL) and anhydrous zinc chloride (0.01 mol). The mixture was refluxed for 8–10 h. The resulting solid was filtered, washed with water, and crystallized from the appropriate solvent [40].
7-Methyl-2,3,6,7,8,9-hexahydro-1H-benzo[4,5]thieno[2,3-b]cyclopenta[e]pyridin-10-amine (2a)
Yield: 84%; M.P. <300 °C; (water); IR (cm−1): 3460, 3356 (NH2), 2943, 2823 (CH aliphatic), 1624 (C=N); 1H NMR (DMSO-d6) δ: 1.03 (d, J = 8.0 Hz, 3H, CH3, at C7), 1.38–1.45 (m, 1H, CH, C7), 1.86 (d, J = 12.0 Hz, 2H, CH2, C6), 1.98–2.06 (qui, J = 8.0 Hz, 2H, CH2, C2), 2.29–2.35 (m, 1H, CH, C8), 2.70 (t, J = 8.0 Hz, 2H, CH2, C9), 2.76 (d, J = 8.0 Hz, 1H, CH, C8), 2.79 (t, J = 8.0 Hz, 2H, CH2, C1), 2.96–3.07 (m, 2H, CH2, C3), 5.65 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 161.4 (C3a, C=N), 159.3 (C10, C=C), 145.7 (C4a, C=C), 128.2 (C5a, C=C), 126.0 (C9b, C=C), 116.9 (C9a, C=C), 115.3 (C10a, C=C), 34.4 (C3, CH2), 32.7 (C8, CH2), 30.0 (C7, CH), 27.9 (C6, CH2), 26.7 (C1, CH2), 25.2 (C2, CH2), 21.9 (CH3, at C7), 20.7 (C9, CH2); Anal. Calcd. for C15H18N2S (258.38): C, 69.73; H, 7.02; N, 10.84; S, 12.41; Found: C, 69.94; H, 7.18; N, 11.07; S, 12.54.
9-Methyl-2,3,6,7,8,9-hexahydro-1H-benzo[4,5]thieno[2,3-b]cyclopenta[e]pyridin-10-amine (2b)
Yield: 60%; M.P. 188 °C; (aqu. ethanol); IR (cm−1): 3502, 3402 (NH2), 2935, 2839 (CH aliphatic), 1612 (C=N); 1H NMR (DMSO-d6) δ: 1.21 (d, J = 8.0 Hz, 3H, CH3, at C9), 1.69–1.88 (m, 4H, 2CH2, C7,C8), 1.99–2.07 (qui, J = 8.0 Hz, 2H, CH2, C2), 2.66–2.75 (m, 4H, 2CH2, C6, C1), 2.78 (t, J = 8.0 Hz, 2H, CH2, C3), 3.39–3.41(broad m, 1H, CH, C9), 5.60 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 161.1 (C3a, C=N), 159.8 (C10, C=C), 144.7 (C4a, C=C), 131.7 (C5a, C=C), 128.1(C9a, C=C), 116.0 (C9b, C=C), 115.4 (C10a, C=C), 33.2 (C3, CH2), 28.9 (C8, CH2), 28.0 (C9, CH), 26.7 (C1, CH2), 24.7 (C6, CH2), 21.8 (C2, CH2), 21.7 (C7, CH2), 16.9 (CH3, at C9); Anal. Calcd. for C15H18N2S (258.38): C, 69.73; H, 7.02; N, 10.84; S, 12.41; Found: C, 69.87; H, 6.97; N, 11.06; S, 12.49.
7-Methyl-1,2,3,4,7,8,9,10-octahydrobenzo[4,5]thieno[2,3-b]quinolin-11-amine (2c)
Yield: 66%; M.P. 203 °C; (water); IR (cm−1): 3506, 3302 (NH2), 2931, 2839 (CH aliphatic), 1631 (C=N); 1H NMR (DMSO-d6) δ: 1.23–1.25 (m, 3H, CH3, at C7), 1.47–1.53 (m, 1H, CH, C8), 1.67–1.71 (m, 1H, CH, C8), 1.77–1.90 (m, 6H, 3CH2, C3,C2, C9), 2.43 (t (appearing as a broad s), 2H, CH2,C4), 2.69 (t (appearing as a broad s), 2H, CH2, C10), 2.79–2.85 (m, 1H, CH, C7), 2.95 (t (appearing as a broad s), 2H, CH2, C1), 5.47 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 157.5 (C6a, C=N), 156.4 (C11, C=C), 147.1 (C5a, C=C), 128.9 (C4a, C=C), 126.3 (C11a, C=C), 116.5 (C11b, C=C), 109.9 (C10a, C=C), 34.9 (C7, CH), 30.2 (C8, CH2), 25.6 (C10, CH2), 24.7 (C4, CH2), 23.1 (C1, CH2), 21.9 (C2, CH2), 21.8 (C3, CH2), 20.7 (CH3, at C7), 19.2 (C9, CH2); Anal. Calcd. for C16H20N2S (272.41): C, 70.55; H, 7.40; N, 10.28; S, 11.77; Found: C, 70.69; H, 7.47; N, 10.51; S, 11.86.
