Abstract
A little more than 10% of the vascular plant flora native to the European Alps is endemic to this area. It has long been noticed that the distribution of endemics across the Alps is very uneven. While most endemics are found along the southern edge of the Alps, with some also on its western, eastern, and northeastern edges, the northern edge of the Alps more or less between Lake Geneva in the west and Lake Traun in the east harbours almost no endemics. The distribution of endemics in the Alps has often been related to the location of glacial refugia. Accordingly, the virtual absence of endemics from the northern edge of the Alps has been explained with the unsuitability of climatic conditions for glacial survival of alpine plants there. After discussing evidence for the existence of glacial refugia for alpine species along the northern edge of the Alps and north of the Alps, I will examine how these refugia differed from refugia along the southern edge of the Alps. I conclude that the uneven distribution of endemics in the Alps is best explained by the different climate through time north and south of the Alps. These climatic differences affected the spatial structure and extent of refugia, the length of isolation of refugial populations, and selective conditions in refugia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following Aeschimann et al.’s (2004) Flora Alpina, the European Alps harbor 3983 native vascular plant taxa (species and subspecies), of which 417 are considered to be endemic to the Alps. It has long been known (Candolle 1875; Chodat and Pampanini 1902; Pawłowski 1970; Favarger 1972; Tribsch and Schönswetter 2003; Tribsch 2004; Aeschimann et al. 2011a, b; Taberlet et al. 2016) that these endemics are not evenly distributed across the Alps, but that a much higher number of endemics is found along the southern edge than along most of the northern edge of the Alps.
According to Tribsch and Schönswetter (2003), the distribution of endemic species in the Alps was first related to the location of glacial refugia by Candolle (1875) and Chodat and Pampanini (1902), and a causal link between their distribution in glacial refugia and their evolutionary divergence and eventually speciation has commonly been assumed. Genetic divergence and subspeciation or speciation in glacial refugia are believed to result from various processes, including geographical isolation, genetic drift in small populations, and adaptive evolution in response to changing abiotic and biotic conditions, where the relative importance of these factors or their combination has been assessed differently by different authors (Hewitt 1996, 2000, 2004; Tribsch and Schönswetter 2003; Tribsch 2004; Hampe and Petit 2005; Carstens and Knowles 2007; Casazza et al. 2008, 2010, 2016b; Stewart et al. 2010; Hampe and Jump 2011; Mee and Moore 2014; Woolbright et al. 2014; Stewart and Stringer 2012; Gentili et al. 2015a, b; Kiedrzyński et al. 2017; Kadereit 2022).
High densities of endemics in particular refugial areas have also been linked to the geomorphological, edaphic, and climatic heterogeneity of these areas (Médail and Verlaque 1997; Médail and Diadema 2009; Casazza et al. 2008, 2010, 2016a; Nieto Feliner 2011, 2014). As such heterogeneity clearly has implications for population size, geographical isolation and variation in selective regimes, environmental heterogeneity should not be considered a separate factor when investigating speciation in refugia the Alps. Also, habitat diversity does not appear to correlate with endemism (Gugerli et al. 2008; Taberlet et al. 2016).
Finally, time available for divergent evolution and speciation needs to be considered (Bennett 1997; Willis and Niklas 2004; Hewitt 2004; Lister 2004; Casazza et al. 2016a; Keppel et al. 2018). While it has been argued that periods of isolation in the Quaternary were never long enough for speciation to be completed (Willis and Niklas 2004; but see Kadereit and Abbott 2021), persistence of interglacial adaptation through glacials has been postulated for Taxus baccata L. by Mayol et al. (2015), and population differentiation has been postulated to persist and accumulate through several climatic cycles by, e.g., Pawłowski (1970), Hewitt (2004), Lister (2004), Smyčka et al. (2017), Jardim de Queiros et al. (2022), and Parisod (2022). Such persistence and accumulation of differentiation implies a certain degree of climatic stability through time in glacial refugia, and such climatic stability has been advocated as a major condition in areas of endemism in general (Jansson 2003; Hampe and Jump 2011; Harrison and Noss 2017; Cai et al. 2023).
Considering the possible role of glacial refugia for evolutionary divergence and speciation, the uneven distribution of endemics across the Alps has been explained by the unsuitability of glacial conditions north of the Alps for the survival and/or diversification of high-elevation species by, e.g., Tribsch (2004), Schönswetter et al. (2005), and Smyčka et al. (2017), where these authors referred to different areas north of the Alps. On the other hand, it has long been known that high-elevation alpine (and high-latitudinal arctic) species were widespread in Quaternary glacials in the lowlands of Central Europe (Birks and Willis 2008). Indeed, Birks and Willis (2008) suggested that the often small interglacial (or postglacial, i.e., extant) ranges rather than the often large glacial ranges of alpine species should be considered refugial (‘warm-stage refugia’).
On the background of these very contradictory views of the presence of glacial refugia for high-elevation plant species north of the Alps, the possible role of refugia for the evident uneven distribution of endemic vascular plant species across the Alps has been reconsidered. After delimiting the area along the northern edge of the Alps where endemics are rare or absent, I will, selecting those endemics which are distributed in refugial areas, examine which of them are most closely related to species from the Alps and which originated in the Quaternary. While this approach concentrates on such species which are relevant when considering the role of refugia for the uneven distribution of endemics, it also aims at establishing that in the Alps Quaternary speciation possibly related to glacial refugia has taken place at all. I will then examine whether the frequency of endemics might be related to the distribution of species diversity, intraspecific genetic diversity or environmental diversity. After discussing evidence for the existence of glacial refugia for high-elevation species along the northern edge of the Alps and north of the Alps and discussing the properties of these refugia, I will examine how these refugia differed from refugia along the southern edge of the Alps. I will conclude that the uneven distribution of endemics distributed in glacial refugia in the Alps is best explained by climatic differences north and south of the Alps. These affected the spatial structure and extent of refugia, the length of isolation of refugial populations, and selective conditions in refugia. In turn, these three factors affected rates of speciation.
Materials and methods
The endemic taxa examined (excl. apomicts) were taken from Aeschimann et al.’s (2004) Flora Alpina. Taxon names used were those used in the respective publication dealing with a taxon even though this partly differed from Aeschimann et al. (2004) and partly differs from current usage. I only considered those endemics which today are distributed in areas regarded glacial refugia (henceforth referred to as ‘refugial endemics’) because only these are relevant when considering the role of refugia for the uneven distribution of endemics. For these endemics, Google Scholar was used to search for phylogenetic and/or phylogeographic literature to identify their closest relatives and to search for information on their estimated age of origin to establish that they are likely to have originated in the Alps in the Quaternary. This approach also served to establish that in the Alps, Quaternary speciation possibly related to glacial refugia has taken place at all. In my search of the literature I encountered additional endemics that were not listed in Aeschimann et al. (2004). However, other endemics may have been missed. Refugial endemics of unknown relationships were not considered further. To assess the distribution of endemics in refugia, I either followed the assessment provided in individual accounts, referred to Tribsch and Schönswetter (2003) for distributional information, or compared their distribution to the distribution of refugial areas as shown in Schönswetter et al. (2005). When necessary, Flora Europaea (Tutin et al. 1968; Tutin et al. 1972; Tutin et al. 1976; Tutin et al. 1980; Tutin et al. 1993) was used to obtain further information on geographical distribution.
Results and discussion
The uneven distribution of endemic species across the Alps
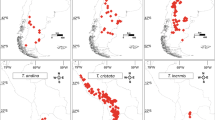
Based on the distribution of endemics as mapped by Aeschimann et al. (2011a, b) and Tribsch and Schönswetter (2003), the area along the northern edge of the Alps that extends more or less from the Haut-Savoie in the West to the Oberösterreichische Voralpen in the East (Fig. 1) is very poor in endemics. While the western limit of this area has not been looked at in great detail recently, but may lie in the area of Lake Geneva (see Vierhapper 1924, 1925; quoted in Aeschimann et al 2011a; Merxmüller 1954; ‘ligne du lac Léman au lac Majeur’ of Aeschimann et al. 2011a), the eastern limit is well known through the work of Merxmüller (1952, 1953, 1954), Niklfeld (1972), Tribsch and Schönswetter (2003), and Tribsch (2004). Merxmüller (1952) and Niklfeld (1972) identified the river Traun in the Oberösterreichische Voralpen as the eastern border (‘Traunlinie’, ‘ligne Traun – Lieser’ of Aeschimann et al. 2011a; Fig. 1) of those parts of the northern Alps poor in endemics. Interestingly, these eastern and western limits also coincide with break zones identified by Thiel-Egenter et al. (2011) and also by earlier authors (see Thiel-Egenter et al. 2011 for discussion) for the distribution of calcifuge species. From the ‘Oberösterreichische Voralpen’ toward the east (‘northeastern calcareous Alps’, ‘northeasternmost Prealps’ and ‘eastermost Central Alps’ following Tribsch (2004)), and particularly in the ‘southern’ and ‘southeastern calcareous Alps’ (following Tribsch (2004)) and the southwestern (Maritime and Ligurian) Alps (Médail and Quezel 1997; Casazza et al. 2008, 2010, 2016a), the number of endemics is much higher. This general pattern also becomes evident when considering the distribution (in the Alps) of monophyletic species-rich lineages such as Moehringia L. sect. Moehringia (Fior and Karis 2007; Aeschimann et al. 2004), Primula L. sect. Auricula Duby (Zhang et al. 2004; Boucher et al. 2016a) or Saxifraga L. sect. Saxifraga subsect. Arachnoideae (Engl. & Irmsch.) Tkach et al. (Gerschwitz-Eidt et al. 2023) where most or all endemics are distributed in the southern Alps. The same applies to, e.g., the diploid and polyploid species of Knautia L. from the Alps—the genus is considered to be of East Mediterranean origin (Resetnik et al. 2014; Frajman et al. 2016)—and to Phyteuma L., a more widespread genus reconstructed to have originated in the Alps (Schneeweiss et al. 2013).
Ice extent in the Alps in the Last Glacial Maximum based on Ivy-Ochs (2015). Map of the Alps: OpenStreetMap https://umap.openstreetmap.fr/de/map/alpen-relief_955504#7/46.108/12.953. The area between the two straight lines on the northern edge of the Alps harbours essentially no endemics. For numbers of endemic species in different parts of the Alps, see Aeschimann et al. (2011a, b), Tribsch and Schönswetter (2003), and Tribsch (2004)
There exist a number of endemics in the endemic-poor area north of the Alps considered here. Thus, Tribsch and Schönswetter (2003), for their ‘northeastern foreland of Austria’, ‘northern foreland of Germany’ and ‘Bodenseegebiet’ list Biscutella laevigata L. subsp. kerneri Mach.-Laur. (best combined with subsp. subaphylla Mach.-Laur. according to Tremetsberger et al. 2002), Onosma helvetica (A. D.C.) Boiss. subsp. austriaca (Beck) Teppner, Tephroseris helenitis (L.) B. Nord. subsp. salisburgensis Cufod., Armeria purpurea W.D.J. Koch and Cochlearia bavarica Vogt. All these, apart from Biscutella laevigata subsp. kerneri, clearly are not most closely related to alpine species, and the status of Onosma helvetica subsp. austriaca (considered a disjunct occurrence of the more widespread O. pseudoarenaria Schur by Kolarčik et al. 2014) has been questioned (Fischer et al. 2005). Tephroseris helenitis subsp. salisburgensis, not recognized taxonomically by Kadereit et al. (2021), has been hypothesized to be of postglacial origin (Pflugbeil et al. 2021), and Cochlearia bavarica is a hybrid taxon of probably very young age (Heubl and Vogt 1985; Koch et al. 1996; Koch 2002). Another few endemics have been identified to the west of the area considered by Tribsch and Schönswetter (2003). Pulmonaria helvetica Bolliger, endemic to the northern edge of the Alps in Switzerland and the Jura Mts., also is a hybrid taxon of likely postglacial origin (Grünig et al. 2021). Interestingly, Arenaria gothica Fr. from Lac de Joux in the Jura Mts. (Info Flora 2022) and Saxifraga oppositifolia L. subsp. amphibia (Sünd.) Braun-Blanq. from Lake Constance (Krause et al. 2017), both discussed by Kadereit (2022), are limited to the shores of lakes covered by ice in the last glacial and may be of only postglacial origin. Armeria purpurea, if indeed endemic to Lake Constance, can be added to this list. The two Lake Constance taxa appear to be extinct (Dienst et al. 2004). Arenaria bernensis Favarger, endemic to the western Prealps in Switzerland, may be a rare example for the possibly Quaternary origin of a refugial endemic east of Lake Geneva (Berthouzoz et al. 2013).
Not all refugial endemics of the Alps are part of alpine lineages and/or of Quaternary age
If the refugial endemics in the Alps originated in glacial refugia, they should have originated in Quaternary times as closest relatives of species from the Alps. Although it will remain unknown whether refugial endemics originated in the area where they are found today, a number of studies which modeled the Last Glacial Maximum (LGM) distribution of refugial endemics in the Alps have shown that the most likely LGM distribution ranges of such endemics were geographically close to or included their current ranges (Schorr et al. 2012, 2013; Casazza et al. 2013; Pan et al. 2020; Guerrina et al. 2022; Adamo et al. 2023). Much has been published on the phylogeography and phylogeny of plant taxa—subspecies, species or larger lineages—from the Alps. However, information on phylogenetic relationships of refugial endemics and their likely time of origin is limited. Partly, taxa have not been analyzed at all; partly, sampling of potential closest relatives is not complete; partly, DNA sequence information generated was not sufficient to resolve relationships; and partly molecular dating, clearly problematical when using, for lack of fossils, secondary calibration in young lineages (Schenk 2016), was not attempted. With respect to the last point, all age estimates reported should be treated very carefully.
