Abstract
Determining how streams develop naturally, particularly the ecological role of newly developed riparian canopy cover, is essential to understanding the factors that structure new stream communities and provides valuable information for restoring highly disturbed ecosystems. However, attempts to understand primary succession in riverine ecosystems have been hindered by a lack of data owing to the infrequent formation of new rivers on the landscape. In the present study, we used five streams formed following the 1980 eruption of Mount St. Helens (WA, USA) to examine the influence of canopy cover development on algal and benthic macroinvertebrate assemblages, biomass, and organic matter processing. Newly established closed canopy reaches had less available light, but no significant differences in algal biomass or macroinvertebrate assemblages compared to open canopy reaches. Instead, algal and macroinvertebrate communities were structured mainly by hydrologic differences among watersheds. In contrast, organic matter processing rates were sensitive to canopy cover development, and rates were faster under closed canopies, especially in late summer or after terrestrial preconditioning. After 40 years of stream and riparian primary successional development, canopy cover strongly influences ecosystem function, but aquatic organism assembly was more influenced by physio-chemical and hydrologic variation. Our findings provide insight into the development of in-stream assemblages and ecosystem functions, which is also relevant to efforts to address major disturbances to stream channels, such as volcanic eruptions, floods, forest fires, and clear-cut logging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the effects of primary succession in rivers and streams is essential for determining how new communities are structured, how streams develop through time (e.g., “evolutionary geomorphology”; Steiger and Corenblit 2012), and to predict restoration outcomes following severe disturbance (e.g., “stage zero” restoration; Wohl et al. 2021). However, efforts to do so are hampered by the infrequent formation of new rivers on the landscape. This information gap can be filled by studying new stream reaches formed following glacial retreat (Milner et al. 2008; Brown and Milner 2012) or new watersheds created following volcanic eruptions (Claeson et al. 2021). For example, the dynamics of stream primary succession have been informed by prior research in Glacier Bay, Alaska, USA (Sidle and Milner 1989; Flory and Milner 1999; Milner and Gloyne-Phillips 2005; Milner et al. 2011) and following volcanic eruptions in South America (Fuentes et al. 2020; Carrillo and Díaz-Villanueva 2021) and the Pacific Northwest (WA, USA; Claeson et al. 2021). As research continues at these primary succession locations, we have unanswered questions about how newly established riparian forests along streams may alter the developmental trajectories of in-stream communities.
The vegetative growth along streams and rivers, termed riparian vegetation, interacts in several key ways with in-stream communities and aquatic ecosystem processes. Riparian forests reduce sediment erosion, promote stream channel stability, provide allochthonous inputs of organic matter, and reduce light availability to streams and rivers (Flory and Milner 1999; Corenblit et al. 2009; D’Souza et al. 2011; Brooks et al. 2012). Light availability can structure in-stream communities, particularly periphytic algae, but also aquatic invertebrate and fish communities from headwaters to deltas (Vannote et al. 1980). By decreasing incident solar radiation, canopy cover can reduce water temperatures and decelerate both primary and secondary production (Feminella et al. 1989; Brooks et al. 2012; Wootton 2012; Wondzell et al. 2019).
Increased canopy cover can lead to increased organic matter inputs and processing rates, and changes to assemblages of organic matter-dependent organisms (Wallace et al. 1997; Flory and Milner 1999; England and Rosemond 2004). Leaf litter decomposition is mediated by detritivore activity (Graça 2001), and shaded stream reaches have led to altered leaf litter decomposition rates. Some studies demonstrate that decomposition rates can be slower in shaded reaches (Mckie and Malmqvist 2009; Lagrue et al. 2011), and other studies show decomposition rates can be faster in shaded reaches (Hladyz et al. 2010; Lecerf and Richardson 2010), but in each case decomposition rates were positively associated with increased shredder densities and/or biomass regardless of shading. Indirectly, canopy cover can also influence organic matter processing through altering microbial communities; for example, light exposure can enhance algal priming of organic matter processing by increasing algal, fungal, and bacterial production on leaf litter (Eckert et al. 2020; Pope et al. 2020). Algal priming accelerates litter decomposition in aquatic settings through primary production of labile carbon and stimulated heterotrophic microbial decomposer activity (Halvorson et al. 2019).
Riparian forests can also influence the preconditioning of organic material—microbial colonization of litter before entering the stream (Abelho and Descals 2019). Not all organic matter enters the stream directly as litter fall, some is preconditioned on the floodplain and moves laterally from upslope terrain in steep-sided valleys, under windy conditions, and in systems with overland flow (France 1995; Benfield 1997; Abelho 2001). Several studies have found that preconditioning in the riparian zone can alter organic matter processing rates, often slowing in-stream decomposition of material that has been exposed longer on land (Bruder et al. 2011; Riedl et al. 2013; Abelho and Descals 2019). On the other hand, sunlight can enhance terrestrial decomposition (via photodegradation mechanisms), but effects appear to vary depending on UV radiation type and degree, environmental and climatic conditions, and litter chemistry (Austin et al. 2016; Pieristè et al. 2020). Studies that have attempted to decouple the influences of terrestrial microbial preconditioning and photodegradation have thus far given mixed results (del Campo et al. 2021).
Riparian zones and streams are ecosystems that are prone to frequent and high-intensity disturbances that sometimes result in the removal of canopy cover. When canopy cover is removed through logging or debris flows, periphyton biomass and invertebrate primary consumers often increase, altering in-stream ecosystem processes (Zimmermann and Death 2002; Kiffney et al. 2003; Snyder and Johnson 2006; Foster et al. 2020). In some cases, like in volcanic landscapes, lahars and other large-scale disturbances can reset stream and river ecosystems to primary stages of succession. The majority of previous research on disturbances in streams has focused on secondary succession, with the role of primary successional riparian plants on stream ecosystem processes relatively understudied (Corenblit et al. 2009). One location where primary succession research has taken place is along chronosequences of glacier retreat in Glacier Bay (AK, USA) where initial growth of moss and the N-fixing forb, Dryas drummondii, is followed by alder, willow, and cottonwood colonization after about 50 years (Sidle and Milner 1989). One study on glacial retreat streams showed that riparian vegetation that extends into streams in the form of branches or roots (“trailing riparian habitat”) is an important habitat for a number of macroinvertebrate taxa, that bed stability influences macroinvertebrate assemblages, and that there was no significant recruitment of woody debris until after 130 years of stream development (Milner and Gloyne-Phillips 2005). Additionally, other studies in these glacial retreat streams found that leaf and root inputs to streams influenced aquatic macroinvertebrates as both habitat and food sources (Flory and Milner 1999). As new watersheds and stream channels develop following major disturbances, we have an opportunity to examine the influence of developing canopy cover in stream systems that otherwise have none, making comparisons more effective.
Several small, new watersheds were created on the Pumice Plain of Mount St. Helens (WA, USA) after the 1980 eruption. As part of the Mount St. Helens National Volcanic Monument, this area has been allowed to develop naturally, without major modification by humans influencing primary succession. Previous surveys of these new watersheds in 2016 provided us with a strong baseline for community assembly of riparian plants, algae, and macroinvertebrates (Claeson et al. 2021). These surveys provided the first evidence that assemblages of all three groups differed among streams and provided some insight into what might be driving primary succession assemblage patterns in stream systems. For example, we found that one watershed with high discharge, cold water, and low canopy cover hosted significantly different assemblages of riparian plants, periphytic algae, diatoms, and benthic macroinvertebrates than most other streams on the Pumice Plain. However, these initial surveys were somewhat confounded with respect to canopy cover as an environmental factor and prompted us to design a new study to isolate the canopy cover variable and address the question of how much influence riparian plant canopy cover can have in structuring aquatic communities and altering ecosystem processes in primary succession watersheds.
