Abstract
In this study, we describe the synthesis of mono- and bis-1H-1,2,3-triazole-tethered β-lactam–isatin conjugates using copper-catalysed azide-alkyne cycloaddition reaction between mono- and di-propargylated azetidin-2-ones and N-alkylazido isatins. The synthesized conjugates were evaluated for their preliminary in vitro analysis against Trichomonas vaginalis at 50 μM. The efficacy of synthesized hybrids was observed to depend on the substituent at N-1 position of β-lactam ring, as well as the presence of single/double 1H-1,2,3-triazole linker. Among the synthesized conjugates, the presence of a p-tolyl substituent at N-1 of β-lactam ring was preferred for good activity profiles while the increase in spacer length did not influence the efficacy of the compounds. Compounds with high levels of potency were further analysed to determine their IC50 values, as well as cytotoxicity profiles against mammalian cells. The most active compound in the synthesized conjugates displayed an IC50 value of 10.49 μM against cultured G3 strain of T. vaginalis and was non-toxic to cultured mammalian HeLa cells at the same concentration.
Similar content being viewed by others
Introduction
With approximately 248 million new cases occurring worldwide annually (WHO, Geneva, 2012), human trichomoniasis is a far more prevalent sexually transmitted disease (STD) than either chlamydia (caused by Chlamydia trachomatis) or gonorrhoea (caused by Neisseria gonorrhoeae) (Soper, 2004). The causative organism for the disease in humans, Trichomonas vaginalis, is primarily acquired through transmission of trophozoites by direct sexual contact, although neonatal infection has also been reported (McLaren et al., 1983). Metronidazole (MTZ), the current and only FDA-approved treatment for this disease, has been used for more than 40 years. However, there are ample reports on the development of resistant isolates to MTZ which in certain cases have shown to be tackled with prolonged therapy and higher dosage (Wright et al., 2010; Upcroft et al., 2009). Further, it is now entrenched that trichomoniasis-infected patients are more susceptible towards human immunodeficiency virus (HIV) as it appeared as a cofactor in HIV transmission and acquisition (Sorvillo et al., 2001; van der Pol et al., 2008). The significant increase in the vulnerability to HIV with trichomoniasis (McClelland et al., 2007; Guenthner et al., 2005) has increased the importance of this disease dramatically. As evident, the identification and development of novel scaffolds with toxicity against T. vaginalis, and minimal cytotoxicity against human cells, is a challenging task and provides a strong impetus for re-engineering and re-positioning of previously characterized drug families (Upcroft et al., 2006).
Isatin is a privileged scaffold with well tolerance in humans and its analogues demonstrate a diverse range of biological and pharmacological properties such as anti-HIV (Bal et al., 2005), anti-viral (Quenelle et al., 2006), anti-cancer (Vine and Locke, 2007; Kopka et al., 2006), anti-fungal (Raj et al., 2003), anticonvulsants (Verma et al., 2004), anti-Parkinson’s disease therapeutic (Igosheva et al., 2005), β-lactamase inhibitors (Casey et al., 1993; Hadfield et al., 2002) and effective SARS coronavirus 3CL protease inhibitor (Chen et al., 2005). The enthralling applications of isatins in organic synthesis, its biological properties, as well as its occurrence in natural products such as spirotryprostatins, horsfiline, gelsemine, gelseverine, rhynchophylline, elacomine, etc. have generated tremendous interest among synthetic organic and medicinal chemists (Fensome et al., 2008; Kumari et al., 2011; Ding et al., 2005; Vintonyak et al., 2010; Rottmann et al., 2010). Particular examples of 2-oxoindole derivatives are SU-5416 (semaxanib) and SU-11248 (Sunitinib) that were reported to have tyrosine kinase inhibitory and anti-angiogenic properties (Ma et al., 2003; Prenen et al., 2006).
Azetidin-2-one (β-lactam) is the crucial structural unit present widely in the β-lactam class of antibiotics (Palomo et al., 1999; Palomo et al., 2004). Following the discovery of penicillin, an array of naturally occurring β-lactam antibiotics has been introduced as chemotherapeutics of incomparable effectiveness for the treatment of bacterial infections. Current interest in this family is focused on the synthesis and modification of the β-lactam ring to obtain compounds with diverse pharmacological potential such as tumour necrosis factor-alpha (TNF-alpha) converting enzyme (TACE) inhibitors (Rao et al., 2007), anti-cancer (O’Boyle et al., 2013; Singh et al., 2011b), anti-coccidial (Liang et al., 2008), cardiovascular (Takai et al., 2004), anti-viral (D’hooghe et al. 2012), mutagenic (Gutierrez et al., 2013), anti-fungal (O’Driscoll et al., 2008) and anti-malarial activities (Jarrahpour et al., 2012). Besides its eminence as a heterocyclic system with numerous biological potential, β-lactam have also been employed as synthetic precursor for the synthesis of a wide variety of heterocyclic scaffolds (Singh, 2003; Alcaide et al., 2007; D’hooghe et al., 2010; Singh et al., 2011a).
Recently, pharmacophore hybridization has been appeared as an attractive paradigm for medicinal chemists. The main incentives for using this strategy relates to the marked improvement in therapeutic potential, potency, mode of action and pharmacokinetics (Meunier, 2008; Muregi and Ishih, 2010; Morphy and Rankovic, 2006).
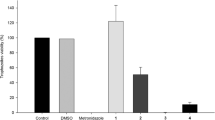
Continuing with our efforts in the synthesis of novel molecular conjugates with biological potential (Raj et al., 2013c; Raj et al., 2013a; Nisha et al., 2013; Kumar et al., 2013; Kumar et al. 2012), we recently discussed the synthesis of 1H-1,2,3-triazole-tethered β-lactam–isatin conjugates and their in vitro evaluation against T. vaginalis Raj et al., 2013b). Most of the synthesized compounds exhibited 100 % growth inhibition at 100 μM with the most potent and non-cytotoxic compound (Fig. 1) displayed an IC50 value of 7.69 μM.
The present communication is an extension of the above approach comprising of the synthesis of mono- and bis-1H-1,2,3-triazole-tethered bifunctional hybrids of isatin with N-1 substituted β-lactams (Fig. 2) and their preliminary in vitro evaluation studies against T. vaginalis. The rationale behind the use of 1H-1,2,3-triazole linker is its active participation in hydrogen bonding, dipole–dipole interaction and stability against hydrolysis and oxidative/reductive conditions (Kolb et al., 2001; Kolb and Sharpless, 2003; Wang et al., 2005; Bock et al. 2006).