Apart from the fact that closer inspection led to the conclusion that some refugial endemics listed by Aeschimann et al. (2004) do not deserve to be recognized taxonomically (Carex norvegica Retz. subsp. pusteriana (Kalela) Chater: Więcław et al. (2017); Galium meliodorum (Beck) Fritsch: Grossfurthner (2018); Oxytropis campestris (L.) DC. subsp. tiroliensis (Sieber ex Fritsch) Leins & Merxm.: Schönswetter et al. (2004a); several species/subspecies of the Papaver alpinum L. complex: Schönswetter et al. (2009); Veronica chamaedrys L. subsp. micans M.A. Fischer: Bardy et al. (2010)), the literature available clearly shows that not all refugial endemics of the Alps originated as closest relatives of species distributed in the Alps, and that not all are of Quaternary age. Thus, there exist phylogenetically isolated and clearly pre-Quaternary refugial endemics such as Berardia subacaulis Vill. (Garcia-Jacas et al. 2016; Guerrina et al. 2022), Gentiana froelichii Rchb. (Favre et al. 2022), Physoplexis comosa (L.) Schur (Cellinese et al. 2009; Schneeweiss et al. 2013), Saxifraga florulenta Moretti (Patsiou et al. 2014; Ebersbach et al. 2017), Valeriana celtica L. (Bell and Donoghue 2005) and Viola argenteria Moraldo & Forneris (Casazza et al. 2016b; Marcussen et al. 2022). Irrespective of their partly high age, however, evidence for a role of Quaternary climatic oscillations in the evolution and distribution of such species has been found. Thus, the subspecific differentiation of Valeriana celtica has been suspected to be of Quaternary age (Weberling et al. 1971), postglacial range contraction has been shown for Berardia subacaulis (Guerrina et al. 2022), and dispersal from a glacial refugium in Saxifraga esculenta has been hypothesized by Szövényi et al. (2009).
Some refugial endemics of the Alps appear to have their closest relatives in geographically very distant areas such as the Eurasian and North American Arctic (Braya alpina Sternb. & Hoppe: Warwick et al. 2004; Chen et al. 2020) or temperate Asia (Callianthemum C.A. Mey.: Kadereit et al. 2019), of which the latter appears to have arrived in Europe in the Quaternary. Others are most closely related to species widespread outside the Alps (Cerastium carinthiacum Vest: Skubic et al. (2018); Chaerophyllum elegans Gaudin: Piwczyński et al. (2015); Odontites luteus (L.) Clairv. subsp. lanceolatus (Gaudin) P. Fourn.: Pinto-Carrasco et al. (2017); Tephroseris integrifolia (L.) Holub subsp. serpentinii (Gáyer) B. Nord. (Kadereit et al. 2021); Teucrium lucidum L.: Salmaki et al. (2016)), of which the latter two appear to have originated in the Quaternary. Several endemics (Table 1) have clear relationships to southern European species or lineages, although it is not always clear whether the Alps were colonized from these southern European areas or vice versa. Many more of those taxa considered to belong to the ‘South European Mountain’ and ‘Mediterranean’ floristic groups of Aeschimann et al. (2011b), the two largest groups of their floristic classification of the flora of the Alps, probably have relationships to southern European taxa. Ranunculus villarsii DC. and R. venetus Huter ex Landolt (Paun et al. 2005: Quaternary; Emadzade and Hörandl 2011: just before onset of Quaternary) are part of a species group widespread in the European Alpine System (EAS), and Alyssum neglectum Magauer et al. is the closest relative of A. montanum L. (Magauer et al. 2014) found across the EAS, both representing a pattern of relationships known from many more taxa (Christ 1867; Engler 1879; Ozenda 1985, 2009; Kadereit 2017).
However, of those species and subspecies endemic to the Alps for which satisfactory data are available, the majority do have their closest relatives in the Alps, and the origin of many of these has been dated to the Quaternary. Undated and dated examples are found in Table 2. In many of the undated examples, limited genetic divergence may imply young and possibly Quaternary age.
In summary, it is very clear that a substantial number of refugial endemics of the southern Alps are the result of Quaternary subspeciation or speciation of lineages distributed in the Alps.
The uneven distribution of refugial endemics in the Alps is not correlated with the distribution of species diversity, intraspecific genetic diversity, or environmental diversity
It is conceivable that the rarity of endemics along the northern edge of the Alps as opposed to their relatively high frequency in the south is proportional to the overall distribution of species, intraspecific genetic, and/or environmental diversity. However, following Gugerli et al. (2008) and Taberlet et al. (2016), this does not seem to be the case. Thus, Gugerli et al. (2008) showed that potential habitat diversity across the Alps generally follows species richness patterns, while Taberlet et al. (2016) demonstrated that species richness does not correlate with areas of endemism. This in turn implies that areas of endemism do not correlate with habitat diversity. Gugerli et al. (2008) also found that highest intraspecific genetic diversity in the Alps occurs along their northern edge, and Taberlet et al. (2016) showed that intraspecific genetic diversity is negatively correlated with species endemism.
The nature of glacial refugia north of the Alps
Various evidence indicates the existence of glacial refugia north of the Alps, i.e., in lowland areas between the ice shields of the Alps and Scandinavia. Birks and Willis (2008) provided a list of 70 ‘alpines’, defined as species that today occur mainly above the altitudinal or beyond the latitudinal treeline (thus including both alpine and arctic species), for which LGM or Weichselian Late Glacial (LG) macrofossils or pollen or spores have been found in northern and Central Europe. However, except for one, all species listed by Birks and Willis (2008) today are distributed in both the Alps (or other parts of the EAS) and in northern Europe, including, for some species, Scotland. As Birks and Willis (2008) clearly stated that ‘Little is known, however, of the LGM distributions of the bulk of the alpines that occur in the Pyrenees and the Alps today because no taxonomically detailed macrofossil analyses have, as far as we know, been done on LGM or LG deposits in Iberia or near the Alps’, it is essentially unknown whether these fossils, in Central Europe, are derived from populations fleeing the Scandinavian ice shield rather than the ice shield of the Alps. Irrespective of this, these fossils certainly illustrate that LGM growing conditions in northern and Central Europe were suitable for plant species which today grow in the Alps and the Arctic. Birks and Willis (2008) also provided a map (based on Väre et al. 2003) of the extant occurrence of alpine species in the potential forest zone of Central Europe, and interpreted these occurrences as ‘cryptic Holocene refugia’. Probably at least those occurrences close to the Alps are likely to be derived from refugial populations of alpine origin.
When considering the glacial distribution of arctic–alpine disjuncts (for reviews see Birks 2008; Schmitt et al. 2010), their wide presence between the Scandinavian and Alps ice shields has been inferred or postulated, based on phylogeographic data, for Arabis alpina L. (Ehrich et al. 2007), Carex atrofusca Schkur (Schönswetter et al. 2006a), Cherleria sedoides L. (Scotland-Alps; Valtueña et al. 2015), Dryas octopetala L. (Skrede et al. 2006), Gentiana nivalis L. (Alvarez et al. 2012), Minuartia biflora (L.) Schinz & Tell. (Schönswetter et al. 2006b), Sibbaldia procumbens L. (Scotland-Alps; Allen et al. 2015), Saxifraga oppositifolia L. (Winkler et al. 2013), and Salix herbacea L. (Alsos et al. 2009). Long-distance dispersal between the Alps and Scandinavia rather than continuous distribution in glacial times between these areas has been found in Comastoma tenellum (Rottb.) Toyok. (Schönswetter et al. 2004b), Ranunculus glacialis L. (Schönswetter et al. 2003b) and Trollius europaeus L. (Després et al. 2002), although a Central European population of the last species was found to be the closest relative of populations from the Alps. Independent colonization of Scandinavia and the Alps from distant areas has been postulated for Ranunculus pygmaeus Wahlenb. (Schönswetter et al. 2006b). The very wide distribution of arctic–alpine species in Europe has also become evident from the analysis of the flora of Upper Teesdale (England), a very well-studied glacial relic site (Pigott 1956; Godwin and Walters 1967; Birks 2015).
Occurrences of alpine species in Germany (and Central Europe) north of the Alps have been looked at in considerable detail by Thorn (1957, 1960). Thorn (1957; see this reference for discussion of earlier authors) provided a list of 30 ‘dealpine’ species for Germany (and Central Europe). In his definition of dealpine Thorn (1957) followed Schustler (1923; quoted in Thorn 1957), who considered species dealpine when they occur mainly at alpine or even nival elevations but have scattered occurrences in Central Europe beyond the northern edge of the Alps. Such occurrences were interpreted as glacial relics by Schustler (1923). In 1960, Thorn provided a list and map of 72 species from lowland Central Europe beyond the edge of the Alps (Alpenvorland) which he considered glacial relics. Most of these species are found in the Black Forest (and Vosges in France), Swabian Alb, Franconian Alb, Upper Palatine and Bavarian Forest, Thuringian-Franconian Highlands and West and East Sudetes, with the northernmost occurrences of alpine species in the Harz. For the edge of the Bavarian Alps (Bayerisches Alpenvorland), Bresinsky (1965) identified ca. 60 species which he considered dealpine. His definition includes species distributed from alpine to lowland elevations. However, for lack of secure knowledge, Bresinsky (1965) abstained from interpreting lowland occurrences of such species as relic occurrences.
Of those species listed by Thorn (1957, 1960) and Bresinsky (1965) as dealpine or glacial relics, a number have been subjected to phylogeographic analyses often aiming at clarifying their relic status. Mainly based on patterns of genetic variation, such as the degree of genetic differentiation among hypothetical glacial relic populations and/or the occurrence of rare alleles, extra-alpine populations of Arabis alpina (Ehrich et al. 2007), Arabidopsis lyrata (L.) O’Kane & Al-Shebaz subsp. petraea (L.) O’Kane & Al-Shebaz (Clauss and Mitchel-Olds 2006; Wernisch 2007), Carduus defloratus L. (Vaupel 2013), Cicerbita alpina (L.) Wallr. (Michel et al. 2010), Draba aizoides L. (Widmer and Baltisberger 1999; Vogler and Reisch 2013), Gentiana pannonica Scop. (Ekrtová et al. 2012), Pulsatilla vernalis Mill. (Ronikier et al. 2008; Kiedrzyński et al. 2017), Saxifraga paniculata Mill. (Reisch et al. 2003; Reisch and Poschlod 2004) and Swertia perennis L. (Urbaniak et al. 2018) were interpreted as glacial relics. When assuming that Biscutella laevigata grew in the Alps in a diploid form before the LGM (Parisod and Besnard 2007), the diploid sublineages outside the Alps of this enigmatic taxon (Manton 1934, 1937) also can be considered glacial relics (Tremetsberger et al. 2002). Evidence for the status of populations as glacial relics was considered inconclusive for Saxifraga aizoides (Lutz et al. 2000) and Tofieldia calyculata (L.) Wahlenb. (Vlasta and Münzbergová 2022), and postglacial colonization of extra-alpine areas beyond the edge of the northern Alps has been postulated for Polygala chamaebuxus L. (Windmaißer et al. 2016), Polygonum viviparum L. (Bauert 1996) and Rosa pendulina L. (Fér et al. 2007; but see Danek et al. 2016). Following Reisch et al. (2002), there is no evidence for glacial relic endemism of Sesleria albicans Kit. ex Schultes, considered a prime example for glacial relic species in Central Europe by Ellenberg (1996).
Ideas about the distribution of trees during the LGM have changed greatly within the last three decades, based on new or reconsidered fossils as well as phylogeographic or species distribution modeling evidence (Gavin et al. 2014). It has become quite clear that at least boreal tree species, most likely in small populations in locally favorable conditions, existed in Central Europe and partly north of the Alps at that time (Willis et al. 2000; Stewart and Lister 2001; Bhagwat and Willis 2008; Birks and Willis 2008; Provan and Bennett 2008; Svenning et al. 2008; Tzedakis et al. 2013). This might provide indirect evidence that growing conditions north of the Alps should also have been good enough for herbaceous alpine plant species. Glacial survival in areas north of the Alps has been inferred from phylogeographic studies even of herbaceous temperate species such as Galium pusillum agg. (Kolář et al. 2013, 2015; Knotek and Kolář 2018), Hippocrepis comosa L. (Leipold et al. 2017), Melica nutans L. (Tyler 2002), Meum athamanticum Jacq. (Huck et al. 2009), Polygonatum verticillatum (L.) All. (Kramp et al. 2009), Sanguisorba minor Scop. (Tausch et al. 2017), Saxifraga rosacea Moench subsp. sponhemica (C.C. Gmel.) D.A. Webb (Walisch et al. 2015) and, in western Europe, Meconopsis cambrica (L.) Vig. (Valtueña et al. 2012). Ohlemüller et al. (2012), in a simulation study, also identified large areas north of the main European mountain ranges as suitable for the growth of temperate plant species during the LGM.
Closer to the Alps, the analysis of distribution patterns of flowering plants led Merxmüller (1952–1954) to infer refugia along the northern edge of the Alps. Genetic evidence in support of Merxmüller (1952–1954) has been provided for, e.g., Androsace lactea L. (Schneeweiss and Schönswetter 2010), Helleborus niger L. (Záveská et al. 2021), Polygala chamaebuxus (Windmaißer et al. 2016), Hornungia alpina (L.) O.Appel (Winkler et al. 2010) and Ranunculus alpestris L. (Paun et al. 2008).
Schönswetter et al. (2005), considering the entire Alps, found that of the several potential calcareous refugia along the northern edge of the Alps identified through the combination of geological and palaeoenvironmental data, only three (their refugia V: northern-Alpine peripheral refugium in central Switzerland; VI: northern-Alpine peripheral refugium in eastern Switzerland; VII: northern-Alpine peripheral refugium in southern Germany) could be verified on the basis of phylogeographic studies of Erinus alpinus L. and Rumex nivalis Hegetschw. (Stehlik 2002; Stehlik et al. 2002). In an earlier study, using the distribution of endemics and the results of phylogeographic studies of alpine plant species, Tribsch and Schönswetter (2003) aimed at identifying refugial areas in the eastern Alps (containing the eastern part of the northern Alps discussed here). A hypothetical refugial area for species of upper alpine and subnival distribution comprising several peripheral parts of the northern calcareous Alps (Berchtesgadener Alpen, Bayerische Voralpen, Wettersteingebirge, Allgäuer Alpen, Säntis, northern Glarner Alpen; their area C8) could not be confirmed because of the absence of narrow endemics from this area.
In conclusion, it seems beyond reasonable doubt that suitable areas for glacial survival of high-elevation species from the Alps were available north of the Alps in much the same way as south of the Alps. If the distribution of populations in refugial areas should be causally linked to their evolutionary divergence resulting in subspeciation or speciation, this implies that northern and southern refugia differed in properties relevant for speciation.
