Abstract
Insect flight along river corridors is a fundamental process that facilitates sustainable succession and diversity of aquatic and terrestrial insect communities in highly dynamic fluvial environments. This study examines variations in the thickness of the insect boundary layer (i.e., the pre-surface atmosphere layer in which air velocity does not exceed the sustained speed of flying insects) caused by interactions between diurnal winds and the heterogenous habitat mosaics in the floodplain of a braided river. Based on advective–diffusive theory, we develop and test a semi-empirical model that relates vertical flux of flying insects to vertical profiles of diurnal winds. Our model suggests that, in the logarithmic layer of wind, the density of insect fluxes decreases exponentially with the altitude due to the strong physical forcing. Inside the insect boundary layer, the insect fluxes can increase with the altitude while the winds speed remains nearly constant. We suggest a hypothesis that there is a close correspondence between the height of discontinuity points in the insect profiles (e.g. points with abrupt changes of the insect flux) and the displacement heights of the wind profiles (e.g. points above which the wind profile is logarithmic). Vertical profiles were sampled during three time-intervals at three different habitat locations in the river corridor: a bare gravel bar, a gravel bar with shrubs, and an island with trees and shrubs. Insects and wind speed were sampled and measured simultaneously over each location at 1.5-m intervals up to approximately 17 m elevation. The results support our working hypothesis on close correspondence between discontinuity and displacement points. The thickness of the insect boundary layer matches the height of the discontinuity points and was about 5 m above the bare gravel bar and the gravel bar with shrubs. Above the island, the structure of the insect boundary layer was more complex and consisted of two discontinuity points, one at the mean height of the trees’ crowns (ca. 15 m), and a second, internal boundary layer at the top of the shrubs (ca. 5 m). Our findings improve the understanding of how vegetation can influence longitudinal and lateral dispersal patterns of flying insects in river corridors and floodplain systems. It also highlights the importance of preserving terrestrial habitat diversity in river floodplains as an important driver of both biotic and abiotic (i.e., morphology and airscape) heterogeneity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural river corridors are highly heterogeneous landscapes with dynamic mosaics consisting of groundwater and surface waters in still and flowing states, alluvial riverbeds shaped into bedforms, and patches of aquatic and riparian vegetation (Ward et al. 1999). Recent decades have seen increasing evidence that elements of such mosaics are interconnected not only longitudinally and laterally, but also vertically. These vertical linkages comprise fluxes of mass and energy that are driven by sedimentary, hydrological, atmospheric and ecological processes (Marston et al. 1995; Karaus et al. 2013). While most organisms living in river ecosystems are influenced by longitudinal and lateral transfer of mass and energy related to the hydrosphere, aquatic insects with an aerial adult stage and terrestrial living in the floodplain are perhaps just as strongly influenced by vertical fluxes in the air (Harrison 1980; Dijkstra et al. 2014; Ulyshen et al. 2010). Immature and adult insects play important ecological roles in riverine ecosystems, including in riparian and terrestrial areas. They are a food resource for fish, amphibians and terrestrial predators, and act as primary consumers, detritivores, predators, and pollinators (Batzer and Wissinger 1996; Dijkstra et al. 2014).
An important aspect in the study of riverine insect communities is to better understand how habitat heterogeneity and insects’ dispersal ability interact to drive community dynamics. For the aquatic insects, dispersal within and among river corridors has been a subject of study for many decades (Dijkstra et al. 2014). Species’ flight behavior is hypothesized to facilitate a bet-hedging strategy that allows offspring to overcome habitat deterioration (McIntyre and Wiens 1999; Holland et al. 2006). The paradigm of “adult replacement for larval displacement” assumes that transition between moving water and air, characteristic of insects with strongly terrestrial evolutionary roots (Freeland and Okamura 2001; Bohonak and Jenkins 2003; Dijkstra et al. 2014), helps to compensate the downstream drift of juveniles in water by adults flying upstream along the river corridor. For aquatic and terrestrial insects, a better understanding of their movement within the floodplain can be obtained by coupling the habitat heterogeneity across the scales with the insects’ dispersal potentials (Delettre and Morvan 2000; Petersen et al. 1999; Petersen et al. 2004; Paetzold et al. 2006; Holland et al. 2006; Dingle and Drake 2007; Didham et al. 2012). Knowledge of the movement of individual insects within heterogenous habitats would be helpful in estimating such dispersal potentials. In this paper we focus on flying insects that have strong dispersal ability (Garcia-Rios et al. 2022). These need to maintain appropriate directions, altitudes and ground speeds in order to realize their dispersal potential in these highly dynamic environments in which strong air flows can influence flight.
Magnitudes of air flows are small near the ground and grow exponentially with height. This provides only a narrow range of altitudes in which insects can maintain sustained controlled flights with ground speeds counteracting the magnitudes of moving air (Haine 1955; Burt and Pedgley 1997; Briers et al. 2003; Combes and Dudley 2009). The layer between a solid surface and the height at which ambient air velocities exceed the sustained speed of flying insects is called the insect boundary layer (Taylor 1974). The definition of this layer involves two components: species-specific biological abilities of insects to fly, and geophysical processes in the atmospheric boundary layer. Intensive research on individual insect active motion has been carried out in controlled laboratory setups (e.g., Naranjo 2019) in which only a very small proportion of insects were aquatic (mostly mosquitoes). Even though laboratory methods have become technically highly sophisticated, their results inevitably remain the surrogates of a real insects’ behavior in the field, particularly in complex and dynamic fluvial environments.