Experimental section
Melting points were determined by open capillary using a Veego precision digital melting point apparatus (MP-D) and are uncorrected. IR spectra were recorded on a Shimadzu D-8001 spectrophotometer. 1H NMR spectra were recorded in deuterochloroform and dimethylsulphoxide-d6 with a Jeol 300 (300 MHz) spectrometer using TMS as an internal standard. Chemical shift values are expressed as parts per million downfield from TMS and J values are in hertz. Splitting patterns are indicated as s singlet, d doublet, t triplet, m multiplet, dd double doublet, ddd doublet of a doublet of a doublet and br broad peak. 13C NMR spectra were recorded on Jeol 300 (75 MHz) spectrometer in deuterochloroform and dimethylsulphoxide using TMS as internal standard. High-resolution mass spectra were recorded on Bruker-micrOTOF-Q II spectrometer. Column chromatography was performed on a silica gel (60-120 mesh).
General method for the preparation of β-lactam–isatin conjugates 6 and 7
To a stirred solution of azide 5 (1 mmol for 2 and 2 mmol for 3) in ethanol–water (10:1) was added in succession appropriate acetylenic lactam 2 or 3 (1 mmol), copper sulphate (0.055 mmol for 2 and 0.1 mmol for 3) and sodium ascorbate (0.13 mmol for 2 and 0.26 for 3) at room temperature. On completion, as monitored by tlc, water was added to the reaction mixture and extracted with chloroform. Combined organic layers were dried over anhydrous sodium sulphate and concentrated under reduced pressure to result in a crude product which was purified by silica gel column chromatography.
1-(2-{4-[(2-Oxo-4-styryl-1-p-tolyl-azetidin-3-ylamino)-methyl]-[1,2,3]triazol-1-yl}-ethyl)-1H-indole-2,3-dione (6a)
Brick red colour, yield 74 %; IR (KBr) νmax: 1738, 1612 cm−1; mp 214–215 °C; 1H NMR (CDCl3, 300 MHz): δ 2.23 (s, 3H, -CH3); 3.76 (s, 2H, –CH2–); 4.10–4.13 (m, 2H, –CH2–); 4.40–4.57 (m, 2H, –CH2–); 4.61 (d, J = 5.1 Hz, 1H, H4); 4.82 (dd, J = 5.1, 8.1 Hz, 1H, H3); 6.16 (dd, J = 8.1, 15.9 Hz, 1H, H2); 6.48 (d, J = 8.1 Hz, 1H, ArH); 6.64 (d, J = 15.9 Hz, 1H, H1); 6.90–7.42 (m, 12H, ArH); 7.58 (s, 1H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 21.1 (CH3), 37.6 (C-13), 46.5 (C-9), 48.3 (C-10), 61.6 (C-15), 71.8 (C-14), 110.1 (C-4), 117.2 (C-16), 117.7 (C-2), 123.6 (C-24), 124.0 (C-19), 124.5 (C-17), 125.3 (C-21), 126.7 (C-20), 128.1 (C-6), 128.7 (C-1), 129.1 (C-25), 133.9 (C-26), 134.5 (C-3), 135.1 (C-11), 135.8 (C-18), 138.6 (C-23), 144.1 (C-5), 151.5 (C-12), 158.1 (C-8), 164.4 (C-22), 182.2 (C-7). HRMS calculated for C31H28N6O3 [M]+ 532.2223 found 532.2230; Anal. Calcd. (%) for: C, 69.91; H, 5.30; N, 15.78, found: C, 69.99; H, 5.38; N, 15.73.
1-(2-{4-[(1-Cyclohexyl-2-oxo-4-styryl-azetidin-3-ylamino)-methyl]-[1,2,3]triazol-1-yl}-ethyl)-1H-indole-2,3-dione (6b)
Brick red colour, yield 65 %; IR (KBr) νmax: 1737, 1616 cm−1; mp 219–220 °C; 1H NMR (CDCl3, 300 MHz): δ 1.26–1.97 (m, 10H, cyclohexyl), 3.48–3.63 (m, 1H, cyclohexyl), 3.70 (s, 2H, –CH2–); 4.12–4.23 (m, 2H, –CH2–); 4.28 (d, J = 5.1 Hz, 1H, H4); 4.60 (dd, J = 5.4, 11.4 Hz, 2H, –CH2–); 4.83 (dd, J = 5.1, 8.4 Hz, 1H, H3); 6.27 (dd, J = 8.4, 15.9 Hz, 1H, H2); 6.71 (d, J = 15.9 Hz, 1H, H1); 6.99–7.44 (m, 9H, ArH); 7.68 (s, 1H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 24.1 (C-25), 24.8 (C-27), 25.4 (C-26), 30.2 (C-24), 31.3 (C-28), 39.4 (C-23), 45.4 (C-13), 47.5 (C-9), 51.6 (C-10), 61.7 (C-15), 72.0 (C-14), 110.3 (C-4), 117.3 (C-16), 117.8 (C-2), 124.1 (C-19), 124.6 (C-17), 124.8 (C-21), 125.5 (C-20), 126.7 (C-6), 128.4 (C-1), 134.2 (C-3), 135.1 (C-11), 135.6 (C-18), 144.4 (C-5), 150.1 (C-12), 158.4 (C-8), 164.1 (C-22), 182.1 (C-7). HRMS calculated for C30H32N6O3 [M]+ 524.2536 found 524.2530; Anal. Calcd. (%) for: C, 68.68; H, 6.15; N, 16.02, found: C, 68.61; H, 6.24; N, 16.10.
1-[2-(4-{[1-(4-Fluoro-phenyl)-2-oxo-4-styryl-azetidin-3-ylamino]-methyl}-[1,2,3]triazol-1-yl)-ethyl]-1H-indole-2,3-dione (6c)
Brick red colour, yield 78 %; IR (KBr) νmax: 1733, 1612 cm−1; mp 203–204 °C; 1H NMR (300 MHz CDCl3): δ 3.92 (s, 2H, –CH2–); 4.16–4.19 (m, 2H, –CH2–); 4.26 (d, J = 5.1 Hz, 1H, H4); 4.47–4.64 (m, 2H, –CH2–); 4.70 (dd, J = 5.1, 7.5 Hz, 1H, H3); 6.19 (dd, J = 7.8, 16.2 Hz, 1H, H2); 6.51 (d, J = 8.1 Hz, 1H, ArH); 6.65 (d, J = 16.2 Hz, 1H, H1); 6.95–7.50 (m, 12H, ArH); 7.55 (s, 1H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 37.1(C-13), 46.4 (C-9), 47.7 (C-10), 61.8 (C-15), 72.3 (C-14), 110.2 (C-4), 117.0 (C-16), 117.6 (C-2), 123.8 (C-25), 124.1 (C-24), 124.6 (C-19), 125.4 (C-17), 126.9 (C-21), 128.1 (C-20), 128.6 (C-6), 129.0 (C-1), 134.4 (C-3), 135.2 (C-11), 135.8 (C-18), 138.9 (C-23), 144.0 (C-5), 148.7 (C-26), 151.1 (C-12), 158.2 (C-8), 164.9 (C-22), 182.1 (C-7). HRMS calculated for C30H25FN6O3 [M]+ 536.1972 found 536.1979; Anal. Calcd. (%) for: C, 67.15; H, 4.70; N, 15.66, found: C, 67.06; H, 4.77; N, 15.60.