Differences between northern and southern glacial refugia and their potential influence on speciation
Climate
Climatic conditions in glacials were very different north and south of the Alps (Birks and Willis 2008). While in the north, climate favored steppe shrub tundra vegetation (and steppe tundra vegetation further north), most likely very similar to interglacial ecological conditions at alpine elevations (Körner 2021), vegetation south of the Alps, below a narrow montane forest zone, was xeric Artemisia–Poaceae–Chenopodiaceae steppe. When refugial populations persisted (in interglacials or the Holocene) in their glacial refugial areas, they are believed to have been exposed to strong selection (Hewitt 1996, 2000, 2004; Ackerly 2003; Hampe and Petit 2005; Stewart et al. 2010; Stewart and Stringer 2012; Mee and Moore 2014). Along these lines, Gerschwitz-Eidt et al. (2023) suggested, for a subgroup of alpine saxifrages, that some speciation in this lineage may be the result of a two-step process, in which glacial climate resulted in geographical isolation in glacial refugia in a first step, and subsequent interglacial climate resulted in adaptive divergence of populations persisting in the refugial area in a second step (see also Kadereit 2022).
Space
Looking at fossil evidence, the often rather wide distribution of glacial relics (dealpines) in Central Europe, and the possibly very wide glacial distribution of arctic–alpine species in Central Europe, it seems very likely that glacial refugia of alpine species north of the Alps will have been very large areas probably containing many populations linked through gene flow. This hypothesis is supported by the fact that ecological conditions in glacials in Central Europe may have been very similar to interglacial ecological conditions at alpine elevations. Much in contrast to this, as mentioned earlier, glacial vegetation south of the Alps, below a narrow montane forest zone, has been characterized as steppe (Birks and Willis 2008) or xeric Artemisia–Poaceae–Chenopodiaceae steppe (Birks 2015). The latter was considered too dry for high-elevation open tundra-like vegetation by Birks (2015). This might imply that much in contrast to the vast size of glacial refugia north of the Alps, glacial refugia south of the Alps may have been spatially very limited where the southern latitudinal limits were imposed by climate. Probably more importantly, there existed large topographical differences at the northern and southern edges of at least the LGM ice shield of the Alps. Looking at this ice shield (Fig. 1), it is obvious that LGM glaciation, dependent on climate, reached into the lowland in most parts north of the Alps. Much in contrast to this, the edge of the LGM ice shield was located in mountainous areas in most parts of the southern Alps. In addition to the latitudinal limitation of southern alpine refugia, this will have resulted in their further topographical fragmentation. For the Maritime and Ligurian Alps (Casazza et al. 2008, 2010, 2016a), the effects of small scale refugia on the degree of among population differentiation have been shown for, e.g., Gentiana ligustica R. Vilm. & Chopinet (Diadema et al. 2005), Saxifraga florulenta (Szövenyi et al. 2009), Primula latifolia Lapeyr. and P. marginata Curtis (Schorr et al. 2013) and Viola argenteria (Casazza et al. 2016b). In summary, glacial refugia for high-elevation species north of the Alps are likely to have been classical macrorefugia, and those south of the Alps may have had similarities with microrefugia (or cryptic refugia; for discussion of terms see Birks 2015).
However, although it seems very likely that expansion of high-elevation plant species further south than the edge of the south Alps was not possible in lowland areas due to climatic conditions there (Birks 2015), it is obvious that a large number of species from high elevations in the Alps also grow in southern European mountains. Accordingly, refugial areas of some high-elevation species south of the Alps also may have been very large.
Following Aeschimann et al. (2011b), 77 species found in the Alps are shared with the Dinarids, 66 with the Apennine, 44 with the Pyrenees, and 40 with the Carpathians, and many more occur in different combinations of these mountain ranges. The alpine flora of south European mountains has also been analyzed by Gentili et al. (2015a, b), who identified suitable habitats of high-elevation alpine species, and Vargas (2003) provided an early comparative analysis of seven plant species ranging from the Alps to south European mountain ranges. Detailed analyses of species distributed from the Alps via the Massif Central and the Pyrenees to the southern Spanish Sierra Nevada have been presented by Kropf et al. (2006, 2008). Examination of six species led to the conclusion that this distribution is clearly best explained by interglacial/postglacial disruption of a continuous range obtained by migration in four of these six species. On the other hand, the occurrence of Phyteuma globulariifolium Sternb. & Hoppe in the Pyrenees has been interpreted as the result of long-distance dispersal from the southwestern Alps by Schönswetter et al. (2002), and Sanz et al. (2017) concluded that the Sierra Nevada population of Artemisia umbelliformis Lam. most likely originated by long-distance dispersal from the Alps. For the Carpathians, most disjunctions with the Alps have been interpreted as the result of vicariance of a once continuous range with some instances of long-distance dispersal (Ronikier 2011; Mráz and Ronikier 2016). Habitat continuity in glacial times between the southwest Alps and the Apennine has been thought likely by Zhang et al. (2004), Dillenberger and Kadereit (2013) and Moore et al. (2013), and genetic discontinuity between the Apennine and the Alps was detected by, e.g., Ansell et al. (2008) and Grassi et al. (2009), again pointing at vicariance of a once continuous range.
All this confirms the very close relationship between the flora of the Alps and those of the Pyrenees, Apennine, Carpathians, Dinarids, and Balkans already pointed out and discussed in some detail long ago by, e.g., Christ (1867) and Engler (1879), and more recently by Ozenda (1985, 2009), Aeschimann et al. (2011b), and Kadereit (2017).
For the many high-elevation species from the Alps which did not expand into southern mountain ranges in glacial times, it seems likely that glacial refugia on the southern edge of the Alps often will have covered substantially smaller areas than glacial refugia north of the Alps because refugia in the south were latitudinally limited by different climate in the south and because they were further fragmented by the location of the edge of the ice shield in mountainous areas. Although such smaller refugial areas do not qualify as one of three types of microrefugia as defined by Rull (2009), because these imply the existence of a macrorefugium, Birks (2015) suggested that a fourth type of microrefugium without the existence of a macrorefugium could be recognized. Whether considered microrefugia or not, small glacial refugia on the southern edge of the Alps are similar to microrefugia in several respects. Most importantly, in a spatial context, refugia were isolated (Cai et al. 2023) and effective population sizes may have been small, favoring evolutionary divergence through processes such as genetic drift (Stewart et al. 2010; Mee and Moore 2014; Woolbright et al. 2014).
Time
The overall duration of the Quaternary was the same north and south of the Alps. However, periods of geographical isolation of refugial populations, a likely prerequisite for speciation, might have differed in length in the north and south. Provided glacial refugia north of the Alps were indeed the vast lowland areas between the northern European ice shield and the ice shield of the Alps, geographical isolation among refugial populations will have been very limited in glacials, and opportunity for geographical isolation will only have arisen in interglacials provided populations at high elevations were geographically isolated. In support of limited geographical isolation in lowland refugia, gene flow among refugial populations in glacial times in Central Europe has been postulated for Carduus defloratus (Vaupel 2013), Cicerbita alpina (Michl et al. 2010), and Saxifraga rosacea subsp. sponhemica (Walisch et al. 2015). In contrast to lack of geographical isolation in the north, geographical isolation among populations occurred in the small and fragmented glacial refugia south of the Alps. As, in sum, interglacials were much shorter than glacials since the so-called Mid-Pleistocene between about 1.25 and 0.7 million years ago (Birks 2019), overall time of isolation will have been longer south than north of the Alps. The possible evolutionary effect of this is similar to what has been concluded from a comparison of the phylogeographic structure of montane Anthyllis montana L. and alpine Hornungia alpina, where genetic differentiation in the former was found to be much stronger than in the latter (Kropf et al. 2003). Kropf et al. (2003) argued that this can be explained by long glacial vicariance in the former and short interglacial vicariance in the latter species.
A potential threefold role for climate in the evolution of refugial endemics
The above considerations show that climate, either (1) directly through natural selection or indirectly through affecting (2) the size of refugial areas and (3) the duration of geographical isolation, may have played a role in the evolution of refugial endemics. Their very uneven distribution in the Alps, with a virtual absence along much of the northern edge of the Alps and the existence of many endemics along the southern edge of the Alps, was the starting point of my argument. Potentially, climate will have had a direct influence on evolutionary divergence when populations persisted in southern refugia and were increasingly subjected to warming conditions and changes in associated abiotic and biotic factors which represented dramatically altered selective conditions. In support of this, Luqman et al. (2023) showed that populations of Dianthus sylvestris Wulfen in glacial refugia at the southern edge of the Alps evolved to their current adaptive optima from an alpine-like refugial state, although far less so than populations expanding from these refugia. The biology and evolution of such stable rear edge (Hampe and Petit 2005) populations or species have been discussed by, e.g., Ackerly (2003), Hampe and Petit (2005), Hampe and Jump (2011), Woolbright et al. (2014), Kiedrzyński et al. (2017), and Kadereit (2022). A potential indirect influence of climate is twofold. First, unsuitable climate may have limited the size of glacial refugia for high-elevation species along the southern edge of the Alps, and the location of the edges of glaciers in mountainous terrain will have further fragmented the spatial extent of refugia. Geographically isolated suitable climatic conditions have recently been hypothesized to be an important explanatory variable for the geographical distribution of species diversity (Coelho et al. 2013). Much in contrast to this, refugial areas in the north of the Alps most likely were of vast size. Such size difference may have favored evolutionary processes typical for isolated small populations (mainly genetic drift) in the south. Second, climate affected the duration of geographical isolation differently in the south and north. While in the south, isolation arose in long glacials, it arose, if at all, in much shorter interglacials in the north. This will have increased the probability of evolutionary divergence in the south. It seems likely that a combination of all three factors will have played a role in the evolution of the endemics found along the southern edge of the Alps.
As pointed out above, the northeastern Alps (‘northeastern calcareous Alps’ and ‘northeasternmost Prealps’) have been identified as an area of endemism by several authors (e.g., Tribsch and Schönswetter 2003; Tribsch 2004; Essl et al. 2009). As climatic differences between the northeastern Alps and endemic-poor areas to the west are less obvious than the climatic differences between north and south of the Alps, the presence of an area of endemism in the northeastern Alps deserves special attention. Probably most importantly, vegetation along the eastern edge of the Alps appears to have been xeric steppe probably in much the same way as along the southern edge of the Alps (Frenzel 1964; Lang 1994), partly, in the area of the Wiener Becken, even containing stands of Pinus nigra J.F. Arnold (Niklfeld 1972). In support of this, the refugial area ‘easternmost Central Alps’ is located on the eastern edge of the Alps (Tribsch 2004). Interestingly, Janská et al. (2017) postulated high habitat suitability for typical steppe vegetation along the easternmost part of the northern edge of the Alps during the LGM.
Of the endemics of the northeastern Alps listed by Tribsch and Schönswetter (2003; their refugial area C1), Campanula beckiana Hayek, probably Campanula pulla L., Euphorbia austriaca A. Kern. and Euphorbia saxatilis Jacq. have their closest relatives in southeast Europe and cannot be considered part of alpine lineages. Many other of the northeastern Alps endemics listed by Tribsch and Schönswetter (2003) have at least some (often few) occurrences along the eastern edge of the Alps when the Rax-Schneeberg area of Tribsch and Schönswetter (2003) is considered part of the xeric steppe area. This seems justified on the basis of the distribution of some taxa (Niklfeld 1972). The endemics listed by Tribsch and Schönswetter (2003) are: Achillea clusiana Tausch, Biscutella laevigata subsp. austriaca, Callianthemum anemonoides (Zahlbr.) Endl., Campanula praesignis Beck, Dianthus alpinus L., Dianthus plumarius L. subsp. hoppei (Port.) Hegi (a taxon not recognized in Aeschimann et al. 2004), Doronicum glaciale (Wulfen) Nyman subsp. calcareum (Vierh.) Hegi, Draba stellata Jacq., Galium meliodorum (a taxon not distinguishable from G. lucidum All. according to Grossfurthner (2018)), Leontodon montaniformis Widder, Leucanthemum atratum (Jacq.) DC., Melampyrum angustissimum Beck, Melampyrum subalpinum (Jur.) A.Kern., Papaver alpinum p.p., Primula clusiana Tausch, Pulsatilla styriaca (Pritz.) Simonk., Soldanella austriaca Vierh., and Thlaspi alpestre Jacq. Also, in much of the northeastern Alps east of the Traunlinie, the edge of the LGM ice shield was located in mountainous area (Fig. 1; Ivy-Ochs 2015; Seguinot et al. 2018).
It is, thus, conceivable that these taxa originated in refugia along the eastern edge of the Alps where the role of climate might have been the same as hypothesized for the southern edge, and then spread westwards. Indeed, Niklfeld (1972) listed a number of taxa distributed in the Pinus nigra area along the eastern edge of the Alps which also grow along the northern edge of the northeastern Alps. Of the endemic species listed above, only Doronicum glaciale has been analyzed phylogeographically (Pachschwöll et al. 2015). Pachschwöll et al. (2015) reported that D. glaciale subsp. calcareum, distributed in unglaciated areas, cannot easily be separated from D. glaciale subsp. glaciale which is distributed in formerly glaciated areas to the west. They identified a primary contact zone between the two and postulated recent westward migration of subsp. glaciale.
However, Tribsch and Schönswetter (2003) also listed some endemics which today do not grow along the eastern edge of the Alps ((Dianthus plumarius subsp. blandus (Rchb.) Hegi, a taxon not recognized in Aeschimann et al. (2004), Festuca versicolor Tausch subsp. brachystachys (Hack.) Markgr.-Dann. and subsp. pallidula (Hack.) Markgr.-Dann., Galium truniacum (Ronninger) Ronninger, Pulmonaria kerneri Wettst.)). Understanding the origin of those taxa found along the northeastern but not the eastern edge of the Alps clearly requires further investigation.
