Abstract
Plant reproduction in alpine environments is affected by climate both directly through climate impacts on growth and phenology, and indirectly through impacts on the biotic interactions affecting pollination success. These effects can be highly variable in time and space. In this study we investigated how different abiotic and biotic factors influence reproductive investment and success in populations of Ranunculus acris across an alpine landscape over a two-year period. In an alpine area at Finse, southern Norway, we measured reproductive investment (total seed mass) and reproductive success (seed-set rate) in 38 sites differing in temperature (related to elevation) and length of the growing season (related to time of snowmelt). To assess biotic interactions, we measured floral density and pollinator visits and conducted a supplemental pollen experiment. Reproductive investment and success increased with temperature, but only when floral density and/or number of pollinator visits was high, and only in the warmer year (2016). Reproduction in R. acris was pollen-limited in both years, especially at warmer temperature and in sites with early snowmelt. Pollinator visits increased with temperature and with higher floral density, suggesting a shift in relative importance of the biotic factors (from plants to pollinators) in limiting reproduction with increasing temperature. Our study shows that reproductive investment and success in R. acris is affected by climate through the interactive effects of abiotic and biotic processes. These effects vary between years and across the landscape, suggesting a potential for larger-scale buffering of climate change effects in heterogeneous landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mountain ecosystems are characterised by having cold and often short growing seasons, and temperature-related climatic factors are known to be the major limiting factors of the growth, phenology, and reproduction of alpine plants (Körner 2003). Accordingly, a number of studies have shown the importance of different climate factors, such as temperature and the timing of snowmelt (Jonas et al. 2008; Delnevo et al. 2018; Rixen et al. 2022) on different aspects of alpine plants’ reproductive success. These climatic factors can affect the reproductive success of plants directly, through physiological constraints on flowering and seed maturation (Billings 1987). Additionally, several studies have shown that climate can also affect reproductive success indirectly, through shifting biotic interactions such as pollination (Totland 1994a; Urbanowicz et al. 2018). The small-scale topography in alpine areas creates large variation in abiotic conditions (i.e. timing of snowmelt and growing season temperature) within short distances (Scherrer and Körner 2011), and these conditions can also vary considerably between years, depending on temperature and snowfall (Rixen et al. 2022). This results in a lot of variation in these abiotic factors, and therefore in the potential net outcome of direct and indirect (via biotic interactions) climate constraints on plant reproduction, in space and time. While a large number of studies have demonstrated the importance of both direct and indirect effects of climate on the reproductive success of alpine plants (Totland and Eide 1999; Urbanowicz et al. 2018), and broad-scale patterns in these effects across climatic gradients (Totland 1997; Lazaro et al. 2009), fewer studies have investigated how these different effects may vary and interact at smaller scales, such as across years or along fine-scale gradients within landscapes (but see: Pardee et al. 2019; Kudo 2021).
Temperature is highly variable in time and space, as is the timing of snowmelt, resulting in considerable fine-scale variation in the climatic conditions that alpine plants are exposed to. As temperatures are generally low and unstable in spring, and increase towards peak growing season, the rate of development in plants will also vary over the growing season (Wipf et al. 2006; Inouye 2008). Early flowering plants thus have more time to complete their life cycle during the growing season, but are often exposed to harsh climatic conditions, while late flowering plants often experience more favourable climatic conditions but have less time and therefore often require faster development to successfully reproduce during the short alpine summer (Kudo and Suzuki 1999; Huelber et al. 2006; Delnevo et al. 2018). The amount of winter precipitation and in combination with the temperature early in the growing season also affects the timing of snowmelt, which in turn determines how much time plants have to grow, flower and develop mature seeds (Kudo and Suzuki 1999; Huelber et al. 2006).
As most alpine plants are obligate outcrossers, and depend on insects as pollen vectors for their reproduction (Kudo 2022), climatic factors that affect pollinator activity or pollination success may also affect plant reproductive success. We refer to these as biotic, or indirect (via biotic interactions) climate effects. The main pollinators in mountains are flies and bumble bees, with flies becoming increasingly important with elevation (Totland 1993). Alpine pollinators overwinter underground and exit their dormancy shortly after snowmelt (Høye and Forchhammer 2008). Pollinator abundance is therefore often low early in the season and increases towards peak season, correlating well with increased abundance of flowering plants in the middle of the growing season (Mizunaga and Kudo 2017). Alpine areas often have heterogenous topography resulting in variation in the timing of snowmelt affecting the flowering period of plants, which provides pollinating insects with floral resources for long periods over relatively short distances. This availability of patches with high floral density throughout the growing season can thus attract pollinators and facilitate pollination in alpine plants (Hegland et al. 2009a). However, if floral densities are too high, intraspecific competition for pollination can result in pollen limitation for individual flowers (Garcia-Camacho and Totland 2009). In addition, pollinator activity and efficiency depend strongly on temperature and the weather, and decreases at low temperatures, with wind and rain (Sieber et al. 2011). Pollen limitation can thus occur due to lack of pollinators in unfavourable weather, which may be independent of or in concurrence with direct abiotic climate limitations, such as low temperatures preventing the development of mature seeds (Zimmerman and Pyke 1988; Totland and Eide 1999; Totland 2001). By studying the complex interactions between abiotic and biotic factors along small-scale gradients, over time, we can get a better understanding of the contemporary challenges on plant phenology and reproduction, but also future challenges that will arise with climate change.
Temperature and precipitation are generally increasing under climate change, and how that affects snowmelt is highly uncertain (IPCC 2023). Contrary to other regions, at high elevations in Nordic oceanic mountains a higher accumulation of snow is expected, which can lead to later snowmelt (Hanssen-Bauer et al. 2017). Natural climatic gradients can act as a space-for-time substitution to study responses to climate change (Pickett 1989), while experimental approaches provide mechanistic understanding of short-term responses to climate (Dunne et al. 2004). To study the complex interactions of abiotic and biotic factors in alpine habitats we need long-term and integrated approaches that combine experiments across climate gradients and years (Dunne et al. 2003; Rustad 2008; Borer et al. 2014; Delnevo et al. 2018). The small-scale heterogeneity in alpine areas exposes plants to different timing of snowmelt, temperatures, and biotic factors throughout the growing season (Stanton et al. 1994), and provide suitable natural gradients to make predictions for long-term consequences of human induced climate change on plant populations and communities (Dunne et al. 2004; Fukami and Wardle 2005; Blois et al. 2013).