1-Methyl-1,2,3,4,7,8,9,10-octahydrobenzo[4,5]thieno[2,3-b]quinolin-11-amine (2d)
Yield: 66%; M.P. 186 °C (aqu. ethanol); IR (cm−1): 3444, 3332 (NH2), 2935, 2862 (CH aliphatic), 1631 (C=N); 1H NMR (DMSO-d6) δ: 1.17 (d, J = 8.0 Hz, 3H, CH3, at C1), 1.77–1.85 (m, 8H, 4CH2, C2,C3,C8,C9), 2.69–2.83 (m, 4H, 2CH2, C4,C10), 3.09–3.11 (m, 1H, CH, C1), 3.56 (t (appearing as a broad s), 2H, CH2, C7), 7.00 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 152.0 (C6a, C=N), 149.7 (C11, C=C), 147.9 (C5a, C=C), 133.1 (C4a, C=C), 131.4 (C11a, C=C), 117.6 (C11b, C=C), 112.1 (C10a, C=C), 29.3(C2, CH2), 28.55 (C1, CH), 28.5 (C7, CH2), 25.3 (C10, CH2), 23.0 (C4, CH2), 22.3 (C3, CH2), 21.8 (C9, CH2), 21.2(C8, CH2), 17.6 (CH3, at C1); Anal. Calcd. for C16H20N2S (272.13): C, 70.55; H, 7.40; N, 10.28; S, 11.77; Found: C, 70.73; H, 7.52; N, 10.43; S, 11.90.
1,7-Dimethyl-1,2,3,4,7,8,9,10-octahydrobenzo[4,5]thieno[2,3-b]quinolin-11-amine (2e)
Yield: 65%; M.P. 191 °C; (aqu. ethanol); IR (cm−1): 3322, 3221 (NH2), 2935, 2866 (CH aliphatic), 1631 (C=N); 1H NMR (DMSO-d6) δ: 1.21–1.27 (m, 6H, 2CH3, at C1,C7),1.46–1.55 (m, 1H, CH, C1), 1.68–1.89 (m, 8H, 4CH2, C2,C3,C8,C9), 2.44–2.46 (m, 2H, CH2, C4), 2.69–2.71 (m, 2H, CH2, C10), 2.76–2.81 (m, 1H, CH, C7), 5.47 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 157.0 (C6a, C=N), 147.3 (C11, C=C), 135.9 (C5a, C=C), 132.3 (C4a, C=C), 129.7 (C11a, C=C), 116.6 (C11b, C=C), 110.9 (C10a, C=C), 35.6 (C7, CH), 31.0 (C8, CH2), 29.8 (C2, CH2), 29.0 (C1, CH), 25.6 (C10, CH2), 23.9 (C4, CH2), 22.4 (C3, CH2), 21.3 (CH3, at C1), 20.0 (CH3, at C7), 17.6 (C1, CH2); Anal. Calcd. for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19; Found: C, 71.42; H, 7.85; N, 9.94; S, 11.34.
1,9-dimethyl-1,2,3,4,7,8,9,10-octahydrobenzo[4,5]thieno[2,3-b]quinolin-11-amine (2f)
Yield: 67%; M.P. 248 °C; (aqu. ethanol); IR (cm−1): 3460, 3321 (NH2), 2951, 2870 (CH aliphatic), 1635; 1H NMR (DMSO-d6) δ: 1.09 (d, J = 8.0 Hz, 3H, CH3, at C9), 1.21–1.24 (m, 3H, CH3, C1), 1.32–1.42 (m, 1H, CH, C9), 1.69–1.98 (m, 6H, 3CH2, C2,C3,C8), 2.01–2.05 (m, 1H, CH, C1), 2.58–2.75 (m, 6H, 3CH2, C4,C7,C10), 5.47 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 158.3(C6a, C=N), 152.6 (C11, C=C), 146.8 (C5a, C=C), 132.0 (C4a, C=C), 128.9 (C11b, C=C), 116.3 (C11a, C=C), 110.4 (C10a, C=C), 32.4 (C10, CH2), 32.2 (C2, CH2), 31.7 (C7, CH2), 31.4 (C1, CH),30.7 (C8, CH2),29.4 (C9, CH), 28.7 (C4, CH2), 25.2 (C3, CH2), 22.0 (CH3, at C1), 17.4 (CH3, at C9); Anal. Calcd. for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19; Found: C, 71.45; H, 7.80; N, 9.89; S, 11.40.