These considerations for the NE Alps may also apply to the western limit of the north Alps area lacking endemics, i.e., to areas west of Lake Geneva (ligne du lac Léman au lac Majeur of Aeschimann et al. 2011a), as steppe vegetation bordered on the Alps to the west but not to the east of Lake Geneva (Lang 1994). Somewhat in accordance with this, Divíšek et al. (2022) identified the northwestern Alps as an LGM source area for steppe species limited to Central Europe. As regards the LGM ice shield west of Lake Geneva, however, this expanded into lowland areas as far south as the Chartreuse/Isere area (Fig. 1; Ivy-Ochs 2015; Seguinot et al. 2018).
Conclusion
Considering glacial refugia in the Alps, Smyčka et al. (2017) concluded that these constitute museums of phylogenetic diversity and served as islands of suitable conditions during glacial periods. Equally, Jardim de Queiros et al. (2022), for terrestrial organisms including vascular plants, hypothesized persistence through glacial climate cycles as a major component for the evolution of endemics in the Alps. Both these explanations clearly emphasize climatic stability as a major factor in the origin and distribution of endemics, as postulated more generally by, e.g., Fjeldså and Lovett (1997), Jansson (2003), Jetz et al. (2004), Hampe and Jump (2011), Svenning et al. (2015), Harrison and Noss (2017), and Cai et al. (2023).
For the specific setting examined here, i.e., the uneven distribution of endemics in the Alps, a more differentiated picture of the role of climate for the origin of endemics emerges. Thus, potentially climate influenced the size of refugia, the duration of geographical isolation and the selective conditions in refugia, and thus influenced both neutral and adaptive evolutionary processes. While a role of both neutral and adaptive processes has been postulated before (Hewitt 1996, 2000, 2004; Tribsch and Schönswetter 2003; Tribsch 2004; Hampe and Petit 2005; Carstens and Knowles 2007; Casazza et al. 2008, 2010, 2016b; Stewart et al. 2010; Hampe and Jump 2011; Mee and Moore 2014; Woolbright et al. 2014; Stewart and Stringer 2012; Gentili et al. 2015a, b; Kiedrzyński et al. 2017), southern Alps endemics may serve as good study systems for assessing the relative roles of these processes. Given the right data and analytical tools, their distinction may be possible (e.g., Luqman et al. 2021; for discussion of analytical approaches see Johri et al. 2022). As emphasized by de Lafontaine et al. (2018), it will be particularly interesting to investigate the role of natural selection, shown by Luqman et al. (2023) and also postulated by Hua and Wiens (2013) when concluding that speciation via climatic-niche divergence may have predominated during the climatic oscillations of the Quaternary. Probably, as postulated by Gerschwitz-Eidt et al. (2023), speciation at the southern edge of the Alps was a two-step process. While in a first step, often small populations became isolated in glacial refugia, adaptive divergence took place in interglacials (or the Holocene) when refugial populations persisted in their refugia. If such scenario could be confirmed, it would be obvious that glacial refugia were more than just sanctuaries where species were preserved from extinction as also concluded by Nieto Feliner (2011).
Here, it is hypothesized that the uneven distribution of refugial endemics in the Alps may be the result of uneven rates of origination north and south of the Alps. The possibility of uneven rates of extinction in these two areas was not considered. When assuming that climatic stability is a major factor in the origin and distribution of endemics (Fjeldså and Lovett 1997; Jansson 2003; Jetz et al. 2004; Hampe and Jump 2011; Svenning et al. 2015; Harrison and Noss 2017; Cai et al. 2023), it is conceivable that the generally harsher climate along the northern edge of the Alps did not allow endemics to persist. However, based on the evidence presented above, the hypothesis that the properties of refugia north of the Alps did not favor the origin of distinct genetic lineages, i.e., endemics, is favored. Also, the possible role of other factors for the uneven distribution of endemics in the Alps was not discussed. For example, as Smyčka et al. (2017) reported that high endemism in glacial refugia is found only on calcareous bedrock, bedrock as a factor affecting endemism clearly deserves further analysis.
Data availability
Not applicable.
References
Ackerly DD (2003) Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci 164:S165–S184. https://doi.org/10.1086/368401
Adamo M, Skokanova K, Bobo-Pinilla J, Glaccone E, de Giles JP, Muicciarelli M (2023) Molecular evidence and environmental niche evolution at the origin of the disjunct distribution in three mountain endemic Tephroseris (Asteraceae) of the Mediterranean basin. Alp Bot 133:117–133. https://doi.org/10.1007/s00035-023-00300-w
Aeschimann D, Lauber K, Moser DM, Theurillat JP (2004) Flora Alpina Vols. 1–3, Haupt Verlag, Bern, Stuttgart, Wien
Aeschimann D, Rasolofo N, Theurillat J-P (2011a) Analyse de la flore des Alpes. 1: Historique et biodiversité. Candollea 66:27–55. https://doi.org/10.15553/c2011v661a2
Aeschimann D, Rasolofo N, Theurillat J-P (2011b) Analyse de la flore des Alpes. 2: Biodiversité et chorologie. Candollea 66:225–253. https://doi.org/10.15553/c2011v662a1
Alarcón M, Vargas P, Sáez L, Molero J, Aldasoro JJ (2012) Genetic diversity of mountain plants: Two migration episodes of Mediterranean Erodium (Geraniaceae). Mol Phylogenet Evol 63:866–876. https://doi.org/10.1016/j.ympev.2012.02.031
Allen GA, Marr KL, McCormick LJ, Hebda RJ (2015) Geographical origins, migration patterns and refugia of Sibbaldia procumbens, an arctic–alpine plant with a fragmented range. J Biogeogr 42:1665–1676. https://doi.org/10.1111/jbi.12543
Alsos IG, Alm T, Normand S, Brochmann C (2009) Past and future range shifts and loss of diversity in dwarf willow (Salix herbacea L.) inferred from genetics, fossils and modelling. Global Ecol Biogeogr 18:223–239. https://doi.org/10.1111/j.1466-8238.2008.00439.x
Alvarez N, Manel S, Schmitt T, the IntraBioDiv Consortium (2012) Contrasting diffusion of Quaternary gene pools across Europe: The case of the arctic–alpine Gentiana nivalis L. (Gentianaceae). Flora 207:408–413. https://doi.org/10.1016/j.flora.2012.03.006
Ansell SW, Grundmann M, Russell SJ, Schneider H, Vogel JC (2008) Genetic discontinuity, breeding system change and population history of Arabis alpina in the Italian Peninsula and adjacent Alps. Mol Ecol 17:2245–2257. https://doi.org/10.1111/j.1365-294X.2008.03739.x
Aparicio A, Martín-Hernanz S, Parejo-Farnés C, Arroyo J, Lavergne S, Yeşilyurt EB, Zhang M-L, Rubio E, Albaladej RG (2017) Phylogenetic reconstruction of the genus Helianthemum (Cistaceae) using plastid and nuclear DNA-sequences. Syst Evolut Infer Taxon 66:868–885. https://doi.org/10.12705/664.5
Bardy KE, Albach DC, Schneeweiss GM, Fischer MA, Schönswetter P (2010) Disentangling phylogeography, polyploid evolution and taxonomy of a woodland herb (Veronica chamaedrys group, Plantaginaceae s.l.) in southeastern Europe. Mol Phylogenet Evol 57:771–786. https://doi.org/10.1016/j.ympev.2010.06.025
Bauert MR (1996) Genetic diversity and ecotypic differentiation in arctic and alpine populations of Polygonum viviparum. Arct Alp Res 28:190–195. https://doi.org/10.1080/00040851.1996.12003165
Bell CD, Donoghue MJ (2005) Phylogeny and biogeography of Valerianaceae (Dipsacales) with special reference to the South American valerians. Org Divers Evol 5:147–159. https://doi.org/10.1016/j.ode.2004.10.014
Bennett KD (1997) Evolution and ecology: the pace of life. Cambridge University Press, Cambridge
Berthouzoz M, Maendly S, Bétrisey S, Mangili S, Prunier P, Lexer C, Kozlowski G (2013) Some like it cold: distribution, ecology and phylogeny of Arenaria bernensis Favarger (Caryophyllaceae) from the western Prealps in Switzerland. Alp Bot 123:65–75. https://doi.org/10.1007/s00035-013-0116-5
Bhagwat SA, Willis KJ (2008) Species persistence in northerly glacial refugia of Europe: a matter of chance or biogeographical traits? J Biogeogr 35:464–482. https://doi.org/10.1111/j.1365-2699.2007.01861.x
Biella P, Akter A, Muñoz-Pajares AJ, Federici G, Galimberti A, Jersáková J, Labra M, Mangili F, Tommasi N, Mangili L (2021) Investigating pollination strategies in disturbed habitats: the case of the narrow-endemic toadflax Linaria tonzigii (Plantaginaceae) on mountain screes. Plant Ecol 222:511–523. https://doi.org/10.1007/s11258-021-01123-7
Birks HH (2008) The Late-Quaternary history of arctic and alpine plants. Plant Ecol Divers 1:135–146. https://doi.org/10.1080/17550870802328652
Birks HJB (2015) Some reflections on the refugium concept and its terminology in historical biogeography, contemporary ecology and global-change biology. Biodiversity 16:196–212. https://doi.org/10.1080/14888386.2015.1117022
Birks HJB (2019) Contributions of Quaternary botany to modern ecology and biogeography. Plant Ecol Divers 12:189–385. https://doi.org/10.1080/17550874.2019.1646831
Birks HJB, Willis KJ (2008) Alpines, trees, and refugia in Europe. Plant Ecol Divers 1:147–160. https://doi.org/10.1080/17550870802349146
Bittkau C, Kadereit JW (2002) Phylogenetic and geographical relationships in Papaver alpinum L. (Papaveraceae) based on RAPD data. Bot Jahrb Syst 123:463–479
Boucher FC, Thuiller W, Roquet C, Douzet R, Aubert S, Alvarez N, Lavergne S (2012) Reconstructing the origins of high-alpine niches and cushion life form in the genus Androsace s.l. (Primulaceae). Evolution 66:1255–1268. https://doi.org/10.1111/j.1558-5646.2011.01483.x
Boucher FC, Zimmermann NE, Conti E (2016a) Allopatric speciation with little niche divergence is common among alpine Primulaceae. J Biogeogr 43:591–602. https://doi.org/10.1111/jbi.12652
Boucher FC, Casazza G, Szövényi P, Conti E (2016b) Sequence capture using RAD probes clarifies phylogenetic relationships and species boundaries in Primula sect. Auricula. Mol Phylogenet Evol 104:60–72. https://doi.org/10.1016/j.ympev.2016.08.003
Bresinsky A (1965) Zur Kenntnis des circumalpinen Florenelements im Vorland nördlich der Alpen. Ber Bayr Bot Ges 38:5–67
Cai L, Kreft H, Taylor A, Schrader J, Dawson W, Essl F, van Kleunen M, Pergl J, Pyšek P, Winter M, Weigelt P (2023) Climatic stability and geological history shape global centers of neo- and paleoendemism in seed plants. Proc Natl Acad Sci USA 120:e2300981120. https://doi.org/10.1073/pnas.2300981120
Carstens BC, Knowles L (2007) Shifting distributions and speciation: species divergence during rapid climate change. Mol Ecol 16:619–627. https://doi.org/10.1111/j.1365-294X.2006.03167.x
Casazza G, Zappa E, Mariotti MG, Médail F, Minuto L (2008) Ecological and historical factors affecting distribution pattern and richness of endemic plant species: the case of the Maritime and Ligurian Alps hotspot. Diversity Distrib 14:47–58. https://doi.org/10.1111/j.1472-4642.2007.00412.x
Casazza G, Zappa E, Mariotti MG, Médail F, Minuto L (2010) Distribution pattern and richness of endemic plant species in Maritime and Ligurian Alps. Boll Mus Ist Biol Univ Genova 72:130–136
Casazza G, Grassi F, Zecca G, Mariotti MG, Guerrina M, Minuto L (2013) Phylogeography of Primula allionii (Primulaceae), a narrow endemic of the Maritime Alps. Bot J Linn Soc 173:637–653. https://doi.org/10.1111/boj.12110
Casazza G, Grassi F, Zecca G, Minuto L (2016b) Phylogeographic insights into a peripheral refugium: The importance of cumulative effect of glaciation on the genetic structure of two endemic plants. PLoS One 11:e0166983. https://doi.org/10.1371/journal.pone.0166983
Casazza G, Barberis, G, Guerrina, M, Zappa E, Mariotti MG, Minuto L (2016a) The plant endemism in the Maritime and Ligurian Alps. Biogeographia J Integ Biogeogr 31:73–88. https://doi.org/10.21426/B631132738
Cellinese N, Smith SA, Edwards EJ, Kim ST, Haberle RC, Avramakis M, Donoghue MJ (2009) Historical biogeography of the endemic Campanulaceae of Crete. J Biogeogr 36:1253–1269. https://doi.org/10.1111/j.1365-2699.2008.02077.x
Cetlová V, Zozomová-Lihová J, Melichárková A, Mártonfiová L, Španiel S (2021) Multiple drivers of high species diversity and endemism among Alyssum annuals in the Mediterranean: The evolutionary significance of the Aegean hotspot. Front Plant Sci 12:627909. https://doi.org/10.3389/fpls.2021.627909
Chen H, German DA, Al-Shaebaz IA, Yue J, Sun H (2020) Phylogeny of Euclidieae (Brassicaceae) based on plastome and nuclear ribosomal DNA data. Mol Phylogenet Evol 153:106940. https://doi.org/10.1016/j.ympev.2020.106940