In this study, we hypothesized that developing canopies of alder and willow would have a strong influence on the physical microclimate and environmental conditions of stream reaches, contributing to differences in aquatic species assembly and ecosystem processes. To assess the influence of canopy cover on in-stream biota and function in a primary successional landscape, we compared algal and macroinvertebrate biomass and assemblages, as well as organic matter processing rates, between open and closed canopy stream reaches across five new streams on Mount St. Helens. We expected closed canopy reaches to reduce light availability to the stream and therefore reduce algal biomass, but to provide increased allochthonous inputs supporting more macroinvertebrate shredders and to have increased in-stream organic matter processing rates. We also evaluated the effects of terrestrial preconditioning and photodegradation on in-stream organic matter processing rates using open and closed canopy riparian terraces adjacent to each stream. We predicted that preconditioning under closed canopies would slow organic matter processing rates but that preconditioning in open canopies would accelerate processing rates. Our ecosystem also includes riparian tree herbivores that alter the timing and quality of leaf litter inputs (LeRoy et al. 2020a, b), so we made comparisons between summer and autumn organic matter processing rates to explore seasonal differences. We predicted that summer rates would be faster than autumn rates based on higher stream water temperatures and increased stream flows. Finally, we measured several stream and reach-scale hydrologic and physio-chemical characteristics to distinguish the effects of canopy cover from other environmental differences among streams.
Material and methods
Site description
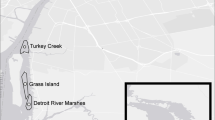
Mount St. Helens (MSH, Lawetlat'la in the Cowlitz language) has long served as a model for studying terrestrial plant primary succession since its catastrophic eruption in 1980 (del Moral 1999); however, there has been less emphasis on in-stream and riparian ecosystem succession in the blast zone (but see Claeson et al. 2021). The massive lateral eruption in 1980 transformed over 600 km2 of forested land and initiated a 2.9-km3 landslide down the north side of the mountain (Swanson and Major 2005). The debris avalanche and subsequent pyroclastic flows deposited a thick layer of pumice, ash, and rocky debris (> 100 m depth in places) in a 15-km2 area now known as the Pumice Plain (Meyer and Martinson 1989), which was entirely reformed geologically and where no vegetation survived. Five new watersheds developed on the Pumice Plain as precipitation fell, snow melted, and springs re-emerged from the new geological material (Fig. 1; N46.243403, W-122.165776). All streams draining those watersheds are undergoing primary succession and very little research has been undertaken to examine their development (but see Claeson et al. 2021). In the time since the eruption, the Pumice Plain has been slowly colonized by early successional plants and patchy riparian zones dominated by Sitka alder (Alnus viridis (Chaix) DC. ssp. sinuata (Regel) A. Löve & D. Löve) and Sitka willow (S. sitchensis; Titus and del Moral 1998; del Moral and Jones 2002).
We established ten study reaches across the Pumice Plain, with two reaches (1 open canopy and 1 closed canopy reach) per stream: Camp-East (Camp-E) Creek, Geothermal-West (Geo-W) Creek, Clear Creek, and two Willow Creek tributaries (Redrock and Forsyth Creeks, respectively) (Fig. 1). Stream water sources vary seasonally, with winter and spring flows fed by a combination of groundwater, snowmelt, and precipitation, while summer baseflows are primarily groundwater fed. Streams on the east side of the Pumice Plain (Forsyth, Redrock, Willow) have a glacial melt signature from the Forsyth Glacier on the northeast side of the mountain and are fed by established spring heads, likely emerging from shallow aquifers supported by higher-elevation ice melt (Claeson et al. 2021). Streams on the west side of the Pumice Plain receive inputs from multiple diffuse springs, geothermal sources, and snowmelt. From our previous surveys of these streams, we know that riparian vegetation is almost continuous in the depositional (lower) area of the watersheds and rare in the erosional (upper) areas, but that many physical and chemical attributes vary considerably between the erosional and depositional areas. Therefore, we selected study reaches in the transitional (middle) area to minimize physical differences between streams and to focus the study on canopy cover. On each stream we chose one open canopy reach (canopy cover < 30%) and the nearest closed canopy reach (> 80%). We measured reach-scale physical and chemical variables and sampled for periphytic algae and benthic macroinvertebrates between July and August 2018. We also measured organic matter processing in the summer (July–August) and autumn (September–October) of 2019.
Physio-chemical variables
At each open and closed canopy site, between 18 and 24 July 2018 we determined reach length as 20-fold the average wetted width or 20 m, whichever was longer (Fitzpatrick et al. 1998), and divided the length by 5 to create six transects where we measured bankfull widths and wetted widths. Substrate composition and the average sediment particle size (D50, in millimeters) were assessed by a modified pebble count of 100 particles randomly selected across each reach (Kaufmann et al. 1999). Slope (%) was measured with a clinometer at 1-m height between the upstream and downstream ends of each reach.
At each reach, water temperature (°C) was recorded hourly from 18 July to 30 September 2018 and again from 15 July to 4 October 2019, with HOBO Pro v2 temperature loggers (Onset, Bourne, MA, USA). However, two of the streams were surface dry by late August or September in 2018, and three streams were surface dry by mid-July or August 2019 which was a low water year for WA (USDA NRCS NWCC, Spirit Lake SNOTEL site #777). In addition, the 2018 Clear Creek open canopy logger was lost in a flood, limiting data for this site to 2019. Between 20 and 24 July 2018 (between 1000 and 1400 hours each day), instantaneous daytime measurements of temperature (°C), temperature-compensated pH (Ion 6+ pH/Ion Meter Kit; Oakton Instruments, Vernon Hills, IL, USA), specific conductance (COND, µS/cm), dissolved oxygen (DO, mg/L; Pro Plus multiparameter meter; YSI Incorporated, Yellow Springs, OH, USA), and colored dissolved organic matter (CDOM, ppb; Cyclops 7 turbidity sensor; Turner Designs, Sunnyvale, CA, USA) were averaged from near the bottom, middle, and top of each reach.
Stream discharge and water nutrients were measured in the open canopy reaches because of flow continuity between the open and closed canopy reaches on each stream. These variables were measured once to help evaluate among-stream differences and were not expected to differ between reaches within streams. Stream discharge (m3/s) was measured between 20 and 24 July 2018 using a Hach FH950.1 flow meter (Hach Co., Loveland, CO, USA). Water samples were collected on 11 July 2019 for dissolved organic carbon (DOC, mg/L), ammonia + ammonium (NH3–N + NH4+–N, mg/L), nitrate + nitrite (NO3−–N + NO2−–N, mg/L), and orthophosphate (PO43−–P, mg/L). Water samples were filtered in situ (Whatman GF/F filters, pore size: 0.7 μm; Whatman International Ltd, Maidstone, UK) and stored in the dark and frozen until laboratory analysis. Water samples were sent to the Oregon State University Cooperative Chemical Analytical Laboratory for the following analyses: DOC using a combustion-infrared method (American Public Health Association [APHA] Standard Method 5310 B); NH3–N + NH4+–N using both APHA Method 4500-NH3 G and US Environmental Protection Agency (EPA) Method 350.1; NO3−–N + NO2−–N using cadmium reduction methods APHA 4500-NO3 F and EPA 353.2; and orthophosphate using ascorbic acid methods APHA 4500-PF and EPA 365.1.
Riparian canopy cover and light availability
Canopy cover (%) was measured at three locations (upstream, middle, and downstream) for each reach, using average values from each cardinal direction at 1-m height with a spherical crown densiometer (Forestry Suppliers, Inc., Jackson, MS, USA). In addition, light availability (lux, lumens m2/day) at the surface of the channel was recorded every 15 min continually from 21 to 25 July 2018 with HOBO Pendant loggers (Onset) secured to large rocks, leveled, and placed in the middle of the stream at each densiometer location. For each logger, we averaged the mean daily light availability (lux) across sampling days.
To assess the growth of vegetation over several decades prior to our study at each of our study reach locations, we determined the Normalized Difference Vegetation Index (NDVI) for each year between 1984 and 2019 based on Landsats 5 and 8 Collection 1 surface reflectance datasets accessed through Google Earth Engine (Gorelick et al. 2017). Following methods described in Albano et al. (2020), we used the median NDVI value from high-quality, cloud-free images between 1 July and 30 September of each year; the window in which annual peak NDVI is expected to occur. Annual NDVI values for each reach were calculated as the average of three 30-m pixels that overlapped each open and closed reach on each stream. NDVI values < 0.1 correspond to barren rock and soil, values between 0.2 and 0.3 represent shrub and grassland, and values 0.6–0.8 represent rainforests (Weier and Herring 2000); therefore, we assumed values > 0.3 were representative of the development of riparian vegetation and data were analyzed as described below.