1-(3-{4-[(2-Oxo-4-styryl-1-p-tolyl-azetidin-3-ylamino)-methyl]-[1,2,3]triazol-1-yl}-propyl)-1H-indole-2,3-dione (6d)
Brick red colour, yield 72 %; IR (KBr) νmax: 1732, 1617 cm−1; mp 197–198 °C; 1H NMR (300 MHz CDCl3): δ 2.24 (s, 3H, –CH3); 2.32 (dd, J = 6.6, 12.9 Hz, 2H, –CH2–); 3.75 (a pair of doublet, J = 15.0 Hz, 2H, –CH2–); 4.10–4.21 (m, 2H, –CH2–); 4.59 (dd, J = 5.1, 11.1 Hz, 2H, –CH2–); 4.68 (d, J = 5.1 Hz, 1H, H4); 4.84 (dd, J = 5.1, 8.4 Hz, 1H, H3); 6.30 (dd, J = 8.4, 15.9 Hz, 1H, H2); 6.54 (d, J = 8.1 Hz, 1H, ArH); 6.73 (d, J = 15.9 Hz, 1H, H1); 6.97 (d, J = 8.1 Hz, 1H, ArH); 7.09–7.45 (m, 11H, ArH); 7.76 (s, 1H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 21.3 (CH3), 28.0 (C-10), 38.1 (C-14), 45.2 (C-9), 47.4 (C-11), 61.2 (C-16), 71.9 (C-15), 109.8 (C-4), 117.1 (C-17), 117.6 (C-2), 124.1 (C-25), 124.6 (C-20), 124.9 (C-18), 125.3 (C-22), 126.7 (C-21), 128.2 (C-6), 128.6 (C-1), 129.5 (C-26), 134.1 (C-27), 135.4 (C-3), 135.6 (C-12), 135.9 (C-19), 138.4 (C-24), 144.2 (C-5), 150.2 (C-13), 158.1 (C-8), 164.6 (C-23), 182.1 (C-7). HRMS Calculated for C32H30N6O3 [M]+ 546.2379 found 546.2372; Anal. Calcd. (%) for: C, 70.31; H, 5.53; N, 15.37, found: C, 70.37; H, 5.59; N, 15.29.
1-(3-{4-[(1-Cyclohexyl-2-oxo-4-styryl-azetidin-3-ylamino)-methyl]-[1,2,3]triazol-1-yl}-propyl)-1H-indole-2,3-dione (6e)
Brick red colour, yield 74 %; IR (KBr) νmax: 1731, 1610 cm−1; mp 211–212 °C; 1H NMR (300 MHz CDCl3): δ 1.27–1.95 (m, 10H, cyclohexyl), 2.33 (dd, J = 6.3, 13.2 Hz, 2H, –CH2–); 3.47–3.63 (m, 1H, cyclohexyl), 3.73 (s, 2H, –CH2–); 4.13–4.24 (m, 2H, –CH2–); 4.34 (d, J = 5.1 Hz, 1H, H4); 4.56 (m, 2H, –CH2–); 4.87 (dd, J = 5.1, 8.4 Hz, 1H, H3); 6.25 (dd, J = 8.4, 15.6 Hz, 1H, H2); 6.69 (d, J = 15.6 Hz, 1H, H1); 7.06–7.53 (m, 9H, ArH); 7.71 (s, 1H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 23.7 (C-26), 24.8 (C-28), 25.5 (C-27), 28.2 (C-10), 31.1 (C-25), 31.9 (C-29), 39.1 (C-24), 45.6 (C-14), 47.4 (C-9), 51.2 (C-11), 62.9 (C-16), 72.4 (C-15), 109.9 (C-4), 117.1 (C-17), 117.8 (C-2), 124.1 (C-20), 124.7 (C-18), 124.8 (C-22), 125.3 (C-21), 126.2 (C-6), 128.5 (C-1), 133.8 (C-3), 135.2 (C-12), 135.8 (C-19), 144.5 (C-5), 150.2 (C-13), 158.6 (C-8), 164.2 (C-23), 182.0 (C-7). HRMS Calculated for C31H34N6O3 [M]+ 538.2692 found 538.2699; Anal. Calcd. (%) for: C, 69.12; H, 6.36; N, 15.60, found: C, 69.19; H, 6.28; N, 15.67.
1-[3-(4-{[1-(4-Fluoro-phenyl)-2-oxo-4-styryl-azetidin-3-ylamino]-methyl}-[1,2,3]triazol-1-yl)-propyl]-1H-indole-2,3-dione (6f)
Brick red colour, yield 81 %; IR (KBr) νmax: 1730, 1616 cm−1; mp 206–207 °C; 1H NMR (300 MHz CDCl3): δ 2.35 (dd, J = 6.6, 13.2 Hz, 2H, –CH2–); 3.84 (s, 2H, –CH2–); 4.10–4.21 (m, 2H, –CH2–); 4.25 (d, J = 5.1 Hz, 1H, H4); 4.46–4.63 (m, 2H, –CH2–); 4.73 (dd, J = 5.1, 8.1 Hz, 1H, H3); 6.24 (dd, J = 8.1, 15.9 Hz, 1H, H2); 6.58 (d, J = 8.1 Hz, 1H, ArH); 6.69 (d, J = 15.9 Hz, 1H, H1); 6.94–7.44 (m, 12H, ArH); 7.66 (s, 1H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 27.6 (C-10), 37.2 (C-14), 46.6 (C-9), 47.8 (C-11), 61.6 (C-16), 72.1 (C-15), 110.0 (C-4), 117.0 (C-17), 117.7 (C-2), 123.5 (C-26), 124.2 (C-25), 124.7 (C-20), 125.4 (C-18), 126.8 (C-22), 128.0 (C-21), 128.8 (C-6), 129.1 (C-1), 134.5 (C-3), 135.1 (C-12), 135.7 (C-19), 138.8 (C-24), 144.2 (C-5), 148.8 (C-27), 151.4 (C-13), 158.3 (C-8), 164.7 (C-23), 182.3 (C-7). HRMS calculated for C31H27FN6O3 [M]+ 550.2129 found 550.2122; Anal. Calcd. (%) for: C, 67.63; H, 4.94; N, 15.26, found: C, 67.70; H, 4.88; N, 15.31.