Chodat R, Pampanini R (1902) Sur la distribution des plantes dans les alpes austro-orientales et plus particulièrement d’un choix de plantes des alpes cadoriques et venitiennes. Le Globe 41:1–70
Christ H (1867) Über die Verbreitung der Pflanzen der alpinen Region der europäischen Alpenkette. Neue Denkschr. Allg Schweiz Ges Ges Naturw 22:1–85
Clauss MJ, Mitchel-Olds T (2006) Population genetic structure of Arabidopsis lyrata in Europe. Mol Ecol 15:2753–2766. https://doi.org/10.1111/j.1365-294X.2006.02973.x
Coelho MTP, Barreto E, Rangel TF, Diniz-Filho JAF, Wüest RO, Bach W, Skeels A, McFadden IR, Roberts DW, Pellissier L, Timmermann NE, Graham CH (2013) The geography of climate and the global patterns of species diversity. Nature 622:537–544. https://doi.org/10.1038/s41586-023-06577-5
Danek H, Fér T, Marhold K (2016) Glacial survival in northern refugia? Phylogeography of the temperate shrub Rosa pendulina L. (Rosaceae): AFLP versus chloroplast DNA variation. Biol J Linn Soc 119:704–718. https://doi.org/10.1111/bij.12619
Day PD, Berger M, Hill L, Fay MF, Leitch AR, Leitch IJ, Kelly LJ (2014) Evolutionary relationships in the medicinally important genus Fritillaria L. (Liliaceae). Mol Phylogenet Evol 80:11–19. https://doi.org/10.1016/j.ympev.2014.07.024
de Candolle AP (1875) Sur les causes de l’inégale distribution des plantes rares dans la chaîne des Alpes. Atti Del Congresso Internazionale Botanico Tenuto in Firenze 1874:92–104
de Lafontaine G, Napier JD, Petit RJ, Hu S (2018) Invoking adaptation to decipher the genetic legacy of past climate change. Ecology 99:1530–1546. https://doi.org/10.1002/ecy.2382
De Castro O, Innangi M, Di Maio A, Menale B, Bacchetta G, Pires M et al (2016) Disentangling phylogenetic relationships in a hotspot of diversity: The butterworts (Pinguicula L., Lentibulariaceae) endemic to Italy. PLoS One 11:e0167610. https://doi.org/10.1371/journal.pone.0167610
De Luca D, Del Guacchio E, Conti F, Iamonico D, Caputo P (2022) Relationships within Mcneillia indicate a complex Eevolutionary history and reveal a new species of Minuartiella (Caryophyllaceae, Alsinoideae). Plants 11:2118. https://doi.org/10.3390/plants11162118
Després L, Loriot S, Gaudeul M (2002) Geographic pattern of genetic variation in the European globeflower Trollius europaeus L. (Ranunculaceae) inferred from amplified fragment length polymorphism markers. Mol Ecol 11:2337–2347. https://doi.org/10.1046/j.1365-294X.2002.01618.x
Diadema K, Bretagnolle F, Affre L, Yuan Y-M, Médail F (2005) Geographic structure of molecular variation of Gentiana ligustica (Gentianaceae) in the Maritime and Ligurian regional hotspot, inferred from ITS sequences. Taxon 54:887–894. https://doi.org/10.2307/25065569
Dienst M, Peintinger M, Strang I (2004) Rückgang der Strandrasen-Arten am Bodenseeufer – Verbreitungskarten von Myosotis rehsteineri, Deschampsia littoralis, Saxifraga oppositifolia subsp. amphibia und Armeria purpurea. AGBU e.V. – Thema des Monats Dezember 2004
Dillenberger MS, Kadereit JW (2013) The phylogeny of the European high mountain genus Adenostyles Cass. (Asteraceae-Senecioneae) reveals that edaphic shifts coincide with dispersal events. Am J Bot 100:1171–1183. https://doi.org/10.3732/ajb.1300060
Dillenberger MS, Kadereit JW (2017) Simultaneous speciation in the European high mountain flowering plant genus Facchinia (Minuartia s.l., Caryophyllaceae) revealed by genotyping-by-sequencing. Mol Phylogenet Evol 112:23–35. https://doi.org/10.1016/j.ympev.2017.04.016
Divíšek J, Večeřa M, Welk E, Danihelka J, Chytrý K, Douda J, Chytrý M (2022) Origin of the central European steppe flora: insights from palaeodistribution modelling and migration simulations. Ecography 2022:e06293. https://doi.org/10.1111/ecog.06293
Dixon CJ, Schönswetter P, Suda J, Wiedermann MM, Schneeweiss GM (2009) Reciprocal Pleistocene origin and postglacial range formation of an allopolyploid and its sympatric ancestors (Androsace adfinis group, Primulaceae). Mol Phylogenet Evol 50:74–83. https://doi.org/10.1016/j.ympev.2008.10.009
Ebersbach J, Schnitzler J, Favre A, Muellner-Riehl AN (2017) Evolutionary radiations in the species-rich mountain genus Saxifraga L. BMC Evol Biol 17:119. https://doi.org/10.1186/s12862-017-0967-2
Ehrendorfer F Guo YP (2006) Multidisciplinary studies on Achillea sensu lato (Compositae-Anthemideae): New data on systematics and phylogeography. Willdenowia 36, Special Issue: Festschrift Werner Greuter: 69–87. https://www.jstor.org/stable/3997683
Ehrich D, Gaudeul M, Assefa A, Koch MA, Mummenhoff K, Nemomissa S, IntraBioDiv Consortium, Brochmann C, Ehrich D, Gaudeul M (2007) Genetic consequences of Pleistocene range shifts: contrast between the Arctic, the Alps and the East African mountains. Mol Ecol 16:2542–2559. https://doi.org/10.1111/j.1365-294X.2007.03299.x
Ekrtová E, Štech M, Fér T (2012) Pattern of genetic differentiation in Gentiana pannonica Scop.: did subalpine plants survive glacial events at low altitudes in Central Europe? Plant Syst Evol 298:1383–1397. https://doi.org/10.1007/s00606-012-0644-2
Ellenberg H (1996) Vegetation Mitteleuropas mit den Alpen, 5th edn. Ulmer, Stuttgart
Emadzade K, Hörandl E (2011) Northern Hemisphere origin, transoceanic dispersal, and diversification of Ranunculeae (DC. (Ranunculaceae) in the Cenozoic. J Biogeogr 38:517–530. https://doi.org/10.1111/j.1365-2699.2010.02404.x
Engler A (1879) Versuch einer Entwicklungsgeschichte der Pflanzenwelt. Wilhelm Engelmann, Leipzig
Essl F, Staudinger M, Stöhr O, Schratt-Ehrendorfer L, Rabitsch W, Niklfeld H (2009) Distribution patterns, range size and niche breadth of Austrian endemic plants. Biol Conserv 142:2547–2558. https://doi.org/10.1016/j.biocon.2009.05.027
Favarger C (1972) Endemism in the montane floras of Europe. In: Valentine DH (ed) Taxonomy, Phytogeography and Evolution. Academic Press, London, pp 191–204
Favre A, Paule J, Ebersbach J (2022) Incongruences between nuclear and plastid phylogenies challenge the identification of correlates of diversification in Gentiana in the European Alpine System. Alp Bot 132:29–50. https://doi.org/10.1007/s00035-021-00267-6
Fér T, Vašák P, Vojta J, Marhold K (2007) Out of the Alps or Carpathians? Origin of Central European populations of Rosa pendulina. Preslia 79:367–376
Fior S, Karis PO (2007) Phylogeny, evolution and systematics of Moehringia (Caryophyllaceae) as inferred from molecular and morphological data: a case of homology reassessment. Cladistics 23:362–372. https://doi.org/10.1111/j.1096-0031.2007.00150.x
Fischer MA, Adler W, Oswald K (2005) Exkursionsflora für Österreich, Liechtenstein und Südtirol, 2. Aufl. Land Oberösterreich, Linz
Fjeldså J, Lovett JC (1997) Geographical patterns of old and young species in African forest biota: the significance of specific montane areas as evolutionary centres. Biodivers Conserv 6:325–346. https://doi.org/10.1023/A:1018356506390
Info Flora (2022) Das nationale Daten- und Informationszentrum der Schweizer Flora. https://www.infoflora.ch. Accessed 8 August 2023.
Frajman B, Rešetnik I, Niketić M, Ehrendorfer F, Schönswetter P (2016) Patterns of rapid diversification in heteroploid Knautia sect. Trichera (Caprifoliaceae, Dipsacoideae), one of the most intricate taxa of the European flora. BMC Evol Biol 16:204. https://doi.org/10.1186/s12862-016-0773-2
Frajman B, Schönswetter P (2017) Amphi-Adriatic distributions in plants revisited: Pleistocene trans-Adriatic dispersal in the Euphorbia barrelieri group (Euphorbiaceae). Bot J Linn Soc 185:240–252. https://doi.org/10.1093/botlinnean/box055
Frenzel B (1964) Über die offene Vegetation der letzten Eiszeit am Ostrande der Alpen. Verh Zool-Bot Ges Wien 103(104):110–137
Garcia-Jacas N, Garnatje T, Susanna A, Vilatersana R (2016) Tribal and subtribal delimitation and phylogeny of the Cardueae (Asteraceae): a combined nuclear and chloroplast DNA analysis. Mol Phylogenet Evol 22:51–64. https://doi.org/10.1006/mpev.2001.1038
Gargiulo R, Del Guacchio E, Caputo P (2015) Phylogenetic reconstruction of Asperula sect. Cynanchicae (Rubiaceae) reveals a mosaic of evolutionary histories. Taxon 64:754–769. https://doi.org/10.12705/644.7
Gavin DG, Fitzpatrick MC, Gugger PF et al (2014) Climate refugia: joint inference from fossil records, species distribution models and phylogeography. New Phytol 204:37–54. https://doi.org/10.1111/nph.12929
Gentili R, Bacchetta G, Fenu G, Cogoni D, Abeli T, Rossi G, Salvatore MC, Baroni C, Citterio S (2015a) From cold to warm-stage refugia for boreo-alpine plants in southern European and Mediterranean mountains: the last chance to survive or an opportunity for speciation? Biodiversity 16:247–261. https://doi.org/10.1080/14888386.2015.1116407
Gentili R, Baroni C, Caccianiga M, Armiraglio S, Ghiani A, Citterio S (2015b) Potential warm-stage microrefugia for alpine plants: Feedback between geomorphological and biological processes. Ecol Complexity 21:87–99. https://doi.org/10.1016/j.ecocom.2014.11.006
Gerschwitz-Eidt MA, Dillenberger MS, Kadereit JW (2023) Phylogeny of Saxifraga section Saxifraga subsection Arachnoideae (Saxifragaceae) and the origin of low elevation shade-dwelling species. Ecol Evol 13:e9728. https://doi.org/10.1002/ece3.9728
Godwin H, Walters SM (1967) The scientific importance of Upper Teesdale. Proc Bot Soc Br Isl 6:348–351
Gómez-Campo C (2003) The genus Guentheria Andr. in Bess. (Brassicaceae, Brassiceae). An Jard Bot Madr 60:301–307. https://doi.org/10.3989/ajbm.2002.v60.i2.93
Grassi F, Minuto L, Casazza G, Labra M, Sala F (2009) Haplotype richness in refugial areas: phylogeographical structure of Saxifraga callosa. J Plant Res 122:377–387. https://doi.org/10.1007/s10265-009-0230-z
Greimler J, Park J-M, Schneeweiss H (2011) Gentianella (Gentianaceae): A model taxon for evolution in the Alps. Taxon 60:427–435. https://doi.org/10.1002/tax.602012
Grossfurthner LP (2018) Phylogenetic relationships of the Galium lucidum complex in the Eastern Alps. Master’s Thesis, Universität Wien
Grünig S, Fischer M, Parisod C (2021) Recent hybrid speciation at the origin of the narrow endemic Pulmonaria helvetica. Ann Bot 127:21–31. https://doi.org/10.1093/aob/mcaa145
Guerrina M, Theodoridis S, Minuto L, Conti E, Casazza G (2022) First evidence of post-glacial contraction of Alpine endemics: Insights from Berardia subacaulis in the European Alps. J Biogeogr 49:79–93. https://doi.org/10.1111/jbi.14282
Gugerli F, Englisch T, Niklfeld H, Tribsch A, Mirek Z, Ronikier M, Zimmermann NE, Holderegger R, Taberlet P, IntraBioDiv Consortium (2008) Relationships among levels of biodiversity and the relevance of intraspecific diversity in conservation – a project synopsis. Perspect Plant Ecol Evol Syst 10:259–281. https://doi.org/10.1016/j.ppees.2008.07.001
Hampe A, Jump AS (2011) Climate relicts: Past, present, future. Annu Rev Ecol Evol Syst 42:313–333. https://doi.org/10.1146/annurev-ecolsys-102710-145015
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467. https://doi.org/10.1111/j.1461-0248.2005.00739.x
Harrison S, Noss R (2017) Endemism hotspots are linked to stable climatic refugia. Ann Bot 119:207–214. https://doi.org/10.1093/aob/mcw248
Heimer V, Frajman B (2023) Polyploidization was not involved in the origin of five endemic species from southern Europe but is otherwise frequent in Euphorbia section Esula (Euphorbiaceae). Bot J Linn Soc 201:260–285. https://doi.org/10.1093/botlinnean/boac040
Hendrichs M, Oberwinkler F, Begerow D, Bauer R (2004) Carex subgenus Carex (Cyperaceae) – A phylogenetic approach using ITS sequences. Plant Syst Evol 246:89–107. https://doi.org/10.1007/s00606-004-0128-0
Heubl GR, Vogt R (1985) Chemosystematische Studien in der Gattung Cochlearia L. (Cruciferae). Bot Jahrb Syst 107:177–194
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58:247–276. https://doi.org/10.1111/j.1095-8312.1996.tb01434.x
Hewitt GM (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913. https://doi.org/10.1038/35016000
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond b, Biol Sci 359:183–195. https://doi.org/10.1098/rstb.2003.1388
Hidalgo O, Garcia-Jacas N, Garnatje T, Susanna A (2006) Phylogeny of Rhaponticum (Asteraceae, Cardueae–Centaureinae) and related genera inferred from nuclear and chloroplast DNA sequence data: Taxonomic and biogeographic implications. Ann Bot 97:705–714. https://doi.org/10.1093/aob/mcl029
Hua X, Wiens JJ (2013) How does climate influence speciation? Am Nat 182:1–12. https://doi.org/10.1086/670690