3-Methyl-1,2,3,4,7,8,9,10-octahydrobenzo[4,5]thieno[2,3-b]quinolin-11-amine (2g)
Yield: 84%; M.P. 231 °C; (water); IR (cm−1): 3483, 3379 (NH2), 2943, 2831 (CH aliphatic), 1616 (C=N); 1H NMR (DMSO-d6) δ: 1.03 (d, 3H, J = 8.0 Hz, CH3, at C3), 1.38–1.47 (m, 1H, CH, C3), 1.72–1.77 (m, 4H, 2CH2, C8,C9), 1.87–1.90 (m, 2H, CH2, C2), 2.30–2.36 (m, 1H, CH, C4), 2.42 (t, 2H, CH2, C1), 2.68–2.74 (m, 2H, CH2, C10), 2.77–2.78 (m, 1H, CH, C4), 2.94–3.08 (m, 2H, CH2, C7), 5.47 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 157.9 (C6a, C=N), 151.0 (C11, C=C), 147.6 (C5a, C=C), 128.7 (C4a, C=C), 126.4 (C11a, C=C), 116.9 (C11b, C=C), 110.6 (C10a, C = C),33.1 (C2, CH2), 32.6 (C3, CH), 30.4 (C4, CH2), 28.3 (C7, CH2), 25.7 (C10, CH2), 22.9 (C8, CH2), 22.53 (C9, CH2), 2.50 (CH3, at C3), 21.1(C1, CH2); Anal. Calcd. for C16H20N2S (272.41): C, 70.55; H, 7.40; N, 10.28; S, 11.77; Found: C, 70.63; H, 7.52; N, 10.44; S, 11.85.
3,7-Dimethyl-1,2,3,4,7,8,9,10-octahydrobenzo[4,5]thieno[2,3-b]quinolin-11-amine (2h)
Yield: 72%; M.P. 195 °C; (water); IR (cm−1): 3502, 3298 (NH2), 2947, 2831 (CH aliphatic), 1631 (C=N); 1H NMR (DMSO-d6) δ: 1.04 (d, J = 4.0 Hz, 3H, CH3, at C3), 1.23–1.26 (m, 3H, CH3, at C7), 1.41–1.53 (m, 2H, CH2, C9), 1.70–1.73 (m, 1H, CH, C3), 1.80–1.92 (broad m, 4H, 2CH2, C2,C8), 2.30–2.36 (m, 1H, CH, C7), 2.42 (t (appearing as a broad s, 2H, CH2, C4), 2.73–2.82 (m, 2H, CH2, C1), 2.97–3.08 (m, 2H, CH2, C10), 5.46 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 158.3 (C6a, C=N), 157.1 (C11, C=C), 147.7 (C5a, C=C), 129.1 (C4a, C=C), 126.5 (C11a, C=C), 116.9 (C11b, C=C), 110.5 (C10a, C=C), 35.5 (C7, CH), 33.4 (C2, CH2), 30.88 (C8, CH2), 30.81 (C3, CH), 30.6 (C4, CH2), 28.5 (C10, CH2), 25.9 (CH3, at C3), 23.7 (C1, CH2), 21.3 (CH3, at C7), 19.7 (C9, CH2); Anal. Calcd. for C17H22N2S (286.43): C, 71.49; H, 7.91; N, 9.85; S, 11.07; Found): C, 71.28; H, 7.74; N, 9.78; S, 11.19.
3,9-Dimethyl-1,2,3,4,7,8,9,10-octahydrobenzo[4,5]thieno[2,3-b]quinolin-11-amine (2i)
Yield: 78%; M.P. 227 °C; (water); IR (cm−1): 3475, 3367 (NH2), 2947, 2827 (CH aliphatic), 1616 (C=N); 1H NMR (DMSO-d6) δ: 1.05 (d, J = 8.0 Hz, 3H, CH3, at C3), 1.10 (d, J = 4.0 Hz, 3H, CH3, at C9), 1.38–1.47 (m, 2H, CH2, C8), 1.86–2.04 (m, 5H, 2CH2, 1CH, C2,C4,C3), 2.33–2.40 (m, 1H, CH, C9), 2.61–2.67 (m, 1H, CH, C10), 2.81–2.82 (m, 1H, CH, C10), 2.85 (t (appearing as a broad s, 2H, CH2, C1), 3.00–3.13 (m, 2H, CH2,C7), 6.76 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 155.3 (C6a, C=N), 151.1 (C11, C=C), 148.5 (C5a, C=C), 129.0 (C4a, C=C), 126.3 (C11a, C=C), 116.9 (C11b, C=C), 110.3 (C10a, C=C), 32.8 (C10, CH2), 31.1 (C2, CH2), 30.8 (C7, CH2), 30.1 (C8, CH2), 29.9 (C3, CH), 28.3 (C9, CH), 28.0 (C4, CH2), 25.4 (C1, CH2), 21.5 (CH3, at C9), 20.9 (CH3, at C3); Anal. Calcd. for C15H18N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19; Found: C, 71.45; H, 7.86; N, 9.94; S, 11.26.
2-Amino-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carbonitrile 3a
Yield: 52%; M.P. 152 °C. The compound was prepared according to the reported procedure [53].
2-Amino-6-methyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carbonitrile 3b
Yield: 51%; M.P. 185 °C. The compound was prepared according to the reported procedure [55].
General procedure for preparation of compounds 4a–e
The respective thiophenes 3a, b (0.01 mol) were mixed with the appropriate ketones (10 mL) and anhydrous zinc chloride (0.01 mol). The mixture was refluxed for 8–10 h. The formed solid was filtered, washed with water, and crystallized from the appropriate solvent [40].