The aim of this study is to identify the direct abiotic and indirect (via biotic interactions) climatic factors regulating the phenology and reproductive investment and success in the widespread and functionally important alpine forb Ranunculus acris L. Due to its high local floral density, R. acris is an important floral resource for alpine pollinator communities across the boreal and arctic biomes. The reproduction of R. acris is well studied, including variation across broad-scale climate gradients (i.e. along elevational gradients; Totland 1994b, 1997, 1999). However we lack knowledge of how reproductive success is affected by small-scale climate variability, and in particular in Nordic oceanic mountains, where the change in timing of snowmelt is uncertain (Hanssen-Bauer et al. 2017). Over two years, we studied phenology, floral density of R. acris, and pollinator visits in 38 sites along local snowmelt and temperature gradients in Finse, southern Norway, and combined these with a hand pollination experiment along the gradients to test for variation in pollen limitation. At the end of each growing season, we measured reproductive investment (total seed mass) and reproductive success (seed-set rate). Specifically, we investigate how abiotic factors (timing of snowmelt and temperature) govern the timing of peak flowering in R. acris, and if biotic factors (visitation rate, pollen limitation, and floral density of surrounding R. acris individuals) interact with abiotic factors to affect reproductive investment and success. Combining studies along local snowmelt and temperature gradients with an experiment to assess pollen limitation will give insights into the selective pressures R. acris is experiencing and understand how the heterogenous alpine topography and climate is shaping the resources available for pollinator communities (Graae et al. 2018).
Materials and methods
Study area and study species
The study was conducted over two growing seasons (2016 and 2017) at Mount Sanddalsnuten (60.6136 °N, 7.5213 °E), near Finse in southwestern Norway (Fig. 1). The area has an alpine-oceanic climate (Moen, 1998), with an average annual temperature of − 2.2 ℃, and an average summer temperature of 6.3 ℃ (June - August). The annual and summer (June – August) precipitation for the area is 990 mm and 257 mm, respectively (www.seklima.met.no).
The study area is situated in the mid-alpine zone, between 1411 and 1489 m a.s.l., in a south-west facing slope. The growing season is short, and snowmelt normally starts in late May, with the whole mountain slope being free of snow by mid-July. As the bedrock is dominated by calcareous phyllite, it supports a species-rich heath community, where common species are Dryas octopetala, Bartsia alpina, Bistorta vivipara, Thalictrum alpinum and Ranunculus acris. In addition to being one of the most common species, R. acris is also very attractive and an important pollen source for the local pollinator community (Totland 1993, 1994a). R. acris is a non-clonal self-incompatible species, pollinated by dipteran insects in alpine area (Totland 1994b).
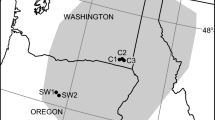
Map of the study area with all 38 sites. The colour represents the average snowmelt day-of-year (DOY) for the whole study period. Most sites were used in both years, while some were only used in one year (dashed line around circle; see methods for details). Due to the heterogeneous topography, the timing of snowmelt varies strongly and independently of elevation across the study area. Contour intervals = 10 m, contour intervals within the box = 1 m. Inset map shows map of Southern Norway with Finse marked in red
Study design
The heterogenous topography in the area naturally causes the snow to melt at different times and also exposes the plants to different temperatures during the growing season, because it is generally colder earlier in the season (Wipf et al. 2006; Inouye 2008). In spring 2016, we established 30 study sites that differed in the timing of snowmelt in which R. acris was abundant (Fig. 1). The sites were selected in three stages with two weeks difference to get sites with a large variation in the timing of snowmelt. The same sites were used in 2017, except for ten sites that had very few flowers due to very late snowmelt in 2016, these were therefore replaced with eight new sites, flowering ca. 2 weeks earlier than the earliest sites from 2016 (2016: n = 30; 2017: n = 28; total across both years: n = 38 see Appendix A for snowmelt dates from all sites).
At each snowmelt stage we established five plots, resulting in 150 plots in 2016 and 140 plots in 2017. Each established plot was marked with metal tubes, divided into two 0.5 × 0.5 m sub-plots, approximately 10 cm apart. The subplots were placed next to each other, where one subplot was used for a pollen supplemental experiment (see below) and the other subplot was left unmanipulated as a control.
Data collection
We calculate growing degree days to obtain the thermal requirement plants use for seed development, using daily mean temperature data, between snowmelt and the last seed collection in 2016 and 2017, from a local weather station (Finsevatn SN25830; 1210 m a.s.l., 2 km south from study site) from the Norwegian Meteorological Institute (http://www.seklima.met.no/). Because the weather station was located c. 250 m below our study sites, we adjusted the daily mean temperature at each site according to the global average adiabatic lapse rate, using a 0.6 ℃ reduction in temperature per 100 m elevation (Körner 2003). To test if the lapse rate correction was appropriate for our sites, we calculated the adiabatic lapse rate of the study sites located between 1411 and 1489 m a.s.l., using temperature data from 30 iButtons (DS1922L-F5) installed at each site in 2016. The iButtons were placed inside a mesh bag under two white plastic cones, to prevent the logger from direct sunlight (Holden et al. 2013) and hung from a stick 0.5 m above ground. The adiabatic lapse rate for our sites measured by local climate loggers was − 0.51 ± 0.14 (t = -3.5, P < 0.001), being within the global average lapse rate.
We calculated the growing degree days above 0 °C for seed development by summing the daily mean temperatures above 0 ℃ from the time the snow melted to the time seeds were ripe and ready to be collected. We selected the threshold of 0 ℃ because many alpine plants can grow at temperatures as low as 0 °C (Wipf et al. 2009; Steinacher and Wagner 2012).
We assessed flowering phenology and floral density of R. acris (i.e. conspecific density) and counted all flowering individuals within each plot (0.5 m2) every other day during the growing season (2016: from 17 June to 18 August; 2017: from 11 June to 22 August). The timing of peak flowering was calculated as the day of the year with the highest number of flowers per site. To estimate floral density, which we used as a correlate of pollinator attraction or competition for pollinators, we calculated the mean number of flowering individuals per site over the whole period of flowering (Dauber et al. 2010).
Pollinator visits of R. acris, which we used as a potential correlate of pollen limitation, were recorded for each site over the whole flowering period. Observations were done on days with no rain and low wind, between 09:30 and 17:00. One observation consisted of counting all pollinator visits to R. acris flowers at one site for a period of five minutes. Up to three such observations were done per site, spread across the day. The pollinators were identified to order (Diptera and Lepidoptera). Mean pollinator visits were calculated for each growing season per site. A total of 235 observations were conducted in 2016 and 396 observations in 2017.