Huck S, Büdel B, Haase P, Kadereit JW, Printzen C (2009) Range wide phylogeography of the European temperate-montane Meum athamanticum: evidence for periglacial persistence and multiple northern refugia. J Biogeogr 36:1588–1599. https://doi.org/10.1111/j.1365-2699.2009.02096.x
Hühn P, Dillenberger MS, Krause S, Kadereit JW (2023) Polyploid hybrid speciation in the Calcarata species complex of Viola sect. Melanium (Violaceae) - relating hybrid species to parent species distribution and ecology. Bot J Linn Soc 201:309–328. https://doi.org/10.1093/botlinnean/boac056
İkinci N, Oberprieler C, Güner A (2006) On the origin of European lilies: phylogenetic analysis of Lilium section Liriotypus (Liliaceae) using sequences of the nuclear ribosomal transcribed spacers. Willdenowia 36:647–656. https://doi.org/10.3372/wi.36.36201
Ivy-Ochs, S. (2015) Glacier variations in the European Alps at the end of the last glaciation. Cuad de Investig Geogr 41:295–315. https://doi.org/10.18172/cig.2750
Janska V, Jimenez-Alfaro B, Chytrý M, Divísek J, Anenkhonov O, Korolyuk A, Lashchinskyi N, Culek M (2017) Palaeodistribution modelling of European vegetation types at the Last Glacial Maximum using modern analogues from Siberia: Prospects and limitations. Quat Sci Rev 159:103e115. https://doi.org/10.1016/j.quascirev.2017.01.011
Jansson R (2003) Global patterns in endemism explained by past climatic change. Proc R Soc Lond B 270:583–590. https://doi.org/10.1098/rspb.2002.2283
Jardim de Queiroz L, Doenz CJ, Altermatt F, Alther R, Borko S, Brodersen J, Gossner MM, Graham C, Matthews B, McFadden IR, Pellissier L, Schmitt T, Selz OM, Villalba S, Rüber L, Zimmermann NE, Seehausen O (2022) Climate, immigration and speciation shape terrestrial and aquatic biodiversity in the European Alps. Proc R Soc B 289:20221020. https://doi.org/10.1098/rspb.2022.1020
Jetz W, Rahbek C, Colwell RK (2004) The coincidence of rarity and richness and the potential signature of history in centres of endemism. Ecol Lett 7:1180–1191. https://doi.org/10.1111/j.1461-0248.2004.00678.x
Johri P, Aquadro CF, Beaumont M, Charlesworth B, Excoffier L, Eyre-Walker A, Keightley PD, Lynch M, McVean G, Payseur BA, Pfeifer SP, Stephan W, Jensen JD (2022) Recommendations for improving statistical inference in population genomics. PLoS Biol 20:e3001669. https://doi.org/10.1371/journal.pbio.3001669
Kadereit JW (2017) The role of in situ species diversification for the evolution of high vascular plant species diversity in the European Alps - a review and interpretation of phylogenetic studies of the endemic flora of the Alps. Perspect Plant Ecol Evol Syst 26:28–38. https://doi.org/10.1016/j.ppees.2017.03.002
Kadereit JW (2022) Adaptive evolutionary divergence of populations persisting in warming cold-stage refugia – candidate examples from the periphery of the European Alps. Alp Bot 133:1–10. https://doi.org/10.1007/s00035-022-00291-0
Kadereit JW, Abbott RJ (2021) Plant speciation in the Quaternary. Plant Ecol Divers 14:105–142. https://doi.org/10.1080/17550874.2021.2012849
Kadereit JW, Griebeler EM, Comes HP (2004) Ouaternary diversification in European alpine plants - pattern and process. Philos Trans R Soc Lond B Biol Sci 359:265–274. https://doi.org/10.1098/rstb.2003.1389
Kadereit JW, Repplinger M, Schmalz N, Uhink CH, Wörz A (2008) The phylogeny and biogeography of Apiaceae subf. Saniculoideae: from south to north and south again. Taxon 57:365–382. https://doi.org/10.2307/25066010
Kadereit JW, Lauterbach M, Kandziora M, Spillmann J, Nyffeler R (2019) Dual colonization of European high altitude areas from Asia by Callianthemum C.A. Mey. (Ranunculaceae). Plant Syst Evol 305:431–443. https://doi.org/10.1007/s00606-019-01583-5
Kadereit JW, Laux P, Dillenberger MD (2021) A conspectus of Tephroseris (Rchb.) Rchb. in Europe outside Russia and notes on the decline of the genus. Willdenowia 51:271–317. https://doi.org/10.3372/wi.51.51209
Keppel G, Ottaviani G, Harrison S, Wardell-Johnson GW, Marcantonio M, Mucina L (2018) Towards an eco-evolutionary understanding of endemism hotspots and refugia. Ann Bot 122:927–934. https://doi.org/10.1093/aob/mcy173
Kiedrzyński M, Zielińska KM, Kiedrzyńska E, Rewicz A (2017) Refugial debate: on small sites according to their function and capacity. Evol Ecol 31:815–827. https://doi.org/10.1007/s10682-017-9913-4
Klein JT, Kadereit JW (2015) Phylogeny, biogeography and evolution of edaphic association in the European oreophytes Sempervivum and Jovibarba (Crassulaceae). Int J Plant Sci 176:44–71. https://doi.org/10.1086/677948
Knotek A, Kolář F (2018) Different low-competition island habitats in Central Europe harbour similar levels of genetic diversity in relict populations of Galium pusillum agg. (Rubiaceae). Biol J Linn Soc 125:491–507. https://doi.org/10.1093/biolinnean/bly126
Koch M (2002) Genetic differentiation and speciation in prealpine Cochlearia: Allohexaploid Cochlearia bavarica Vogt (Brassicaceae) compared to its diploid ancestor Cochlearia pyrenaica DC. in Germany and Austria. Plant Syst Evol 232:35–49. https://doi.org/10.1007/s006060200025
Koch M, Hurka H, Mummenhoff K (1996) Chloroplast DNA restriction site variation and RAPD-analyses in Cochlearia (Brassicaceae): Biosystematics and speciation. Nord J Bot 16:585–603. https://doi.org/10.1111/j.1756-1051.1996.tb00276.x
Kolář F, Lučanová M, Vít P, Urfus T, Chrtek J, Fér T, Ehrendorfer F, Suda J (2013) Diversity and endemism in deglaciated areas: ploidy, relative genome size and niche differentiation in the Galium pusillum complex (Rubiaceae) in Northern and Central Europe. Ann Bot 111:1095–1108. https://doi.org/10.1093/aob/mct074
Kolář F, Píšová S, Záveská E, Fér T, Weiser M, Ehrendorfer F, Suda J (2015) The origin of unique diversity in deglaciated areas: traces of Pleistocene processes in north-European endemics from the Galium pusillum polyploid complex (Rubiaceae). Mol Ecol 24:1311–1334. https://doi.org/10.1111/mec.13110
Kolarčik V, Zozomová-Lihová J, Ducár E, Mártonfi P (2014) Evolutionary significance of hybridization in Onosma (Boraginaceae): analyses of stabilized hemisexual odd polyploids and recent sterile hybrids. Biol J Linn Soc 112:89–107. https://doi.org/10.1111/bij.12270
Konowalik K, Wagner F, Tomasello S, Vogt R, Oberprieler C (2015) Detecting reticulate relationships among diploid Leucanthemum Mill. (Compositae, Anthemideae) taxa using multilocus species tree reconstruction methods and AFLP fingerprinting. Mol Phylogenet Evol 92:308–328. https://doi.org/10.1016/j.ympev.2015.06.003
Körner C (2021) Alpine Plant Life. Springer Nature, Heidelberg
Kramp K, Huck S, Niketić M, Tomović G, Schmitt T, (2009) Multiple glacial refugia and complex postglacial range shifts of the obligatory woodland plant Polygonatum verticillatum (Convallariaceae). Plant Biol 11:392–404. https://doi.org/10.1111/j.1438-8677.2008.00130.x
Krause C, Wörz A, Thiv M (2017) Molecular analysis of the extinct Central European endemic Saxifraga oppositifolia subsp. amphibia and its implications on glaciation biogeography. Alp Bot 127:85–95. https://doi.org/10.1007/s00035-016-0183-5
Kropf M, Kadereit JW, Comes HP (2003) Differential cycles of range contraction and expansion in European high mountain plants during the Late Quaternary: insights from Pritzelago alpina (L.) O. Kuntze (Brassicaceae). Mol Ecol 12:931–949. https://doi.org/10.1046/j.1365-294X.2003.01781.x
Kropf M, Comes HP, Kadereit JW (2006) Long-distance dispersal versus vicariance: the origin and genetic diversity of alpine plants in the Spanish Sierra Nevada. New Phytol 172:169–184. https://doi.org/10.1111/j.1469-8137.2006.01795.x
Kropf M, Comes HP, Kadereit JW (2008) Causes of the genetic architecture of southwest European high mountain disjuncts. Plant Ecol Divers 1:217–228. https://doi.org/10.1080/17550870802331938
Kuzmanović N, Lakušić D, Frajman B, Alegro A, Schönswetter P (2017) Phylogenetic relationships in Seslerieae (Poaceae) including resurrection of Psilathera and Sesleriella, two monotypic genera endemic to the Alps. Taxon 66:1349–1370. https://doi.org/10.12705/666.5
Lang G (1994) Quartäre Vegetationsgeschichte Europas. Gustav Fischer Verlag, Jena, Stuttgart
Lega M, Fior S, Prosser F, Bertolli A, Li M, Varotto C (2012) Application of the unified species concept reveals distinct lineages for disjunct endemics of the Brassica repanda (Brassicaceae) complex. Biol J Linn Soc 106:482–497. https://doi.org/10.1111/j.1095-8312.2012.01887.x
Leipold M, Tausch S, Poschlod P, Reisch C (2017) Species distribution modeling and molecular markers suggest longitudinal range shifts and cryptic northern refugia of the typical calcareous grassland species Hippocrepis comosa (horseshoe vetch). Ecol Evol 7:1919–1935. https://doi.org/10.1002/ece3.2811
Ley AC, Nissen J, Wölk A, Röser M (2018) Glacial refugia and speciation in a group of wind-pollinated and -dispersed, endemic Alpine species of Helictotrichon (Poaceae). PLoS ONE 13:e0205354. https://doi.org/10.1371/journal.pone.0205354
Lister AM (2004) The impact of Quaternary Ice Ages on mammalian evolution. Philos Trans R Soc Lond b, Biol Sci 359:221–241. https://doi.org/10.1098/rstb.2003.1436
López-Alvarado J, Sáez L, Filigheddu R, Garcia-Jacas N, Susanna A (2014) The limitations of molecular markers in phylogenetic reconstruction: The case of Centaurea sect. Phrygia (Compositae). Taxon 63:1079–1091. https://doi.org/10.12705/635.6
Luqman H, Widmer A, Fior S, Wegmann D (2021) Identifying loci under selection via explicit demographic models. Mol Ecol Resour 21:2719–2737. https://doi.org/10.1111/1755-0998.13415
Luqman H, Wegmann D, Fior S, Widmer A (2023) Climate-induced range shifts drive adaptive response via spatio-temporal sieving of alleles. Nat Commun 14:1080. https://doi.org/10.1038/s41467-023-36631-9
Lutz E, Schneller JJ, Holderegger R (2000) Understanding population history for conservation purposes: population genetics of Saxifraga aizoides (Saxifragaceae) in the lowlands and lower mountains north of the Alps. Am J Bot 87:583–590. https://doi.org/10.2307/2656602
Magauer M, Schönswetter P, Jang T-S, Frajman B (2014) Disentangling relationships within the disjunctly distributed Alyssum ovirense/A. wulfenianum group (Brassicaceae), including description of a novel species from the North-Eastern Alps. Bot J Linn Soc 176:486–505. https://doi.org/10.1111/boj.12214
Manton I (1934) The problem of Biscutella laevigata L. Z Indukt Abstammungs- Vererbungsl 67:41–57. https://doi.org/10.1007/BF01744017
Manton I (1937) The problem of Biscutella laevigata L. II. Evidence from meiosis. Ann Bot 1:439–462. https://www.jstor.org/stable/42906562
Marcussen T, Ballard HE, Danihelka J, Flores AR, Nicola MV, Watson JM (2022) A revised phylogenetic classification for Viola (Violaceae). Plants 11:2224. https://doi.org/10.3390/plants11172224
Mayol M, Riba M, González-Martínez SC, Bagnoli F, de Beaulieu J-L, Berganzo E, Burgarella C, Dubreuil M, Krajmerová D, Paule L, Romšaková I, Vettori C, Vincenot L, Vendramin GG (2015) Adapting through glacial cycles: insights from a long-lived tree (Taxus baccata). New Phytol 208:973–986. https://doi.org/10.1111/nph.13496
Médail F, Diadema K (2009) Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J Biogeogr 36:1333–1345. https://doi.org/10.1111/j.1365-2699.2008.02051.x
Médail F, Quézel P (1997) Hot-spots analysis for conservation of plant biodiversity in the Mediterranean basin. Ann Missouri Bot Gard 84:112–127. https://doi.org/10.2307/2399957
Médail F, Verlaque R (1997) Ecological characteristics and rarity of endemic plants from southeast France and Corsica: implications for biodiversity conservation. Biol Conserv 80:269–281. https://doi.org/10.1016/S0006-3207(96)00055-9
Mee JA, Moore J-S (2014) The ecological and evolutionary implications of microrefugia. J Biogeogr 41:837–841. https://doi.org/10.1111/jbi.12254
Merxmüller H (1952) Untersuchungen zur Sippengliederung und Arealbildung in den Alpen. Teil I. Jahrb Ver Schutze Alpenpfl -Tiere 17:96–133
Merxmüller H (1953) Untersuchungen zur Sippengliederung und Arealbildung in den Alpen. Teil II. Jahrb Ver Schutze Alpenpfl -Tiere 18:135–158
Merxmüller H (1954) Untersuchungen zur Sippengliederung und Arealbildung in den Alpen. Teil III Schluß. Jahrb Ver Schutze Alpenpfl -Tiere 19:97–139
Michel T, Huck S, Schmitt T, Liebrich A, Haase P, Büdel B (2010) The molecular population structure of the tall forb Cicerbita alpina (Asteraceae) supports the idea of cryptic glacial refugia in central Europe. Bot J Linn Soc 164:142–154. https://doi.org/10.1111/j.1095-8339.2010.01079.x