6-Methyl-2,3,6,7,8,9-hexahydro-1H-cyclopenta[4,5]thieno[2,3-b]quinolin-10-amine (4a)
Yield: 65%; M.P. 211 °C; (water); IR (cm−1): 3414, 3325(NH2), 2935, 2862 (CH aliphatic), 1631 (C=N); 1H NMR (DMSO-d6) δ: 1.25 (d, J = 8.0 Hz, 3H, CH3, at C6), 1.48–1.54 (m, 1H, CH, C7), 1.67–1.74 (m, 1H, CH, C7), 1.83–1.89 (m, 2H, CH2, C8), 2.35–2.42(m, 2H, CH2, C2), 2.46 (atypical t, 2H, CH2, C9), 2.79–2.82 (m, 1H, CH, C6), 2.87 (t, J = 8.0 Hz, 2H, CH2,C3), 3.07 (t, J = 8.0 Hz, 2H, CH2, C1), 5.48 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 164.0 (C5a, C=N), 157.4 (C10, C=C), 147.1 (C4a, C=C), 135.8 (C3a, C=C), 134.9 (C10a, C=C), 114.6 (C10b, C=C), 112.0 (C9a, C=C), 35.9 (C6, CH), 31.1 (C3, CH2), 29.6 (C7, CH2), 29.5 (C9, CH2), 27.5 (C2, CH2), 23.9 (C1, CH2), 21.6 (CH3 at C6), 20.0 (C8, CH2); Anal. Calcd. for C15H18N2S (258.38): C, 69.73; H, 7.02; N, 10.84; S, 12.41; Found: C, 69.96; H, 7.25; N, 11.08; S, 12.37.
2-Methyl-1,2,3,4,7,8-hexahydro-6H-cyclopenta[e]thieno[2,3-b;5,4-c’]dipyridin-5-amine (4b)
Yield: 76%; M.P. 233 °C; (water); IR (cm−1): 3344, 3224 (NH2), 2947, 2846 (CH aliphatic), 1643 (C=N); 1H NMR (DMSO-d6) δ: 2.05 (qui, J = 8.0 Hz, 2H, CH2, C7), 2.72 (t, J = 8.0 Hz, 2H, CH2, C3), 2.84 (atypical t, J = 8.0 Hz, 2H, CH2, C4), 2.89 (s, 3H, CH3, N-CH3), 3.50–3.51 (m, 4H, 2CH2, C6, C8), 4.39 (s, 2H, CH2, C1), 5.99 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 161.9 (C8a, C=N), 158.9 (C5, C=C), 146.1 (C9a, C=C), 123.8 (C10a, C=C), 120.0 (C4b, C=C), 115.7 (C4a, C=C), 115.3 (C5a, C=C), 50.2 (C3, CH2), 49.1 (N-CH3), 41.0 (C1, CH2), 32.9 (C6, CH2), 26.6 (C4, CH2), 22.9 (C7, CH2), 21.6 (C8, CH2); Anal. calcd. for C14H17N3S (259.37): C, 64.83; H, 6.61; N, 16.20; S, 12.36; Found: C, 65.02; H, 6.78; N, 16.43; S, 12.49.
2-Methyl-1,2,3,4,6,7,8,9-octahydro-1H-pyrido[4’,3’:4,5]thieno[2,3-b]quinolin-5-amine (4c)
Yield: 73%; M.P. 223 °C; (water); IR (cm−1): 3444, 3352 (NH2), 2931, 2846 (CH aliphatic), 1620 (C=N); 1H NMR (DMSO-d6) δ: 1.73–1.79 (m, 4H, 2CH2, C7,C8), 2.36 (s, 3H, CH3, N-CH3), 2.44 (atypical t, 2H, CH2,C6), 2.67 (atypical t, 2H, CH2, C3), 2.72 (atypical t, 2H, CH2, C4), 3.06 (atypical t, 2H, CH2, C9), 3.49 (s, 2H, CH2, C1), 5.51(s, 2H, NH2, D2O exchangeable);13C NMR (DMSO) δ: 158.3 (C9a, C=N), 153.6 (C5, C=C), 147.6 (C10a, C=C), 127.0 (C11a, C=C), 125.2 (C4b, C=C), 116.7 (C4a, C=C), 110.9 (C5a, C=C), 53.9 (C1, CH2), 51.8 (C3, CH2), 45.2 (N-CH3), 32.9(C9, CH2), 26.6 (C6, CH2), 23.1 (C4, CH2), 22.7 (C7, CH2), 22.6 (C8, CH2); Anal. Calcd. for C15H19N3S (273.40): C, 65.90; H, 7.00; N, 15.37; S, 11.73; Found: C, 65.73; H, 7.21; N, 15.59; S, 11.81.