3-{Bis-[1-(3-[2,3-dioxo-1H-indol-1-yl]-ethyl)-1H-[1,2,3]triazol-4-ylmethyl]-amino}-4-styryl-1-p-tolyl-azetidin-2-one (7a)
Brick red colour, yield 75 %; IR (KBr) νmax: 1730, 1621 cm−1; mp >230 °C; 1H NMR (300 MHz CDCl3): δ 2.27 (s, 3H, -CH3); 3.73 (a pair of doublet, J = 15.0 Hz, 4H, 2x–CH2–); 4.10–4.21 (m, 5H, H4 + 2x–CH2–); 4.56 (dd, J = 5.1, 10.8 Hz, 4H, 2x–CH2–); 4.82 (dd, J = 5.1, 8.1 Hz, 1H, H3); 6.29 (dd, J = 8.4, 15.9 Hz, 1H, H2); 6.56 (d, J = 7.8 Hz, 2H, ArH); 6.69 (d, J = 16.2 Hz, 1H, H1); 6.97 (t, J = 7.8 Hz, 2H, ArH); 7.07 (d, J = 8.1 Hz, 2H, ArH); 7.25–7.47 (m, 11H, ArH); 7.68 (s, 2H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 20.8 (CH3), 40.6 (C-13), 45.3 (C-9), 47.5 (C-10), 61.3 (C15), 71.5 (C14), 109.4 (C-4), 117.0 (C-16), 117.4 (C-2), 124.0 (C-24), 124.7 (C-19), 124.8 (C-17), 125.5 (C-21), 126.6 (C-20), 128.3 (C-6), 128.7 (C-1), 129.5 (C-25), 133.9 (C-26), 135.1 (C-3), 135.3 (C-11), 135.9 (C-18), 138.5 (C-23), 144.3 (C-5), 149.9 (C-12), 158.4 (C-8), 164.7 (C-22), 182.5 (C-7). HRMS calculated for C44H38N10O5 [M]+ 786.3027 found 786.3034; Anal. Calcd. (%) for: C, 67.16; H, 4.87; N, 17.80, found: C, 67.07; H, 4.90; N, 17.87.
3-{Bis-[1-(3-[2,3-dioxo-1H-indol-1-yl]-ethyl)-1H-[1,2,3]triazol-4-ylmethyl]-amino}-1-cyclohexyl-4-styryl-azetidin-2-one (7b)
Brick red colour, yield 75 %; IR (KBr) νmax: 1739, 1614 cm−1; mp >230 °C; 1H NMR (300 MHz CDCl3): δ 1.27–1.98 (m, 10H, cyclohexyl), 3.47–3.62 (m, 1H, cyclohexyl), 3.71 (a pair of doublet, J = 15.0 Hz, 4H, 2x–CH2–); 4.11-4.22 (m, 4H, 2x–CH2–); 4.27 (d, J = 5.1 Hz, 1H, H4); 4.58 (dd, J = 5.4, 11.1 Hz, 4H, 2x–CH2–); 4.84 (dd, J = 5.1, 8.4 Hz, 1H, H3); 6.31 (dd, J = 8.4, 15.9 Hz, 1H, H2); 6.68 (d, J = 15.9 Hz, 1H, H1); 7.03–7.46 (m, 13H, ArH); 7.75 (s, 2H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 24.4 (C-25), 24.9 (C-27), 25.3 (C-26), 30.1 (C-24), 31.4 (C-28), 39.8 (C-23), 45.2 (C-13), 47.3 (C-9), 51.5 (C-10), 61.6 (C-15), 71.8 (C-14), 110.2 (C-4), 117.1 (C-16), 117.5 (C-2), 124.2 (C-19), 124.7 (C-17), 124.9 (C-21), 125.7 (C-20), 126.6 (C-6), 128.5 (C-1), 134.0 (C-3), 135.2 (C-11), 135.8 (C-18), 144.5 (C-5), 150.2 (C-12), 158.6 (C-8), 164.3 (C-22), 182.0 (C-7). HRMS calculated for C43H42N10O5 [M]+ 778.3340 found 778.3347; Anal. Calcd. (%) for: C, 66.31; H, 5.44; N, 17.98, found: C, 66.37; H, 5.39; N, 17.93.
3-{Bis-[1-(3-[2,3-dioxo-1H-indol-1-yl]-ethyl)-1H-[1,2,3]triazol-4-ylmethyl]-amino}-1-(4-fluoro-phenyl)-4-styryl-azetidin-2-one (7c)
Brick red colour, yield 66 %; IR (KBr) νmax: 1732, 1618 cm−1; mp >230 °C; 1H NMR (300 MHz CDCl3): δ 3.98 (s, 4H, –CH2–); 4.10–4.21 (m, 5H, H4 + 2x–CH2–); 4.39 (d, J = 5.1 Hz, 1H, H4); 4.56 (dd, J = 5.1, 10.8 Hz, 4H, 2x–CH2–); 4.92 (dd, J = 5.1, 8.4 Hz, 1H, H3); 6.32 (dd, J = 8.4, 15.6 Hz, 1H, H2); 6.50 (d, J = 7.8 Hz, 2H, ArH); 6.71 (d, J = 16.2 Hz, 1H, H1); 6.99-7.49 (m, 15H, ArH); 7.59 (s, 2H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 41.2 (C-13), 45.8 (C-9), 47.6 (C-10), 61.1 (C-15), 72.3 (C-14), 110.2 (C-4), 117.1 (C-16), 117.5 (C-2), 124.1 (C-25), 124.6 (C-24), 124.8 (C-19), 125.7 (C-17), 126.8 (C-21), 128.1 (C-20), 128.6 (C-6), 129.4 (C-1), 135.2 (C-3), 135.6 (C-11), 136.0 (C-18), 138.4 (C-23), 144.1 (C-5), 149.0 (C-26), 150.4 (C-12), 159.2 (C-8), 164.7 (C-22), 181.9 (C-7). HRMS calculated for C43H35FN10O5 [M]+ 790.2776 found 7990.2770; Anal. Calcd. (%) for: C, 65.31; H, 4.46; N, 17.71, found: C, 65.37; H, 4.53; N, 17.64.
