Abstract
While the position of alpine and arctic treelines can be predicted by climatic data, the underlying biological mechanisms are still unclear. In a recent paper in this journal (Körner C, Lenz A, Hoch G (2023) Chronic in situ tissue cooling does not reduce lignification at the Swiss treeline but enhances the risk of 'blue' frost rings. Alpine Botany https://doi.org/10.1007/s00035-023-00293-6) we presented results of an in situ stem-cooling experiment at a Swiss treeline site. The experiment provided answers to two entirely different questions, related to xylogenesis at treeline: (a) the absence of chronic effects of low temperature on lignification, and (b) a high time resolution insight into the rare occurrence of damages in young, still undifferentiated, and thus, non-lignified cells at the occasion of an exceptional early season frost event. In the last issue of Alpine Botany (August 7, 2023), our data had been re-interpreted by (Büntgen, Alpine Botany, 2023) by confusing (b) with (a). Cell death before secondary wall formation interrupts all metabolism, and thus, cannot exert a specific limitation of lignification. For the xylem to lignify, it requires a secondary cell wall in the first place. A frost damage in young tracheid cells is unsuitable for a dendrological treeline hypothesis based on a low-temperature threshold for lignification. Generally, the global pattern of treeline position is not associated with local freezing conditions.
Similar content being viewed by others
Introduction
All organisms reach low-temperature range limits, and so do plants. In trees, the range limit becomes particularly obvious by their forming a high elevation or high latitude treeline. As was pointed out many times in the past, trees have not undergone an inferior evolutionary adaptation of their metabolism to low temperatures, but they become ‘victims’ of their tall stature that enforces aerodynamic coupling with the free atmosphere that is becoming colder with altitude (Körner 1998; Körner 2012; Körner and Hoch 2023). In contrast, the low-stature alpine vegetation profits from aerodynamic decoupling from atmospheric conditions that ensures warmer life conditions during the day and permits growth far above treeline at otherwise similar physiological response characteristics to low temperature (Körner 2008, 2021).
It had been discussed that cormophytes may face a common limit at around 5–6 °C for any significant growth, while monocellular algae in cold water or melting snow may thrive at 0–2 °C because of their lack of complex tissues and lignification (Körner 2008). Later experimental evidence has falsified this explanation. It turned out that growth gradually approaches zero at 0 °C in all plant species from periodically cold environments (Nagelmüller et al. 2017; Schenker et al. 2014; Körner et al. 2019), however, the approach is asymptotic, and growth rates become so low below 5–6 °C that they do not contribute significantly to biomass production, hence, the often assumed 5–6 °C growth limit for crops (Körner 2008). An important observation is that the metabolism associated with xylem lignification is not inferior to any other metabolism, given that xylem was found always lignified in plant stems that grew (very slowly, though) at constant 0 °C for several months (Körner et al. 2019). The biochemical constraints that are slowing growth at low temperature in cold-adapted plants are still not fully resolved, with protein turnover, ATP production, cell wall formation, physiological dryness in cold soils, or a combination of several limitations discussed as possibilities (Körner 2021, Chap. 13). In line with such a broad syndrome of growth limitation, exposure to cold conditions has been found to activate a surprisingly wide spectrum of genes (e.g. Lee et al. 2008).
So, while the possibility that lignification constrains plant growth under low temperatures had been listed amid different candidates for growth limits of plants in cold climates (Körner 2008), the evidence for a single, most critical driver of tissue formation at low temperature vanished. Since the final step of cell wall lignification is an auto-polymerization (not metabolism), in essence, a polymerization of mono-lignols in the matrix of fully developed secondary walls (Chantreau and Tuominen 2022), it can even occur in dead cells (Pesquet et al. 2013). This makes sense, because lignification makes the cell wall impermeable for water. Lignified xylem conduits can be produced under constant 0 °C (Körner et al. 2019), growth conditions more severe than what any higher plant would ever experience within its growing season.
To test whether trees at treeline would produce tree rings that are less lignified when exposed to climatic conditions several hundred meters above the treeline, we applied cooling coats to stems, using Peltier technology to simulate a 3 K cooling below ambient of the meristematic region throughout a growing season (corresponding to a c. 500–600 shift in treeline elevation; Lenz et al. 2013; Körner et al. 2023a). We found no effect on lignification. In a response to this latter publication by Büntgen (Alpine Botany, August 7, 2023), these findings had been mistaken as supporting Büntgen’s idea that treelines are formed because of a constraint in lignification (Crivellaro et al. 2022).