2,7-Dimethyl-1,2,3,4,6,7,8,9octahydropyrido[4’,3’:4,5]thieno[2,3-b]quinolin-5-amine (4d)
Yield: 70%; M.P. <300 °C; (water); IR (cm−1): 3491, 3305 (NH2), 2943, 2850 (CH aliphatic), 1631 (C=N); 1H NMR (DMSO-d6) δ: 1.09 (d, J = 4.0 Hz, 3H, CH3, C7), 1.32–1.42 (m, 1H, CH, C7), 1.83–1.84 (m, 2H, CH2, C8), 1.95–2.02 (m, 1H, CH, C6), 2.36 (s, 3H, CH3, N-CH3), 2.61–2.62 (m, 1H, CH, C6), 2.67 (atypical t, 2H, CH2, C3), 2.75 (atypical t, J = 4.0 Hz, 2H, CH2, C9), 3.03–3.07 (broad m, 2H, CH2, C4), 3.50 (s, 2H, CH, C1), 5.51(s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 157.7 (C9a, C=N), 152.7 (C5, C=C), 147.1 (C10a, C=C), 126.3 (C11a, C=C), 124.5 (C4b, C=C),116.0 (C4a, C=C), 110.0 (C5a, C=C), 53.2 (C1, CH2),51.2 (C3, CH2), 44.6 (N-CH3), 31.9 (C6, CH2), 31.1 (C9, CH2), 30.2 (C8, CH2), 28.2 (C7, CH), 25.9 (CH3 at C7), 21.5 (C4, CH2); Anal. Calcd. for C16H21N3S (287.42): C, 66.86; H, 7.36; N, 14.62; S, 11.16; Found: C, 66.98; H, 7.45; N, 14.89; S, 11.08.
2,9-Dimethyl-1,2,3,4,6,7,8,9-octahydropyrido[4’,3’:4,5]thieno[2,3-b]quinolin-5-amine (4e)
Yield: 67%; M.P.< 300 °C; (water); IR (cm−1): 3502, 3313 (NH2), 2943, 2843 (CH aliphatic), 1620 (C=N); 1H NMR (DMSO-d6) δ: 1.24 (d, J = 8.0 Hz, 3H, CH2, C9), 1.45–1.54 (m, 1H, CH, C8), 1.67–1.74 (m, 1H, CH, C8), 1.85–1.90 (m, 2H, CH2, C7), 2.35 (s, 3H, CH3, N-CH3), 2.44 (atypical t, 2H, CH2, C3), 2.67 (atypical t, 2H, CH2, C6), 2.78–2.83 (m, 1H, CH, C9), 3.04 (atypical t, 2H, CH2, C4), 3.50 (s, 2H, CH2, C1), 5.48 (s, 2H, NH2, D2O exchangeable); 13C NMR (DMSO) δ: 158.0 (C9a, C=N), 157.0 (C5, C=C), 147.3 (C10a, C=C), 126.8 (C11a, C=C), 124.7 (C4b, C=C), 116.1 (C4a, C=C), 110.2 (C5a, C=C), 53.5 (C1, CH2), 51.4 (C3, CH2), 44.8 (N-CH3), 35.2 (C9, CH), 30.4 (C8, CH2), 26.1 (C6, CH2), 23.2 (CH3 at C9), 20.8 (C7, CH2), 19.3 (C4, CH2); Anal. Calcd. for C16H21N3S (287.42): C, 66.86; H, 7.36; N, 14.62; S, 11.16; Found: C, 67.04; H, 7.49; N, 14.88; S, 11.23.
Biology
Both AChE and BuChE enzyme brain contents were assessed, then the inhibitory effects of series 2 and 4 were determined for different concentrations of each compound. Aβ content was also measured. The inhibition properties are reported as IC50 values and were determined graphically from inhibition curves of log inhibitor concentration vs. percent of inhibition. IC50 values represent the concentration of inhibitor required for 50% inhibition of the enzyme [35, 56]. Donepezil and tacrine, as two well-established anti-dementia drugs with potent AChE and BuChE inhibitory effects, were used as the reference drugs in this study. In addition, the effects of the newly synthesized 14 compounds on Aβ were also estimated. Male Wistar rats (200–250 g) were used in the present experiment. Animals were housed at the animal facility of the Faculty of Pharmacy, Cairo University, for 1 week prior to experimentation in an ambient temperature of 22 ± 2 °C. Rats were maintained on a standard pellet diet and given tap water ad libitum. The study was carried out in accordance with the APA ethical standards and with the approval of the Ethics Committee for Animal Experimentation at the Faculty of Pharmacy, Cairo University. Rats were randomly assigned to 18 treatment groups (each group of 6 rats) divided as follows; Group I: Rats received vehicle (1% tween 80, orally) and served as a normal control group. Group II: Rats received AlCl3 (100 mg/kg, orally) and served as Alzheimer’s control group. Groups III & IV: Rats were given tacrine (200, 100, 50, 25 mg/kg, orally) and donepezil (200, 100, 50, 25 mg/kg, orally), respectively, serving as standard control groups, Groups V–XVIII: Rats were treated with the 14 new compounds (200, 100, 50, 25 mg/kg, orally). Oral administration of AlCl3 daily for 30 consecutive days was used to induce Alzheimer’s disease, in all groups except for the normal control group. Treatments started on the 31st day and for 21 days. Thereafter, one hour after the last drug administration, animals were euthanized, under thiopental anesthesia, by decapitation. Brains were removed and homogenized immediately in ice-cold saline to obtain 10% (w/v) homogenate using a glass homogenizer (Glas-Col homogenizer). The homogenates were centrifuged at 15,000 rpm for 20 min. The supernatants were used for estimation of AChE, BuChE, and Aβ that were measured using enzyme-linked immunosorbent assay (ELISA) with the following catalog numbers and suppliers’ names; LS-F22804 (Lifespan Biosciences, USA), DBCHE0 (Quantikine, USA), and CEA946Mu (Cloud-Clone Corp., USA), respectively. The assays’ procedures were performed according to the manufacturer’s instructions. The results are reported as mean ± standard error (SEM).