We experimentally hand pollinated individuals of R. acris to test for pollen limitation. Four individual plants per plot were marked and randomly assigned to one of two treatments: supplemental pollination (i.e. natural and supplemental pollination; n = 355) or untouched controls (i.e. only natural pollination; n = 401). In a few cases where too few R. acris individuals were found within the plots, individuals were selected within 0.5 m outside the plot. The first flowering individuals of R. acris in the plots were avoided because they are often sterile (Ø. Totland, pers. com). The four individuals per plot were selected to be in the same developmental stage, i.e., ready to receive pollen, to ensure that all individuals from the same plot had approximately the same time for seed development. For the supplemental pollination treatment, we applied pollen from three anthers from five different individuals found in the surroundings of the plot to one flower of each R. acris individual. The supplemental pollination was applied on two different days to increase the chance of pollination success. Almost all individuals of R. acris had only one flower and for consistency we therefore decided to only pollinate one flower per individual. The control plants were naturally pollinated, and we did not have bagged flowers to assess the efficiency of the supplemental pollination treatment.
Seeds from the marked individuals in both years were collected and left to air dry in the lab for two to four weeks. In 2016, the number of ripe seeds and the number of ovules in each gynoecium were counted separately. In both years, total seed mass of both mature seeds and undeveloped seeds including unfertilized ovules was weighed for individual flowers, and it was defined as reproductive investment. In 2016, the proportion of ovules that developed into mature seeds (i.e., seed-set rate) per individual flower was counted, and it was defined as reproductive success.
Statistical analyses
We tested how abiotic and biotic factors affect reproductive investment and success in R. acris in two steps. First, we ran a model including only the climate factors (timing of snowmelt, growing degree days above 0 °C, hereafter temperature, and their interaction) to test for the direct abiotic effects of climate (abiotic model). Second, we ran three models, each including the interaction of the two abiotic climate factors and one biotic factor at a time (floral density, pollinator visits, or supplemental pollination) to test for effects of, or climate interactions with, biotic processes related to pollination (attraction of or competition for pollinators; observational and experimental assessment of pollen limitation; abiotic and biotic models). We were interested in the interaction between each abiotic and one biotic factor and did not include the three-way interaction.
For this, we used linear mixed-effects models (LMM) and generalised linear mixed-effects models (GLMM) in the nlme and lme4 package (Bates et al. 2015; Pinheiro and Bates 2023) in R version 4.2.2 (R Core Team 2022). We fitted separate models with reproductive investment (total seed mass) as response for 2016 and 2017, and one model with reproductive success (seed-set rate) as response from 2016. For the analysis of reproductive investment we used a LMM, with a normal error distribution, and homoscedasticity was achieved after log transforming the response variable. For the analysis of reproductive success, we used a GLMM with a binomial error distribution, and weighted by the total number of ovules. To test what affected pollinator visits on the day of observation we used a LMM, with a normal error distribution, which is justified when the mean is high enough and approximates a normal error distribution. Because all predictor variables were on different scales, we rescaled all numeric predictors to mean 0 and standard deviation 1, to avoid convergence issues in the models.
Finally, because pollinator visits also depend on the timing of snowmelt, temperature and floral density, we tested their relationship. For this, we ran an additive LMM, with a normal error distribution, and total pollinator visits as response, as well as timing of snowmelt, mean daily temperature, and daily number of flowers as predictors. We did not expect a linear relationship with timing of snowmelt and thus included both a linear and quadratic term.
The data are available on the Open Science Framework data repository (Halbritter et al. 2024) and the code to process and analyse the data is available on GitHub: https://github.com/linnvassvik/Mismatch2022.
Results
Annual variations in abiotic and biotic factors and reproduction
Snowmelt was on average 11.1 days earlier in 2017 (P < 0.001), mean daily temperature was on average 0.7 °C higher in 2016 compared to 2017 (t120 = 3.07, P < 0.002; Fig. 2a, b) and R. acris plants reached peak flowering 12 days earlier in the first year (difference is calculated from the 20 sites shared between years). In 2017, the floral density of R. acris was on average 3.1 times higher during the whole growing season compared to 2016 (t629 = 6.2, P < 0.001, n2016 = 5563, n2017 = 17,295; Fig. 2c). R. acris plants were almost exclusively visited by dipteran insects in 2016 (Diptera: 99,6% of the visits, 0,4% Lepidoptera, belonging to the families Zygaenidae and Argynnini). In 2017, we only observed dipteran insects. Pollinator visits were 1.9 times higher in 2016 compared to 2017 (t292 = 10.89, P < 0.001).
R. acris plants that emerged early in the growing season, corresponding with early timing of snowmelt, required higher cumulative temperature to reach peak flowering, and thus had a longer pre-flowering period (i.e. time between snowmelt and peak flowering; Fig. 2d). In contrast, plants growing in late-snowmelt sites and thus emerging later in the growing season, required less cumulative temperature, reaching peak flowering faster. The time from pollination (i.e. the timing of supplemental pollination) to mature seeds was on average 5.6 days longer in the first year (2016: 34.9 days, 2017: 29.3 days). The average reproductive investment (total seed mass) was 1.6 times higher in 2016 (9 ± 6 mg) compared to 2017 (6 ± 7 mg, t680 = 7.4, P < 0.001).
(a) Daily mean temperature across the growing season in day of the year (DOY) for 2016 (rose circle) and 2017 (violet triangle). (b) Mean growing season temperature for both years. (c) Floral density of Ranunculus acris across the growing season in day of the year (DOY) for 2016 (rose circle) and 2017 (violet triangle). The lines are drawn by geom_smooth function in the ggplot2 package. (d) Temperature (degree days above 0 °C) versus the number of days between snowmelt and peak flowering (days since snowmelt) in both years. The colour gradient indicates the time of snowmelt in days of the year (DOY). Dashed line around symbols indicates sites only used in one of the years
Reproductive investment is affected indirectly by climate via biotic factors
The abiotic model showed that reproductive investment (total seed mass) in 2016 decreased with higher temperature (t130 = -2.25, P = 0.026; Fig. 3a; Table 1a), while abiotic factors alone did not affect reproductive investment in 2017 (Fig. 3b; Table 1a).