3-{Bis-[1-(3-[2,3-dioxo-1H-indolw-1-yl]-propyl)-1H-[1,2,3]triazol-4-ylmethyl]-amino}-4-styryl-1-p-tolyl-azetidin-2-one (7d)
Brick red colour, yield 70 %; IR (KBr) νmax: 1737, 1610 cm−1; mp >230 °C; 1H NMR (300 MHz CDCl3): δ 2.26 (s, 3H, –CH3); 2.32 (dd, J = 6.6, 13.2 Hz, 4H, 2x–CH2–); 3.70–3.75 (m, 4H, –CH2–); 4.04–4.10 (m, 4H, 2x–CH2–); 4.27 (t, J = 6.6 Hz, 4H, 2x–CH2–); 4.66 (d, J = 5.1 Hz, 1H, H4); 4.82 (dd, J = 5.1, 8.1 Hz, 1H, H3); 6.31 (dd, J = 8.4, 15.9 Hz, 1H, H2); 6.68 (d, J = 15.9 Hz, 1H, H1); 6.87 (d, J = 8.4 Hz, 2H, ArH); 7.04-7.60 (m, 15H, ArH); 7.82 (s, 2H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 20.8 (CH3), 27.5 (C-10), 37.3 (C-14), 46.0 (C-9), 47.3 (C-11), 61.8 (C-16), 72.5 (C-15), 110.1 (C-4), 117.1 (C-17), 117.6 (C-2), 123.9 (C-25), 124.2 (C-20), 124.8 (C-18), 125.5 (C-22), 126.5 (C-21), 128.2 (C-6), 128.7 (C-1), 129.5 (C-26), 133.8 (C-27), 134.8 (C-3), 135.3 (C-12), 135.9 (C-19), 138.5 (C-24), 144.3 (C-5), 150.2 (C-13), 158.3 (C-8), 164.8 (C-23), 182.9 (C-7). HRMS calculated for C46H42N10O5 [M]+ 814.3340 found 814.3347; Anal. Calcd. (%) for: C, 67.80; H, 5.19; N, 17.19, found: C, 67.85; H, 5.24; N, 17.11.
3-{Bis-[1-(3-[2,3-dioxo-1H-indol-1-yl]-propyl)-1H-[1,2,3]triazol-4-ylmethyl]-amino}-1-cyclohexyl-4-styryl-azetidin-2-one (7e)
Brick red colour, yield 78 %; IR (KBr) νmax: 1739, 1614 cm−1; mp >230 °C; 1H NMR (300 MHz CDCl3): δ 1.29–1.97 (m, 10H, cyclohexyl), 2.34 (dd, J = 6.3, 12.9 Hz, 4H, 2x–CH2–); 3.46-3.61 (m, 1H, cyclohexyl); 3.84 (s, 4H, –CH2–); 4.15–4.26 (m, 4H, 2x–CH2–); 4.58 (m, 4H, 2x–CH2–); 4.67 (d, J = 5.1 Hz, 1H, H4); 4.91 (dd, J = 5.1, 8.7 Hz, 1H, H3); 6.28 (dd, J = 8.7, 15.6 Hz, 1H, H2); 6.70 (d, J = 15.6 Hz, 1H, H1); 7.01-7.51 (m, 13H, ArH); 7.63 (s, 2H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 24.1 (C-26), 24.6 (C-28), 25.5 (C-27), 27.9 (C-10), 30.8 (C-25), 31.6 (C-29), 39.3 (C-24), 45.1 (C-14), 47.5 (C-9), 51.7 (C-11), 62.8 (C-16), 72.1 (C-15), 109.8 (C-4), 117.2 (C-17), 117.7 (C-2), 124.0 (C-20), 124.6 (C-18), 124.8 (C-22), 125.4 (C-21), 126.3 (C-6), 128.4 (C-1), 133.7 (C-3), 135.3 (C-12), 135.9 (C-19), 144.6 (C-5), 150.1 (C-13), 158.8 (C-8), 164.1 (C-23), 182.3 (C-7). HRMS calculated for C45H46N10O5 [M]+ 806.3653 found 806.3645; Anal. Calcd. (%) for: C, 66.98; H, 5.75; N, 17.36, found: C, 66.90; H, 5.82; N, 17.49.
3-{Bis-[1-(3-[2,3-dioxo-1H-indol-1-yl]-propyl)-1H-[1,2,3]triazol-4-ylmethyl]-amino}-1-(4-fluoro-phenyl)-4-styryl-azetidin-2-one (7f)
Brick red colour, yield 69 %; IR (KBr) νmax: 1734, 1613 cm−1; mp >230 °C; 1H NMR (300 MHz CDCl3): δ 2.34 (dd, J = 6.0, 12.6 Hz, 4H, 2x–CH2–); 3.88 (s, 4H, –CH2–); 4.07–4.18 (m, 4H, 2x–CH2–); 4.33 (d, J = 5.1 Hz, 1H, H4); 4.56 (m, 4H, 2x–CH2–); 4.90 (dd, J = 5.1, 8.4 Hz, 1H, H3); 6.34 (dd, J = 8.4, 15.6 Hz, 1H, H2); 6.51 (d, J = 8.1 Hz, 2H, ArH); 6.68 (d, J = 15.6 Hz, 1H, H1); 7.04–7.46 (m, 15H, ArH); 7.62 (s, 2H, triazole-H); 13C NMR (CDCl3, 75 MHz): δ ppm = 27.4 (C-10), 41.5 (C-14), 45.9 (C-9), 47.1 (C-11), 62.4 (C-16), 72.0 (C-15), 110.3 (C-4), 117.2 (C-17), 117.6 (C-2), 124.2 (C-26), 124.6 (C-25), 124.8 (C-20), 125.5 (C-18), 126.7 (C-22), 128.0 (C-21), 128.5 (C-6), 129.5 (C-1), 135.1 (C-3), 135.4 (C-12), 136.3 (C-19), 138.1 (C-24), 144.5 (C-5), 148.4 (C-27), 149.8 (C-13), 159.1 (C-8), 164.4 (C-23), 182.3 (C-7). HRMS calculated for C45H39FN10O5 [M]+ 818.3089 found 818.3095; Anal. Calcd. (%) for: C, 66.00; H, 4.80; N, 17.11, found: C, 66.07; H, 4.74; N, 17.18.