In silico molecular studies
The physicochemical in silico ADME and pharmacokinetic properties’ prediction studies were conducted using the Swiss ADME predictor [57]. The Swiss Institute of Bioinformatics (SIB) invented such a free web tool to predict the pharmacokinetics, physicochemical properties, and drug-likeness of newly investigated leads. Accordingly, this study was applied to the most potent newly synthesized compounds (2c, 2d, 2e, 2h, 2i, and 4e). The compound structures were imported, and the structures’ smiley was entered to run the Swiss ADME study.
All the docking studies were performed using Molecular Operating Environment (MOE-2008) software. The X-ray crystal structure of both the tacrine–AChE complex (PDB ID: 1ACJ) [58] and the tacrine–BuChE complex (PDB ID: 4bds) [59] were downloaded from Protein Data Bank (PDB). All minimizations were carried out until an RMSD gradient of 0.05 kcal mol−1 Å−1 with Merk Molecular Force Field (MMFF94x). Docking of the most stable conformers was carried out using Triangle Matcher Replacement and London dG scoring function. Moreover, to ensure the accuracy of the docking study, validation was carried out by re-docking the co-crystallized ligand (tacrine) into the AChE active site resulting in a very close alignment with the original ligand with an RMSD and an energy score of 0.3398 and −10.8740 kcal/mol, sequentially. Furthermore, the re-docking validation of the co-crystallized ligand (tacrine) into the BuChE active site was successfully carried out and produced a binding pattern with an RMSD and an energy score of 0.2398 and −10.5068 kcal/mol, respectively.
Conclusion
Two tacrine analogs series 2a–i and 4a–e were synthesized and eventually structurally elucidated. The new members were screened against their AChE and BuChE enzymes in addition to the Aβ protein inhibition. Both tacrine and donepezil were considered reference drugs in the assays. All compounds showed better inhibitory effects on both AChE and BuChE enzymes than tacrine. On other hand, All Compounds except 2a, 2b, 2f, 2g, 4a, 4b, and 4c displayed an inhibitory effect on both AChE and BuChE enzymes better than donepezil. Finally, compounds 2c, 2d, 2e, 2h, 2i, and 4e resulted in more decrement of Aβ compared to both tacrine and donepezil. The six common promising members in all the assays, 2c, 2d, 2e, 2h, 2i, and 4e, were subjected to molecular modeling and in silico Swiss ADME investigations. All the predicted pharmacokinetic properties revealed moderate water solubility, high human intestinal absorption, and passing blood–brain barrier with a bioavailability score of 0.55. Moreover, the molecular docking results showed extra water-mediated H-bonding with the Asp72 residue in the AChE active gorge. More specifically, 4e possessed cation–π interaction between its protonated tetrahydrothienopyridine nitrogen and Trp432. Thus, the tetracyclicthienopyridine fused system has proven to be a promising start for the discovery of new anti-Alzheimer multi-targeting leads.
References
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet 2021;397:1577–90.
Omura JD, Mcguire LC, Patel R, Baumgart M, Lamb R, Jeffers EM, et al. Modifiable risk factors for Alzheimer disease and related dementias among adults aged ≥45 years. MMWR Morb Mortal Wkly Rep. 2022;71:680–5.
Ibrahim MM, Gabr MT. Multitarget therapeutic strategies for Alzheimer’s disease. The challenge of development of therapeutics for multifactorial conformational diseases. Neural Regen Res. 2018;14:437–40. http://www.nrronline.org.
Briggs R, Kennelly SP, O’neill D. Drug treatments in Alzheimer’s disease. Clin Med. 2016;16:247–53.
Atri A. Current and future treatments in Alzheimer’s disease. Semin Neurol. 2019;39:227–40.
Gong CX, Liu F, Iqbal K. Multifactorial hypothesis and multi-targets for Alzheimer’s disease. J Alzheimer’s Dis 2018;64:S107–17.
Van Harten AC, Jelle P, Pijnenburg YAL, Teunissen CE, Blankenstein MA, Scheltens P, et al. Cerebrospinal fluid A b 42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimer’s Dement. 2013;9:481–7. https://doi.org/10.1016/j.jalz.2012.08.004.
Chalupova K, Korabecny J, Bartolini M, Monti B, Lamba D, Caliandro R, et al. Novel tacrine-tryptophan hybrids: multi-target directed ligands as potential treatment for Alzheimer’s disease. Eur J Med Chem. 2019;168:491–514.