Log transformed reproductive investment (total seed mass) with increasing growing degree days above 0 °C for early (yellow) and late (red) snowmelt. The points show the raw data while the line is the model prediction with confidence intervals. Line type indicates non-significant (dashed) relationship between temperature and reproductive investment. Day of the year (DOY) is shown in parentheses in the colour legend
When also accounting for biotic factors (abiotic and biotic models), we found that floral density in 2016 and supplemental pollination depended on temperature (i.e. interactions between abiotic and biotic factors; Fig. 4; Table 1). Reproductive investment was higher with increasing temperature, but only when floral density was high, and only in 2016 (interaction of temperature and floral density: t130 = 1.99, P = 0.049; Fig. 4a and b; Table 1b). In 2016, when accounting for pollinator visits, plants growing early in the season had higher reproductive investment (timing of snowmelt: t130 = -3.57, P = 0.002; Fig. 4c and d). In addition, pollinator visits in an interaction with temperature had a marginal effect in the first year, where reproductive investment increased with temperature, but only when pollinator visits were high (interaction of temperature and pollinator visits; t130 = 1.80, P = 0.075). When accounting for supplemental pollination, R. acris plants growing in sites with early snowmelt had higher reproductive investment compared to plants growing in sites with late snowmelt in 2016 (timing of snowmelt: t269 = -3.93, P < 0.001). In both years, reproductive investment increased in response to supplemental pollination, but only when temperature was above a certain threshold (interaction of temperature and supplemental pollination; 2016: ca. 375-degree days above 0 °C, t269 = 2.72, P = 0.007; 2017: ca. 550-degree days above 0 °C, t425 = 3.17, P = 0.002; Fig. 4e and f; Table 1b).
Log transformed reproductive investment (total seed mass (mg)) with increasing growing degree days above 0 °C for floral density (count) in (a) 2016 and (b) 2017, pollinator visits (count) in (c) 2016, and (d) 2017, and supplemental pollination in (e) 2016, and (f) 2017. The points show the raw data while the lines are the model predictions with confidence intervals. Line type indicates significant (solid) and non-significant (dashed) relationship between temperature and reproductive investment and an interaction with a biotic factor. The colours indicate the low (light blue) and high (dark blue) floral density, low (light pink) and high (dark pink) pollinator visits, and control (light green) and supplemental pollination (dark green) pollen source. For floral density (a, b) and pollinators visits (c, d) the predicted low and high value is shown in parentheses in the colour legend
Reproductive success is affected by both climate and biotic factors
The number of ovules and seeds were only counted in 2016, and each individual R. acris flower produced on average 12.2 ± 8 fully developed seeds and additionally had 10.6 ± 8 undeveloped ovules, resulting in 55% of the gynoecium fully developed to ripe seeds.
When only accounting for abiotic factors, reproductive success (seed-set rate) generally decreased with temperature but increased with temperature in late-snowmelt sites (interaction temperature and timing of snowmelt: Z = 3.69, P < 0.001; Fig. 5., Table 2a).
Similarly to reproductive investment, when accounting for both abiotic and biotic factors, there was a temperature dependent effect of floral density and supplemental pollination on reproductive success (Fig. 6, Table 2b). When accounting for floral density, reproductive success increased with temperature and higher floral density, but only when floral density was high (interaction of temperature and floral density: Z = 8.46, P < 0.001; Fig. 6a; Table 2b). Reproductive success decreased with temperature and was higher in early-snowmelt sites, when including pollinator visits in the model. Similarly to reproductive investment, there was a non-significant trend for a temperature dependent effect of pollinator visits, where reproductive success increased with temperature, when pollinator visits were high (trend for interaction of temperature and pollinator visits: Z = 1.66, P = 0.096; Fig. 6b; Table 2b). Reproductive success also increased with supplemental pollination, but only above a certain temperature threshold (interaction of temperature and supplemental pollination: ca. 375-degree days above 0 °C; Z = 8.17, P = < 0.001; Fig. 6c; Table 2b). Reproductive success was higher in early-snowmelt sites and this effect was stronger with supplemental pollination (interaction of timing of snowmelt and supplemental pollination: Z = 2.23, P = 0.026; Fig. 6d).
Reproductive success (seed-set rate) with increasing growing degree days above 0 °C for (a) floral density (count) (b) pollinator visits (count), and (c) supplemental pollination in 2016. (d) Reproductive success (seed-set rate) with increasing timing of snowmelt for supplemental pollination in 2016. The points show the raw data while the lines are the model predictions with confidence intervals. The colours indicate the low (light blue) and high (dark blue) floral density, low (light pink) and high (bright pink) pollinator visits, and control (light green) and supplemental pollination (dark green). For floral density (a) and pollinators visits (b) the predicted low and high value is shown in parentheses in the colour legend
Pollinator visits depend on temperature and floral density
We tested how pollinator visits depend on the timing of snowmelt, daily mean temperature and number of flowers on the day of the observations. In both years, pollinator visits increased with a higher mean daily temperature (2016: t204 = 5.87, P = < 0.001, 2017: t366 = 2.8, P = 0.006) and higher number of flowers (2016: t204 = 10.8, P = < 0.001, 2017: t366 = 12.8, P = < 0.001; Fig. 7; Table 3), while the timing of snowmelt had no effect on the number of pollinator visits.
Discussion
Our study shows that reproductive investment (total seed mass) and success (seed-set rate) in R. acris populations in a cold and wet alpine environment are governed by the interactive effects of abiotic and biotic factors. Specifically, temperature, timing of snowmelt, floral density, and pollen limitation were all found to affect reproduction, resulting in substantial variation in reproduction between years and across the landscape. While we did not find a significant effect of pollinator visitation per se, we found that the dipteran insects that pollinate R. acris are more active in warmer weather and are attracted to areas with a higher floral density. This indicates that higher temperatures can act through increased pollinator activity, and that patches of high R. acris density can attract pollinators, leading to variation in visitation that can potentially underlie some of the variation in reproductive success across the landscape and over time. These results suggest that either biotic or abiotic factors alone are not sufficient to understand and predict variation in reproduction in heterogeneous alpine landscapes, and that explicitly studying the interactions of these factors to understand variation in reproductive investment and success across the landscape and over time is key.