Periphytic algal biomass and community structure
At each reach between 18 and 24 July 2018, a composite periphytic algae sample was collected from five random substrates representing the dominant types present (cobbles or sand, following methods described in Claeson et al. 2021). Briefly, the area sampled on cobble was determined by measuring each rock along three axes and calculating the half surface area (Dall 1979). Sand was collected from the top 1-cm layer of substrate using a 6-cm-diameter petri dish with a surface area of 28.27-cm2 and a large plastic spatula. Algae were removed from rocks and sand by scrubbing with toothbrushes to create a pooled slurry. We measured the total volume of the slurry, then split it into the following aliquots: (1) 50 mL for quantitative analysis of soft-bodied algae; (2) 50 mL for quantitative analysis of diatoms (both aliquots 1 and 2 were preserved with 2.5% glutaraldehyde); and (3) 10–25 mL filtered for algal biomass, as measured using chlorophyll-a (Chl-a, mg/m2). The Chl-a aliquots were filtered onto pre-combusted (500 °C) and pre-weighed GF/C filters, then stored in the dark and frozen (− 80 °C) until spectrophotometric analysis following Steinman et al. (2017).
Algal identification and quantification were completed following the method of Stancheva et al. (2012), with modification of the biovolume formula to address the specifics of the sampling method (Claeson et al. 2021). This laboratory method for algae allows species-level identification of soft-bodied algae and individual biovolume estimates for each taxon (µm3/cm2; which was converted to mm3/m2 for ease of reporting and comparisons). The method was originally designed for soft-bodied algae, but for the purposes of this study, the living diatom cells with protoplasts were included as well. All diatoms with intact protoplasts observed in the sample were identified to the genus level and their biovolumes calculated.
Aquatic macroinvertebrate community structure
Benthic macroinvertebrates were collected between 18 and 24 July 2018 at each reach from eight 0.09-m2 Surber samples (500-μm mesh) from haphazardly chosen microsites while working from the bottom to the top of the reach. Subsamples were pooled on site (0.72-m2 total per reach) and preserved in 80% ethanol. In the laboratory, each sample was subsampled for a minimum of 600 individuals. Insects were identified to genus or species, except Chironomidae, which were identified to subfamily or tribe (Merritt et al. 2019). Non-insects were identified to class, except snails, which were identified to family. To estimate insect biomass (mg/m2), the body length of each insect was measured with a micrometer and converted using family-specific length to dry mass regressions (Benke et al. 1999). Subsample results were divided by the fraction subsampled to obtain full-sample estimates standardized to 1 m2. Aquatic macroinvertebrates were classified into functional feeding groups (FFG: gatherer, filterer, scraper, shredder, predator; Merritt et al. 2019). The sample proportion composed of each FFG was calculated, along with habitat degradation-sensitive taxa in the orders Ephemeroptera, Plecoptera, and Trichoptera (%EPT) and non-insects (%).
Organic matter processing
Organic matter processing was determined in 2019 for both summer (15 July to 14 August 2019) and autumn (5 September to 4 October 2019) using canvas strips as a proxy. Canvas strips have been used to measure organic matter processing in many different environments and under various circumstances with success (Tiegs et al. 2007, 2013, 2019) and especially when comparing the influence of environmental conditions on organic matter processing since they are a standardized material and result in less variation than leaf litter decomposition methods. In this system, two prevalent herbivores (Cryptorhynchus lapathi, Coleoptera, stem-boring willow weevil and Paranthrene robinae, Lepidoptera, western Poplar clearwing moth) cause premature leaf abscission and litter fall in the summer season (Che-Castaldo et al. 2015; LeRoy et al. 2020b). We assessed organic matter processing rates in both summer and autumn seasons to determine the relative potentials for organic matter processing at open and closed canopy sites in both seasons. We prepared 500 canvas strips according to the precise specifications outlined in Tiegs et al. (2013), assigning each a unique numbered metal tag. Canvas strips were transported to the field in individual envelopes and attached using cable ties to lengths of rope, which were then anchored to the streambed with rebar. On 15 July 2019, 200 canvas strips were installed across all five streams in the open and closed canopy reaches for two summer harvests (n = 10 strips/reach/harvest; SH1 = 15 days, SH2 = 30 days). Additionally, on 24 July 2019, 100 canvas strips were installed on the riparian forest floor for preconditioning across all five streams and along both open and closed canopy reaches at each stream (n = 5 strips/reach/harvest). On 5 September 2019, all 100 riparian strips were moved into their respective stream reaches and an additional 200 canvas strips were installed across all five streams in the open and closed canopy reaches for two fall harvests (n = 10 strips/reach/harvest; FH1 = 15 days, FH2 = 29 days). The riparian strips were preconditioned on land for 43 days prior to being harvested after 15 days (riparian RH1) and 29 days (riparian RH2) in each respective in-stream location. On harvest dates, each cable tie was cut to remove the strip from the rope, then each canvas strip was rinsed in 95% ethanol and placed into an individual coin envelope for transport. Envelopes and canvas strips were dried at 50 °C for 48 h. Dry canvas strips were analyzed for tensile strength (TS; measured in kilo-pound per square inch, ksi) on a tensiometer (Instron, Norwood MA, USA; Satec series, 150LX using 50kN maximum load calipers) and using Partner software version 9.4b (Illinois Tool Works, Inc., Glenview, IL, USA). Percent tensile strength loss per day (%TS loss/day) was calculated by dividing the tensile strength of each sample strip by the average tensile strength of eight control strips, subtracting from 1.0, multiplying by 100, then dividing by the number of days incubated. We made comparisons of percent tensile strength loss per day and per degree-day, but degree-days did not improve the models and were dropped from the analyses.
Statistical analyses
Differences between canopy types (open or closed reaches) and among streams (5 streams) were assessed by either analysis of variance (ANOVA) or linear mixed effects models performed with R v. 4.1.2 and the packages aov, lme4, multcomp, and ggplot2 (R Core Team 2021). The Shapiro-Wilks test was used to determine if each response variable needed to be ln-transformed, or arc-sine square root-transformed (% data) in order to meet the assumptions for normality and homoscedasticity. The algal and macroinvertebrate response variables were measured as a single sample from each open versus closed reach. Thus two-way permutational ANOVAs with no interaction were used to examine differences between canopy types and among streams. Tukey’s honestly significant difference (HSD) post hoc tests were used to compare differences among streams when ANOVA effects were significant.
The response variables bankfull width, % canopy cover, light, and %TS loss were tested with linear mixed effects models, with canopy type, stream, and their interaction as fixed effects, and within-reach replicates as random effects to control for pseudoreplication within reaches. ANOVAs were performed on the models to test for significance of fixed effects, producing an analysis of deviance table with Type II Wald's Chi-square (χ2) values (Canopy with 1 degree of freedom [df], Stream with 4 df, Canopy × Stream with 4 df). Specific contrasts with adjusted p-values were used to test for differences between open and closed canopies within each stream (not all pairwise comparisons). Differences in NDVI over time (1984–2019) were also tested with a linear mixed effects model, with canopy type, stream, and their interaction as fixed effects, and within-reach replicates nested within year (as a continuous variable) as random effects. Organic matter processing experiments in the summer and fall were tested separately as comparisons between season were not important to this study. We used an additional linear mixed effects model to test for differences in %TS loss by including conditioning (none or terrestrially preconditioned) as a fixed effect along with canopy type, stream, and their interactions, and within-reach replicates as a random effect.
Water temperatures at each reach were not statistically analyzed due to irregular stream drying and the loss of a temperature logger for 1 year, resulting in an unbalanced dataset. A few variables (discharge and water nutrients) were measured for use as co-variates at the stream level only and could not be statistically tested among streams or sites.
Periphytic algae and benthic macroinvertebrate assemblages were analyzed with non-metric multidimensional scaling (NMDS) ordinations using Bray–Curtis distance measures (PC-Ord v.7; MjM Software, Gleneden Beach, OR, USA). Permutational multivariate analyses of variance (PerMANOVAs) were used to test for community differences among canopy types and streams. The algal ordination was created using log10-transformed taxa biovolume (mm3/m2), and the macroinvertebrate ordination was created using log10-transformed taxa density (individuals/m2). Pearson correlations (r ≥ |0.5|) in ordination space were used to assess the influences of physio-chemical factors on community structure across the reaches and streams, with significant correlations shown by vector lines on ordination plots.