The biology of tree ring formation at low temperature
The following three biological criteria underpin that this ‘alternative’ interpretation of the results published by Körner et al. (2023a) is neither matching the facts nor does it meet established knowledge about xylogenesis.
(1) Low temperatures may act on plants in three ways: (a) as a gradual driver of metabolism, including chronic limitations (rates of processes, including growth), (b) as a threshold for tissue survival (freezing tolerance), and (c) as a driver of season length (setting the time frame for development). The alternative interpretation of our data by Büntgen (2023) mixes up (b) with (a), and the assumption that our experimental site is not within the treeline ecotone, rests on references that neglect the significance of (c); see Büntgen et al. (2021, 2022). While it is a truism that long-lived trees at any given treeline will rarely be in equilibrium with the climate, we show that a 3 K season-long cooling clearly exceeds relevant climatic shifts.
(2) Treeline is a global phenomenon, not confined to the northern temperate zone or to conifers, as implied by several statements and citations by Büntgen (2023; comments on ‘tracheids’ therein). Treelines occur at the same isotherm of seasonal mean air temperature in Siberia, with freezing temperatures down to – 60 °C, and in regions without any freezing such as on Kinabalu in Borneo (Körner 2012). Hence, there is no association between tissue freezing tolerance and treeline position, as it does exist for tree species range limits below treeline (Körner et al. 2016). This does not preclude occasional tissue damage, as it can be observed in emerging shoot tips in Picea abies or Larix decidua in the Alps, or in developing treerings with no severe long-term drawbacks for these trees (Fig. 10.4 in Körner 2012; Rixen et al. 2012; Klisz et al. 2023), an insight, already Däniker (1923) arrived at. The life-form tree reaches a low-temperature limit at a common seasonal mean isotherm around the globe in areas with widely diverging freezing risk, including the humid tropics (for references, see Körner and Hoch 2023).
(3) The references in presumed support of the idea that lignification restricts tree growth at treeline in Büntgen (2023) altogether come from non-treeline sites (except for Körner et al. 2023a) and from point observations. The unsubstantiated statements from Crivellaro et al. (2022) are repeated, although these anatomical data are unsuitable to make such inferences (one sample per species), nor did that data set include any samples from trees even close to treeline, as was explained in Körner et al. (2023b). Crivellaro et al. (2022) took the greater fraction of herbaceous species at high alpine elevations as an indication for a failure of upright woody species due to lack of lignin, and thus, neglect the evolutionary selection of small herbaceous plant stature in cold climates for microclimatic and life history benefits. Herbs also occur in many other, low elevation environments.
The afore-mentioned continuous 3 K cooling of tree branches over the entire growing season at treeline (Körner et al. 2023a) revealed no effect on the lignification of differentiated cells in tree rings by the end of the season (blue rings in the sense of Piermattei et al. 2015; Klisz et al. 2023). Hence, a thermal elevation shift of the branch tissue to an elevation c. 500–600 m higher than the current tree limit, did not affect cell wall lignification (see the comment on treeline elevation below). Cells need to first complete the secondary cell wall, before lignification comes into action (Chantreau and Tuominen 2022; Shinshov et al. 2023). This is why, the failure of rings to lignify in otherwise fully differentiated cells, as occasionally observed, are all related to exceptionally cool summer and/or autumn climates, causing the tissue maturation to become incomplete (potentially enhanced by preceding dry summer conditions as for instance in Europe in 1976). In all these cases, the secondary cell wall thickening was completed or nearly completed, but the last step (Chantreau and Tuominen 2022), the lignification, failed. As was explained in Körner et al. (2023a), the most likely reason for this failure is the overall transition of trees into winter dormancy (dominant photoperiod and hormonal control), before all tissue-level processes had been completed, because of cool late-season weather. Hardening (preparedness for freezing) is likely to have priority over the developmental states of certain tissues at latitudes with a pronounced thermal seasonality.