The airscapes along river corridors are linked tightly to the riverine landscapes, with their distinctive longitudinal elevation gradients (i.e., altitudinal differences between river sources and downstream sections). These differences cause daily gradients in temperature and pressure that drive local winds. When the gradients are strong, as in mountainous and piedmont river valleys, the winds reverse during their diurnal cycle and are affected by orography and stability (Atkinson 1995; Atkinson and Shahub 1994). Up-valley winds occur during daytime and reverse to down-valley winds at night (Zardi and Whiteman 2013). When the lower boundary layer is nearly neutral (e.g. vertical thermal convection is negligible), the wind is quasi-unidirectional with a logarithmic profile of mean wind speed that can be derived from the Prandtl’s mixing length theory (Prandl 1925). Under non-neutral conditions, which in river corridors can occur when the bare-gravel river floodplains are heated (Tonolla et al. 2010), the wind profile can be better described by Monin–Obukhov similarity theory which accounts for relative contributions from buoyant and shear productions (Obukhov 1971; Panofsky 1974). Although these semi-empirical theories provide a basis for micrometeorological experiments and measurements, the description must be modified to account for changes in the character of turbulence occurring close to a hydrodynamically rough surface (Finnigan et al 2020) such as an exposed riverbed with patches of riparian vegetation.
The last two decades have seen a spectacular expansion of research addressing effects of roughness in atmospheric and aquatic boundary layer flows over complex hydraulically rough topography (Weringa 1993; Nikora 2010; Finnigan et al. 2020), within and above the vegetation canopies (Nepf 2012). The roughness of surfaces in dynamically active river corridors is formed by the interplay among hydrological, morphological and ecological processes (Montgomery and Buffington 1997; Ward et al. 1999; Tockner et al. 2006; Karaus et al. 2013). These processes, including frequent inundation of the floodplain, and the active transport of sediments and its interactions with aquatic and riparian vegetation, maintain complex and dynamic riverine landscapes (Hubert and Huggenberger 2015). As a result, river floodplains evolve into highly heterogenous spatio-temporal mosaics composed of areas with bare alluvial deposits shaped into bedforms during floods (Fig. 1a), areas of exposed alluvial deposits with patches of pioneering riparian vegetation represented by shrubs with patchy accumulations of fine graded sediments (Fig. 1b), and islands which include patches of riparian forest with trees and shrubs (Fig. 1c). Even though the latest research is progressively focusing on smaller scales like flows over hills covered with tall plant canopies, these scales remain substantially larger than typical scales of fluvial bedforms (Finnigan et al. 2020). Furthermore, most research in vegetation canopy is focusing on a meadow type canopies rather than patchy mosaics, which remain mostly studied theoretically or in laboratory experiments (Poggi et al. 2004; Nepf 2012).
Observations of insect flights suggest that the insect boundary layer occupies the first few meters above the ground (Taylor 1960; Su and Wood 2001). Much less is known about the location of the layers upper edge, though physical reasoning suggests that its location is at the altitudes where vertical variation of turbulent fluxes of momentum is small and wind direction is nearly constant (Drake and Farrow 1988). Above this layer the wind speed increases exponentially and an insect entrained into the wind can be rapidly transported over large distances, possibly in the opposite directions to those preferable for active movements, even though studies suggest that insects can regulate the lift by interacting with air flow (Taylor 1974; Reynolds and Reynolds 2009). The elevation of the upper edge represents a physical threshold allowing for the differentiation of the areas of active and nearly passive modes of insect flight. Knowledge of this threshold is crucial for running simulations with agent-based models of insects dispersal that can be combined with Lagrangian stochastic models which represent turbulent transport in the atmosphere more realistically (Grübler et al. 2008; Reynolds and Reynolds 2009; Bell et al. 2013; Donkin et al. 2017; Leitch et al. 2021).
Based on analysis of vertical profiles of terrestrial insect density, Taylor (1974) suggested that the location of the upper edge of the insect boundary layer is where the insect concentration profile sharply changes the gradient – e.g. in other words the profile features a discontinuity point (DP). Above such point, the density of insects is strongly decreasing with altitude according to the expectation from increasing effect of wind. Johnson (1957) attributes the log-linear decrease in density to the fact that at higher altitudes the spread of passively flying insects follows physical laws of turbulent diffusion. However, to match the log-linear range the wind profile requires a downward displacement to fit the location of the DP (Johnson 1957; Taylor 1974). A similar empirical technique was widely used in engineering and meteorology to fit an empirical log-linear profile of flow velocity to the semi-theoretical logarithmic law by subtracting displacement height (DH), which is interpreted as an altitude of location of the total shear stress (Jackson 1981). Although the relation between these characteristic points in the insect density and wind profiles seems to have physical reasons and is not incidental, it has been not examined hitherto. This might be also due to the fact that in engineering practice the empirical fitting technique involving DH was largely replaced by methods that avoid discontinuity by combining the logarithmic law in the upper layer with a mixing layer in the roughness subrange (Finnigan 2000; Raupach et al. 1996; Nepf 2012).
In this study we investigated the interactions of flying insects with air flows in the natural setting of a heterogenous river corridor. We hypothesize that the displacement height of the logarithmic layer in the wind profile is correlated with the height of the discontinuity point in the vertical profiles of flying-insect density. We tested this hypothesis in the field with a sampling campaign during which density profiles of insects and profiles of mean and turbulent characteristics of wind were simultaneously measured. To assist the analysis of such profiles sampled over short periods, during which characteristics of diurnal winds were nearly constant, we developed a semi-empirical model based on the advective–diffusive theory. By allowing a direct comparison of sampled profiles with the theoretical predictions, this model substitutes the traditional statistical approach to analysis of data lacking replication due to methodological constrains.
Theoretical background
A two-dimensional diffusion equation in a boundary layer relates vertical flux of substance \(F_{w} = \overline{w^{\prime}c^{\prime}}\) to the vertical gradient of concentration (or insect density) as (Csanady 1973).
where \(c = \overline{c} + c^{\prime}\,\) is the concentration (the number of insects in a unit volume; the overbar denotes averaging and apostrophe refers to fluctuation about mean values), \(D_{T} = \kappa u_{*} z\) is the diffusion coefficient, \(u_{*}\) is shear velocity, \(\kappa = 0.41\) is the von Karman constant,\(w = \overline{w} + w^{\prime}\) is the vertical velocity component of air flow, and \(z\) is the vertical coordinate. In the boundary layer the vertical turbulent flux of momentum \(- \overline{u^{\prime}w^{\prime}} = u_{*}^{2}\) is constant and related to the vertical gradient of mean velocity as
where \(u\) is the horizontal velocity component of air flow. Integration of (2) yields the logarithmic profile for the horizontal velocity component (Jackson 1981).
where \(h\) is the distance from the ground or height, \(d\) is the displacement height, and \(z_{0}\) is the hydrodynamic roughness height, \(\Pi\) is the wake parameter (Coles 1956), and \(\delta_{w}\) is the thickness of the wake zone.