Biological evaluation
In vitro protozoal parasite susceptibility assay
Protozoal parasites were cultured for 24 h at 37 °C. To perform the initial susceptibility screens on T. vaginalis, compounds were suspended in DMSO to obtain concentrations of 100 μM; 5 μL aliquots of these suspensions were diluted in 5 mL of TYM diamond’s media to obtain a final concentration of 100 μM. After 24 h, cells were counted using a hemacytometer. Cell counts were normalized to the DMSO controls, in order to allow direct comparison and averaging of the various trials. These data sets were then transformed using Prism Software by Graphpad, by taking the log of the drug concentrations for the trials, and inputting this transform into a log(inhibitor) versus response—variable slope regression option. Within this non-linear regression, constraints were set to force the maximum value (top) to 1 and the minimum value (bottom) to 0. The slope was left variable, and then determined through which regression was performed. The sample size consists of 4 independent trials carried out on four different days (to account for possible variation in parasite culture). The assays were performed in 15 mL culture tubes, with both WT and DMSO control tubes to normalize for the effects of the solvent and in vitro conditions. The IC50 value for active compounds were determined by running assays of increasing drug concentrations, 5–40 μM, and performing a regression analysis using Prism software, from GraphPad.
Cytotoxic evaluation of 6a, the most potent compound in the library, on cultured mammalian cells
The HeLa cells were maintained in Dulbecco’s modified eagle medium that contained 1 % penicillin/streptomycin and 10 % foetal bovine serum in a humified 5 % CO2 atmosphere at 37 °C. Doxorubicin, bleomycin, and 6a (the most potent compound in the library) were added to the medium of cells 24 h after culture. A trypan blue assay was used 24 h after drug treatment to calculate cell viability. This was done in three separate trials to ensure that cytotoxicity results were consistent. The accuracy of our cytotoxic assay was further validated by using etoposide as a positive control which exhibited an IC50 value of 0.61 μM comparable to its reported value (Travelli et al., 2011).
Result and discussions
Chemistry
The mono- and di-propargylated precursors 2 and 3 were prepared via our recently reported protocol involving the treatment of 3-amino-2-azetidinone 1 (Singh et al., 2011c), with 1.1 and 2.1 mmol of propargyl bromide, respectively. The treatment with 1.1 mmol of propargyl bromide led to a mixture of 2 and 3 in the ratio of 75:25, as evidenced by the 1H NMR analysis of the crude reaction mixture while the use of 2.1 mmol of propargyl bromide resulted in the isolation of exclusive di-propargylated product 3. The observed coupling constant J = 5.4 Hz between H3 and H4 confirmed the cis-stereochemistry of the products (Scheme 1).
N-alkyl azido isatin 5, another precursor required for the synthesis of target scaffolds, was prepared by an initial base-assisted N-alkylation of isatin with dibromoalkane followed by subsequent reaction with sodium azide in DMF at 60 °C (Scheme 2) (Singh et al., 2012).
The synthesized precursors 2 and 3 were utilized in the synthesis of desired mono- and bis-1H-1,2,3-triazole-tethered β-lactam–isatin conjugates. Thus, the reaction of 2 with 5 (1 mmol) in the presence of copper sulphate and sodium ascorbate in ethanol–water (10:1) mixture led to the isolation of 6 (Scheme 3), while the reaction of 3 with 5 (2 mmol) under similar conditions led to the formation of 7 in good to excellent yields (Scheme 4).
The structures to the hybrids 6 and 7 were assigned on the basis of spectral data and analytical evidence. Compound 7d, for example, showed a molecular ion peak [M]+ 814.8892 along with the characteristic peaks in 1H and 13C NMR spectra. The 1H NMR spectrum exhibited the presence of a singlet at δ 2.26 corresponding to methyl protons along with characteristic peaks at δ 2.32, 3.72, 4.05 and 4.27 corresponding to methylene protons, and a singlet at δ 7.82 corresponding to triazole ring proton. The presence of a requisite number of carbons in 13C NMR spectrum along with two characteristic peaks at δ 164.8 and 182.9 assigned to isatin ring carbonyls further corroborated the assigned structure.
In vitro activity against T. vaginalis
The synthesized mono- and bis-1H-1,2,3-triazole-tethered β-lactam–isatin conjugates were evaluated for their inhibitory influence on the axenic in vitro growth of T. vaginalis strain G3 cultured in TYM Diamond’s media for 24 h at 37 °C. Table 1 lists the data obtained from the initial percentage inhibition screens at 50 μM. As evident from Table 1, the activity profiles of test compounds showed dependence on the substituent at N-1 of β-lactam ring and the presence of single/double 1H-1,2,3-triazole linker. The increase in spacer length from n = 1 to n = 2 does not have any considerable effect on the efficacy of test compounds.
The most potent of the test compounds viz. 6a and 6d have been selected from % age inhibition data for determining their IC50 values, which is defined as the minimum concentration required for 50 % growth inhibition. These compounds have exhibited an IC50 values of 10.49 (6a) and 25.60 μM (6d), respectively, as shown in Table 2, while their dose–response curves are depicted in Fig. 3.
The most potent compound 6a was then further evaluated for its cytotoxicity against HeLa cells. Results of these cytotoxicity tests consistently showed between 80 and 90 % viability compared with untreated and DMSO-treated cells. The same passage of cells was also tested with bleomycin and doxorubicin (at the same concentration) as posititive controls for toxicity. We also carried out these assays on three independent days with multiple trials in each experiment. Compound 6a consistently showed comparable toxicities to untreated and DMSO-treated HeLa cells when tested at 10 μM.
Conclusion
The present communication describes the synthesis of mono- and bis-1H-1,2,3-triazole-tethered β-lactam–isatin conjugates along with their preliminary in vitro evaluation against T. vaginalis at 50 μM. The synthesized scaffolds have shown a preference for a p-tolyl substituent at N-1 of β-lactam ring for good activity with the most potent and non-cytotoxic compounds 6a and 6d exhibiting an IC50 of 10.49 and 25.60 μM, respectively. However, the exact inhibition site (β-lactam or isatin) responsible for the activity of these conjugates is still uncertain and further studies are currently underway.