Reproductive investment and success depend on both abiotic and biotic factors
Previous studies on pollen limitation in R. acris show contradicting results, as some studies find pollen limitation on reproductive investment (total seed mass; Totland 1997; Hegland and Totland 2007), while others do not (Totland and Eide 1999). We are, however, not aware of any previous study showing that pollen limitation carries through to impact reproductive success (seed-set rate) in R. acris (Totland and Birks 1996; Totland 1997). Interestingly, we found context-dependencies in pollination limitation, supplemental pollination was only beneficial for reproductive investment and success when the plants had accumulated enough temperature (growing degree days). This suggests that plants experiencing lower temperatures were not able to utilize the additional pollen to increase their reproductive investment or success, while plants experiencing higher temperatures potentially could have increased their reproductive investment or success if they were offered better pollination services. This temperature-dependence in pollen limitation may potentially explain differences between the previous studies, and we speculate that the previous studies may have varied in temperature or other important co-limiting factors. This illustrates the importance of considering interactions between biotic and abiotic factor when assessing the limiting factors for vital rates such as reproduction, especially in heterogeneous landscapes.
We also found that reproductive investment and success increased with increasing temperature, but only in areas with high floral density and only under relatively high visitation rates. These effects were only seen in 2016, which was the warmer year. Pollinating dipteran insects are ectothermic and depend on favourable weather to perform optimal pollination services (Totland 1994a; Sieber et al. 2011). With higher temperatures, dipteran insects are able to move between flowers at higher rate and also move between flower patches. Accordingly, we found more visits on R. acris individuals when the daily temperature was higher, and when there were more R. acris individuals in the surroundings (also found by Totland 1994a). Since we found pollen limitation in both years and especially under warmer conditions (see above), this could indicate that the limiting factor for reproduction changes with temperature, which has previously been suggested by Totland (2001). Specifically, under colder conditions, the plants are not able to utilise pollination services they receive, and additional pollination has relatively little effect. In contrast, under relatively warm conditions pollinating insects are more active and pollination service delivery is higher, but the plant’s capacity to utilise pollination services is also higher, hence pollination limitation is actually higher (as seen by the higher effect of supplemental pollination). This could also explain why a higher floral density was only important for reproductive investment and success when temperatures were high, as larger and denser flowering patches attract more pollinators, that are more active in warmer weather when plants are better able to utilise the pollination service.
Reproductive investment and success vary in space and time
Our results showed that the biotic and abiotic factors that affected reproductive investment varied between years, and those that affected reproductive success varied along local snowmelt gradients. While both floral density and pollinator visits interacted with temperature to affect reproductive investment in 2016, there was no such effect in 2017, which was a colder year. A higher floral density is often associated with a higher floral display attracting more pollinators (Dauber et al. 2010), which was supported in 2016 (see above). However, too many flowers can cause the pollinators to be oversaturated with resources, so that pollinator visits per flower will decrease due to increased competition between flowers (Steven et al. 2003). In 2017 the floral density was about three times higher whereas there were only about half as many pollinator visits per flower compared to 2016, suggesting that R. acris flowers may have experienced competition for pollinators in the second year. In support of this, the average reproductive investment was almost halved in 2017 compared to 2016. The mean daily temperature was on average 0.7 °C lower in 2017 compared to 2016, and it could also have been too cold for the pollinating insects to be active. We did however not survey the pollinator community in this study and can therefore not with certainty conclude that there were less pollinators in 2017.
The reason for contradicting effects of floral density between years could also be because we were not able to capture the factors limiting reproductive investment and success in 2017. We found that R. acris required about 175 °C more growing degrees days to utilise the supplemental pollination in 2017 compared to 2016. The second year was on average colder and could therefore put stronger temperature limitations on the plants across the entire study system (Hatfield and Prueger 2015). Overall, it seems like the colder temperature in 2017 was the main limiting factor for pollinator activity, furthermore, affecting the lack of effect on reproductive investment this year.
Our results showed that reproductive success in R. acris also varied across the landscape. Reproductive success was higher at early-snowmelt sites, and pollen limitation affected reproductive success only in plants from early-snowmelt sites. Despite more pollen limitation, earlier emerging plants had more time to accumulate more growing degree days to develop mature seeds and complete the life cycle (Kudo and Suzuki 1999). Plants in alpine habitats are adapted to different selective pressures on different life-history stages, some of which may vary over the growing season (Molau 1993), and even though we found higher pollen limitation early in the season, overall reproductive success was higher in earlier compared to later flowering individuals, indicating that flowering earlier could entail an overall benefit under current climate.
Importance of interactive models and caveats of our study
Reproduction in R. acris depended on both abiotic and biotic factors, and if we had only focused on the abiotic factors in this study, our results would have been misleading. For example, assessment of abiotic factors alone in 2016 would suggest that higher temperature decreases reproductive investment and success. A possible explanation for this could be a frost event that occurred late in the growing season (12 individuals had visual frost damage with brown and withered seeds, personal obs. Linn Vassvik), causing less seeds to develop in late emerging plants as they were accumulating more temperature. This highlights the importance of investigating both the abiotic (direct) and biotic (indirect) effects on reproductive investment and success in alpine plants.
We are aware that there are some caveats to the interpretation of our results. We did not include bagged control flowers to estimate the pollination dependency of R. acris and to what extent it was able to self-pollinate in the absence of pollinators. We did, however, find that supplemental pollination increased reproductive success in R. acris, suggesting seed set was pollinator limited. We pollinated only one flower per plant, which can be problematic for plants that produce several flowers, because resources can be reallocated among flowers (Zimmerman and Pyke 1988) and increased reproductive success in one flower might not be representative for the entire plant and therefore cannot be associated with pollen limitation alone. Almost all R. acris individuals in our study only produced one flower, suggesting that the increase in reproduction with supplemental pollination in our study is mainly due to pollen limitation.