Integration of Eq. (1)-(2) yields the normalized vertical profile of concentration.
where \(\overline{c}_{0}\) is the concentration at the height \(h_{0}\), which corresponds to the reference concentration of the population near the ground (source concentration). Equation (4) is the semi-theoretical analog of the empirical profile proposed by Johnson (1957)
where \(f\left( h \right)\) is the concentration at the height \(h\),\(C\) is the scaling factor denoting the general size of the population, and \(\lambda\) is the regression coefficient. Although both Eqs. (4) and (5) predict log-linear decline of insect concentration, empirical evidence shows that, near the ground, the amount of insects is much less than expected from the linear trend. The point at which the sampled profile deviates from Eqs. (4, 5) is the discontinuity point, which can be offset in the modified height as \(h - \delta\), where \(\delta\) is the height of discontinuity point, which is interpreted as the height of insect boundary layer and in fact equals to \(h_{0}\).
Apparent similarity between Eq. (6) and (3) based on physical considerations supports our working hypothesis that displacement height can be equal to the height of the insect boundary layer \(d \approx \delta\). This has important implications because, if valid, the displacement height can be obtained without direct measurements of wind and insect profiles, using only topographical data and information about type, height and spatial distribution of the riparian vegetation.
Profiles of wind measured in forest canopies often contain a secondary velocity maximum \(u_{\max }\) at the region with a trunk space free of branches (Shaw 1977). Vertical restriction of air flow between tree crowns and shrubs at the level of a ground can produce a venturi-like effect responsible for the formation of momentum excess in such regions (Anderson 2017). Flows with a local excess of momentum are called jets (Pope 2000) and their velocity profiles are described by an exponential function (Dracos et al. 1992)
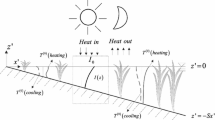
where \(z_{\max }\) is vertical coordinate (height) of velocity maximum, and \(b\) is half width of a jet. Excess of momentum and related increase in turbulence due to venturi jetting inside riparian forest canopy can also affect the insects in flight and produce a secondary discontinuity point in the concentration profile of insects. The link between the elements of floodplain mosaic (Fig. 1) and theoretical predictions based on the hypothesis \(d \approx \delta\) and Eqs. (1)-(7) is illustrated by a conceptual scheme shown in Fig. 2. This schematization is used in the analysis of empirical results obtained in our study.
Conceptual scheme of airscape structure over the floodplain mosaic and insects density profiles. a Wind speed and density profiles over gravel bars. b Wind speed and density profiles over a gravel bar with shrubs. c Wind speed and density profiles over the island with trees and shrubs (black lines = wind speed and red lines = insect density; DP = discontinuity point)
Methods
Sampling design
Our sampling protocols followed the recommendations of Johnson (1969) that: (1) vertical concentration profiles of insects should be measured simultaneously at different heights, and (2) such profiles should be sampled over short time periods. This would yield a profile that can be interpreted in terms of advection–diffusion Eq. (6). Some additional intuitive rules are also straightforward to anticipate. The sampling period should be as long as the period of quasi-steady wind conditions. In this case, the profile of wind and turbulence can be measured by anemometers sampling at several locations using point by point scheme. At each point, the wind speed vector should be measured long enough (7000 speed sample at 20 Hz sampling rate) to ensure statically stable values of mean and turbulent values of wind speed. Because wind and insect profiles depend on the local conditions, the sampling program also needs simultaneous replication of measurements at locations which are representative of different roughness elements and sources of insects (Johnson 1969). All these recommendations were considered and implemented in the sampling design of our study.
Study site and sampling locations
The field study was carried out on the floodplain of the Tagliamento River, near the village of Flagogna in northeast Italy (46°12′2’N 12°58′12’E). The Tagliamento River catchment is bounded by the Carnic and Julian Alps on the northwest and northeast, respectively. The hydrologic regime of the river is of a torrential character, which together with frequent landslides supplies ample quantities of coarse-grained bed load and supports a braided morphological channel in the central part of the catchment. This river system is considered to be the last morphologically intact river in the Alps (Lippert et al. 1995; Müller 1995) and therefore constitutes a model ecosystem for large temperate rivers (Tockner et al. 2003). The corridor of the river is morphologically intact along its entire length and has a total active corridor area of 61.7 km2. The bare gravel areas comprise about 63% of the active corridor area, islands occupy about 17%, and about 20% is covered by the river channel network (Tockner et al. 2003). At the base flow of about 20 m3s−1 at the study area, the active river corridor area is about 1.45 km2 of which bare gravel bed area constitutes 46%. Willows (Salix elaeagnos, S. alba, S. purpurea) and poplars (Populus nigra) up to 5.5 m tall, called shrubs hereafter, occupy about 30% of the total area. Islands with trees (Populus nigra, Alnus incana) about 18 m tall and ground covered with shrubs constitute another 10% of the total area, while the remaining 14% is occupied by the channel network. To replicate the measured profiles and to explore the effects of different elements of the floodplain mosaic, the measurements were simultaneously performed at three locations (Fig. 3a): (1) bare gravel bar (Fig. 3b), (2) gravel bar with shrubs (Fig. 3c), and (3) island with trees and shrubs (Fig. 3d).