Results
Physio-chemical variables
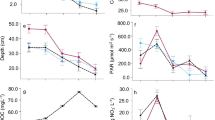
Field measurements of canopy cover (%) for each reach confirmed significantly more cover in the closed canopy reaches compared to open canopy reaches (Wald χ2(1) = 386.09, p < 0.0001) and that canopy cover was not different among streams (Wald χ2(4) = 6.22, p = 0.1835). As expected, in each stream, open canopy reaches experienced significantly greater light intensity (lux) at the stream surface compared to closed canopy reaches during mostly cloud-free days in late July 2018 (Fig. 2; Wald χ2(1) = 150.77, p < 0.0001). Light intensity was not significantly different among streams (Wald χ2(4) = 8.36, p = 0.0793) and was not influenced by an interaction between canopy × stream (Wald χ2(4) = 8.80, p = 0.0662).
Mean daily light intensity (lux) at open (white boxes) and closed (gray boxes) canopy locations at five different early successional streams on the Pumice Plain (Camp-E, Geo-W, Clear, Forsyth, and Redrock Creeks). Boxes represent medians ± quartiles. Asterisks denote significant differences (p < 0.001) between open and closed reaches within a stream. Light was recorded every 15-min from 21 to 26 July 2018
In general, Redrock and Forsyth Creeks were the coldest of the streams, with little diurnal variation. Water temperatures were moderately cool in Clear Creek, moderately warm in Camp-E Creek, and variably warm to very warm in Geo-W Creek (Table 1; Supplementary Information Fig. S1). Several of the stream sites went dry in early to mid-summer in 2019, resulting in fewer wet days for Camp-E, Redrock, and Forsyth Creeks in particular. Throughout the 2019 summer and fall canvas strip study periods, open canopy reaches were dry (i.e., no surface water) for a longer period of time (mean 47% of the days) compared to closed canopy reaches across all streams (mean 38% of the days). Bankfull width was greater in open than closed reaches in each stream (Wald χ2(4) = 188.25, p < 0.0001) and significantly narrower at Camp-E Creek compared to the other four streams (Wald χ2(4) = 299.35, p < 0.0001).
Riparian vegetation
Riparian vegetation cover through time, as measured by NDVI, was significantly influenced by stream (Wald χ2(4) = 459.54, p < 0.0001), canopy type (Wald χ2(1) = 3.92, p = 0.0478), and the stream × canopy interaction (Wald χ2(4) = 43.13, p < 0.0001). Overall, NDVI was highest at streams Clear and Geo-W, and lowest at streams Redrock and Camp-E. Contrasts of canopy type within each stream found significantly higher NDVI at closed, compared to open, canopy reaches along Forsyth and Redrock (p = 0.0011 and p < 0.0001, respectively), but higher NDVI at the open reach along Camp-E (p = 0.0001), and not significantly different along Clear or Geo-W (p = 0.64 and p = 0.62, respectively). Patterns across time (Fig. 3) showed that it was not until about 2005 that canopy cover values started rising quickly at some sites (Forsyth and Geo-W), and much later for others (2009 at Clear and Redrock, 2015 at Camp-E).
Normalized difference vegetation index (NDVI) across all study reaches between 1984 and 2019 based on remotely sensed data. Open (gray lines) and closed (black lines) canopy sites across five streams: Camp-E, Geo-W, Clear, Forsyth, and Redrock Creeks. The horizontal line at NDVI of 0.3 represents a general threshold between grasslands and shrubs (the potential start of riparian vegetation that could provide canopy cover). Mean NDVI values are plotted
Periphytic algae
Algal taxa richness was relatively low, ranging from a single species (Chamaesiphon minutus) in the Redrock-closed canopy reach to 28 taxa in the Camp-E-open canopy reach (Table 1), but was not significantly different between open and closed canopy reaches, nor among streams (Canopy: F(1,4) = 1.47, p = 0.3706; Stream: F(4,4) = 3.42, p = 0.0918). The total algal biovolume estimated per sampling reach ranged from 0.02 to 52.746 mm3/m2 and Chl-a ranged from 0.17 to 24.44 mg/m2. Total algal biovolume and Chl-a trended higher in the open canopy reaches compared to the closed canopy reaches within each stream (except in Geo-W); however, high variability within and among streams led to non-significant differences between open and closed canopy reaches (biovolume: F(1,4) = 2.02, p = 0.2362; Chl-a: F(1,4) = 3.61, p = 0.1677). Neither algal biovolume nor Chl-a varied significantly among streams (biovolume: F(4,4) = 7.26, p = 0.0466; Chl-a: F(4,4) = 3.39, p = 0.1131). The correlation between algal biovolume (as measured by microscope methods) and Chl-a was low (r = 0.24) due to the presence of nonvascular aquatic plants (predominantly mosses) in some samples that contributed to the Chl-a concentrations only.
Overall algal community composition was not significantly different between open and closed canopy reaches (F(1,8) = 0.46, p = 0.9286), but was different among streams (F(4,5) = 2.98, p = 0.0024). Diatoms were abundant in both Redrock and Forsyth Creeks (mostly the long filamentous colonies of Odontidium), and in Geo-W (Aulacoseira, Epithemia, Rhopalodia). N2-fixing heterocystous cyanobacteria and diatoms belonging to Epithemia and Rhopalodia were common in the three western streams, but not observed in Redrock or Forsyth Creeks, indicating potentially different nutrient conditions. The final NMS ordination of periphyton taxa biovolumes (Fig. 4a; stress = 0.0200) shows communities from Forsyth and Redrock to be opposite Camp-E along axis-1 (r2 = 0.42). Along axis-2 (r2 = 0.22), Redrock and Forsyth communities are opposite each other, as are Geo-W and Clear. Axis-3 (not shown, r2 = 0.17) shows a weak separation of the open and closed canopy reaches within Redrock and Forsyth, but no correlations with any physical habitat metrics. Algal communities from Forsyth and Redrock Creeks, both tributaries of the Willow Creek drainage, were significantly (p < 0.05) and positively correlated (r > |0.5|) with Chl-a, DO, and channel slope. The other three streams (Clear, Camp-E, and Geo-W) had algal communities significantly and positively correlated with water temperature, specific conductance, CDOM, and pH. Forsyth and Clear Creek communities were also significantly correlated with greater discharge, wetted stream width, and substrate size (D50).
Non-metric multidimensional scaling (NDMS) ordinations of periphytic algal communities by taxa biovolume (a) and of benthic macroinvertebrate communities by taxa density (b). Open (gray) and closed (black) canopy sites across five streams: Camp-E, Geo-W, Clear, Forsyth, and Redrock Creeks. Reach-level physio-chemical variables correlated with NMDS axes 1 or 2 (r > |0.5|) are overlaid on each plot as vectors that denote the correlation direction and strength. Chl.a chlorphyll a, CDOM chromophoric dissolved organic matter, D50 average sediment particle size (mm), DO dissolved oxygen,
Benthic macroinvertebrates
Macroinvertebrate community metrics differed among streams, however macroinvertebrate richness, density, biomass, and % shredders were not significantly different between open and closed canopy reaches (p > 0.05). Macroinvertebrate taxa richness ranged from 18 to 27 taxa (Table 1) and was significantly lower in Redrock and Forsyth Creeks compared to Camp-E and Geo-W Creeks (F(4,4) = 17.67, p = 0.0132). Camp-E and Geo-W, the warmest streams, also contained proportionately more non-insects (e.g., mites, oligochaetes, ostracods, and snails). Although Redrock had low algae and invertebrate taxa richness, this stream had the highest density of invertebrates due to an abundance of Chironomidae midges. In each of the stream reaches, except in Redrock, EPT density was typically ≥ 50% and EPT biomass was ≥ 75%. Invertebrate density and insect biomass were significantly lower in Clear than in the other streams, except for similar biomass at Geo-W (density: F(4,4) = 14.07, p = 0.0274; biomass F(4,4) = 11.08, p = 0.0258), but unresponsive to canopy cover (p > 0.05). Insect biomass, mainly large bodied Rhyacophila caddisflies, was highest at two open sites, Forsyth and Redrock. Functional feeding groups, including % shredder density or biomass (primarily Nemouridae and Capniidae stoneflies), were unresponsive to either canopy type or stream differences due to high variability (p > 0.05).