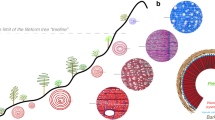
In our experiment (Körner et al. 2023a), two out of nine cooled branches showed a collapse of a few immature, thin-walled cell rows (newly produced, expanding cells with hardly any secondary cell wall) in response to a late June frost event that we documented. That frost was exaggerated by the experimental branch cooling (beyond past 30 yr experience). Control branches exposed to ambient temperatures remained undamaged. Killing undifferentiated cells (so-called frost rings, e.g. Klisz et al. 2023) is very different from delaying final maturation owing to exceptionally cool weather (Fig. 1). These damaged cells did not remain unlignified because of low temperature-constrained lignification, but simply because they were killed in a premature stage by the frost event. Under the pressure of surrounding tissue, such frozen, undifferentiated cells may collapse entirely later on, leaving only a distorted tissue zone among lignified cells (Rixen et al. 2012; Klisz et al. 2023). Such frost rings (commonly only fractions of a ring) are also well known from the literature (see the early account by Däniker 1923) and are not confined to treeline (Büntgen 2023). It appears that there is a stage during the early xylogenesis, during which cells are sensitive to late frost. For that reason, such damages are commonly not affecting an entire ring, but a few developing cell rows only. The cambium itself is not affected (commonly the most freezing tolerant tissue, see Sakai and Larcher 1987; Larcher 2003 p. 390), hence it keeps producing further cell rows that complete their developmental cycle after such a frost event, as was the case in our samples.
Stochastic freezing damage in a few very young cells (1b) must not be confused with a chronic effect of low temperature on the final stages of cell wall differentiation (1a). This distinction is important (Fig. 1), because plants commonly can cope with rare, partial tissue losses due to extreme events, while chronically low temperature constrains the entire organism’s development and growth in a given season. There is no evidence that such rare frost events do occur systematically at treelines around the globe, and they are not confined to treeline, and trees keep growing quite normally after such events.
Our study thus disclosed answers to two entirely different questions related to xylogenesis at treeline: (1) the absence of chronic effects of artificially lowered temperature on lignification, and (2) a high-time resolution insight into the rare occurrence of damages of very young expanding cells at the occasion of an exceptional (and experimentally boosted) late spring frost event. It was one of the tasks of our paper to explain these physiologically different processes and to offer experimental evidence for both. The point made by Büntgen et al. (2023) that evidence for a lignification-controlled treeline position cannot be found in the field, because, if trees were affected, they would die and thus, could not be studied, is neglecting the gradual nature of such effects, and the entire stress physiological literature that builds upon environmental gradients for distilling mechanisms of plant survival (drought gradients, temperature gradients). We are not aware of any publication that evidenced a systematic reduction of lignification as one approaches the tree limit. The likely reason is that there is no mechanism that would place lignification in an inferior (more limiting) position compared to the multitude of other (secondary) metabolic activities (Körner 2008; Körner et al. 2019).
Climatic treelines in a global warming context
It was questioned by Büntgen (2023) whether the treeline research site at Stillberg near Davos (where we performed our experiment) is a treeline site. At this experimental site, a grand afforestation trial was established in 1975 with uppermost trees planted at 2180 m, c. 30 m above the then regional climatic tree limit. Not surprisingly, many of the uppermost trees died and the ones that survived were still growing very slowly and often rather crooked, reaching 1.5 m height 30 years after planting (for references see Handa et al. 2005). Recent climatic warming has reduced these constraints. Since planting in 1975, the linear regression of growing season temperatures on Stillberg indicates a rise of 1.5 K by the year 2009, when we conducted our experiment (Rixen et al. 2012; the year 2003 representing an outlier in the chronology). By this regression, the treeline isotherm reached half of the elevation shift we simulated in our experiment (500–600 m).
Based on the analysis presented by Körner and Paulsen (2004) the Stillberg treeline operated at 7.3 °C seasonal mean temperature in 1997/98, which is very close to the 7.0 °C mean for 12 sites in the Alps during that period, and presumably includes already a 0.5–1.0 K climatic warming effect. Our own data for the experimental trees shown in Fig. 3 of Lenz et al. (2013), indicate that the seasonal mean temperature at 10 cm soil depth under complete shade was around 7 °C in 2009, the study year, again close to the global treeline isotherm, and clearly cooler than predicted by Büntgen (2023). The calculations by Büntgen et al. (2021 and 2022), rest on estimates of statistical models that regressed tree ring width from many N-hemisphere tree ring chronologies (mostly not from treeline) against concurrent air temperature from climate data-bases for selected summer months, not for a defined growing season (as we apply it in our standard protocol; see Körner and Paulsen 2004). In a space-for-time approach, shifts in treeline elevation had been estimated, referring to a potential versus actual limit of tree distribution, a valid and important differentiation (Körner 2021).