Study site and sampling locations: a The view of the floodplain near Flagogna and sampling locations (flags show positions of the sampling towers; the river flows from right to left). b Sampling tower on the bare gravel bar (location 1, Fig. 3a). c Sampling tower on the gravel bar with shrubs (location 2, Fig. 3a). d Sampling tower on an island (location 3, Fig. 3a). e Instrumental setup of a sampling tower (item 1 is an insect trap, item 2 is an anemometer, item 3 is a temperature sensor, and item 4 is a carriage)
Instrumentation
The sampling of both insect and wind profiles was carried out using 20-m tall portable towers assembled on a basis of the Will-Burt Expedition Series Field Masts (Will-Burt/GEROH GmbH, Germany). Each tower section is made of carbon composite material. The tower has a carriage to hoist and position an anemometer and a temperature sensor (Fig. 3e). Each tower had a pair of pulleys at the top to hoist a cable with attached 10 custom-made insect traps spaced 1.5 m apart (Fig. 3e). Each trap was a hollow Polypropylene cylinder of 9 cm in diameter and 30 cm in length. The traps were covered with a transparent polythene film that was coated with the Oecotac insect trapping adhesive (Ryan and Molyneux 1981). Three-dimensional wind velocity vectors were measured with Gill Ultrasonic Anemometer Windmasters (Gill Instruments, Ltd., UK). The sensor is capable of measuring wind in the range from 0 to 50 ms−1 with an output rate of 20 or 32 Hz (Fig. 3e, item 2).
Airscapes during sampling
The airscape conditions over the floodplain are controlled by diurnal winds, which are maintained by temperature-induced pressure differences between the lowland littoral Adriatic part of the Tagliamento catchment and the Alpine upper catchments. In the morning, colder air masses move down the river valley and reverse during the day. At the study site the wind direction is highly controlled by orography of the river valley flanked by steep and high mountain ridges, which drive the wind in the pre-surface layer along the river corridor. During summer, heterogeneous heating of gravel, riparian vegetation and flowing water produce lateral temperature gradients of up to 20 °C (Tonolla et al 2010), which drive vertical convective cells that can locally dominate over the diurnal winds. To explore the simpler situation with the predominantly horizontal airscape conditions, the sampling campaign of this study was performed in October 2012. During this period, the mean air temperature was 16.4 ± 0.8 °C with variations during the day in the range of 5 °C, while between different landscape units the temperature differences were about ± 0.2 °C.
Sampling design
Sampling of insect, wind and temperature profiles were performed from 11.10.2012 until 12.10.2012 during three time-intervals. Each time interval was 2 h long and corresponded to a characteristic phase in diurnal winds: morning (10:30–12:30, 12.10.12), midday (14:00–16:00, 11.10.12), and evening (17:30–19:30, 11.10.12). During these intervals the wind speed and direction were relatively stable in the logarithmic layer. In the morning there was a down-valley wind of about 2 ms−1 in magnitude corresponding to a light breeze on the Beaufort Scale (BS), which changed to light air (BS) up-valley wind of about 1 ms−1 during the midday. In the evening the wind remained as a light air (BS) up-valley wind with the magnitude of about 0.5 ms−1. At each of the three sampling towers, sampling of insects was performed simultaneously at 10 points equally spaced from the ground up to about 17 m height with a span of 1.5 m. After sampling, the adhesive film was removed from each trap, stored and replaced by a fresh one. Profiles of wind velocity were measured simultaneously with the sampling of insects. These profiles were sampled as repeated point-by-point measurements at 12 points of which 10 were located at the same altitudes as the insect traps. One sampling point was located between ground and the first trap and another one above the last trap. The sampling period at each point was 5 min at the sampling rate of 20 Hz thereby yielding about 6 thousand samples of wind speed and temperature.
Post-processing
Insects were collected from the trap films with entomological forceps and then preserved in 70% ethanol for further identification in the laboratory. The taxonomic level of family was reached for most individuals. Exceptions were individuals of Hymenoptera and Psocoptera (assigned to order), Auchenorrhyncha (suborder) and Chironomidae (subspecies and species) (see taxa list in Appendix A). Insect catches in the profiles were normalized by trap surface area and sampling duration in order to represent the insect community metrics as a flux, i.e., count of insects flying through a unit area (m2) per unit of time (second). This provides estimates of the total amount of insects that transited at a given wind speed and altitude.
The post-processing of wind profiles included rotation of instant horizontal components of wind speed vector \(u = \overline{u} + u^{\prime}\) and \(v = \overline{v} + v^{\prime}\) measured at each point so that averaged cross-flow speed \(\overline{v}\) equals to zero, where \(u\) and \(v\) are the primary and cross-flow components respectively, the overbar denotes time-averaged values and the primes refer to turbulent fluctuations. Shear stresses components values in kinematic units were calculated as \(- \overline{u}^{\prime}_{i} \;u^{\prime}_{j} = {1 \mathord{\left/ {\vphantom {1 T}} \right. \kern-0pt} T}\int\limits_{o}^{T} {\left( {u_{i} \left( t \right) - \overline{u}_{i} } \right)} \left( {u_{j} \left( t \right) - \overline{u}_{j} } \right)dt\), where subscripts i, j = 1, 2, 3 refer to the components of wind speed vector and T is the period of averaging. Velocity and shear stress profiles were smoothed using a 3-point box-averaging filter in order to further reduce short-term variability.
Results
Insect communities
The sampling locations 2 (shrub-covered) and 3 (shrub-tree-covered) were characterized by a higher abundance of flying insects and higher taxa richness and diversity compared to location 1 with bare gravel (Fig. 4a, Appendix A). Only four taxa were common at all locations including aphids (Aphididae), were the most abundant taxa overall. Other than these four taxa, assemblages at location 3 shared no taxa with either location 2 or location 1. Location 1 had a larger proportion (66%) of larger insects (> 6 mm total body length) compared to the location 2 with shrubs (51%) and location 3 with trees and shrubs (59%) on the island (Fig. 4b). The mean size of all collected insects was 5.1 mm. Herbivorous insects, mostly aphids, were more abundant at locations 2 and 3. Saprophagous insects were mainly present at location 3, while coprophagous insects were abundant only at location 2 and 3 (Fig. 4c). Although the three locations were equally distant from the water, non-biting midges and sandflies represented about 10% of total catch in the trees locations compared to 20% in locations 1 and 2. Terrestrial insects (e.g. aphids and flies) represented the majority of the insects collected during sampling.