Overall macroinvertebrate community composition did not differ between open and closed canopy reaches (F(1,8) = 0.5811, p = 0.8372), but did differ among streams (F(4,5) = 3.23, p = 0.0006). The final NMS ordination of invertebrate taxa densities (Fig. 4b; stress = 0.0285) shows a cluster of communities from Forsyth and Redrock Creeks to be opposite Camp-E and Geo-W Creeks along axis-1 (r2 = 0.36) and separate from Clear along axis-2 (r2 = 0.35). Axis 3 (not shown, r2 = 0.18) shows a weak separation of Forsyth from Redrock but is not correlated with any physical habitat metrics. For Forsyth and Redrock, located in the same drainage, invertebrate communities were positively correlated with Chl-a, DO, wetted stream width, slope, and larger substrate (D50). Communities from Camp-E and Geo-W, neighboring streams on the western side of the Pumice Plain, were positively correlated with water temperature, specific conductance, CDOM, and pH. Clear Creek invertebrates were positively correlated with discharge.
Organic matter processing
Canvas strips were a sensitive measure of organic matter processing after 30 days in the streams; however, strips that were incubated for only 15 days did not vary among any treatments (p > 0.05) and, therefore, we only present the results from 30-day incubations. During the summer incubation experiment, tensile strength loss (%/day) was significantly greater in closed than open reaches for all streams, except Camp-E which went dry mid-summer (Wald χ2(1) = 118.88, p < 0.0001; Fig. 5a). In addition, canvas strips differed significantly among streams, with the highest tensile strength loss in Forsyth and lowest in Camp-E (Wald χ2(4) = 304.66, p < 0.0001), and there was a significant stream × canopy interaction (Wald χ2(4) = 80.11, p < 0.0001). In the autumn experiment, tensile strength loss was also greater in closed canopy reaches (Wald χ2(1) = 10.60, p = 0.0011), was highest in Geo-W and Redrock Creeks (Wald χ2(4) = 42.54, p < 0.0001), but showed no stream × canopy interaction (Wald χ2(4) = 4.98, p = 0.2889; Fig. 5b). The autumn incubation suffered from prolonged dry periods in the autumn season of 2019 which likely slowed organic matter processing.
Percent tensile strength loss per day (%TS loss/day) for canvas strips used as a proxy for organic matter processing rates in five early successional streams: Camp-E, Geo-W, Clear, Forsyth, and Redrock Creeks. Canvas strips were incubated in open (white boxes) and closed (gray boxes) reaches in the summer season (a), fall season (b), and terrestrially preconditioned strips in the fall season (c) across all five streams. Boxes represent medians ± quartiles. Asterisks denote significant differences (p < 0.001) between open and closed reaches within a stream
Canvas strips preconditioned on the riparian forest floor and exposed to either open canopy (high incident radiation) or closed canopy (low incident radiation) terrestrial conditions for 43 days, prior to being submerged in the stream for 30 days during the autumn incubation, lost more tensile strength than non-preconditioned strips (Wald χ2(1) = 33.90, p < 0.0001); canopy type and stream effects were also significant (canopy: Wald χ2(1) = 25.05, p < 0.0001; stream: Wald χ2(4) = 41.42, p < 0.0001). When examining just the pre-conditioned strips, after incubaton in the stream, tensile strength loss was still greater in closed than open canopy reaches (Wald χ2(1) = 21.69, p < 0.0001), was highest in Clear Creek (Wald χ2(4) = 47.34, p < 0.0001), and had a significant stream × canopy interaction (Wald χ2(4) = 88.28, p < 0.0001; Fig. 5c). In particular, post-hoc comparisons showed faster processing rates for canvas strips that were preconditioned under closed canopies compared to those preconditioned under open canopies within Camp-E, Geo-W, and Clear Creeks, but similar processing rates in Forsyth, and slower processing rates under closed canopies in Redrock.
Discussion
In these early successional streams on MSH, closed canopy conditions influenced a major ecosystem process, organic matter processing, and altered stream morphology, through reduced bankfull width. Although we saw a strong effect of canopy cover on light availability, it did not influence many physio-chemical variables nor significantly affect either algal or aquatic invertebrate assemblages or biomass. In contrast, the strongest drivers of algal and macroinvertebrate assemblages were environmental conditions, such as water temperature (St-Hilaire et al. 2000) and water chemistry (nitrate, specific conductance, CDOM, pH, DO), which vary across the five replicate streams and four watersheds on the Pumice Plain, likely based on differing hydrologic sources. These patterns confirm the results of a 2016 study that compared 21 sites across these same streams and found similar trends across watersheds, with few longitudinal changes moving downstream in any subsystem (Claeson et al. 2021). This earlier study did not control for canopy cover, and the present follow-up study was an attempt to isolate one important potential driver of in-stream succession.
Influences on in-stream community structure
Algal community structure in early successional streams at MSH was not significantly influenced by canopy cover. Algal diversity was relatively low across sites, and assemblages were composed mainly of early successional diatoms and cyanobacteria, which is similar to earlier findings (Rushforth et al. 1986; Steinman and Lamberti 1988; Claeson et al. 2021). However, the algal community structure differed significantly among streams, corroborating findings from 2016 surveys (Claeson et al. 2021). These differences in terms of algal assemblages could be due to differences in physical disturbance regimes across streams, which has been found to influence algal community structure (Robinson and Rushforth 1987). In addition, the algal community in one stream (Geo-W) is very scarce, and samples were dominated by fungal mycelium, significantly influencing the algal community at both the open and closed canopy sites. Large differences among streams in terms of water quality (particularly nitrogen [N]), substrate size, and hydrological variables more strongly influenced algal community structure than the hypothesized role of canopy cover. Although nitrate was not a significant correlate with the NMDS ordination, sites with higher nitrate concentrations tended to have no N-fixing algae, while sites with low nitrate concentrations tended to have higher abundances of N-fixing algae. Differences in nitrate concentrations may be due to different water sources for these streams, but further research is required.
Early successional macroinvertebrate assemblages were also not sensitive to canopy cover, or at least were not different in open versus nearby closed canopy reaches. This contrasts with other studies that have shown an influence of canopy cover on macroinvertebrate assemblages (Behmer and Hawkins 1986; Robinson and Minshall 1986; de Nadaï-Monoury et al. 2014). This result is somewhat unsurprising given that open and closed canopy reaches in the present study were in close proximity. It is possible that greater physical separation could have allowed for more development of different communities, but it could also have incorporated more confounding variables due to differences in other physical factors across the landscape. Macroinvertebrate communities in similar studies were also responsive to differences among watersheds in terms of flow intermittence, which can influence stream temperatures (Datry et al. 2014). Since reductions in riparian forest cover can weaken terrestrial-aquatic linkages (England and Rosemond 2004), it is possible that the developing, early successional riparian forest cover on the MSH Pumice Plain has not been established long enough to strongly influence aquatic macroinvertebrate assemblages. In addition, these riparian zones are narrower than many other temperate headwater stream ecosystems. The watersheds more closely resemble post glacial retreat or high desert ecosystems, where riparian zones are narrow, the transition to the surrounding landscape is dramatic, and uplands are sparse in vegetation (Milner and Gloyne-Phillips 2005).
Implications for riparian canopy cover development
Patterns in NDVI at each study reach varied both spatially and temporally. For example, riparian vegetation at all study sites was extremely limited until 2005 (25 years after the eruption). Some streams showed similar patterns at both the open and closed canopy reaches across time, with Geo-W and Forsyth reaching NDVI values of 0.3 (a threshold commonly used to indicate mesic and/or more well-developed vegetation) in 2004–2005 and Clear Creek reaching this threshold in 2009. In the cases of Clear and Geo-W, vegetation greenness signals detected by Landsat were showing similar trajectories in both open and closed canopy reaches, but canopy cover conditions characterized during field visits in 2018 indicated clear differences that were not detected with Landsat. At the other streams, the patterns in NDVI diverged strongly between open and closed canopy reaches. For example, both Redrock and Forsyth had more vegetation at closed canopy reaches starting in 1990 and continuing through 2018, while Camp-E Creek actually had more vegetation at our “open” sites from 1994 onward. It is possible that recent flooding or stream channel shifts caused vegetation death and canopy openings at Camp-E in 2018–2019. These NDVI data demonstrate that riparian vegetation development and dynamics differed among streams, but that Landsat data did not always provide good discrimination between closed and open canopy sites even for contemporary timescales. Landsat imagery is relatively coarse-resolution (30-m) and so has the potential to capture signals from un-vegetated areas outside the riparian corridor, which likely explains the lower NDVI values at Redrock and Camp-E reaches, which have relatively narrow riparian corridors. Moreover, while NDVI provides a good measure of vegetation biomass production, it cannot necessarily differentiate canopy cover from understory vegetation (Loranty et al. 2018). Despite this limitation, Landsat data captured vegetation trajectories over time, as well as variability among years, which may have been caused by climate variability and/or secondary disturbances, such as flood-driven avulsion events, destruction of riparian vegetation, or stranding of established vegetation over time (Swanson and Major 2005).