Moore AJ, Merges D, Kadereit JW (2013) The origin of the serpentine endemic Minuartia laricifolia subsp. ophiolitica by vicariance and competitive exclusion. Mol Ecol 22:2218–2231. https://doi.org/10.1111/mec.12266
Mráz P, Ronikier M (2016) Biogeography of the Carpathians: evolutionary and spatial facets of biodiversity. Biol J Linn Soc 119:528–559. https://doi.org/10.1111/bij.12918
Nieto Feliner G (2011) Southern European glacial refugia: a tale of tales. Taxon 60:365–372. https://doi.org/10.1002/tax.602007
Nieto Feliner G (2014) Patterns and processes in plant phylogeography in the Mediterranean Basin. A review. Perspect Plant Ecol Evol Syst 16:265–278. https://doi.org/10.1016/j.ppees.2014.07.002
Niklfeld H (1972) Der niederösterreichische Alpenostrand – ein Glazialrefugium montaner Pflanzensippen. Jahrb Ver Schutze Alpenpfl -Tiere 37:1–54
Ohlemüller R, Huntley B, Normand S, Svenning J-C (2012) Potential source and sink locations for climate-driven species range shifts in Europe since the Last Glacial Maximum. Global Ecol Biogeogr 21:152–163. https://doi.org/10.1111/j.1466-8238.2011.00674.x
Ozenda P (1985) La végétation de la chaîne alpine dans l’espace montagnard européen. Masson, Paris
Ozenda P (2009) On the genesis of the plant population in the Alps: New or critical aspects. C R Biologies 332:1092–1103. https://doi.org/10.1016/j.crvi.2009.09.018
Pachschwöll C, Escobar García P, Winkler M, Schneeweiss GM, Schönswetter P (2015) Polyploidisation and geographic differentiation drive diversification in a European high mountain plant group (Doronicum clusii Aggregate, Asteraceae). PLoS ONE 10(3):e0118197. https://doi.org/10.1371/journal.pone.0118197
Pan D, Hülber K, Willner W, Schneeweiss GM (2020) An explicit test of Pleistocene survival in peripheral versus nunatak refugia in two high mountain plant species. Mol Ecol 29:172–183. https://doi.org/10.1111/mec.15316
Pan D, Schönswetter P, Moser T, Vitek E, Schneeweiss GM (2019) Ancestral remnants or peripheral segregates? Phylogenetic relationships of two narrowly endemic Euphrasia species (Orobanchaceae) from the eastern European Alps. AoB Plants https://doi.org/10.1093/aobpla/plz007
Parisod C (2022) Plant speciation in the face of recurrent climate changes in the Alps. Alp Bot 132:21–28. https://doi.org/10.1007/s00035-021-00259-6
Parisod C, Besnard G (2007) Glacial in situ survival in the Western Alps and polytopic autopolyploidy in Biscutella laevigata L. (Brassicaceae). Mol Ecol 16:2755–2767. https://doi.org/10.1111/j.1365-294X.2007.03315.x
Park J-M, Kovačić S, Liber Z, Eddie WMM, Schneeweiss GM (2006) Phylogeny and biogeography of isophyllous species of Campanula (Campanulaceae) in the Mediterranean Area. Syst Bot 31:862–880. https://doi.org/10.1600/036364406779695924
Patsiou TS, Conti E, Zimmermann NE, Theodoridis S, Randin CF (2014) Topo-climatic microrefugia explain the persistence of a rare endemic plant in the Alps during the last 21 millennia. Glob Chang Biol 20:2286–2300. https://doi.org/10.1111/gcb.12515
Paun O, Lehnebach C, Johansson JT, Lockhart P, Hörandl E (2005) Phylogenetic relationships and biogeography of Ranunculus and allied genera (Ranunculaceae) in the Mediterranean region and in the European Alpine System. Taxon 54:911–930. https://doi.org/10.2307/25065478
Paun O, Schönswetter P, Winkler M, Intrabiodiv Consortium, Tribsch A (2008) Historical divergence vs. contemporary gene flow: evolutionary history of the calcicole Ranunculus alpestris group (Ranunculaceae) in the European Alps and the Carpathians. Mol Ecol 17:4263–4275. https://doi.org/10.1111/j.1365-294X.2008.03908.x
Pawłowski B (1970) Remarques sur l’endémisme dans la flore des Alpes et des Carpates. Vegetatio 21:181–243. https://doi.org/10.1007/BF02269663
Pflugbeil G, Affenzeller M, Tribsch A, Comes HP (2021) Primary hybrid zone formation in Tephroseris helenitis (Asteraceae), following postglacial range expansion along the central Northern Alps. Mol Ecol 30:1704–1720. https://doi.org/10.1111/mec.15832
Pigott CD (1956) The vegetation of Upper Teesdale in the North Pennines. J Ecol 44:545–586. https://doi.org/10.2307/2256835
Pinto-Carrasco D, Scheunert A, Heubl G, Rico E, Martínez-Ortega M (2017) Unravelling the phylogeny of the root-hemiparasitic genus Odontites (tribe Rhinantheae, Orobanchaceae): Evidence for five main lineages. Taxon 66:886–908. https://doi.org/10.12705/664.6
Piwczyński M, Puchałka R, Spalik K (2015) The infrageneric taxonomy of Chaerophyllum (Apiaceae) revisited: new evidence from nuclear ribosomal DNA ITS sequences and fruit anatomy. Bot J Linn Soc 178:298–313. https://doi.org/10.1111/boj.12282
Provan J, Bennett KD (2008) Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol 23:564–571. https://doi.org/10.1016/j.tree.2008.06.010
Reisch C, Poschlod P (2004) Clonal diversity and subpopulation structure in central European relict populations of Saxifraga paniculata MILL. (Saxifragaceae). Feddes Repert 115:239–247. https://doi.org/10.1002/fedr.200311040
Reisch C, Poschlod P, Wingender R (2002) Genetic variation of Sesleria albicans Kit. ex Schultes (Poaceae): Lack of evidence for glacial relict endemism in Central Europe. Plant Biol 4:711–719. https://doi.org/10.1055/s-2002-37402
Reisch C, Poschlod P, Wingender R (2003) Genetic variation of Saxifraga paniculata Mill. (Saxifragaceae): molecular evidence for glacial relict endemism in central Europe. Biol J Linn Soc 80:11–21. https://doi.org/10.1046/j.1095-8312.2003.00215.x
Rešetnik I, Frajman B, Bogdanović S, Ehrendorfer F, Schönswetter P (2014) Disentangling relationships among the diploid members of the intricate genus Knautia (Caprifoliaceae, Dipsacoideae). Mol Phylogenet Evol 74:97–110. https://doi.org/10.1016/j.ympev.2014.01.028
Ronikier M (2011) Biogeography of high-mountain plants in the Carpathians: An emerging phylogeographical perspective. Taxon 60:373–389. https://doi.org/10.1002/tax.602008
Ronikier M, Costa A, Fuertes Aguilar J, Nieto Feliner G, Küpfer P, Mirek Z (2008) Phylogeography of Pulsatilla vernalis (L.) Mill. (Ranunculaceae): chloroplast DNA reveals two evolutionary lineages across central Europe and Scandinavia. J Biogeogr 35:1650–1664. https://doi.org/10.1111/j.1365-2699.2008.01907.x
Rull V (2009) Microrefugia. J Biogeogr 36:481–484. https://doi.org/10.1111/j.1365-2699.2008.02023.x
Salmaki Y, Kattari S, Heubl G, Bräuchler C (2016) Phylogeny of non-monophyletic Teucrium (Lamiaceae: Ajugoideae): Implications for character evolution and taxonomy. Taxon 65:805–822. https://doi.org/10.12705/654.8
Samuel R, Gutermann W, Stuessy TF, Ruas CF, Lack H-W, Tremetsberger K, Talavera S, Hermanowski B, Ehrendorfer F (2006) Molecular phylogenetics reveals Leontodon (Asteraceae, Lactuceae) to be diphyletic. Am J Bot 93:1193–1205. https://doi.org/10.3732/ajb.93.8.1193
Sanz M, Schönswetter P, Vallès J, Vilatersana R (2017) Glacial survival in and recent long-distance dispersal to the Iberian Mountains: the phylogeographic history of Artemisia umbelliformis (Asteraceae). Bot J Linn Soc 183:587–599. https://doi.org/10.1093/botlinnean/box002
Schenk JJ (2016) Consequences of secondary calibrations on divergence time estimates. PLoS One 11:e0148228. https://doi.org/10.1371/journal.pone.0148228
Schmitt T, Muster C, Schönswetter P (2010) Are disjunct alpine and arctic-alpine animal and plant species in the Western Palearctic really “relics of a cold past”? In: Habel JC, Assmann T (eds) Relict Species: Phylogeography and Conservation Biology. Springer-Verlag, Berlin Heidelberg, pp 239–252
Schneeweiss GM, Schönswetter P (2010) The wide but disjunct range of the European mountain plant Androsace lactea L. (Primulaceae) reflects Late Pleistocene range fragmentation and post-glacial distributional stasis. J Biogeogr 37:2016–2025. https://doi.org/10.1111/j.1365-2699.2010.02350.x
Schneeweiss GM, Schönswetter P, Kelso S, Niklfeld H (2004) Complex biogeographic patterns in Androsace (Primulaceae) and related genera: Evidence from phylogenetic analyses of nuclear internal transcribed spacer and plastid trnL-F sequences. Syst Biol 53:856–876. https://doi.org/10.1080/10635150490522566
Schneeweiss GM, Pachschwöll C, Tribsch A, Schönswetter P, Barfuss MHJ, Esfeld K, Weiss-Schneeweiss H, Thiv M (2013) Molecular phylogenetic analyses identify Alpine differentiation and dysploid chromosome number changes as major forces for the evolution of the European endemic Phyteuma (Campanulaceae). Mol Phylogenet Evol 69:634–652. https://doi.org/10.1016/j.ympev.2013.07.015
Schneeweiss GM, Winkler M, Schönswetter P (2017) Secondary contact after divergence in allopatry explains current lack of ecogeographical isolation in two hybridizing alpine plant species. J Biogeogr 44:2575–2584. https://doi.org/10.1111/jbi.13071
Schönswetter P, Schneeweiss GM (2009) Androsace komovensis sp. nov., a long mistaken local endemic from the southern Balkan Peninsula with biogeographic links to the Eastern Alps. Taxon 58:544–549. https://doi.org/10.1002/tax.582018
Schönswetter P, Tribsch A, Barfuss M, Niklfeld H (2002) Several Pleistocene refugia detected in the high alpine plant Phyteuma globulariifolium Sternb. & Hoppe (Campanulaceae) in the European Alps. Mol Ecol 11:2637–2647. https://doi.org/10.1046/j.1365-294X.2002.01651.x
Schönswetter P, Tribsch A, Schneeweiss GM, Niklfeld H (2003a) Disjunctions in relict alpine plants: phylogeography of Androsace brevis and A. wulfeniana (Primulaceae). Bot J Linn Soc 141:437–446. https://doi.org/10.1046/j.0024-4074.2002.00134.x
Schönswetter P, Paun O, Tribsch A, Niklfeld H (2003b) Out of the Alps: colonisation of the Arctic by East Alpine populations of Ranunculus glacialis (Ranunculaceae). Mol Ecol 12:3371–3381. https://doi.org/10.1046/j.1365-294X.2003.01984.x
Schönswetter P, Tribsch A, Niklfeld H (2004a) Amplified Fragment Length Polymorphism (AFLP) reveals no genetic divergence of the Eastern Alpine endemic Oxytropis campestris subsp. tiroliensis (Fabaceae) from widespread subsp. campestris. Plant Syst Evol 244:245–255. https://doi.org/10.1007/s00606-003-0096-9
Schönswetter P, Tribsch A, Niklfeld H (2004b) Amplified fragment length polymorphism (AFLP) suggests old and recent immigration into the Alps by the arctic-alpine annual Comastoma tenellum (Gentianaceae). J Biogeogr 31:1673–1681. https://doi.org/10.1111/j.1365-2699.2004.01103.x
Schönswetter P, Stehlik I, Holderegger R, Tribsch A (2005) Molecular evidence for glacial refugia of mountain plants in the European Alps. Mol Ecol 14:3547–3555. https://doi.org/10.1111/j.1365-294X.2005.02683.x
Schönswetter P, Popp M, Brochmann C (2006a) Central Asian origin of and strong genetic differentiation among populations of the rare and disjunct Carex atrofusca (Cyperaceae) in the Alps. J Biogeogr 33:948–956. https://doi.org/10.1111/j.1365-2699.2006.01462.x
Schönswetter P, Popp M, Brochmann C (2006b) Rare arctic-alpine plants of the European Alps have different immigration histories: the snowbed species Minuartia biflora and Ranunculus pygmaeus. Mol Ecol 15:709–720. https://doi.org/10.1111/j.1365-294X.2006.02821.x
Schönswetter P, Solstad H, Escobar García P, Elven R (2009) A combined molecular and morphological approach to the taxonomically intricate European mountain plant Papaver alpinum s.l. (Papaveraceae) — taxa or informal phylogeographical groups? Taxon 58:1326–1343. https://doi.org/10.1002/tax.584020
Schorr G, Holstein N, Pearman PB, Guisan A, Kadereit JW (2012) Integrating species distribution models (SDMs) and phylogeography for two species of Alpine Primula. Ecol Evol 2:1260–1277. https://doi.org/10.1002/ece3.100
Schorr G, Pearman PB, Guisan A, Kadereit JW (2013) Combining palaeodistribution modelling and phylogeographical approaches for identifying glacial refugia in Alpine Primula. J Biogeogr 40:1947–1960. https://doi.org/10.1111/jbi.12132
Schustler F (1923) The dealpines, their nature and importance. Věstnik I. sjezdu českoslov. botaniků v Praze. Prag
Seguinot J, Ivy-Ochs S, Jouvet G, Huss M, Funk M, Preusser F (2018) Modelling last glacial cycle ice dynamics in the Alps. Cryosphere 12:3265–3285. https://doi.org/10.5194/tc-12-3265-2018
Skrede I, Eidesen PB, Piñeiro Portela R, Brochmann C (2006) Refugia, differentiation and postglacial migration in arctic-alpine Eurasia, exemplified by the mountain avens (Dryas octopetala L.). Mol Ecol 15:1827–1840. https://doi.org/10.1111/j.1365-294X.2006.02908.x
Skubic M, Schönswetter P, Frajman B (2018) Diversification of Cerastium sylvaticum and C. subtriflorum on the margin of the south-eastern Alps. Plant Syst Evol 304:1101–1115. https://doi.org/10.1007/s00606-018-1535-y