In conclusion we found that reproductive investment and success in alpine populations of R. acris are affected by the interactive effects of abiotic and biotic factors. Our results have three important implications. First, as we found that reproductive investment and success was affected by the interactive effects of abiotic and biotic factors, we advocate for designs that enable disentangling such interactions when studying reproduction in alpine plants. Second, the role of these abiotic and biotic factors also varied in time and space, suggesting that study designs should account for and allow assessment of complex interactions and context-dependencies in order to understand and making predictions of reproduction in species such as R. acris in a warmer climate. While warmer temperatures can often benefit reproduction, the net outcome also depend on the timing between R. acris flowering and the emergence of pollinating insects. While R. acris is a pollinator-generalist, potentially making it less vulnerable to climate change (Biesmeijer et al. 2006; Memmott et al. 2007), our study suggests that the interplay between plants and the pollinator community is nevertheless variable in time and space and depends on complex abiotic-biotic interactions. Our study illustrates that a phenological mismatch could occur if the plant and the pollinator community at large respond differently to climatic variation or climate change (Hegland et al. 2009b). In addition to warming, the future climate in alpine habitats is also predicted to be more extreme, with more rainfall and frost events (Inouye 2008), which can affect both plants and insects and thus reproduction (Memmott et al. 2007). And third, the small-scale heterogeneity in alpine habitats (e.g. timing of snowmelt, temperature) could make alpine populations less vulnerable to climate change, because populations in patchy landscapes have experienced selective pressures that have increased their resistance and resilience to climate change (Graae et al. 2018). Variable landscapes may contain climate refugia where reproduction can successfully occur under a range of climate conditions, and insects are relatively mobile and so may track these shifting floral patches across the landscape (Ohler et al. 2020).
References
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Biesmeijer JC, Roberts SPM, Reemer M, Ohlemuller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, Kunin WE (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354. https://doi.org/10.1126/science.1127863
Billings WD (1987) Constraints to Plant Growth, Reproduction, and establishment in Arctic environments. Arct Alp Res 19:357–365. https://doi.org/10.1080/00040851.1987.12002616
Blois JL, Williams JW, Fitzpatrick MC, Jackson ST, Ferrier S (2013) Space can substitute for time in predicting climate-change effects on biodiversity. Proc Natl Acad Sci USA 110:9374–9379. https://doi.org/10.1073/pnas.1220228110
Borer ET, Harpole WS, Adler PB, Lind EM, Orrock JL, Seabloom EW, Smith MD (2014) Finding generality in ecology: a model for globally distributed experiments. Methods Ecol Evol 5:65–73. https://doi.org/10.1111/2041-210X.12125
Dauber J, Biesmeijer JC, Gabriel D, Kunin WE, Lamborn E, Meyer B, Nielsen A, Potts SG, Roberts SPM, Sõber V, Settele J, Steffan-Dewenter I, Stout JC, Teder T, Tscheulin T, Vivarelli D, Petanidou T (2010) Effects of patch size and density on flower visitation and seed set of wild plants: a pan-european approach. J Ecol 98:188–196. https://doi.org/10.1111/j.1365-2745.2009.01590.x
Delnevo N, Petraglia A, Carbognani M, Vandvik V, Halbritter AH (2018) Plastic and genetic responses to shifts in snowmelt time affects the reproductive phenology and growth of Ranunculus acris. Perspect Plant Ecol Evol Syst 30:62–70. https://doi.org/10.1016/j.ppees.2017.07.005
Dunne JA, Harte J, Taylor KJ (2003) Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecol Monogr 73:69–86. https://doi.org/10.1890/0012-9615(2003)073[0069:Smfprt]2.0.Co;2:
Dunne JA, Saleska SR, Fischer ML, Harte J (2004) Integrating experimental and gradient methods in ecological climate change research. Ecology 85:904–916. https://doi.org/10.1890/03-8003
Fukami T, Wardle DA (2005) Long-term ecological dynamics: reciprocal insights from natural and anthropogenic gradients. Proc Royal Soc B-Biological Sci 272:2105–2115. https://doi.org/10.1098/rspb.2005.3277
Garcia-Camacho R, Totland O (2009) Pollen limitation in the Alpine: a Meta-analysis. Arct Antarct Alp Res 41:103–111. https://doi.org/10.1657/1938-4246(08-028)[garcia-camacho]2.0.co;2
Graae BJ, Vandvik V, Armbruster WS, Eiserhardt WL, Svenning JC, Hylander K, Ehrlén J, Speed JDM, Klanderud K, Bråthen KA, Milbau A, Opedal OH, Alsos IG, Ejrnæs R, Bruun HH, Birks HJB, Westergaard KB, Birks HH, Lenoir J (2018) Stay or go - how topographic complexity influences alpine plant population and community responses to climate change. Perspect Plant Ecol Evol Syst 30:41–50. https://doi.org/10.1016/j.ppees.2017.09.008
Halbritter AH, Vassvik L, Östman SAH, Vandvik V (2024) Plant-pollinator interactions along snowmelt gradients at Finse in Norway. https://doi.org/10.17605/OSF.IO/GW39Z
Hanssen-Bauer I, Førland E, Haddeland I, Hisdal H, Lawrence D, Mayer S, Nesje A, Nilsen JE, Sandven S, Sandø A, Sorteberg A, Ådlandsvik B (2017) Climate in Norway 2100.