Profiles of wind speed, turbulence and insect fluxes
The sampling towers were located in the central part of the river corridor within a ca. 200 m wide transect and around 100 m distant from neighboring locations. The ratio between the width of the river corridor and the width of the transect was 4.5, which allowed the external boundary layer above the transect to develop as laterally uniform across the central part. This design consideration is confirmed by the measured vertical profiles of the wind speed which show that extrapolation of logarithmic law to the altitudes d + 20 m provides values of wind speed consistently close at those heights for all three locations (Figs. 5–7, mid panels). In contrast to the morning and midday observations (Figs. 5, 6), the profiles sampled in the evening additionally indicate a strong wake effect (Fig. 7, mid panels), which was decreasing the wind speed at location 1 (above the bare gravel; Fig. 7a, Table 1) but increasing the wind speed at location 2 (above shrub vegetation; Fig. 7b, Table 1). This shows that at small wind speed magnitudes the local cross-valley circulation from the sides of the valley had a strong effect on the external wind structure. For instance, the reduction of the wind speed at location 1 can be explained by the sheltering effect caused by trees on the island at the river left (Fig. 3a). At location 2, the increase in wind speed can be explained by the jetting of colder air masses from the tributary valley of the Arzino River. The measured wind profiles also indicate the altitudes at which the logarithmic layer is interfaced with the roughness sub-layer, allowing for the estimation of the values of displacement height d (Figs. 5–7, mid panels).
Vertical profiles of insect fluxes (left panels, DP is a discontinuity point, solid line is Eq. (6), and blue dashed line indicates the altitude of DP), wind speed (middle panels, solid line is Eq. (3) with \(\Pi = 0\)), and turbulent shear stresses (right panels, in kinematic units) sampled in the morning 10:30 12:30 (green colour is vegetation layer). a Location 1 on the bare gravel bar. b Location 2 on the gravel bar with shrubs. c Location 3 on the island with trees and shrubs (subscripts i and e refer to internal and external discontinuity points, the red line is Eq. (7))
Vertical profiles of insect fluxes (left panels, DP is a discontinuity point, solid line is Eq. (6), and blue dashed line indicates the altitude of DP), wind speed (middle panels, solid line is Eq. (3) with \(\Pi = 0\)), and turbulent shear stresses (right panels, in kinematic units) sampled in the midday 14:00–16:00 (green colour is vegetation layer). a Location 1 on the bare gravel bar. b Location 2 on the gravel bar with shrubs. c Location 3 on the island with trees and shrubs (subscripts i and e refer to internal and external discontinuity points, the red line is Eq. (7))
Vertical profiles of insect fluxes (left panels, DP is a discontinuity point, solid line is Eq. (6), and blue dashed line indicates the altitude of DP), wind speed (middle panels, solid line is Eq. (3), and turbulent shear stresses (right panels, in kinematic units) sampled in the evening 17:30–19:30(green colour is vegetation layer). a Location 1 on the bare gravel bar. b Location 2 on the gravel bar with shrubs. c Location 3 on the island with trees and shrubs (subscripts i and e refer to internal and external discontinuity points, the red line is Eq. (7))
Turbulent shear stress is related to the magnitudes of wind speed and to the roughness of the underlying surface. The measured vertical profiles of turbulent shear stress showed that its values in the external logarithmic layer were larger during periods with larger wind speed (Fig. 5, 6, right panels). When wind speed was reduced in the external layer, the local effects became stronger and the bed shear velocity was relatively large inside the roughness layer (Table 1). The upper edge of the roughness layer also coincided with the inflection points in the profiles of shear stress.
Profiles of insect fluxes clearly demonstrate the presence of primary and secondary discontinuity points (Fig. 5–7 left panels). Generally, profiles sampled at location 1 with bare gravel and at location 2 with gravel bars covered with shrubs indicated a single (primary) discontinuity point (Figs. 5a,b and 7a,b left panels); conversely, the profiles sampled at location 3 in the island with trees and shrubs had both primary and secondary points of discontinuity (Figs. 5c, 5c and 7a,c left panels). The altitude of the primary (external or upper) discontinuity point is close to the altitudes of the external airscape layer with the logarithmic wind speed profile (Table 1).
Vertical profiles of wind speed and insect fluxes (Figs. 5–7) provide the values for altitudes of both displacement heights and discontinuity points (Table 1). Their direct comparison indicates a strong correlation (Fig. 8) and thereby supports our working hypothesis.
The diurnal variation of the vertical structuring in small and large insects was substantial only in location 3 (Fig. 9). Larger insects tended to fly higher, at the crown level (Fig. 9c). At location 1 during the morning hours, most of the sampled insects were smaller than 6 mm. Aquatic and terrestrial insects differed in their preferred flight heights and effective flight layer widths (Fig. 10). Aquatic insects were found at lower elevations above gravel and shrubs units. Within the trees, the vertical distribution of the aquatic insects was bimodal, with maxima at 7.8 m and 16.8 m.
Discussion and conclusions
The primary motivation of our pilot study was to better understand the interactions of flying insects with air flows in natural settings. Current knowledge is limited due to a lack of documented patterns of flying insects coupled with the patterns of physical variables in the pre-surface atmosphere (Pasek 1988; Pedgley 1990; Ortega-Jimenez et al. 2013; Ravi et al. 2013; Vance et al. 2013). Although general guidelines for such coupling had been known for long, empirical research was hampered by serious methodological challenges in simultaneously sampling insect and wind profiles over short periods at quasi-steady airscape conditions (Johnson 1969).
We combined advective–diffusive theory with empirical field data to develop a semi-empirical framework and test the hypothesis that coupling can be achieved by identifying and inter-relating characteristic aspects of insect and wind profiles. These are the displacement heights in profiles of wind and discontinuity points in the vertical distributions of insect fluxes. These two are important because: (1) they separate the regions in the pre-surface atmosphere with stronger and weaker physical forcing on flying insects; and (2) they separate the regions affected by smaller-scale interactions of air flow with elements of the floodplain mosaic from regions influenced by larger-scale air dynamics at higher altitudes. We demonstrate that the displacement height of the logarithmic layer in the wind profile closely correlates with the height of the discontinuity point in the vertical profiles of flying insect concentration.