References
Alcaide B, Almendros P, Aragoncillo C (2007) Beta-lactams: versatile building blocks for the stereoselective synthesis of non-beta-lactam products. Chem Rev 107:4437–4492
Bal TR, Anand B, Yogeeswari P, Sriram D (2005) Synthesis and evaluation of anti-HIV activity of isatin beta-thiosemicarbazone derivatives. Bioorg Med Chem Lett 15:4451–4455
Bock VD, Perciaccante R, Jansen TP, Hiemstra H, Maarseveen JH (2006) Click chemistry as a route to cyclic tetrapeptide analogues: synthesis of cyclo-[Pro-Val-psi(triazole)-Pro-Tyr]. Org Lett 8:919–922
Casey LA, Galt R, Page MI (1993) The mechanisms of hydrolysis of the y-lactam lsatin and its derivatives. J Chem Soc Perkin Trans 2:23–28
Chen LR, Wang YC, Lin YW, Chou SY, Chen SF, Liu LT, Wu YT, Kuo CJ, Chen TS, Juang SH (2005) Synthesis and evaluation of isatin derivatives as effective SARS coronavirus 3CL protease inhibitors. Bioorg Med Chem Lett 15:3058–3062
D’hooghe M, Dekeukeleire S, Leemans E, De Kimpe N (2010) Use of functionalized β-lactams as building blocks in heterocyclic chemistry. Pure Appl Chem 82:1749–1759
D’hooghe M, Mollet K, De Vreese R, Jonckers TH, Dams G, De Kimpe N (2012) Design, synthesis, and antiviral evaluation of purine-β-lactam and purine-aminopropanol hybrids. J Med Chem 55:5637–5641
Ding K, Lu Y, Nikolovska-Coleska Z, Qui S, Ding Y, Gao W, Stuckey J, Krajewski K, Roller PP, Tomita Y, Parrish DA, Deschamps JR, Wang S (2005) Structure-based design of potent non-peptide MDM2 inhibitors. J Am Chem Soc 127:10130–10131
Fensome A, Adams WR, Adams AL, Berrodin TJ, Cohen J, Huselton C, Illenberger A, Karen JC, Hudak VA, Marella MA, Melenski EG, McComas CC, Mugford CA, Slayeden OD, Yudt M, Zhang Z, Zhang P, Zhu Y, Winneker RC, Wrobel JE (2008) Design, synthesis, and SAR of new pyrrole-oxindole progesterone receptor modulators leading to 5-(7-fluoro-3,3-dimethyl-2-oxo-2,3-dihydro-1H-indol-5-yl)-1-methyl-1H-pyrrole-2-carbonitrile (WAY-255348). J Med Chem 51:1861–1873
Guenthner PC, Secor WE, Dezzutti CS (2005) Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infect Immun 73:4155–4160
Gutierrez A, Laureti L, Crussard S, Abida H, Rodríguez-Rojas A, Blázquez J, Baharoglu Z, Mazel D, Darfeuille F, Vogel J, Matic I (2013) β-lactam antibiotics promote bacterial mutagenesis via an RpoS-mediated reduction in replication fidelity. Nat Commun 4:1610
Hadfield PS, Casey LA, Galt RHB, Vilanova B, Page MI (2002) Imide and isatin derivatives as γ-lactam mimics of β-lactam antibiotics. Arkivoc vi:125–144
Igosheva N, Lorz C, O’Conner E, Glover V, Mehmet H (2005) Isatin, an endogenous monoamine oxidase inhibitor, triggers a dose- and time-dependent switch from apoptosis to necrosis in human neuroblastoma cells. Neurochem Int 47:216–224
Jarrahpour A, Ebrahimi E, Khalifeh R, Sharghi H, Sahraei M, Sinou V, Latour C, Brunel JM (2012) Synthesis of novel β-lactams bearing an anthraquinone moiety, and evaluation of their antimalarial activities. Tetrahedron 68:4740–4744
Kolb HC, Sharpless KB (2003) The growing impact of click chemistry on drug discovery. Drug Discov Today 8:1128–1137
Kumar K, Singh P, Kremer L, Guérardel Y, Biot C, Kumar V (2012) Synthesis and in vitro anti-tubercular evaluation of 1,2,3-triazole tethered β-lactam-ferrocene and & #x03B2;-lactam-ferrocenylchalcone chimeric scaffolds. Dalton Trans 41:5778–5781
Kumar K, Carrère-Kremer S, Kremer L, Guérardel Y, Biot C, Kumar V (2013) 1H-1,2,3-Triazole-tethered isatin–ferrocene and isatin–ferrocenylchalcone conjugates: synthesis and in vitro anti-tubercular evaluation. Organometallics 32:5713–5719
Kumari G, Nutan ModiM, Gupta SK, Singh RK (2011) Rhodium(II) acetate-catalyzed stereoselective synthesis, SAR and anti-HIV activity of novel oxindoles bearing cyclopropane ring. Eur J Med Chem 46:1181–1188
Liang GB, Qian X, Feng D, Fisher M, Crumley T, Darkin-Rattray SJ, Dulski PM, Gurnett A, Leavitt PS, Liberator PA, Misura AS, Samaras S, Tamas T, Schmatz DM, Wyvratt M, Biftu T (2008) N-alkyl-4-piperidinyl-2,3-diarylpyrrole derivatives with heterocyclic substitutions as potent and broad spectrum anticoccidial agents. Bioorg Med Chem Lett 18:2019–2022
Ma J, Li S, Reed K, Guo P, Gallo JM (2003) Pharmacodynamic-mediated effects of the angiogenesis inhibitor SU5416 on the tumor disposition of temozolomide in subcutaneous and intracerebral glioma xenograft models. Ther J Pharmacol Exp 305:833–839
McClelland RS, Sangare L, Hassan WM (2007) Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 195:698–702
McLaren LC, Davis LE, Healy GR, James CG (1983) Isolation of Trichomonas vaginalis from the respiratory tract of infants with respiratory disease. Pediatrics 71:888–890
Meunier B (2008) Hybrid molecules with a dual mode of action: dream or reality? Acc Chem Res 41:69–77
Morphy JR, Rankovic Z (2006) The physicochemical challenges of designing multiple ligands. J Med Chem 49:4961–4970
Muregi FW, Ishih A (2010) Next-generation antimalarial drugs: hybrid molecules as a new strategy in drug design. Drug Dev Res 71:20–32
Nisha MehraV, Hopper M, Patel N, Hall D, Wrischnik LA, Land KM, Kumar V (2013) Design and synthesis of β-amino alcohol based β-lactam–isatin chimeras and preliminary analysis of in vitro activity against the protozoal pathogen Trichomonas vaginalis. MedChemComm 4:1018–1024
O’Boyle NM, Greene LM, Keely NO, Wang S, Cotter TS, Zisterer DM, Meegan MJ (2013) Synthesis and biochemical activities of antiproliferative amino acid and phosphate derivatives of microtubule-disrupting β-lactam combretastatins. Eur J Med Chem 62:705–721
O’Driscoll M, Greenhalgh K, Young A, Turos E, Dickey S, Lim DV (2008) Studies on the antifungal properties of N-thiolated β-lactams. Bioorg Med Chem 16:7832–7837
Palomo C, Aizpurua JM, Ganboa I, Oiarbide M (1999) Asymmetric synthesis of β-lactams by Staudinger ketene–imine cycloaddition reaction. Eur J Org Chem 12:3223–3235