The two streams with the most developed canopies through time were Geo-W and Clear Creeks, and both of these streams have more fine-grained sediments (sand/silt and small gravel) than the other streams. It is possible that fine-scale differences in substrate type across the Pumice Plain might influence riparian vegetation establishment and persistence, or vice versa, as outlined from other watershed-scale comparisons that show faster riparian tree development for streams with smaller substrates and lower slopes to the wetted edge (Swanson et al. 1982). There is growing interest in exploring how plants influence stream channel dynamics and geomorphology, altering habitats and ecosystem conditions, but also potentially altering their own fitness landscapes (Steiger and Corenblit 2012). For example, at our study sites, bankfull width was narrower in closed canopy reaches and closed canopy reaches had surface water for more of the summer season, providing some evidence that streamside riparian plants may alter the geomorphology and hydrology of the streams themselves.
Influences on organic matter processing
Organic matter processing through a canvas strip proxy was a sensitive measure of ecosystem function in this study. Both direct and indirect effects of canopy cover influenced the loss of tensile strength of the canvas strips. Time spent on the riparian forest floor increased organic matter processing rates (these cotton strips showed more tensile strength loss). This is different from other studies that found increasing time leaves spent conditioning on the forest floor led to slower rates of leaf litter decomposition (Abelho and Descals 2019). It is somewhat difficult to compare the loss of tensile strength of cotton strips to leaf litter decomposition, and it may be that cotton strips are more sensitive to differing environmental conditions than leaf decomposition rates (Tiegs et al. 2007). However, it was not clear if either cotton strips incubated in open canopy reaches were positively influenced by algal priming (Halvorson et al. 2019) or that cotton strips preconditioned on the riparian forest floor at open canopy reaches were positively influenced by photo-degradation (Austin et al. 2016). All strips (preconditioned or not) tended to show more tensile strength loss in closed canopy reaches. These patterns provide further evidence that organic matter processing rates may be influenced by a variety of environmental conditions and create mosaics of complexity for detrital food webs (LeRoy 2019).
The use of canvas strips to measure organic matter processing across sites allows us to compare our rates of tensile strength loss per day to other studies and place organic matter processing on the Pumice Plain into a broader context. The range of average values of tensile strength loss/day across Pumice Plain streams (0.25–2.1% in summer; 0.1–0.95% in autumn) is similar to the range in averages seen in a pioneering paper across Michigan, the Midwest USA, and New Zealand (0.1–3.0%), but our average values were lower (0.93%/day in summer, 0.43%/day in autumn) compared to 1.7%/day in Tiegs et al. (2013). When converted to kD (decomposition rate/day), we can compare our rates of organic matter processing with a larger, global study (Tiegs et al. 2019). We find that our range of values (0.002–0.038 for summer, and 0.001–0.011 for autumn) are much lower than the values for cotton strips incubated at locations across the globe (0.04–0.5).
We suggest that the extreme conditions of the post-eruption landscape, such as intense solar radiation, abrasive young rock and ash, and large variation in water temperatures and water chemistries, might limit detritivore communities, which could slow overall decomposition rates for organic material across the Pumice Plain. Over time, as the streams on the Pumice Plain continue to develop, we may see increased capacities for organic matter processing as well as potential convergence of conditions and more similarity across sites. Prior to the eruption, this was a conifer-dominated second growth forest, and although conifers are establishing in the uplands, it will take many decades for closed canopy conifer forests to develop on the Pumice Plain. One of the only other locations where primary succession of riverine communities has been explored has shown that conifers like Sitka spruce and western hemlock may take over 100 years to establish (Milner and Gloyne-Phillips 2005).
Since organic matter processing was strongly associated with the presence of canopy cover, this makes other factors about the riparian forests at this site even more influential. For example, about half of the riparian cover across the Pumice Plain comprises Sitka willow, which is a dioecious plant. In willow-dominated riparian forests, female willows often grow significantly closer to the stream edge (Ueno and Seiwa 2003; LeRoy et al. 2020b), and in a one study, female leaves were found to decompose slower than male leaves (LeRoy et al. 2020b). Consequently, reaches with high canopy cover are likely also dominated by female willows, contributing female leaf litter as well as female catkins, which are larger, more complex, and support more invertebrates than female leaves (Garthwaite et al. 2021). In combination, these factors would serve to prolong the afterlives of fallen organic material and create closed canopy environments dominated by both female canopies and female organic matter inputs.
Conclusions
Our results support a strong influence of canopy cover on organic matter processing rates across the Pumice Plain as hypothesized, but canopy cover did not directly influence algae or benthic macroinvertebrate assemblages. As early successional streams develop post-eruption, the ecosystem function of organic matter processing was promoted by the presence of riparian vegetation. The lack of a strong influence of canopy cover on in-stream assemblages provides further evidence that the successional assembly of these young communities is driven more strongly by physical factors like hydrologic sources and water quality (Claeson et al. 2021). Understanding how the streams on the Pumice Plain develop over time is important to the basic understanding of in-stream primary succession and for testing hypotheses about the factors that structure communities and ecosystems. These results will inform our efforts to restore other lotic landscapes post disturbance, such as former reservoirs and downstream reaches following dam removals, landscapes exposed to wildfires, and streams recovering from landslides, debris flow events, or lahars.
Data availability
Data and code for statistical analysis is available at GitHub: https://github.com/carrileroy/canopy_cover_2018. GoogleEarthEngine code available at: https://code.earthengine.google.com/e9eb624d4299edb0b125331a9109b158
References
Abelho M (2001) From litterfall to breakdown in streams: a review. Sci World J 1:656–680. https://doi.org/10.1100/tsw.2001.103
Abelho M, Descals E (2019) Litter movement pathways across terrestrial–aquatic ecosystem boundaries affect litter colonization and decomposition in streams. Funct Ecol 33:1785–1797. https://doi.org/10.1111/1365-2435.13356
Albano CM, McGwire KC, Hausner MB et al (2020) Drought sensitivity and trends of riparian vegetation vigor in Nevada, USA (1985–2018). Remote Sens 12:1362. https://doi.org/10.3390/rs12091362
Austin AT, Méndez MS, Ballaré CL (2016) Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems. Proc Natl Acad Sci USA 113:4392–4397. https://doi.org/10.1073/pnas.1516157113
Behmer DJ, Hawkins CP (1986) Effects of overhead canopy on macroinvertebrate production in a Utah stream. Freshw Biol 16:287–300. https://doi.org/10.1111/j.1365-2427.1986.tb00972.x
Benfield EF (1997) Comparison of litterfall input to streams. J North Am Benthol Soc 16:104–108. https://doi.org/10.2307/1468242
Benke AC, Huryn AD, Smock LA, Wallace JB (1999) Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J North Am Benthol Soc 18:308–343. https://doi.org/10.2307/1468447