This finding has important practical implications because displacement height could be estimated directly from digital elevation models coupled with remote sensing surveys. The relationship between displacement height and the height of the discontinuity point will provide a way for quantitative prediction of the insect boundary layer thickness over topographically complex terrain (e.g. floodplains and riparian areas with heterogenous vegetation) without the need for routine sampling of insects. Huber and Huggenberger (2015, see their Fig. 7) reported the characteristics of gravel bars from the Tagliamento River in the same study area using a LiDAR-derived digital elevation model. From this model along the middle of the braided plain on the study area the elevation of bars ranges within 5 m with their length from 25 to 50 m. The grain size ranges from cobbles (256 mm) to pebbles (16 mm). Our study found a good correspondence between the displacement height of the outer logarithmic layer in the wind profiles and gravel bars – the characteristic roughness elements in the floodplain morphology (Figs. 5–8). For location 1, \(d\) closely matches the amplitude of the gravel bars estimated by Huber and Huggenberger (2015). The roughness sublayer is also matching \(d\), which is composed of internal boundary layers, recirculation zones and staked wakes separating above the bar crests (Fig. 2a). In location 2 the elevation of \(d\) corresponds to an average upper boundary of shrubs height. This roughness sublayer includes bleeding flow inside the shrubs vegetation (Fig. 2b).
In location 3 the profiles of flying insects indicate multiple discontinuity points while corresponding wind profiles feature a secondary maximum. We attribute multiple discontinuity points and secondary wind maximum to the complexity of the vertical structure of riparian vegetation. The riparian vegetation with trees and shrubs has a separation between their crowns and understory areas covered with shrubs (Fig. 3d). The porous media of the area between crowns and shrubs has a lower solid fraction and acts as a conduit for accelerated jetting flow. This effect also increases the local turbulence due to the shedding of turbulent vortices from the tree trunks (Fig. 2c). Such hydrodynamic conditions most likely explain the local reduction of insects in these areas. The average elevation of tree crowns matches with the elevation of displacement height of external logarithmic layer \(d_{e}\). The roughness sublayer corresponds to the external displacement height and includes an internal displacement point \(d_{i}\), which is a separated by jet-like venturi flow between shrubs and bleed flow at the trees crown.
In our study, the airscape over the floodplain can be characterized as a unidirectional quasi-uniform hydrodynamically rough, turbulent air flow. In the roughness sublayer the wind speed is significantly smaller than the characteristic wind speed in the external logarithmic layer. Insects, and in particular herbivorous aphids, may prefer vegetated locations as feeding sites as well as due to suitable micro-climatic conditions. Our results concord to previous studies reporting that vegetated habitats offer, besides food and shelter, a spot buffering against strong wind conditions (i.e., as windbreaker spot), collecting insects from less sheltered neighboring areas (Lewis 1969; Whitaker et al. 2000).
Wind speed and turbulence have different effects on the smaller and larger insects because the insect flight speed depends on their size. In this study we found substantial differences in flight heights of smaller and larger insects only at location 3 (tree-covered island). There, wind speed and turbulence were reduced to the largest extent and the layer where insect flight speed exceeded wind velocity was also the largest. The presence of tree vegetation at the flight heights likely enabled fast sheltering. In this landscape unit, where the dispersal of insects must be active, we found that the second mode in the insect vertical distribution (at the crown level) was formed mostly by insects larger than 6 mm. Flight characteristic of each taxon may have also be an important factor to determine the vertical distribution of insects; especially at the island location where a wider wind-sheltered vertical displacement was allowed. Small insects (e.g. aphids and small Nematocera) exploit areas close to the ground. Likely, this might reflect their limited flight ability. Furthermore, they can camouflage with the ground vegetation with higher chances of avoiding potential predators (e.g. spiders). On the other hand, larger insects, most of which represent important pollinators (e.g. tachina flies), exploit higher layer due to their better flight ability and to availability of flowers (e.g. black locust) on which they feed.
In this pilot study, with airscape conditions without strong thermal vertical convection due to inhomogeneous temperature distribution on the floodplain surface, the thickness of insect boundary layer is strongly depending on the local roughness. The characteristics of roughness (e.g. the heights of local protrusions) can be evaluated form the spatial topographical and vegetation surveys without complex simultaneous sampling of wind and insect profiles. However, interrelation between insect flight and complex spatiotemporal patterns of microclimatic conditions with strong vertical convection over braided river floodplains need further investigation. Better understanding of processes related to the flight and dispersal of winged insects in river valleys will provide better understanding of impacts of river regulation and land-use changes on biological and ecological processes along river corridors. Indeed, the adult phase of semi-terrestrial insects is a very vulnerable phase affecting the fitness of insect populations. Up to now, knowledge about insect behavior and mortality rates during the flying phase remains limited; even though it has major ramifications for conservation and restoration planning. Topography and roughness of rivers-floodplain system not only determine the width of a river corridor (Muehlbauer et al. 2014; Gurnell et al. 2016) but also defines its height (this pilot study). The present study provides first insights into a 3-D multi-spherical (e.g. hydro-, litho-, atmo-, and biosphere) perspective of riverine landscapes and may stimulate further airscape research.
Data availability
The data sets collected in this study are available from the corresponding author on a reasonable request.
References
Anderson J (2017) Fundamentals of Aerodynamics (6th ed.) New York, McGraw-Hill Education. P. 218.
Atkinson BW (1995) Orographic and stability effects on valley-side drainage flows. Bound-Layer Meteor 75:403–428
Atkinson BW, Shahub AN (1994) Orographic and stability effects on day-time, valley-slope flows. Bound-Layer Meteor 68:275–300
Batzer DP, Wissinger SA (1996) Ecology of insect communities in nontidal wetlands. Annu Rev Entomol 41:75–100
Bell JR, Aralimarad P, Lim K-S, Chapman JW (2013) Predicting insect migration density and speed in the daytime convective boundary layer. PLoS ONE 8:e54202
Bohonak AJ, Jenkins DG (2003) Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecol Lett 6:783–796
Briers RA, Cariss HM, Gee JHR (2003) Flight activity of adult stoneflies in relation to weather. Ecol Entomol 28:31–40
Burt PJA, Pedgley DE (1997) Nocturnal insect migration: effect of local winds. Advances in ecological research. Academic Press, USA, pp 61–92
Coles D (1956) The law of the wake in the turbulent boundary layer. J Fluid Mech 1:191–226
Combes SA, Dudley R (2009) Turbulence-driven instabilities limit insect flight performance. Proc Natl Acad Sci 106:9105–9108
Csanady GT (1973) Turbulent Diffusion in the Environment. 3, Reidel, Boston.