The treeline (the thermal limit for tree growth) is rarely identical to the actual position of the uppermost trees in a region (Körner and Hoch 2023). The global treeline isotherm temperatures as calculated by Körner and Paulsen (2004) and Paulsen and Körner (2014) from either on-site data (6.7 °C) or data bases (6.4 °C) respectively, do already account for the climatic warming that had occurred by the years for which these analyses had been performed. Irrespective of whether we use as a reference the 1.5 K warming trend by Rixen et al. (2012), the actual site data for 1997/1978 (7.3 °C), or an assumed equilibrium treeline temperature of 6 °C (as in the tropics), our cooling treatment was simulating an upslope shift in equilibrium treeline position by at least 1.5 K.
The models in Büntgen et al. (2022) also revealed that the responsiveness of tree ring width to temperature declines with rising temperature, confirming previous reports by Briffa et al. (1998) and Paulsen et al. (2000). The latter study clearly illustrated that ring-width near treeline becomes rather unresponsive to temperature, once a certain (warm) temperature threshold is surpassed, causing linear regressions across a wider range of temperatures to become doubtful.
Conclusion
In summary, we conclude that the re-interpretation of our data offered by Büntgen (2023) confuses stress physiology with growth physiology, it repeats (without data for treeline) already falsified links between low temperature and xylem lignification (Körner et al. 2019, 2023a, 2023b), it infers tree responses from the occurrences of herbs, (thus dismissing the functional difference among the life forms tree and herb). It is biased by N-hemisphere temperate and boreal tree ring data, overlooks the global nature of the treeline phenomenon that includes humid tropical treelines. Tropical treelines often experience chronically low temperature without a critical freezing risk as it is associated with a ‘spring-flush’ of growth in the temperate zone. Any experimental falsification of the so far evidenced absence of a specific low-temperature constraint of lignification would best adopt widely accepted sampling design criteria and analysis principles that were not met in Crivellaro et al. (2022) as was explained in Körner et al. (2023b). In our view, separating stress physiological (spring frost) from growth physiological (chronic) effects (Fig. 1) is essential for explaining the biology of such low temperature-related phenomena in tree rings.
Data availability
Not applicable in this opinion article.
References
Briffa KR, Schweingruber FH, Jones PD, Osborne TJ, Shiyatov SG, Vaganov EA (1998) Reduced sensitivity of recent tree growth to temperature at high northern latitudes. Nature 391:678–682
Büntgen U (2023) Experimental evidence for a thermal limitation of plant cell wall lignification at the alpine treeline. Alp Bot. https://doi.org/10.1007/s00035-023-00299-0
Büntgen et al (2021) The influence of decision making in tree ring-based climate reconstructions. Nature Comm 12:3411
Büntgen U, Piermatei A, Dolezal J, Crivellaro A (2023) Reply to: Biogeographic implications of plant stature and microclimate in cold regions. Comms Biol. 6:662. https://doi.org/10.1038/s42003-023-05033-4
Büntgen U, Piermattei A, Crivellaro A, Reinig F, Krusic PJ, Trnka M, Torbenson M, Esper J (2022) Common Era treeline fluctuations and their implications for climate reconstructions. Global Planet Change 219:103979
Chantreau M, Tuominen H (2022) Spatio-temporal regulation of lignification. Adv Biol Res 104:271–283. https://doi.org/10.1016/bs.abr.2022.03.006
Crivellaro A, Piermattei A, Dolezal J, Dupree P, Büntgen U (2022) Biogeographic implication of temperature-induced plant cell wall lignification. Comms Biol 5:767
Däniker A (1923) Biologische Studien über Baum- und Waldgrenze, insbesondere über die klimatischen Ursachen und deren Zusammenhänge. Vierteljahrsschrift Naturf Ges Zürich 68:1–102
Handa T, Körner C, Hättenschwiler S (2005) A test of the treeline carbon limitation hypothesis by in situ CO2 enrichment and defoliation. Ecology 86:1288–1300
Klisz M, Chakraborty D, Cvjetkovic B, Grabner M, Lintunen A, Mayer K, George JP, Rossi S (2023) Functional traits of boreal species and adaptation to local conditions. In: Girona MM, Morin H, Gauthier S, Bergeron Y (eds) Boreal forests in the face of climate change. Springer, Cham, pp 323–349. https://doi.org/10.1007/978-3-031-15988-6