Brooks RT, Nislow KH, Lowe WH et al (2012) Forest succession and terrestrial–aquatic biodiversity in small forested watersheds: a review of principles, relationships and implications for management. Forestry 85:315–328. https://doi.org/10.1093/forestry/cps031
Brown LE, Milner AM (2012) Rapid loss of glacial ice reveals stream community assembly processes. Glob Change Biol 18:2195–2204. https://doi.org/10.1111/j.1365-2486.2012.02675.x
Bruder A, Chauvet E, Gessner MO (2011) Litter diversity, fungal decomposers and litter decomposition under simulated stream intermittency. Funct Ecol 25:1269–1277. https://doi.org/10.1111/j.1365-2435.2011.01903.x
Carrillo U, Díaz-Villanueva V (2021) Impacts of volcanic eruptions and early recovery in freshwater environments and organisms. Biol Rev 96:2546–2560. https://doi.org/10.1111/brv.12766
Che-Castaldo C, Crisafulli CM, Bishop JG, Fagan WF (2015) What causes female bias in the secondary sex ratios of the dioecious woody shrub Salix sitchensis colonizing a primary successional landscape? Am J Bot 102:1309–1322. https://doi.org/10.3732/ajb.1500143
Claeson SM, LeRoy CJ, Finn DS et al (2021) Variation in riparian and stream assemblages across the primary succession landscape of Mount St. Helens, U.S.A. Freshw Biol 66:1002–1017. https://doi.org/10.1111/fwb.13694
Corenblit D, Steiger J, Gurnell AM, Naiman RJ (2009) Plants intertwine fluvial landform dynamics with ecological succession and natural selection: a niche construction perspective for riparian systems. Glob Ecol Biogeogr 18:507–520. https://doi.org/10.1111/j.1466-8238.2009.00461.x
D’Souza LE, Reiter M, Six LJ, Bilby RE (2011) Response of vegetation, shade and stream temperature to debris torrents in two western Oregon watersheds. For Ecol Manag 261:2157–2167. https://doi.org/10.1016/j.foreco.2011.03.015
Dall PC (1979) A sampling technique for littoral stone dwelling organisms. Oikos 33:106–112. https://doi.org/10.2307/3544518
Datry T, Larned ST, Fritz KM et al (2014) Broad-scale patterns of invertebrate richness and community composition in temporary rivers: effects of flow intermittence. Ecography 37:94–104. https://doi.org/10.1111/j.1600-0587.2013.00287.x
de Nadaï-Monoury E, Gilbert F, Lecerf A (2014) Forest canopy cover determines invertebrate diversity and ecosystem process rates in depositional zones of headwater streams. Freshw Biol 59:1532–1545. https://doi.org/10.1111/fwb.12364
del Moral R (1999) Plant succession on pumice at Mount St. Helens, Washington. Am Midl Nat 141:101–114
del Moral R, Jones C (2002) Vegetation development on pumice at Mount St. Helens, USA. Plant Ecol 162:9–22. https://doi.org/10.1023/A:1020316503967
del Campo R, Martí E, Bastias E et al (2021) Floodplain preconditioning of leaf litter modulates the subsidy of terrestrial C and nutrients in fluvial ecosystems. Ecosystems 24:137–152. https://doi.org/10.1007/s10021-020-00508-5
Eckert RA, Halvorson HM, Kuehn KA, Lamp WO (2020) Macroinvertebrate community patterns in relation to leaf-associated periphyton under contrasting light and nutrient conditions in headwater streams. Freshw Biol 65:1270–1287. https://doi.org/10.1111/fwb.13473
England LE, Rosemond AD (2004) Small reductions in forest cover weaken terrestrial-aquatic linkages in headwater streams. Freshw Biol 49:721–734. https://doi.org/10.1111/j.1365-2427.2004.01219.x
Feminella JW, Power ME, Resh VH (1989) Periphyton responses to invertebrate grazing and riparian canopy in three northern California coastal streams. Freshw Biol 22:445–457. https://doi.org/10.1111/j.1365-2427.1989.tb01117.x
Fitzpatrick F, Waite I, D’Arconte P et al (1998) Revised methods for characterizing stream habitat in the NATIONAL Water Quality Assessment Program. U.S. Geological Survey, Denver, CO
Flory EA, Milner AM (1999) Influence of riparian vegetation on invertebrate assemblages in a recently formed stream in Glacier Bay National Park, Alaska. J North Am Benthol Soc 18:261–273. https://doi.org/10.2307/1468464
Foster AD, Claeson SM, Bisson PA, Heimburg J (2020) Aquatic and riparian ecosystem recovery from debris flows in two western Washington streams, USA. Ecol Evol 10:2749–2777. https://doi.org/10.1002/ece3.5919
France RL (1995) Empirically estimating the lateral transport of riparian leaf litter to lakes. Freshw Biol 34:495–499. https://doi.org/10.1111/j.1365-2427.1995.tb00907.x
Fuentes N, Goméz L, Venegas H, Rau JR (2020) Total devastation of river macroinvertebrates following a volcanic eruption in southern Chile. Ecosphere 11:e03105. https://doi.org/10.1002/ecs2.3105
Garthwaite IJ, Froedin-Morgensen A, Hartford SH et al (2021) Summer flower pulses: catkin litter processing in headwater streams. Fundam Appl Limnol 195:243–254. https://doi.org/10.1127/fal/2021/1384
Gorelick N, Hancher M, Dixon M et al (2017) Google earth engine: planetary-scale geospatial analysis for everyone. Remote Sens Environ 202:18–27. https://doi.org/10.1016/j.rse.2017.06.031
Graça MAS (2001) The role of invertebrates on leaf litter decomposition in streams—a review. Int Rev Hydrobiol 86:383–393. https://doi.org/10.1002/1522-2632(200107)86:4/5%3c383::AID-IROH383%3e3.0.CO;2-D
Halvorson HM, Barry JR, Lodato MB et al (2019) Periphytic algae decouple fungal activity from leaf litter decomposition via negative priming. Funct Ecol 33:188–201. https://doi.org/10.1111/1365-2435.13235
Hladyz S, Tiegs SD, Gessner MO et al (2010) Leaf-litter breakdown in pasture and deciduous woodland streams: a comparison among three European regions. Freshw Biol 55:1916–1929
Kaufmann PR, Levine P, Robison EG et al (1999) Quantifying Physical Habitat in Wadeable Streams. US Environmental Protection Agency, Washington DC
Kiffney PM, Richardson JS, Bull JP (2003) Responses of periphyton and insects to experimental manipulation of riparian buffer width along forest streams. J Appl Ecol 40:1060–1076. https://doi.org/10.1111/j.1365-2664.2003.00855.x
Lagrue C, Kominoski JS, Danger M et al (2011) Experimental shading alters leaf litter breakdown in streams of contrasting riparian canopy cover. Freshw Biol 56:2059–2069. https://doi.org/10.1111/j.1365-2427.2011.02637.x
Lecerf A, Richardson JS (2010) Litter decomposition can detect effects of high and moderate levels of forest disturbance on stream condition. For Ecol Manag 259:2433–2443. https://doi.org/10.1016/j.foreco.2010.03.022
LeRoy CJ (2019) Aquatic–terrestrial interactions: mosaics of intermittency, interconnectivity and temporality. Funct Ecol 33:1583–1585. https://doi.org/10.1111/1365-2435.13397
LeRoy CJ, Fischer D, Schweitzer JA, Bailey JK (2020a) Aphid gall interactions with forest tree genotypes influence leaf litter decomposition in streams. Forests 11:182. https://doi.org/10.3390/f11020182
LeRoy CJ, Ramstack Hobbs JM, Claeson SM et al (2020b) Plant sex influences aquatic–terrestrial interactions. Ecosphere 11:e02994. https://doi.org/10.1002/ecs2.2994
Loranty M, Davydov S, Kropp H et al (2018) Vegetation indices do not capture forest cover variation in upland Siberian larch forests. Remote Sens 10:1686. https://doi.org/10.3390/rs10111686
Mckie BG, Malmqvist B (2009) Assessing ecosystem functioning in streams affected by forest management: increased leaf decomposition occurs without changes to the composition of benthic assemblages. Freshw Biol 54:2086–2100. https://doi.org/10.1111/j.1365-2427.2008.02150.x
Merritt RW, Cummins KW, Berg MB (eds) (2019) An introduction to the aquatic insects of North America, 4th edn. Kendall Hunt Publishing, Dubuque
Meyer DF, Martinson HA (1989) Rates and processes of channel development and recovery following the 1980 eruption of Mount St. Helens, Washington. Hydrol Sci J 34:115–127. https://doi.org/10.1080/02626668909491318
Milner AM, Gloyne-Phillips IT (2005) The role of riparian vegetation and woody debris in the development of macroinvertebrate assemblages in streams. River Res Appl 21:403–420. https://doi.org/10.1002/rra.815
Milner AM, Robertson AL, Monaghan KA et al (2008) Colonization and development of an Alaskan stream community over 28 years. Front Ecol Environ 6:413–419. https://doi.org/10.1890/060149
Milner AM, Robertson AL, Brown LE et al (2011) Evolution of a stream ecosystem in recently deglaciated terrain. Ecology 92:1924–1935. https://doi.org/10.1890/10-2007.1