Saify ZS, Sultana N. Role of acetylcholinesterase inhibitors and Alzheimer disease. In: Atta-ur-Rahman, Muhammad Iqbal Choudhary, editors. Drug design and discovery in Alzheimer’s disease. Ch. 7. Pakistan. Elsevier; 2014. p. 387–425. https://doi.org/10.1016/B978-0-12-803959-5.50007-6.
Scarpini E, Scheltens P.Feldman H, Treatment of Alzheimer’s disease: current status and new perspectives. vol. 2. Lancet neurology. Lancet Publishing Group. 2003. p. 539–47.
Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982;217:408–17.
Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11:315–35. http://www.ncbi.nlm.nih.gov/pubmed/24179466.
Jbilo O, L’hermite Y, Talesa V, Toutant JP, Chatonnet A. Acetylcholinesterase and butyrylcholinesterase expression in adult rabbit tissues and during development. Eur J Biochem 1994;225:115–24.
Massoulié J, Pezzementi L, Bon S, Krejci E, Vallette FM. Molecular and cellular biology of cholinesterases. Prog Neurobiol. 1993;41:31–91.
Luo Z, Sheng J, Sun Y, Lu C, Yan J, Liu A, et al. Synthesis and evaluation of multi-target-directed ligands against Alzheimer’s disease based on the fusion of donepezil and ebselen. J Med Chem. 2013;56:9089–99.
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992;256:184–5.
Bandyopadhyay S, Goldstein LE, Lahiri DK, Rogers JT. Role of the APP non-amyloidogenic signaling pathway and targeting-secretase as an alternative drug target for treatment of Alzheimer’s disease. Curr Med Chem. 2007;14:2848–64.
Takahashi RH, Nagao T, Gouras GK. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol Int. 2017;67:185–93.
Zheng W, Tsai MY, Wolynes PG. Comparing the aggregation free energy landscapes of amyloid beta(1-42) and amyloid beta(1-40). J Am Chem Soc. 2017;139:16666–76.
Harkany T, Ábrahám I, Timmerman W, Laskay G, Tóth B, Sasvári M, et al. β-Amyloid neurotoxicity is mediated by a glutamate-triggered excitotoxic cascade in rat nucleus basalis. Eur J Neurosci. 2000;12:2735–45.
Sun BL, Chen Y, Fan DY, Zhu C, Zeng F, Wang YJ. Critical thinking on amyloid-beta-targeted therapy: challenges and perspectives. Sci China Life Sci. 2021;64:926–37.
Nisticò R, Borg JJ. Aducanumab for Alzheimer’s disease: a regulatory perspective. Pharmacol Res 2021;171:105754.
Makhaeva GF, Kovaleva NV, Boltneva NP, Lushchekina S V, Rudakova EV, Stupina TS, et al. Conjugates of tacrine and 1,2,4-thiadiazole derivatives as new potential multifunctional agents for Alzheimer’s disease treatment: synthesis, quantum-chemical characterization, molecular docking, and biological evaluation. Bioorg Chem 2020;94:103387.
Zhang X, Rakesh KP, Bukhari SNA, Balakrishna M, Manukumar HM, Qin HL. Bioorganic Chemistry Multi-targetable chalcone analogs to treat deadly Alzheimer’ s disease: current view and upcoming advice. Bioorg Chem. 2018;80:86–93.
Roldán-Peña JM, Romero-Real V, Hicke J, Maya I, Franconetti A, Lagunes I, et al. Tacrine-O-protected phenolics heterodimers as multitarget-directed ligands against Alzheimer’s disease: selective subnanomolar BuChE inhibitors. Eur J Med Chem. 2019;181:111550–66.
Kumar A, Singh A, Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67:195–203.
Watkins PB, Zimmerman HJ, Knapp MJ, Gracon SI, Lewis KW. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. J Am Med Assoc. 1994;271:992–8. http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.1994.03510370044030.
Mimica N, Presecki P. Side effects of approved antidementives. Psychiatr Danub. 2009;21:108–13. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L354412095
Cacabelos R. Donepezil in Alzheimer’s disease: from conventional trials to pharmacogenetics. Neuropsychiatr Dis Treat. 2007;3:303–33.
Mahmoud Z, Sayed HS, Mohamed LW, Mohamed KO. Development of new donepezil analogs: synthesis, biological screening and in silico study rational. Med Chem Res. 2022;31:1754–70.
Bullock R, Touchon J, Bergman H, Gambina G, He Y, Rapatz G, et al. Rivastigmine and donepezil treatment in moderate to moderately-severe Alzheimer’s disease over a 2-year period. Curr Med Res Opin. 2005;21:1317–27. http://www.tandfonline.com/doi/full/10.1185/030079905X56565
Ismaili L, Refouvelet B, Benchekroun M, Brogi S, Brindisi M, Gemma S, et al. Multitarget compounds bearing tacrine- and donepezil-like structural and functional motifs for the potential treatment of Alzheimer’s disease. Prog Neurobiol 2017;151:4–34.
Wang Y, Wang H, Chen HZ. AChE inhibition-based multi-target-directed ligands, a novel pharmacological approach for the symptomatic and disease-modifying therapy of Alzheimer’s disease. Curr Neuropharmacol. 2016;14:364–75.