Slovák M, Melichárková A, Gbúrová Štubňová E, Kučera J, Mandáková T, Smyčka J, Lavergne S, Passalacqua NG, Vďačný P, Paun O (2022) Pervasive introgression during rapid diversification of the European mountain genus Soldanella (L.) (Primulaceae). Syst Biol 72:491-504. https://doi.org/10.1093/sysbio/syac071
Smyčka J, Roquet C, Renaud J, Thuiller W, Zimmermann NE, Lavergne S (2017) Disentangling drivers of plant endemism and diversification in the European Alps – A phylogenetic and spatially explicit approach. Perspect Plant Ecol Evol Syst 28:19–27. https://doi.org/10.1016/j.ppees.2017.06.004
Španiel S, Juillerat P, Kaplan K, Bovio M, Bäumler B, Perret M, Mártonfiová L, Zozomová-Lihová J (2022) Out of the Balkans and Anatolia to the Western Alps? Surprising phylogenetic implications for two endemic Alyssum (Brassicaceae) species: A. cognense sp. nov. and A. rossetii. Bot J Linn Soc 201:286–308. https://doi.org/10.1093/botlinnean/boac041
Sramkó G, Laczkó L, Volkova PA, Bateman RM, Mlinarec J (2019) Evolutionary history of the Pasque-flowers (Pulsatilla, Ranunculaceae): molecular phylogenetics, systematics and rDNA evolution. Mol Phylogenet Evol 135:45–61. https://doi.org/10.1016/j.ympev.2019.02.015
Stehlik I (2002) Glacial history of the alpine Rumex nivalis (Polygonaceae): a comparison of traditional phylogeographic methods with nested clade analysis. Am J Bot 89:2007–2016. https://doi.org/10.3732/ajb.89.12.2007
Stehlik I, Blattner FR, Holderegger R, Bachmann K (2002) Multiple glacial nunatak survival of the high alpine Eritrichium nanum (L.) Gaudin within the European Alps. Mol Ecol 11:2027–2036. https://doi.org/10.1046/j.1365-294X.2002.01595.x
Stewart JR, Lister AM (2001) Cryptic northern refugia and the origins of modern biota. Trends Ecol Evol 16:608–613. https://doi.org/10.1016/S0169-5347(01)02338-2
Stewart JR, Stringer CB (2012) Human evolution out of Africa: the role of refugia and climate change. Science 335:1317–1321. https://doi.org/10.1126/science.1215627
Stewart JR, Lister AM, Barnes I, Dalén L (2010) Refugia revisited: individualistic responses of species in space and time. Proc R Soc Lond B 277:661–671. https://doi.org/10.1098/rspb.2009.1272
Svenning J-C, Normand S, Kageyama M (2008) Glacial refugia of temperate trees in Europe: insights from species distribution modelling. J Ecology 96:1117–1127. https://doi.org/10.1111/j.1365-2745.2008.01422.x
Svenning J-C, Eiserhardt WL, Normand S, Ordonez A, Sandel B (2015) The influence of paleoclimate on present-day patterns of biodiversity and ecosystems. Annu Rev Ecol Evol Syst 46:551–572. https://doi.org/10.1146/annurev-ecolsys-112414-054314
Szövényi P, Arroyo K, Guggisberg A, Conti E (2009) Effects of Pleistocene glaciations on the genetic structure of Saxifraga florulenta (Saxifragaceae), a rare endemic of the Maritime Alps. Taxon 58:532–543. https://doi.org/10.1002/tax.582017
Taberlet P, Zimmermann NE, Englisch T et al (2016) Genetic diversity in widespread species is not congruent with species richness in alpine plant communities. Ecol Lett 15:1439–1448. https://doi.org/10.1111/ele.12004
Tausch S, Leipold M, Poschlod P, Reisch C (2017) Molecular markers provide evidence for a broad-fronted recolonisation of the widespread calcareous grassland species Sanguisorba minor from southern and cryptic northern refugia. Plant Biol 19:562–570. https://doi.org/10.1111/plb.12570
Thiel-Egenter C, Alvarez N, Holderegger R et al (2011) Break zones in the distributions of alleles and species in alpine plants. J Biogeogr 38:772–782. https://doi.org/10.1111/j.1365-2699.2010.02441.x
Thorn K (1957) Praealpin - Dealpin. Wandlung eines Arealbegriffes. Mitt. Florist.-Soziol. Arb.gem. 6/7:79–89
Thorn K (1960) Bemerkungen zu einer Übersichtskarte vermutlicher Glazialreliktpflanzen Deutschlands. Mitt. Florist.-Soziol. Arb.gem. 8:81–85
Tremetsberger K, König C, Samuel R, Pinsker W, Stuessy TF (2002) Infraspecific genetic variation in Biscutella laevigata (Brassicaceae): new focus on Irene Manton’s hypothesis. Plant Syst Evol 233:163–181. https://doi.org/10.1007/s00606-002-0189-x
Tribsch A (2004) Areas of endemism of vascular plants in the Eastern Alps in relation to Pleistocene glaciation. J Biogeogr 31:747–760. https://doi.org/10.1111/j.1365-2699.2004.01065.x
Tribsch A, Schönswetter P (2003) Patterns of endemism and comparative phylogeography confirm environmental evidence for Pleistocene refugia in the Eastern Alps. Taxon 52:477–497. https://doi.org/10.2307/3647447
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (eds) (1968) Flora Europaea, vol 2. Cambridge University Press, Cambridge
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (eds) (1972) Flora Europaea, vol 3. Cambridge University Press, Cambridge
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (eds) (1976) Flora Europaea, vol 4. Cambridge University Press, Cambridge
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (eds) (1980) Flora Europaea, vol 5. Cambridge University Press, Cambridge
Tutin TG, Burges NA, Chater AO, Edmondson JR, Heywood VH, Moore DM, Valentine DH, Walters SM, Webb DA (eds) (1993) Flora Europaea Ed. 2, Vol. 1. Cambridge University Press, Cambridge
Tyler T (2002) Large-scale geographic patterns of genetic variation in Melica nutans, a widespread Eurasian woodland grass. Plant Syst Evol 236:73–87. https://doi.org/10.1007/s00606-002-0235-8
Tzedakis PC, Emerson BC, Hewitt GM (2013) Cryptic or mystic? Glacial tree refugia in northern Europe. Trends Ecol Evol 28:696–704. https://doi.org/10.1016/j.tree.2013.09.001
Urbaniak J, Kwiatkowski P, Pawlikowski P (2018) Phylogeography of Swertia perennis in Europe based on cpDNA markers. PeerJ 6:e5512. https://doi.org/10.7717/peerj.5512
Valtueña FJ, Preston CD, Kadereit JW (2012) Phylogeography of a Tertiary relict plant, Meconopsis cambrica (Papaveraceae) implies the existence of northern refugia for a temperate herb. Mol Ecol 21:1423–1437. https://doi.org/10.1111/j.1365-294X.2012.05473.x
Valtueña FJ, Dillenberger MS, Kadereit JW, Moore AJ, Preston CD (2015) What is the origin of the Scottish populations of the European endemic Cherleria sedoides L. (Caryophyllaceae)? New J Botany 5:13–25. https://doi.org/10.1179/2042349715Y.0000000002
Väre H, Lampinen R, Humphries C, Williams P (2003) Taxonomic diversity of vascular plants in the European Alpine areas. In: Nagy L, Grabherr G, Körner C, Thompson DBA (eds) Alpine biodiversity in Europe. Springer, Berlin, pp 133–148
Vargas P (2003) Molecular evidence for multiple diversification patterns of alpine plants in Mediterranean Europe. Taxon 52:463–476. https://doi.org/10.2307/3647383
Vaupel A (2013) The ecology and genetics of central and peripheral populations of Carduus defloratus. Dissertation, Philipps-Universität Marburg
Vierhapper F (1924) Über endemische Alpenpflanzen. Alpenfreund 147–148:181–184
Vierhapper F (1925) Über endemische Alpenpflanzen. Alpenfreund:15–16, 47–48, 63–64, 79–80
Vlasta T, Münzbergová Z (2022) Genetic variation in lowland and mountain populations of Tofieldia calyculata and their ability to survive within low levels of genetic diversity. Conserv Genet 23:605–622. https://doi.org/10.1007/s10592-022-01439-5
Vogler F, Reisch C (2013) Vital survivors: low genetic variation but high germination in glacial relict populations of the typical rock plant Draba aizoides. Biodivers Conserv 22:1301–1316. https://doi.org/10.1007/s10531-013-0471-y
Wagner F, Ott T, Zimmer C, Reichhart V, Vogt R, Oberprieler C (2019) ‘At the crossroads towards polyploidy’: genomic divergence and extent of homoploid hybridization are drivers for the formation of the ox-eye daisy polyploid complex (Leucanthemum, Compositae-Anthemideae). New Phytol 223:2039–2053. https://doi.org/10.1111/nph.15784
Walden N, German DA, Wolf EM, Kiefer M, Rigault P, Huang X-C, Kiefer C, Schmickl R, Franzke A, Neuffer B, Mummenhoff K, Koch MA (2020) Nested whole-genome duplications coincide with diversification and high morphological disparity in Brassicaceae. Nat Commun 11:3795. https://doi.org/10.1038/s41467-020-17605-7
Walisch TJ, Matthies D, Hermant S, Colling G (2015) Genetic structure of Saxifraga rosacea subsp. sponhemica, a rare endemic rock plant of Central Europe. Plant Syst Evol 301:251–263. https://doi.org/10.1007/s00606-014-1070-4
Warwick SI, Al-Shehbaz IA, Sauder C, Harris JG, Koch M (2004) Phylogeny of Braya and Neotorularia (Brassicaceae) based on nuclear ribosomal internal transcribed spacer and chloroplast trnL intron sequences. Can J Bot 82:76–392. https://doi.org/10.1139/b04-012
Weberling F, Endlich B, Engel K (1971) Zur systematischen Stellung von Valeriana pancicii Halácsy & Baldacci und V. bertiscea Pančič. Österr Bot Z 119:94–101. https://doi.org/10.1007/BF01373111
Wernisch MM (2007) Molecular systematics, phylogeography and evolution of the genus Cardaminopsis Hayek (Brassicaceae), the closest relatives of the model plant Arabidopsis thaliana (L.) Heynh. Dissertation, Universität für Bodenkultur Wien
Widmer A, Baltisberger M (1999) Extensive intraspecific chloroplast DNA (cpDNA) variation in the alpine Draba aizoides L. (Brassicaceae): haplotype relationships and population structure. Mol Ecol 8:1405–1415. https://doi.org/10.1046/j.1365-294x.1999.00700.x
Więcław H, Kurnicki B, Bihun M, Białecka B, Koopman J (2017) Carex section Racemosae (Cyperaceae) in Europe: morphological diversity, taxonomy and phylogenetic relationships Bot J Linn Soc 183:124–145. https://doi.org/10.1111/boj.12490
Willis KJ, Niklas KJ (2004) The role of Quaternary environmental change in macroevolution: the exception or the rule? Philos Trans R Soc Lond b, Biol Sci 359:159–172. https://doi.org/10.1098/rstb.2003.1387
Willis KJ, Rudner E, Sümegi P (2000) The full-glacial forests of Central and Southeastern Europe. Quat Res 53:203–213. https://doi.org/10.1006/qres.1999.2119
Windmaißer T, Kattari S, Heubl G, Reisch C (2016) Glacial refugia and postglacial expansion of the alpine–prealpine plant species Polygala chamaebuxus. Ecol Evol 6:7809–7819. https://doi.org/10.1002/ece3.2515
Winkler M, Tribsch A, Paun O, Englisch T, IntraBioDiv-Consortium, Schönswetter P (2010) Pleistocene distribution range shifts were accompanied by breeding system divergence within Hornungia alpina (Brassicaceae) in the Alps. Mol Phylogenet Evol 54:571–82. https://doi.org/10.1016/j.ympev.2009.08.009
Winkler M, Tribsch A, Schneeweiss GM, Brodbeck S, Gugerli F, Holderegger R, Schönswetter P (2013) Strong nuclear differentiation contrasts with widespread sharing of plastid DNA haplotypes across taxa in European purple saxifrages (Saxifraga section Porphyrion subsection Oppositifoliae). Bot J Linn Soc 173:622–636. https://doi.org/10.1111/boj.12104
Woolbright SA, Whitham TG, Gehring CA, Allan GJ, Bailey JK (2014) Climate relicts and their associated communities as natural ecology and evolution laboratories. Trends Ecol Evol 29:406–416. https://doi.org/10.1016/j.tree.2014.05.003
Záveská E, Kirschner P, Frajman B, Wessely J, Willner W, Gattringer A, Hülber K, Lazić D, Dobeš C, Schönswetter P (2021) Evidence for glacial refugia of the forest understorey species Helleborus niger (Ranunculaceae) in the southern as well as in the northern limestone Alps. Front Plant Sci 12:683043. https://doi.org/10.3389/fpls.2021.683043
Zhang L-B, Comes HP, Kadereit JW (2004) The temporal course of Quaternary speciation in the European high mountain endemic Primula sect. Auricula (Primulaceae). Int J Plant Sci 165:191–207. https://doi.org/10.1086/380747
Acknowledgements
The author is much indebted to Hans-Peter Comes (Salzburg/Austria), Elizabeth M. Joyce (Munich/Germany) and Richard J. Abbott (St. Andrews/Scotland) as well as to three anonymous reviewers and the editor Christian Parisod (Fribourg/Switzerland) for their comments which greatly helped to improve this manuscript. Many thanks to Leonie Hinderhofer (Munich/Germany) for preparation of Fig. 1.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JWK conceptualized, wrote and revised this paper.
Corresponding author
Ethics declarations
Conflict of interest
The author did not receive support from any organization for the submitted work and has no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadereit, J.W. The uneven distribution of refugial endemics across the European Alps suggests a threefold role of climate in speciation of refugial populations. Alp Botany 134, 29–50 (2024). https://doi.org/10.1007/s00035-024-00306-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00035-024-00306-y