Delettre YR, Morvan N (2000) Dispersal of adult aquatic Chironomidae (Diptera) in agricultural landscapes. Freshwater Biol 44:399–411
Didham RK, Blakely TJ, Ewers RM, Hitchings TR, Ward JB, Winterbourn MJ (2012) Horizontal and vertical structuring in the dispersal of the adult aquatic insects in a fragmented landscape. Fundam Appl Limnol 180:27–40
Dijkstra K-DB, Monaghan MT, Pauls SU (2014) Freshwater biodiversity and aquatic insect diversification. Ann Rev Entom 59:143–163
Dingle H, Drake VA (2007) What is migration? Bioscience 57(2):113–121
Donkin E, Dennis P, Ustalakov A, Warren J, Clare A (2017) Replicating complex agent based models, a formidable task. Environ Model Softw 92:142–151
Dracos T, Giger M, Jirka GH (1992) Plane turbulent jets in a bounded fluvial layer. J Hydraul Res 54:263–274
Drake VA, Farrow RA (1988) The influence of atmospheric structure and motions on insect migration. Ann Rev Entomol 33:183–210
Finnigan J (2000) Turbulence in plant canopies. Annu Rev Fluid Mech 32:519–571
Finnigan J, Ayotte K, Harman I, Katul G, Oldroyd H, Patton E, Poggi D, Ross A, Taylor P (2020) Boundary-layer flow over complex topography. Bound-Layer Meteorol 177:247–313
Freeland JR, Okamura B (2001) Dispersal in freshwater invertebrates. Annu Rev Ecol Syst 32:159–181
Garcia-Rios R, Moi DA, Melo AS, Mormul RP (2022) Insect dispersal ability is crucial to overcome limitations in patch colonization of Eichhornia crassipes floating meadows. Limnology 23:287–289
Grübler MU, Morand M, Naef-Daenzer B (2008) A predictive model of the density of airborne insects in agricultural environments. Agric Ecosyst Environ 123:75–80
Gurnell AM, Bertoldi W, Tockner K, Wharton G, Zolezzi G (2016) How large is a river? conceptualizing river landscape signatures and envelopes in four dimensions. Water 3:313–325
Haine E (1955) Aphid take-off in controlled wind speeds. Nature 175:474–475
Harrison RG (1980) Dispersal polymorphism in insects. Annu Rev Ecol Syst 11:95–118
Holland RA, Wikelski M, Wilcove DS (2006) How and why do insects migrate? Science 313:794–796
Huber E, Huggenberger P (2015) Morphological perspective on the sedimentary characteristics of a coarse, braided reach: Tagliamento RIVER (NE Italy). Geomorphology 248:111–124
Huffaker CB, Gutierres AP (1999) Ecological entomology. Toronto John Wiley and Sons
Jackson PS (1981) On the displacement height in the logarithmic velocity profile. J Fluid Mech 111:15–25
Johnson CG (1957) The distribution of insects in the air and the empirical relation of density to height. J Anim Ecol 26:479–494
Johnson CG (1969) Migration and dispersal of insects by flight. Methuen, London
Karaus U, Larsen S, Guillong H, Tockner K (2013) The contribution of the lateral aquatic habitat to insect diversity along river corridors in the Alps. Landscape Ecol 28:1755–1767
Leitch KJ, Ponce FV, Dickson WB, van Breugel F, Dickinson MH (2021) The long-distance flight behavior of Drosophila supports an agent-based model for wind-assisted dispersal in insects. Proc Nat Acad Sci 118(17):e2013342118
Lewis T (1969) The distribution of flying insects near a low hedgerow. J Appl Ecol 6:443–452
Lippert W, Müller N, Rossel S, Schauer T, Vetter G (1995) Der Tagliamento – Flussmorphologie und Auenvegetation der grössen Wildflusslandschaft in den Alpen. Jahrb Ver Schutz Bergwelt 60:11–70
Marston R, Girel J, Pautou G, Piégay H, Bravard J, Arneson C (1995) Channel metamorphosis, floodplain disturbance, and vegetation development: Ain River, France. Geomorphology 13:121–131
McIntyre N, Wiens JA (1999) Interactions between landscape structure and animal behavior: the roles of heterogeneously distributed resources and food deprivation on movement patterns. Landscape Ecol 14:437–447
Montgomery DR, Buffington JM (1997) Channel-reach morphology in mountain drainage basins. Geol Soc Am Bull 109:596–611
Muehlbauer JD, Collins SF, Doyle MW, Tockner K (2014) How wide is a stream? spatial extent of the potential “stream signature” in terrestrial food webs using meta-analysis. Ecol 95:44–55
Müller N (1995) River dynamics and floodplain vegetation and their alteration due to human impacts. Arch Hydrobiol Supp 101:477–512
Naranjo SE (2019) Assessing insect flight behavior in the laboratory: a primer on flight mill methodology and what can be learned. Ann Entomol Soc Am 112:182–199
Nepf HM (2012) Hydrodynamics of vegetated channels. J Hydraul Res 50:262–279
Nikora V (2010) Hydrodynamics of aquatic ecosystems: an interface between ecology, biomechanics and environmental fluid mechanics. Riv Res Appl 26:367–384
Obukhov AM (1971) Turbulence in an atmosphere with a non-uniform temperature. Bound-Layer Meteorol 2:7–29
Ortega-Jimenez VM, Greeter SM, Mittal R, Hedrick TL (2013) Hawkmoth flight stability in turbulent vortex streets. J Exp Biol 216:4567–4579
Paetzold A, Bernet JF, Tockner K (2006) Consumer-specific responses to riverine subsidy pulses in a riparian arthropod assemblage. Freshwater Biol 51:1103–1115
Panofsky H (1974) The atmospheric boundary layer below 150 meters. Ann Rev Fluid Mech 6:147–177
Pasek JE (1988) Influence of wind and windbreaks on local dispersal of insects. Agric Ecosyst Environ 22(23):539–554
Pedgley DE (1990) Concentration of flying insects by the wind. Phil Trans R Soc Land B 328:631–653