Ismail MM, Kamel MM, Mohamed LW, Faggal SI, Galal MA. Synthesis and biological evaluation of thiophene derivatives as acetylcholinesterase inhibitors. Molecules 2012;17:7217–31.
Mohamed LW, Abuel-Maaty SM, Mohammed WA, Galal MA. Synthesis and biological evaluation of new oxopyrrolidine derivatives as inhibitors of acetyl cholinesterase and β amyloid protein as anti-Alzheimer’s agents. Bioorg Chem. 2018;76:210–7. https://doi.org/10.1016/j.bioorg.2017.11.008.
Ceschi MA, da Costa JS, Lopes JPB, Câmara VS, Campo LF, Borges AC, et al. Novel series of tacrine-tianeptine hybrids: synthesis, cholinesterase inhibitory activity, S100B secretion and a molecular modeling approach. Eur J Med Chem. 2016;121:758–72.
Soukup O, Jun D, Zdarova-Karasova J, Patocka J, Musilek K, Korabecny J, et al. A Resurrection of 7-MEOTA: a comparison with tacrine. Curr Alzheimer Res. 2013;10:893–906.
Recanatini M, Cavalli A, Belluti F, Piazzi L, Rampa A, Bisi A, et al. SAR of 9-amino-1,2,3-4-tetrahydroacridine-based acetylcholinesterase inhibitors: synthesis, enzyme inhibitory activity, QSAR, and structure-based CoMFA of tacrine analogues. J Med Chem. 2000;43:2007–18.
Arnold H. Synthesis of thiophene analogues of the tacrine series. Synthesis. 2007;7:1027–32.
Badran MM, Hakeem MA, Abuel-Maaty SM, El-Malah A, Salam RMA. Design, synthesis, and molecular-modeling study of aminothienopyridine analogues of tacrine for Alzheimer’s disease. Arch Pharm. 2010;343:590–601. http://doi.wiley.com/10.1002/ardp.200900226.
El-Malah A, Abouelatta AIY, Mahmoud Z, Salem HH. New cyclooctathienopyridine derivatives in the aim of discovering better anti-Alzheimer’s agents. J Mol Struct. 2019;1196:162–8.
Mohamed LW, Mohamed KO, Sayed HS, Mahmoud Z. Recent modifications of anti-dementia agents focusing on tacrine and/or donepezil analogs. Med Chem. 2022;1–14. https://doi.org/10.2174/1573406418666220827155615.
de los Ríos C, Marco-Contelles J. Tacrines for Alzheimer’s disease therapy. III. The pyridotacrines. Eur J Med Chem. 2019;166:381–9.
Pe E, Soriano E. Recent advances in the Friedlander reaction. Chem Rev. 2012;109:2652–71.
Chaki H, Yamabe H, Sugano M, Morita S, Bessho T. Desgin and syntheses of 4-acylaminopyridine derivatives: novel high affinity choline uptake enhancers II. Bioorg Med Chem Lett. 1995;5:1495–500.
Hamama WS, Waly SM, Said SB, Zoorob HH. Annulation of o-aminoquinoxaline-1,4-dioxidenitrile with ketonic compounds under Friedländer-type cyclocondensation and its biological evaluation. J Heterocycl Chem. 2018;55:1554–63.
Hu H, Zhang A, Ding L. Facile synthesis of novel tacrine analogues. J Chem Res. 2009;2009:562–4.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;64(Suppl):4–17.
Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1:55–68.
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–23.
Egan WJ, Merz KM, Baldwin JJ. Prediction of drug absorption using multivariate statistics. J Med Chem. 2000;43:3867–77.
Muegge I, Heald SL, Brittelli D. Simple selection criteria for drug-like chemical matter. J Med Chem. 2001;44:1841–6.
Karl G, Elfriede S, Horst B. 2-Amino-thiophene aus methylenaktiven Nitrilen, Carbonylverbindungen und Schwefel. Chem Ber. 1966;99:94–100.
Xu F, Li Y, Xu F, Ye Q, Han L, Gao J, et al. Solvent-free synthesis of 2-aminothiophene-3-carbonitrile derivatives using high-speed vibration milling. J Chem Res. 2014;38:450–2.
Aurelio L, Valant C, Figler H, Flynn BL, Linden J, Sexton PM, et al. 3- and 6-Substituted 2-amino-4,5,6,7-tetrahydrothieno[2,3-c]pyridines as A1 adenosine receptor allosteric modulators and antagonists. Bioorg Med Chem. 2009;17:7353–61.
Washington PM, Morffy N, Parsadanian M, Zapple DN, Burns MP. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J Neurotrauma. 2014;31:125–34.
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:1–13. https://doi.org/10.1038/srep42717.
Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, et al. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci USA. 1993;90:9031–5.
Nachon F, Carletti E, Ronco C, Trovaslet M, Nicolet Y, Jean L, et al. Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem J. 2013;453:393–9.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoud, Z., Mohamed, L.W., Mohamed, K.O. et al. New tetracyclic systems integrated thienopyridine scaffold as an anti-dementia lead: in silico study and biological screening. Med Chem Res 32, 571–586 (2023). https://doi.org/10.1007/s00044-022-03013-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-03013-7