Hatfield JL, Prueger JH (2015) Temperature extremes: Effect on plant growth and development. Weather Clim Extremes 10:4–10. https://doi.org/10.1016/j.wace.2015.08.001
Hegland SJ, Totland O (2007) Pollen limitation affects progeny vigour and subsequent recruitment in the insect-pollinated herb Ranunculus acris. Oikos 116:1204–1210. https://doi.org/10.1111/j.2007.0030-1299.15694.x
Hegland SJ, Grytnes JA, Totland O (2009a) The relative importance of positive and negative interactions for pollinator attraction in a plant community. Ecol Res 24:929–936. https://doi.org/10.1007/s11284-008-0572-3
Hegland SJ, Nielsen A, Lazaro A, Bjerknes AL, and O. Totland (2009b) How does climate warming affect plant-pollinator interactions? Ecol Lett 12:184–195. https://doi.org/10.1111/j.1461-0248.2008.01269.x
Holden ZA, Klene AE, Keefe RF, Moisen GG (2013) Agric For Meteorol 180:281–286. https://doi.org/10.1016/j.agrformet.2013.06.011. Design and evaluation of an inexpensive radiation shield for monitoring surface air temperatures
Høye TT, Forchhammer MC (2008) Phenology of high-Arctic arthropods: effects of Climate on spatial, Seasonal, and inter-annual variation. Advances in Ecological Research. Academic, pp 299–324
Huelber K, Gottfried M, Pauli H, Reiter K, Winkler M, Grabherr G (2006) Phenological responses of snowbed species to snow removal dates in the Central Alps: implications for climate warming. Arct Antarct Alp Res 38:99–103. https://doi.org/10.1657/1523-0430(2006)038[0099:Prosst]2.0.Co;2
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89:353–362. https://doi.org/10.1890/06-2128.1
IPCC (2023) Synthesis report of the IPCC sixth assessment report (AR6). Long report
Jonas T, Rixen C, Sturm M, Stoeckli V (2008) How alpine plant growth is linked to snow cover and climate variability. J Geophys Research-Biogeosciences 113:1–10. https://doi.org/10.1029/2007jg000680
Körner C (2003) Alpine plant life: functional plant ecology of high mountain ecosystems. Springer, Berlin
Kudo G (2021) Habitat-specific effects of flowering advance on fruit-set success of alpine plants: a long-term record of flowering phenology and fruit-set success of Rhododendron Aureum. Alp Bot 131:53–62. https://doi.org/10.1007/s00035-021-00248-9
Kudo G (2022) Outcrossing syndrome in alpine plants: implications for flowering phenology and pollination success. Ecol Res 37:288–300. https://doi.org/10.1111/1440-1703.12314
Kudo G, Suzuki S (1999) Flowering phenology of alpine plant communities along a gradient of snowmelt timing. Polar Biosci 12:100–113
Lazaro A, Lundgren R, Totland O (2009) Co-flowering neighbors influence the diversity and identity of pollinator groups visiting plant species. Oikos 118:691–702. https://doi.org/10.1111/j.1600-0706.2008.17168.x
Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant–pollinator interactions. Ecol Lett 10:710–717. https://doi.org/10.1111/j.1461-0248.2007.01061.x
Mizunaga Y, Kudo G (2017) A linkage between flowering phenology and fruit-set success of alpine plant communities with reference to the seasonality and pollination effectiveness of bees and flies. Oecologia 185:453–464. https://doi.org/10.1007/s00442-017-3946-9
Molau U (1993) Relationships between flowering phenology and life history strategies in Tundra plants. Arct Alp Res 25:391–402. https://doi.org/10.1080/00040851.1993.12003025
Ohler L-M, Lechleitner M, Junker RR (2020) Microclimatic effects on alpine plant communities and flower-visitor interactions. Sci Rep 10:1366. https://doi.org/10.1038/s41598-020-58388-7
Pardee GL, Jensen OJ, Inouye DW, Irwin RE (2019) The individual and combined effects of snowmelt timing and frost exposure on the reproductive success of montane forbs. J Ecol. https://doi.org/10.1111/1365-2745.13152
Pickett STA (1989) Space-for-time substitution as an alternative to long-term studies. Springer, New York
Pinheiro J, Bates D (2023) Nlme: Linear and nonlinear mixed effects models. R Core Team R package version 3.1–164 https://CRAN.R-project.org/package=nlme
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rixen C, Hoye TT, Macek P, Aerts R, Alatalo JM, Anderson JT, Arnold PA, Barrio IC, Bjerke JW, Björkman MP, Blok D, Blume-Werry G, Boike J, Bokhorst S, Carbognani M, Christiansen CT, Convey P, Cooper EJ, Cornelissen JHC, Coulson SJ, Dorrepaal E, Elberling B, Elmendorf SC, Elphinstone C, Forte TGW, Frei ER, Geange SR, Gehrmann F, Gibson C, Grogan P, Halbritter AH, Harte J, Henry GHR, Inouye DW, Irwin RE, Jespersen G, Jónsdóttir IS, Jung JY, Klinges DH, Kudo G, Lämsä J, Lee H, Lembrechts JJ, Lett S, Lynn JS, Mann HMR, Mastepanov M, Morse J, Myers-Smith IH, Olofsson J, Paavola R, Petraglia A, Phoenix GK, Semenchuk P, Siewert MB, Slatyer R, Spasojevic MJ, Suding K, Sullivan P, Thompson KL, Väisänen M, Vandvik V, Venn S, Walz J, Way R, Welker JM, Wipf S, Zong SW (2022) Winters are changing: snow effects on Arctic and alpine tundra ecosystems. Arctic Science 8:572–608. https://doi.org/10.1139/as-2020-0058
Rustad LE (2008) The response of terrestrial ecosystems to global climate change: towards an integrated approach. Sci Total Environ 404:222–235. https://doi.org/10.1016/j.scitotenv.2008.04.050
Scherrer D, Körner C (2011) Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J Biogeogr 38:406–416. https://doi.org/10.1111/j.1365-2699.2010.02407.x
Sieber Y, Holderegger R, Waser NM, Thomas VFD, Braun S, Erhardt A, Reyer H-U, Wirth LR (2011) Do alpine plants facilitate each other’s pollination? Experiments at a small spatial scale. Acta Oecol 37:369–374. https://doi.org/10.1016/j.actao.2011.04.005
Stanton ML, Rejmanek M, Galen C (1994) Changes in vegetation and soil fertility along a predictable snowmelt gradient in the Mosquito Range, Colorado, USA. Arct Alp Res 26:364–374. https://doi.org/10.2307/1551798
Steinacher G, Wagner J (2012) Effect of temperature on the progamic phase in high-mountain plants. Plant Biol 14:295–305. https://doi.org/10.1111/j.1438-8677.2011.00498.x
Steven JC, Rooney TP, Boyle OD, Waller DM (2003) Density-dependent Pollinator Visitation and Self-Incompatibility in Upper Great Lakes Populations of Trillium grandiflorum. J Torrey Bot Soc 130:23–29. https://doi.org/10.2307/3557522
Totland O (1993) Pollination in alpine Norway - flowering phenology, insect visitors, and visitation rates in 2 plant-communities. Can J Botany-Revue Canadienne De Botanique 71:1072–1079. https://doi.org/10.1139/b93-124
Totland O (1994a) Influence of climate, time of day and season, and Flower Density on Insect Flower Visitation. Alp nor Ecography 17:159–165. https://doi.org/10.1111/j.1600-0587.1994.tb00089.x
Totland O (1994b) Intraseasonal variation in pollination intensity and seed set in an alpine population of Ranunculus-acris. Southwest nor Ecography 17:159–165. https://doi.org/10.1111/j.1600-0587.1994.tb00089.x
Totland O (1997) Limitations on reproduction in alpine Ranunculus acris. Can J Botany-Revue Canadienne De Botanique 75:137–144. https://doi.org/10.1139/b97-016
Totland O (1999) Effects of temperature on performance and phenotypic selection on plant traits in alpine Ranunculus acris. Oecologia 120:242–251. https://doi.org/10.1007/s004420050854
Totland O (2001) Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82:2233–2244. https://doi.org/10.1890/0012-9615(2003)073[0069:Smfprt]2.0.Co;210.1890/0012-9658(2001)082[2233:edplas]2.0.co;2
Totland O, Birks HJB (1996) Factors influencing inter-population variation in Ranunculus acris seed production in an alpine area of southwestern Norway. Ecography 19:269–278. https://doi.org/10.1111/j.1600-0587.1996.tb00236.x
Totland O, Eide W (1999) Environmentally-dependent pollen limitation on seed production in alpine Ranunculus acris. Ecoscience 6:173–179. https://doi.org/10.1080/11956860.1999.11682518
Urbanowicz C, Virginia RA, Irwin RE (2018) Pollen limitation and reproduction of three plant species across a temperature gradient in western Greenland. Arct Antarct Alp Res 50. https://doi.org/10.1080/15230430.2017.1414485
Wipf S, Rixen C, Mulder CPH (2006) Advanced snowmelt causes shift towards positive neighbour interactions in a subarctic tundra community. Glob Change Biol 12:1496–1506. https://doi.org/10.1111/j.1365-2486.2006.01185.x
Wipf S, Stoeckli V, Bebi P (2009) Winter climate change in alpine tundra: plant responses to changes in snow depth and snowmelt timing. Clim Change 94:105–121. https://doi.org/10.1007/s10584-009-9546-x
Zimmerman M, Pyke GH (1988) Reproduction in Polemonium: assessing the factors limiting seed set. Am Nat 131:723–738. https://doi.org/10.1086/284815
Acknowledgements
We thank Finse Alpine Research Center, and its station manager at the time, Erika Leslie, for the hospitality throughout the two years of this study. Also, a special thanks to Signe Svensson and Deborah Davy for help with fieldwork and Ørjan Totland for help with the study design. We are also grateful for the detailed comments from two anonymous reviewers. This work was supported by Olaf Grolle Olsen og Bødtkers legat (University of Bergen) ID 13808, 2016.