Palomo C, Aizpurua JM, Ganboa I, Oiarbide M (2004) Asymmetric synthesis of β-lactams through the Staudinger reaction and their use as building blocks of natural and nonnatural products. Curr Med Chem 11:1837–1872
Prenen H, Cools J, Mentens N, Folens C, Sciot R, Schoffski P, Van Oosterom A, Marynen P, Debiec-Rychter M (2006) Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin Cancer Res 8:262–2627
Quenelle DC, Keith KA, Kern ER (2006) In vitro and in vivo evaluation of isatin-beta-thiosemicarbazone and marboran against vaccinia and cowpox virus infections. Antiviral Res 71:24–30
Raj AA, Raghunathan R, Sridevikumaria MR, Raman N (2003) Synthesis, antimicrobial and antifungal activity of a new class of spiro pyrrolidines. Bioorg Med Chem 11:407–419
Raj R, Biot C, Carrère-Kremer S, Kremer L, Guérardel Y, Gut J, Rosenthal PJ, Kumar V (2013a) 4-aminoquinoline-β-lactam conjugates: synthesis, antimalarial and anti-tubercular evaluation. Chem Biol Drug Des 83(2):191–197. doi:10.1111/cbdd.12225
Raj R, Singh P, Haberkern NT, Faucher RM, Patel N, Land KM, Kumar V (2013b) Synthesis of 1H-1,2,3-triazole linked β-lactam–isatin bifunctional hybrids and preliminary analysis of in vitro activity against the protozoal parasite Trichomonas vaginalis. Eur J Med Chem 63:897–906
Raj R, Singh P, Singh P, Gut J, Rosenthal PJ, Kumar V (2013c) Azide-alkyne cycloaddition en route to 1H-1,2,3-triazole-tethered 7-chloroquinoline–isatin chimeras: synthesis and antimalarial evaluation. Eur J Med Chem 62:590–596
Rao BG, Bandarage UK, Wang T, Come JH, Perola E, Wei Y, Tian SK, Saunders JO (2007) Novel thiol-based TACE inhibitors: rational design, synthesis, and SAR of thiol-containing aryl sulphonamides. Bioorg Med Chem Lett 17:2250–2253
Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, Gonzalez-Paez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT (2010) Spiroindolones, a potent compound class for the treatment of malaria. Science 329:1175–1180
Singh GS (2003) Recent progress in the synthesis and chemistry of azetidinones. Tetrahedron 59:7631–7649
Singh GS, D’hooghe M, De Kimpe N (2011a) Synthesis and reactivity of spiro-fused β-lactams. Tetrahedron 67:1989
Singh P, Raj R, Kumar V, Mahajan MP, Bedi PMS, Kaur T, Saxena AK (2011b) 1,2,3-Triazole tethered β-lactam-chalcone bifunctional hybrids: synthesis and anticancer evaluation. Eur J Med Chem 47:594–600
Singh P, Sachdeva S, Raj R, Kumar V, Mahajan MP, Nasser S, Vivas L, Gut J, Rosenthal PJ, Feng TS, Chibale K (2011c) Antiplasmodial and cytotoxicity evaluation of 3-functionalized 2-azetidinone derivatives. Bioorg Med Chem Lett 21:4561–4563
Singh P, Sharma P, Anand A, Bedi PMS, Kaur T, Saxena AK (2012) Azide-alkyne cycloaddition en route to novel 1H-1,2,3-triazole tethered isatin conjugates with in vitro cytotoxic evaluation. Eur J Med Chem 55:455–461
Soper D (2004) Trichomoniasis: under control or undercontrolled? Am J Obstet Gynecol 190:281–290
Sorvillo F, Smith L, Kerndt P, Ash L (2001) Trichomonas vaginalis, HIV, and African-Americans. Emerg Infect Dis 7:927–932
Takai S, Jin D, Muramatsu M, Okamoto Y, Miyazaki M (2004) Therapeutic applications of chymase inhibitors in cardiovascular diseases and fibrosis. Eur J Pharm 501:1–8
Travelli C, Drago V, Maldi E, Kaludercic N, Galli U, Boldorini R, Lisa FD, Tron GC, Canonico PL, Genazzani AA (2011) Reciprocal potentiation of the antitumoral activities of FK866, an inhibitor of nicotinamide phosphoribosyltransferase, and etoposide or cisplatin in neuroblastoma cells. J Pharmacol Exp Ther 338:829–840
Upcroft JA, Dunn L, Wright J, Benakli K, Upcroft P, Vanelle P (2006) 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob Agents Chemother 50:344–347
upcroft JA, Dunn LA, Wal T, Tabrizi S, Delgadillo-Correa MG, Johnson PJ, Garland S, Siba P, Upcroft P (2009) Metronidazole resistance in Trichomonas vaginalis from highland women in Papua New Guinea. Sex health 6:334–338
van der Pol B, Kwok C, Pierre-Louis B et al (2008) Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis 197:548–554
Verma M, Pandeya SN, Singh KN, Stables JP (2004) Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharm 54:49–56
Vintonyak VV, Warburg K, Kruse H, Grimme S, Hubel K, Rauh D, Waldmann H (2010) Identification of thiazolidinones spiro-fused to indolin-2-ones as potent and selective inhibitors of the Mycobacterium tuberculosis protein tyrosine phosphatase B. Angew Chem Int Ed 49:5902
Wang Q, Chittaboina S, Barnhill HN (2005) Advances in 1,3-dipolar cycloaddition reaction of azides and alkynes—a prototype of click chemistry. Lett Org Chem 2:293–301
World Health Organization (2012) Global incidence and prevalence of selected curable sexually transmitted infections—2008. Geneva, Switzerland. http://apps.who.int/iris/handle/10665/75181. Accessed 22 Oct 2013
Wright JM, Webb RI, O’donoghue P, upcroft P, upcroft JA (2010) Hydrogenosomes of laboratory-induced metronidazole-resistant Trichomonas vaginalis lines are downsized while those from clinical metronidazole-resistant isolates are not. J Eukaryo Microbiol 57:171–176
Acknowledgments
Financial assistance from Board of Research in Nuclear Sciences under DAE Research Award for Young Scientist Scheme (VK) is gratefully acknowledged. KML was supported by the Office of Sponsored Programs and Research, and the Department of Biological Sciences, University of the Pacific.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raj, R., Sharma, V., Hopper, M.J. et al. Synthesis and preliminary in vitro activity of mono- and bis-1H-1,2,3-triazole-tethered β-lactam–isatin conjugates against the human protozoal pathogen Trichomonas vaginalis . Med Chem Res 23, 3671–3680 (2014). https://doi.org/10.1007/s00044-014-0956-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-0956-6