Pieristè M, Neimane S, Solanki T et al (2020) Ultraviolet radiation accelerates photodegradation under controlled conditions but slows the decomposition of senescent leaves from forest stands in southern Finland. Plant Physiol Biochem 146:42–54. https://doi.org/10.1016/j.plaphy.2019.11.005
Pope CA, Halvorson HM, Findlay RH et al (2020) Light and temperature mediate algal stimulation of heterotrophic activity on decomposing leaf litter. Freshw Biol 65:1210–1222. https://doi.org/10.1111/fwb.13465
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org. Accessed 21 Nov 2022
Riedl HL, Marczak LB, McLenaghan NA, Hoover TM (2013) The role of stranding and inundation on leaf litter decomposition in headwater streams. Riparian Ecol Conserv 1:3–10. https://doi.org/10.2478/remc-2013-0002
Robinson CT, Minshall GW (1986) Effects of disturbance frequency on stream benthic community structure in relation to canopy cover and season. J North Am Benthol Soc 5:237–248. https://doi.org/10.2307/1467711
Robinson CT, Rushforth SR (1987) Effects of physical disturbance and canopy cover on attached diatom community structure in an Idaho stream. Hydrobiologia 154:49–59. https://doi.org/10.1007/BF00026830
Rushforth SR, Squires LE, Cushing CE (1986) Algal communities of springs and streams in the Mt. St. Helens region, Washington, USA following the May 1980 eruption. J Phycol 22:129–137. https://doi.org/10.1111/j.1529-8817.1986.tb04155.x
Sidle RC, Milner AM (1989) Stream development in Glacier Bay National Park, Alaska, USA. Arct Alp Res 21:350–363. https://doi.org/10.1080/00040851.1989.12002749
Snyder CD, Johnson ZB (2006) Macroinvertebrate assemblage recovery following a catastrophic flood and debris flows in an Appalachian mountain stream. J North Am Benthol Soc 25:825–840. https://doi.org/10.1899/0887-3593(2006)025[0825:MARFAC]2.0.CO;2
Stancheva R, Fetscher AE, Sheath RG (2012) A novel quantification method for stream-inhabiting, non-diatom benthic algae, and its application in bioassessment. Hydrobiologia 684:225–239. https://doi.org/10.1007/s10750-011-0986-8
Steiger J, Corenblit D (2012) The emergence of an 'evolutionary geomorphology’? Cent Eur J Geosci 4:376–382. https://doi.org/10.2478/s13533-011-0075-6
Steinman AD, Lamberti GA (1988) Lotic algal communities in the Mt. St. Helens region six years following the eruption. J Phycol 24:482–489. https://doi.org/10.1111/j.1529-8817.1988.tb04251.x
Steinman AD, Lamberti GA, Leavitt PR, Uzarski DG (2017) Biomass and pigments of benthic algae. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology, 3rd edn. Academic Press, Boston, pp 223–241
St-Hilaire A, Morin G, El-Jabi N, Caissie D (2000) Water temperature modelling in a small forested stream: implication of forest canopy and soil temperature. Can J Civ Eng 27:1095–1108. https://doi.org/10.1139/l00-021
Swanson FJ, Major JJ (2005) Physical events, environments, and geological—ecological interactions at Mount St. Helens: March 1980–2004. In: Dale VH, Swanson FJ, Crisafulli CM (eds) Ecological responses to the 1980 eruption of Mount St. Helens. Springer, New York, pp 27–144
Swanson F, Gregory S, Sedell J, Campbell A (1982) Land-water interactions: The riparian zone. In: Edmonds R (ed) Analysis of coniferous forest ecosystems in the western United States. Hutchinson Ross Publishing Co., Stroudsburg, pp 267–291
Tiegs SD, Langhans SD, Tockner K, Gessner MO (2007) Cotton strips as a leaf surrogate to measure decomposition in river floodplain habitats. J North Am Benthol Soc 26:70–77. https://doi.org/10.1899/0887-3593(2007)26[70:CSAALS]2.0.CO;2
Tiegs SD, Clapcott JE, Griffiths NA, Boulton AJ (2013) A standardized cotton-strip assay for measuring organic-matter decomposition in streams. Ecol Indic 32:131–139. https://doi.org/10.1016/j.ecolind.2013.03.013
Tiegs SD, Costello DM, Isken MW et al (2019) Global patterns and drivers of ecosystem functioning in rivers and riparian zones. Sci Adv 5:eaav0486. https://doi.org/10.1126/sciadv.aav0486
Titus JH, del Moral R (1998) The role of mycorrhizal fungi and microsites in primary succession on Mount St. Helens Am J Bot 85:370–375. https://doi.org/10.2307/2446330
Ueno N, Seiwa K (2003) Gender-specific shoot structure and functions in relation to habitat conditions in a dioecious tree, Salix sachalinensis. J For Res 8:0009–0016. https://doi.org/10.1007/s103100300001
Vannote RL, Minshall GW, Cummins KW et al (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137. https://doi.org/10.1139/f80-017
Wallace JB, Eggert SL, Meyer JL, Webster JR (1997) Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277:102–104. https://doi.org/10.1126/science.277.5322.102
Weier J, Herring D (2000) Measuring vegetation (NDVI & EVI). https://earthobservatory.nasa.gov/features/MeasuringVegetation. Accessed 20 Jul 2022
Wohl E, Castro J, Cluer B et al (2021) Rediscovering, reevaluating, and restoring lost river-wetland corridors. Front Earth Sci. https://doi.org/10.3389/feart.2021.653623
Wondzell SM, Diabat M, Haggerty R (2019) What matters most: Are future stream temperatures more sensitive to changing air temperatures, discharge, or riparian vegetation? J Am Water Resour Assoc 55:116–132. https://doi.org/10.1111/1752-1688.12707
Wootton JT (2012) River food web response to large-scale riparian zone manipulations. PLoS ONE 7:e51839. https://doi.org/10.1371/journal.pone.0051839
Zimmermann EM, Death RG (2002) Effect of substrate stability and canopy cover on stream invertebrate communities. NZ J Mar Freshw Res 36:537–545. https://doi.org/10.1080/00288330.2002.9517109
Acknowledgements
Support for this study was provided by the National Science Foundation, through grant DEB #1836387 to CJL with a supplemental ROA grant to DSF and CJL. The Pacific Northwest Research Station of the USDA Forest Service provided in-kind support to SMC and the project as a whole. We thank Philip Dodge, Mechanical Engineering Department Lab Tech at Olympic College-Washington State University in Bremerton, WA for collaborating with IJG and CJL and providing assistance with tensile strength measurements. We thank The Evergreen State College’s Summer Undergraduate Research Fellows (SURF) program for funding for undergraduates IJG and AMFM who collaborated on field and laboratory work. A large team of Evergreen undergraduates helped with field surveys: AMFM, IJG, BKK, VM, Jordan Moffett, Lauren Walker, Lily Messinger, and Maya Nabipoor. Evergreen undergraduates also helped with canvas strip deployments and analysis: IJG, BKK, MAT, LJT, VM, and Jo Feldman. Mount St. Helens Institute student scientists helped with fieldwork during the summer High School Ecology Program and Upward Bound Program. Staff at Evergreen’s Science Support Center provided field gear and lab assistance. We thank two anonymous reviewers and the editor for their thoughtful comments that improved this paper.
Funding
This research was supported by the National Science Foundation, through grant DEB #1836387.
Author information
Authors and Affiliations
Contributions
SMC, JMRH, and CJL collaborated closely on all aspects of this research project. CJL and JMRH coordinated student collaborators. CJL organized field trips, collected data, and took the lead on writing the manuscript. SMC, JMRH, and CJL collected data, quality-checked data, and analyzed data. MAT and LJT wrote an initial draft of the abstract, helped with lab analysis, and helped write early sections of the paper. IJG took the lead on canvas strip tensile strength analysis. IJG, BKK, AMFM, and VM made major contributions in the field, collected data, collaborated on lab analysis, and/or contributed to the manuscript. CMA completed NDVI analyses and wrote sections of the manuscript. RS identified species from algal samples and wrote sections of the manuscript. DSF collaborated on fieldwork and wrote sections of the manuscript. All authors edited multiple drafts of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
LeRoy, C.J., Claeson, S.M., Garthwaite, I.J. et al. Canopy development influences early successional stream ecosystem function but not biotic assemblages. Aquat Sci 85, 77 (2023). https://doi.org/10.1007/s00027-023-00972-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-00972-w