Funding
Open access funding provided by Norwegian Institute of Bioeconomy Research
Author information
Authors and Affiliations
Contributions
We follow the CreDiT taxonomy(and recognize the following author contributions, Conceptualization (co), Data curation (da), Formal analysis (fo), Funding acquisition (fu), Investigation (in), Methodology (me), Project administration (pr), Resources (re), Software (so), Supervision (su), Validation (va), Visualization (vi), Writing – original draft (wo), and Writing – review & editing (wr), as follows: LV (co, da, fo, in, me, va, vi, wo); VV (co, me, pr, re, su, wo), SAHÖ (in, me, wr); AN (su, wr); AHH (co, da, fo, fu, in, me, pr, re, su, va, vi, wo).
Corresponding authors
Ethics declarations
competing interests
The authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A – site information
Appendix A – site information
Overview of all sites, with siteID, day of snowmelt in the two years of the study (2016 and 2017), area (m2), elevation above sea level (m a.s.l.), and the laps rate correction for each site. This correction is the value subtracted from the temperature data from the weather station at 1200 m a.s.l. to fit the adiabatic lapse rate of a decrease in -0.6 ℃ per 100 m elevation (see methods).
SiteID | Day of snowmelt in 2016 (doy) | Day of snowmelt in 2017 (doy) | Area(m2) | Elevation m a.s.l. | Laps rate correction |
|---|---|---|---|---|---|
1-a | Not used | 140 | 15.8 | 1458 | 1.548 |
1-b | Not used | 140 | 10.0 | 1459 | 1.554 |
1-c | Not used | 150 | 15.0 | 1453 | 1.518 |
1-d | Not used | 150 | 10.0 | 1452 | 1.512 |
1-e | Not used | 150 | 15.0 | 1450 | 1.500 |
1-f | Not used | 154 | 13.5 | 1467 | 1.602 |
1-g | Not used | 140 | 8.0 | 1429 | 1.374 |
1-h | Not used | 140 | 18.0 | 1428 | 1.368 |
2-a | 169 | 161 | 22.5 | 1448 | 1.488 |
2-b | 169 | 161 | 29.8 | 1448 | 1.488 |
2-c | 169 | 161 | 24.0 | 1468 | 1.608 |
2-d | 169 | 162 | 17.5 | 1475 | 1.650 |
2-e | 169 | 161 | 15.0 | 1476 | 1.656 |
2-f | 169 | 161 | 30.0 | 1489 | 1.734 |
2-g | 169 | 161 | 24.0 | 1481 | 1.686 |
2-h | 169 | 161 | 22.5 | 1456 | 1.536 |
2-i | 169 | 171 | 23.0 | 1433 | 1.398 |
2-j | 169 | 171 | 21.0 | 1434 | 1.404 |
3-a | 186 | 162 | 28.0 | 1435 | 1.410 |
3-b | 186 | 172 | 73.5 | 1436 | 1.416 |
3-c | 186 | 167 | 18.0 | 1459 | 1.554 |
3-d | 186 | 172 | 18.0 | 1460 | 1.560 |
3-e | 186 | 162 | 26.3 | 1468 | 1.608 |
3-f | 186 | 167 | 110.5 | 1481 | 1.686 |
3-g | 186 | 172 | 24.0 | 1467 | 1.602 |
3-h | 186 | 170 | 28.0 | 1447 | 1.482 |
3-i | 186 | 175 | 59.5 | 1419 | 1.314 |
3-j | 186 | 178 | 88.0 | 1428 | 1.368 |
4-a | 197 | Not used | 60.0 | 1428 | 1.368 |
4-b | 197 | Not used | 35.0 | 1427 | 1.362 |
4-c | 197 | Not used | 45.0 | 1431 | 1.386 |
4-d | 197 | Not used | 21.0 | 1443 | 1.458 |
4-e | 197 | Not used | 38.5 | 1470 | 1.620 |
4-f | 197 | Not used | 23.0 | 1462 | 1.572 |
4-g | 197 | Not used | 16.3 | 1458 | 1.548 |
4-h | 197 | Not used | 32.0 | 1442 | 1.452 |
4-i | 197 | Not used | 22.5 | 1411 | 1.266 |
4-j | 197 | Not used | 48.0 | 1412 | 1.272 |
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vassvik, L., Vandvik, V., Östman, S.A.H. et al. Temporal and spatial variation in the direct and indirect effects of climate on reproduction in alpine populations of Ranunculus acris L. Alp Botany (2024). https://doi.org/10.1007/s00035-024-00317-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00035-024-00317-9