Körner C (1998) A re-assessment of high elevation treeline positions and their explanation. Oecologia 115:445–459
Körner C (2008) Winter crop growth at low temperature may hold the answer for alpine treeline formation. Plant Ecol Divers 1:3–11
Körner C (2012) Alpine treelines. Springer, Basel
Körner C (2021) The cold range limit of trees. Trends Ecol Evol 36:979–989
Körner C, Hoch G (2023) Not every high latitude or high elevation forest edge is a treeline. J Biogeogr 50:838–845. https://doi.org/10.1111/jbi.14593
Körner C, Paulsen J (2004) A world-wide study of high altitude treeline temperatures. J Biogeogr 31:713–732
Körner C, Basler D, Hoch G, Kollas C, Lenz A, Randin CF, Vitasse Y, Zimmermann NE (2016) Where, why and how? Explaining the low temperature range limit of temperate tree species. J Ecol 104:1076–1088. https://doi.org/10.1111/1365-2745.12574
Körner C, Riedl S, Keplinger T, Richter A, Wiesenbauer J, Schweingruber F, Hiltbrunner E (2019) Life at 0 °C: the biology of the alpine snowbed plant Soldanella pusilla. Alp Bot 129:63–80. https://doi.org/10.1007/s00035-019-00220-8
Körner C, Lenz A, Hoch G (2023a) Chronic in situ tissue cooling does not reduce lignification at the Swiss treeline but enhances the risk of ‘blue’ frost rings. Alp Bot. https://doi.org/10.1007/s00035-023-00293-6
Körner C, Fajardo A, Hiltbrunner E (2023b) Biogeographic implications of plant stature and microclimate in cold regions. Comms Biol. 6:663. https://doi.org/10.1038/s42003-023-05032-5
Larcher W (2003) Physiological plant ecology, 4th edn. Springer, Berlin
Lee YP, Fleming AJ, Körner C, Meins F (2008) Differential expression of the CBF pathway and cell cycle related genes in Arabidopsis accessions in response to chronic low-temperature exposure. Plant Biol 11:273–283. https://doi.org/10.1111/j.1438-8677.2008.00122.x
Lenz A, Hoch G, Körner C (2013) Early season temperature controls cambial activity and total tree ring width at the alpine treeline. Plant Ecol Divers 6:365–375
Nagelmüller S, Hiltbrunner E, Körner C (2017) Low temperature limits for root growth in alpine species set by cell differentiation. AoB PLANTS 9:plx054. https://doi.org/10.1093/aobpla/plx054
Paulsen J, Weber UM, Körner C (2000) Tree growth near treeline: abrupt or gradual reduction with altitude? Arct Antarct Alp Res 32:14–20
Pesquet E, Zhang B, Gorzsas A, Puhakainen T, Serk H, Escamez S, Barbier O, Gerber L, Courtois-Moreau C, Alatalo E, Paulin L, Kangasjarvi J, Sundberg B, Goffner D, Tuominen H (2013) Noncell-autonomous postmortem lignification of tracheary elements in Zinnia elegans. Plant Cell 25:1314–1328
Piermattei A, Crivellaro A, Carrer M, Urbinati C (2015) The ‘blue ring’: anatomy and formation hypothesis of a new tree-ring anomaly in conifers. Trees Struct Funct 29:613–620
Rixen C, Dawes MA, Wipf S, Hagedorn F (2012) Evidence of enhanced freezing damage in treeline plants during six years of CO2 enrichment and soil warming. Oikos 121:1532–1543
Sakai A, Larcher W (1987) Frost survival of plants: responses and adaptation. Ecol Studies 62, Springer, Berlin
Schenker G, Lenz A, Körner C, Hoch G (2014) Physiological minimum temperatures for root growth in seven common European broad-leaved tree species. Tree Phys 34:302–313. https://doi.org/10.1093/treephys/tpu003
Shivanov VV, Arzac A, Popkova MI, Yang B, He M, Vaganov EA (2023) Experimental and theoretical analysis of tree ring growth in cold climates. In: Girona MM, Morin H, Gauthier S, Bergeron Y (eds) Boreal forests in the face of climate change. Springer, Cham, pp 295–321. https://doi.org/10.1007/978-3-031-15988-6_11
Acknowledgements
We gratefully acknowledge the help with and discussion of local climate data (the climate station is located 100 m below the experimental site) by the Stillberg-team at SLF Davos (P. Bebi, E. Frei, C. Rixen).
Funding
Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Contributions
This opinion article was written jointly by all three authors.
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Körner, C., Hiltbrunner, E. & Hoch, G. Experimental evidence, global patterns of treeline position and climate provide no substance for a lignin limitation hypothesis of tree growth. Alp Botany (2024). https://doi.org/10.1007/s00035-023-00305-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00035-023-00305-5