Petersen I, Winterbottom JH, Orton S, Friberg N, Hildrew AG, Spiers DC, Gurney WSC (1999) Emergence and lateral dispersal of adult Plecoptera and Trichoptera from broadstone stream, U.K. Freshw Biol 42:401–416
Petersen I, Masters Z, Hildrew AG, Ormerod SJ (2004) Dispersal of adult aquatic insects in catchments of differing land use. J Appl Ecol 41:934–950
Poggi D, Porporato A, Ridolfi L, Albertson JD, Katul GG (2004) The effect of vegetation density on canopy sub-layer turbulence. Bound-Layer Meteor 111:565–587
Pope SB (2000) Turbulent flows. University Press, Cambridge, p 771
Prandtl L (1925) Bericht über Untersuchungen zur ausgebildeten Turbulenz". Z Angew Math Mech 5:136–139
Raupach MR, Finnigan JJ, Brunet Y (1996) Coherent eddies and turbulence in vegetation canopies: the mixing layer analogy. Bound-Layer Meteor 78:351–382
Ravi S, Crall JD, Fisher A, Combes S (2013) Rolling with the flow: bumblebees flying in unsteady wakes. J Exp Biol 216:4299–4309
Reynolds AM, Reynolds DR (2009) Aphid aerial density profiles are consistent with turbulent advection amplifying flight behaviors: abandoning the epithet “passive.” Proc Royal Soc b: Biol Sciences 276:137–143
Ryan L, Molyneux DH (1981) Non-setting adhesives for insect traps. Int J Trop Insect Sci 1:349–355
Shaw RH (1977) Secondary wind speed maxima inside plant canopies. J Appl Meteor 16:514–521
Su JC, Woods SA (2001) Importance of sampling along a vertical gradient to compare the insect fauna in managed forests. Environ Entomol 30:400–408
Taylor L (1960) The distribution of insects at low levels in the air. J Anim Ecol 29:45–63
Taylor LR (1974) Insect migration, flight periodicity and the boundary layer. J Anim Ecol 43:225–238
Tockner K, Ward JV, Arscott DB, Edwards PJ, Kollmann J, Gurnell AM, Petts GE, Maiolini B (2003) The Tagliamento river: a model ecosystem of European importance. Aquat Sci 65:239–253
Tockner K, Paetzold A, Karaus U, Claret C, Zettel J (2006) Ecology of Braided Rivers. In: Sambrook Smith GH, Best JL, Bristow CS, Petts GE (eds) Braided rivers: process, deposits, ecology and management. Blackwell Publishing Ltd., Oxford, UK, pp 339–359
Tonolla D, Acuña V, Uelinger U, Frank T, Tockner K (2010) Thermal heterogeneity in river floodplains. Ecosystems 13:727–740
Ulyshen MD, Horn S, Hanula JL (2010) Response of beetles (Coleoptera) at three heights to the experimental removal of an invasive shrub, Chinese privet (Ligustrum sinense), from floodplain forest. Biol Invasions 12:1573–1579
Vance JT, Faruque I, Humbert JS (2013) Kinematic strategies for mitigating gust perturbations in insects. Bioinspir Biomim 8:016004
Ward JV, Tockner K, Schiemer F (1999) Biodiversity of floodplain river ecosystems: ecotones and connectivity. Regul Rivers: Res Mgmt 15:125–139
Whitaker DM, Carroll AL, Montevecchi WA (2000) Elevated numbers of flying insects and insectivorous birds in riparian buffer strips. Can J Zool 78:740–747
Wieringa J (1993) Representative roughness parameters for homogeneous terrain. Bound-Layer Meteor 63:323–363
Winterbourn MJ, Chadderton WL, Entrekin SA, Tank JL, Harding JS (2007) Distribution and dispersal of adult stream insects in a heterogeneous montane environment. Arch Hydrobiol 168:127–135
Zardi D and Whiteman CD (2013) Diurnal mountain wind systems In: Fotini K. Chow, Stephan F.J. De Wekker, Bradley J. Snyder (Eds) Mountain weather research and forecasting. Springer, USA, pp. 35-119
Acknowledgements
Alessandro Manfrin and Oleksandra Shumilova were supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, grants 326210499/GRK2360 and SU 405/10, respectively). Tatiana Sukhodolova is thanked for a great contribution to organization of field studies and help with post-processing of the results. Claudio Cruciat and Alejandro Nardin are thanked for helping in field sampling campaigns. Viktor Baranov helped with identification of insects.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
A.S. and K.T. have designed the study. A.S. organized and participated field works and post-processed and analyzed the results. He also wrote and revised the manuscript in accordance with the suggestions of other authors. A.M: and S.L. participated in field works, performed post-processing of insects, wrote the parts of the manuscript and edited the drafts. O.S. assisted with plotting the results and editing of the manuscript. M.T.M. and K.T. provided valuable discussions of the results and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
Appendix 1 Taxa list for the identified samples
Taxa |
|---|
Acalyptrata |
Anthomidae |
Aphidae |
Aranea |
Auchenorrhyncha |
Bryophaenocladius sp. |
Cecidomyiidae |
Ceratopogonidae |
Chironominae |
Chrysididae |
Dolichopodidae |
Drosophilidae |
Emyptera |
Hymenoptera |
Lonchaeidae |
Miridae |
Muscidae |
Nematocera |
Orthocladinae |
Phoridae |
Pipunculidae |
Polypedium laetum |
Psocoptera |
Psychodidae |
Scatopsidae |
Sciaridae |
Simuliidae |
Staphylinidae |
Symphyta |
Tachinidae |
Tipulidae |
Tvetenia verralli |
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sukhodolov, A.N., Manfrin, A., Larsen, S. et al. Interactions between diurnal winds and floodplain mosaics control the insect boundary layer in a river corridor. Aquat Sci 85, 109 (2023). https://doi.org/10.1007/s00027-023-01002-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-01002-5